Abstract
The standard treatment guideline for stage IIIA/N2 non-small cell lung cancer (NSCLC) remains controversial despite years of research, and the necessity of surgery is still debated. This study aims to explore optimal treatment and surgical methods for stage IIIA/N2 NSCLC patients.
We obtained data from the Taiwan Cancer Registry (TCR) to compare the overall survival rates of different subgroups of stage IIIA/N2 NSCLC patients, as well as the overall survival rates of different treatment strategies and surgical methods.
Our study included 2,237 stage IIIA/N2 NSCLC patients. Neoadjuvant chemotherapy followed by surgery and adjuvant chemotherapy led to significantly higher survival rates. For T1N2 patients, surgery alone showed better survival (P < .001). Neoadjuvant chemotherapy with surgery and adjuvant chemotherapy provided higher survival for T2N2 and T3N2 patients (P < .001). Among surgical types, lobectomy had the lowest mortality rate, which was statistically significant.
In conclusion, our study recommends neoadjuvant therapy followed by surgery and adjuvant therapy for improved survival in stage IIIA/N2 NSCLC patients. Surgery should not be excluded for resectable stage IIIA/N2 NSCLC patients, with lobectomy being the preferred option due to its significantly higher survival rate.
Similar content being viewed by others
Introduction
In 2020 lung cancer was the second most diagnosed cancer and the leading cause of cancer mortality, accounting for 18% of cancer deaths1. Risk factors include smoking, pulmonary fibrosis, human immunodeficiency virus disease, alcohol consumption, and exposure to environmental toxins like asbestos. Common symptoms include cough, hemoptysis, chest pain, dyspnea and paraneoplastic syndromes2. The 5-year survival rate for lung cancer is typically low, ranging from 10 to 20% between 2010 and 2014, and late diagnosis is often a contributing factor1. In a study by Rodak et al., 65.33% of men diagnosed with lung cancer were already at advanced stages3.
Lung cancer is a major cause of cancer-related deaths in Taiwan, with increasing prevalence4. In Taiwan, adenocarcinoma is the most common subtype of lung cancer, and over half of the lung cancer patients are diagnosed at stage IV. In 2014, the 5-year survival rates were 26.86% for lung cancer overall and 25.13% for stage III lung cancer. From 2010 to 2016, there was a decrease in chemotherapy usage (from 66.6 to 42.4%) and an increase in surgery (from 21.0 to 34.4%) in Taiwan5.
Stage III non-small cell lung cancer (NSCLC) is a highly heterogeneous disease with variations in tumor size and lymph node location. Treatments can vary and are chosen based on factors like tumor size, tumor burden, and patient considerations. One option is the “trimodal treatment” involving chemotherapy or radiation therapy, followed by surgery for selected stage IIIA/N2 (cT1-3N2) NSCLC patients. Currently, there are accepted treatments for NSCLC at stages I, II, IIIB (T4N2 and N3), and IV. Surgical resection followed by systemic therapy is standard for stages I and II and some suitable stage IIIA cases6,7. Despite years of research, there is no consensus on the standard treatment for stage IIIA/N2 NSCLC, and the necessity of surgery remains debatable. This study aims to investigate treatment and surgical methods for stage IIIA/N2 NSCLC using data from the Taiwan Cancer Registry (TCR), comparing overall survival rates among different subgroups and treatment strategies.
Materials and methods
Data source and ethic method
The methods were based on a previous experiment5. This study was approved by the Changhua Christian Hospital Institutional Review Board (Internal Review Board number 190410). Methods used in the following research were all in accordance with relevant guidelines and regulations.
Our data was obtained from the Taiwan Cancer Registry (TCR), which is maintained by the Ministry of Health and Welfare of Taiwan under the supervision of the government. The TCR is linked to the National Health Insurance system of Taiwan and provides detailed information on cancer patients, including chest/abdominal computed tomography scans, laboratory data, and treatment.
When researchers were utilizing enrollers’ sensitive data, files, documents, information, or specimens obtained etc. everything was de-identified and unable to back trace to end-users. According to the consents requirements by the authorities. When applying the program, TCR researchers only need to grant by the authorities when applying, therefore, the need for retrieving consent of all the enroller of TCR is waived.
The data that support the findings of this study are available from Taiwan Cancer Registry (TCR) but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the corresponding authors upon reasonable request and with permission of Taiwan Cancer Registry (TCR).
Study population
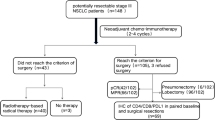
In this study, we collected data on lung cancer patients from the TCR using the International Classification of Disease for Oncology codes C34.0, C34.1, C34.2, C34.8, and C34.9. Tumors were graded using the World Health Organization’s classification system and staged in accordance with the 7th tumor, node, metastasis (TNM) staging system. Out of 41,947 lung cancer patients diagnosed between 2010 and 2016, we excluded individuals with metastatic NSCLC, stages other than stage IIIA, nodal stages other than N2, ages below 18, or missing clinical stage information. This resulted in 2,237 lung cancer patients enrolled in our study.
Main outcome and comorbidities
The main outcome of this study was overall survival, which was defined as the length of time from the tissue diagnosis date to the date of death or December 31, 2018. The Taiwan death certificate database provided the death date and cause of death. The initial treatment for lung cancer refers to the therapy initiated within three months of diagnosis. Follow-up data was collected from diagnosis until death or December 31, 2018. Preexisting comorbidities were measured using the Charlson Comorbidity Index (CCI).
Statistical analysis
We used the chi-square test to analyze the distributions of age, sex, CCI score, cell type, clinical T, clinical N, clinical M, clinical stage, grading, treatment strategy, and survival data. The Kaplan-Meier method was used to generate overall survival curves, and the log-rank test was used to determine significant differences. All statistical analyses were conducted using SAS software, version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Our study included 2,237 patients with stage IIIA/N2 NSCLC. As shown in Table 1, the majority of patients were male. The mean age of the T1N2, T2N2, and T3N2 groups were 66.7 ± 12.0, 68.0 ± 12.1 and 69.3 ± 12.7, respectively. Most patients had a CCI score of ≤ 2. Adenocarcinoma was the predominant cell type in the T1N2 and T2N2 groups, followed by squamous cell carcinoma. In contrast, squamous cell carcinoma was the most common cell type in the T3N2 group, followed by adenocarcinoma. The main treatment for the T1N2 and T2N2 groups was operation plus adjuvant chemotherapy, whereas chemotherapy plus other treatment was the main therapy for the T3N2 group. Lobectomy was the most frequently performed surgical method used among all N2 patients.
Tables 2 and 3 demonstrate the overall survival of the patients through univariate analysis and multivariate analysis. Patients aged 70 years or older had a significantly lower survival rate compared to the 50–69 age group (HR = 1.934 by univariate analysis and HR = 1.408 by multivariate analysis, P < .001). Female patients had a significantly higher survival rate (HR = 0.645 by univariate analysis and HR = 0.773 by multivariate analysis, P < .001). Higher CCI scores (3–5 and ≥ 5) were associated with lower survival rates. Squamous cell carcinoma and large cell carcinoma had significantly lower survival rates compared to adenocarcinoma. The survival rate was also significantly lower in stage T2N2 and T3N2 patients compared to the T1N2 group. Lobectomy had the lowest mortality rate, which was significant (HR = 0.290 by univariate analysis and HR = 0.354 by multivariate analysis, P < .001).
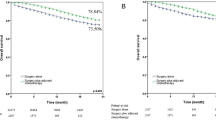
Figure 1 illustrates the survival curve of all stage IIIA/N2 NSCLC patients, with the 5-year overall survival rate being 28.39% (95% CI = 0.2650–0.3028). Figure 2 displays the survival curves for stage IIIA/N2 NSCLC patients stratified by clinical T stage. The 5-year overall survival rates are 45.67% (95% CI = 0.4048–0.5086), 29.17% (95% CI = 0.2639–0.3195), and 19.78% (95% CI = 0.1704–0.2252), respectively, for T1N2, T2N2, and T3N2 (P < .001).
Figure 3a demonstrates the survival curves for all stage IIIA/N2 NSCLC patients stratified by treatment, revealing a higher survival rate for those who received neoadjuvant chemotherapy plus operation and adjuvant chemotherapy (P < .001). Figure 3b shows that T1N2 patients had a higher survival rate with operation alone (P < .001). Figure 3c and Fig, 3d demonstrate that neoadjuvant chemotherapy plus operation and adjuvant chemotherapy resulted in a higher survival rate for T2N2 and T3N2 patients (P < .001). Figure 4a demonstrates the survival curves for all stage IIIA/N2 NSCLC patients stratified by operative method, indicating a higher survival rate for those who underwent lobectomy (P < .001). Figure 4b and c, and 4d also revealed a trend showing that lobectomy was associated with a higher survival rate in T1N2, T2N2, and T3N3 NSCLC patients, respectively (P < .001).
a Kaplan-Meier survival curves for all stage IIIA/N2 NSCLC patients stratified by treatment method (P < .001). b Kaplan-Meier survival curves for T1N2 NSCLC patients stratified by treatment method (P < .001). c Kaplan-Meier survival curves for T2N2 NSCLC patients stratified by treatment method (P < .001). d Kaplan-Meier survival curves for T3N2 NSCLC patients stratified by treatment method (P < .001).
a Kaplan-Meier survival curves for all stage IIIA/N2 NSCLC patients stratified by operative method (P < .001). b Kaplan-Meier survival curves for T1N2 NSCLC patients stratified by operative method (P < .001). c Kaplan-Meier survival curves for T2N2 NSCLC patients stratified by operative method (P < .001). d Kaplan-Meier survival curves for T3N2 NSCLC patients stratified by operative method (P < .001).
Discussion
Patient demographic data
We categorized the stage IIIA/N2 NSCLC patients in Taiwan between 2010 and 2016 into T1N2, T2N2 and T3N2 subgroups, with T2N2 being the largest subgroup, followed by T1N2 and T3N2. There were no significant differences in CCI scores and mean age among the subgroups. A recent research had reported that males have a nearly 2-fold higher incidence and mortality rate of lung cancer than females1. In line with that previous study, more than half of the stage IIIA/N2 NSCLC patients in Taiwan were male.
NSCLC can be further classified into adenocarcinoma, squamous cell carcinoma, and large cell carcinoma. Adenocarcinoma is the most prevalent subtype, accounting for approximately 40% of lung cancer cases, while squamous cell carcinoma represents 25–30% and large cell carcinoma accounts for 5–10% of cases2. A review article had reported a shift towards adenocarcinoma as the main cell type in stage III NSCLC, although some studies still reported epidermoid cell carcinoma as the major type8. Our result is generally in line with previous studies, in that adenocarcinoma is the most common cell type in stage IIIA/N2 NSCLC, followed by squamous cell carcinoma. Minor differences may arise from the inclusion criteria and ethnic variations in our study population.
Much research has been conducted on the treatment of stage IIIA/N2 NSCLC; however, the standard treatment remains controversial. Currently, multimodality treatment is widely accepted worldwide for stage IIIA/N2 NSCLC. In our study, T1N2 and T2N2 patients primarily received surgery followed by adjuvant therapy, while chemotherapy was the main treatment for T3N2 patients. Interestingly, although surgery was not the primary treatment for T2N2 and T3N2 patients, lobectomy was the most common surgical option for all N2 patients. The overall survival rates of different treatments and surgical types will be detailed in the subsequent sections.
Survival patterns of stage IIIA/N2 NSCLC
Previous research has identified several factors associated with higher survival in stage III NSCLC, including female sex, younger age at diagnosis, better performance status, and adenocarcinoma8. Lin et al. conducted a study in Taiwan from 2002 to 2014, which demonstrated higher 5-year survival rates for large cell carcinoma and adenocarcinoma. They also mentioned the predominance of adenocarcinoma as the main histological subtype in Asian countries, particularly among women and non-smokers, unlike the prevalence of small cell lung cancer (SCLC) and squamous cell carcinoma in Western countries9. Our study is in line with these findings, as we observed significantly lower survival rates among patients aged ≥ 70 years and those with a CCI score of 3–5 or > 5. In contrast, women and patients with adenocarcinoma had a significantly higher survival rate. Additionally, we found that lobectomy had the significantly lowest mortality rate compared to other surgical methods. A further detailed discussion of surgical methods will be presented in a following subsection.
Few researches have reported the global 5-year survival rate from NSCLC. In the United States, the 5-year relative survival rate improved from 16.5% in 1975–1977 to 25.1% in 2009–20158. More recently, the 5-year overall survival rate in stage IIIA/N2 NSCLC patients in America increased from 20% in 1988–1996 to 35% in 2006-201410. The 5-year survival rate of lung cancer is only 10–20% in most countries1, while the global 5-year survival rate of stage IIIA/N2 NSCLC patients was 20–25%11. In our study, we found that the 5-year survival rate was 28.39%, which is better than the global average.
Role of surgery in trimodal treatment of stage IIIA/N2 NSCLC
For years, great effort has been devoted to the management of stage IIIA/N2 NSCLC patients. However, there is still controversy as to whether surgery plays an indispensable role in the management of these patients12. In the 1980s through the early 1990s, surgical treatment often resulted in poor local control and early distant metastasis, with a 5-year survival rate around 10%13. However, advancements in diagnostic tools, chemotherapy regimens and surgical techniques have enabled more patients to undergo surgery, leading to increased research on its impact in stage IIIA/N2 NSCLC patients.
A recent phase III clinical trial by Eberhardt et al. showed that in resectable stage IIIA/N2 and selected IIIB NSCLC patients treated with induction chemotherapy and concurrent chemoradiotherapy, there was no significant difference in long-term survival between the surgical group and the definitive chemoradiotherapy group14. It is worth noting that since that study included some selected stage IIIB NSCLC patients, its result might lead to a different interpretation of the treatments compared to studies of only stage IIIA/N2 NSCLC patients.
On the other hand, another multicenter study revealed that neoadjuvant chemotherapy or chemoradiotherapy followed by surgery had a better overall survival rate than definitive chemoradiotherapy in stage IIIA/N2 NSCLC15. In a Japanese cohort study by Yoshino et al., the 5-year survival rate was 30.1% in patients with clinical stage IIIA/N2 NSCLC who underwent surgery in 2004. The 5-year survival rate was 33.7% in patients who received surgery alone13. While the higher 5-year survival rate in the surgery group may be due to the exclusion of advanced cases in the study, it still reflects the impact of surgery on stage IIIA/N2 NSCLC.
Nowadays, the current clinical standard for the treatment of resectable stage IIIA NSCLC is induction chemotherapy with/without concurrent radiation therapy, followed by surgery, with/without adjuvant therapy16. In a single center retrospective study by Uy et al., stage IIIA/N2 NSCLC patients receiving trimodal therapy had a 3-year survival rate of 52.3%. They suggested that in highly selected patients, undergoing chemoradiotherapy before surgical treatment could lead to better outcomes in trimodal therapy17.
It is worth noting that treatments including operation had the top three overall survival rates in the T1 ~ T3 subgroups. In a recent paper by Cheng et al., the 1-year, 3-year and 5-year survival rates were 74.6%, 41.0%, and 27.7% in T1N2 patients, 67.6%, 33.2%, and 21.8% in T2N2 patients, and 61.1%, 27.8%, and 19.1% in T3N2 patients in the United States7. Our results go beyond the report mentioned above, showing a higher 5-year survival rate. Additionally, it is notable that the operation rate is higher in Taiwan compared to the United States7, suggesting that surgery may be associated with a higher survival rate. From this standpoint, we suggest that surgery may provide better outcomes in stage IIIA/N2 NSCLC patients, and neoadjuvant therapy plus operation and adjuvant therapy is a reasonable treatment option. It is also important that patients with resectable stage IIIA/N2 NSCLC consider having an operation because they may be potential candidates for curative surgical treatment.
Choice of surgical type
A previous multi-institutional randomized trial by Ginsberg et al. had demonstrated that limited pulmonary resection in T1N0 NSCLC patients led to higher mortality and a higher locoregional recurrence rate18. Consequently, lobectomy is now widely considered as the standard surgical procedure of choice for T1N0 NSCLC. However, which type of pulmonary resection can be considered the first choice for stage IIIA/N2 NSCLC patients remains controversial. A recent phase III clinical trial (INT0139) showed that there was no significant improvement in overall survival from surgery after induction chemoradiotherapy compared with completion of definitive-dose radiotherapy. However, the overall survival rate was significantly increased in the lobectomy subgroup, whereas there was a non-significant higher mortality rate in the pneumonectomy subgroup. The authors mentioned that the high mortality rate following pneumonectomy may affect the outcome of induction chemoradiotherapy, leading to the absence of an overall survival benefit in the trial19. A similar result was found by Arndt et al., who showed that lobectomy was associated with lower morbidity and mortality than pneumonectomy20. Another recent review article also revealed that pneumonectomy was inversely correlated with the 5-year survival rate and post-pneumonectomy status was a disadvantage when treating recurrent disease21. Also, a review article by Niki Iranpour et al. demonstrated that low FEV1(forced expiratory volume in one second), right-sided pneumonectomy, extended resection, and increased blood loss were related to the increased risk of postoperative complication. The authors speculated that those who underwent pneumonectomy had more advanced disease involving more lobes and bronchovascular structures compared to those who received lobectomy, leading to more postoperative complications. They also favored lobectomy over pneumonectomy when operable22. In a prospective study conducted by Bram Balduyck et al., they found that life quality returned to original physical function 1 month after sleeve lobectomy. On the other hand, there was no return to baseline function 12 months after pneumonectomy, with significant increase of postoperative dyspnea, thoracic pain and shoulder dysfunction, leading to worsen quality of life23. However, Gillaspie et al., in a review article, considered that pneumonectomy cannot be excluded from multimodality treatment if performed at experienced centers with careful patient selection and postoperative care24.
In our study, it is notable that for each type of surgery, the overall survival rate was higher than the survival rate for the group that did not undergo surgery. Comparing our results to previous studies, a similar pattern of results was found in that lobectomy had the significantly highest overall survival rate and lowest mortality rate among all stage IIIA/N2 NSCLC patients. We also found that sublobectomy, which includes segmentectomy and wedge resection, had the lowest overall survival rate among all surgical types performed in stage IIIA/N2 patients. In a recent cohort study, both lobectomy and sublobectomy were associated with higher overall survival rates than non-surgery. Furthermore, lobectomy was found to have a significantly better survival rate than sublobectomy25. As discussed, our finding supported that surgery and a clear surgical margin can improve the overall survival rate. Regarding the choice of surgical type, complete resection produced a better survival rate, and lobectomy is a more viable option than pneumonectomy. Pneumonectomy may be performed under certain conditions but should be avoided if future therapy might be necessary in the case of disease recurrence. Further research on the choice of surgical type in resectable stage IIIA/N2 NSCLC patients is still needed.
Challenges and advances in the treatment of stage IIIA/N2 NSCLC: the role of imaging and personalized therapy approaches”
According to the current NCCN guideline, treatment of stage IIIA/N2 NSCLC includes surgical resection plus lymph node dissection following adjuvant chemotherapy in operable cases, CCRT in high surgical risk group, systemic therapy with or without radiotherapy following surgery and adjuvant chemotherapy. Among these strategies, selected patients such as fit, single station non-bulky N2, requiring only lobectomy, are recommended for systemic therapy following operation12. It is important to determine whether N2 disease is present before the initiation of therapy since it has a great impact on prognosis and treatment decisions, which is strongly related to the imaging accuracy. However, studies on applying imaging technology to NSCLC are very rare currently, but there are still some studies that provide some new imaging applications that can be used to accurately locate tumors and metastatic lymph nodes in NSCLC. Chenyu You et al. developed several novel models which can enhance the accurate delineation of anatomical structures in medical images such as CT or MRI scans26,27. These advanced imaging techniques could be applied to improve the segmentation of tumor and lymph nodes in stage IIIA/N2 NSCLC, which is important for staging, treatment and follow-up.
Other concerns include the heterogeneity of stage IIIA/N2 NSCLC patients and the life quality after trimodal therapy. Owing to the high heterogeneity of N2 patients, surgery should be carefully considered in suitable cases of N2 patients. Moreover, trimodal therapy including preoperative or postoperative chemotherapy and radiotherapy may cause serious side effects, such as fatigue, bone marrow suppression, and increased infection risk, leading to poor quality of life. Hence it is a challenge of making the treatment decision of N2 patients. Hans-Stefan Hofmann et al. studied the subclassified stage IIIA/N2 NSCLC patients and showed that surgery should be applied in patients who had sensitivity to systemic treatment and non-bulky tumors28.
Strengths and limitations
Our research is a nationwide study that obtained data from the TCR, which provided detailed information about cancer patients, including images, laboratory data, and treatments. One of the strengths of our study is that it included a large number of stage IIIA/N2 patients in Taiwan. As far as we know, it is the first study to investigate the overall survival rates of different treatment strategies and surgical methods in stage IIIA/N2 NSCLC patients. This study can provide useful insights for clinical decision-making regarding the treatment of stage IIIA/N2 NSCLC patients.
However, there are several limitations to our research. First, our study is a retrospective study, which means bias and confounding variables are unavoidable as the historical data may be incomplete or inaccurate. Secondly, this is a single-country study, and our data was obtained from TCR, which is linked to the National Health Insurance system of Taiwan. There were some potential limitations of TCR, such as limited generalizability and delayed data availability. Hence it should be kept in mind when applying our results to different populations.Furthermore, the ability to perform surgery or other therapies may be limited by the medical resources provided by different hospitals. Finally, although the outcomes of different treatments and surgical types on overall survival are clarified in this study, it may be challenging to directly apply the conclusions of our results to all stage IIIA/N2 NSCLC patients due to high patient heterogeneity within a population. Further research is still required, and medical teams need to consider the individual conditions of each patient to make appropriate decisions.
Conclusion
Our study revealed that neoadjuvant therapy plus surgery and adjuvant therapy resulted in a higher survival rate among stage IIIA/N2 NSCLC patients. Patients who underwent surgery had higher overall survival rates in the T1-T3 subgroups. Surgery appears to provide a better outcome for patients with resectable stage IIIA/N2 NSCLC. Lobectomy is the optimal surgical procedure, with significantly lower mortality rates than other surgical types. Therefore, we recommend that patients with resectable stage IIIA/N2 NSCLC not be excluded from surgery, and that lobectomy may be the preferred surgical option due to its higher survival rate.
Data availability
The data that support the findings of this study are available from Taiwan Cancer Registry (TCR) but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the corresponding authors upon reasonable request and with permission of Taiwan Cancer Registry (TCR).
References
Sung, H. et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249. https://doi.org/10.3322/caac.21660 (2021).
Duma, N., Santana-Davila, R. & Molina, J. R. Non-small Cell Lung Cancer: Epidemiology, Screening, diagnosis, and treatment. Mayo Clin. Proc. 94, 1623–1640. https://doi.org/10.1016/j.mayocp.2019.01.013 (2019).
Rodak, O., Peris-Díaz, M. D., Olbromski, M., Podhorska-Okołów, M. & Dzięgiel, P. Current Landscape of Non-small Cell Lung Cancer: Epidemiology, histological classification, targeted therapies, and Immunotherapy. Cancers (Basel). 13. https://doi.org/10.3390/cancers13184705 (2021).
Chang, Y. J., Huang, J. Y., Lin, C. H. & Wang, B. Y. Survival and treatment of Lung Cancer in Taiwan between 2010 and 2016. J. Clin. Med. 10 https://doi.org/10.3390/jcm10204675 (2021).
Tsai, H. C., Huang, J. Y., Hsieh, M. Y. & Wang, B. Y. Survival of Lung Cancer patients by histopathology in Taiwan from 2010 to 2016: a Nationwide Study. J. Clin. Med. 11 https://doi.org/10.3390/jcm11195503 (2022).
Mithoowani, H. & Febbraro, M. Non-small-cell Lung Cancer in 2022: a review for General practitioners in Oncology. Curr. Oncol. 29, 1828–1839. https://doi.org/10.3390/curroncol29030150 (2022).
Cheng, Y. F. et al. Comparison of treatment strategies for patients with clinical stage T1-3/N2 Lung Cancer. J. Natl. Compr. Canc Netw. 18, 143–150. https://doi.org/10.6004/jnccn.2019.7353 (2020).
Casal-Mouriño, A. et al. Epidemiology of stage III lung cancer: frequency, diagnostic characteristics, and survival. Transl Lung Cancer Res. 10, 506–518. https://doi.org/10.21037/tlcr.2020.03.40 (2021).
Lin, H. T. et al. Epidemiology and Survival Outcomes of Lung Cancer: A Population-Based Study. BioMed Research International 8148156, doi: (2019). https://doi.org/10.1155/2019/8148156 (2019).
Zeng, W. Q. et al. Postoperative Radiotherapy for Resected Stage IIIA-N2 non-small-cell Lung Cancer: a Population-Based Time-Trend Study. Lung. 197, 741–751. https://doi.org/10.1007/s00408-019-00284-7 (2019).
Nastase, A. et al. Molecular markers for long-term survival in Stage IIIA (N2) NSCLC patients. Cancer Genomics Proteom. 19, 94–104. https://doi.org/10.21873/cgp.20306 (2022).
Riely, G. J. et al. Non-small Cell Lung Cancer, Version 4.2024, NCCN Clinical Practice guidelines in Oncology. J. Natl. Compr. Canc Netw. 22, 249–274. https://doi.org/10.6004/jnccn.2204.0023 (2024).
Yoshino, I. et al. Surgical outcome of stage IIIA- cN2/pN2 non-small-cell lung cancer patients in Japanese lung cancer registry study in 2004. J. Thorac. Oncol. 7, 850–855. https://doi.org/10.1097/JTO.0b013e31824c945b (2012).
Eberhardt, W. E. et al. Phase III study of surgery Versus Definitive Concurrent Chemoradiotherapy Boost in patients with Resectable Stage IIIA(N2) and selected IIIB non-small-cell lung Cancer after induction chemotherapy and concurrent chemoradiotherapy (ESPATUE). J. Clin. Oncol. 33, 4194–4201. https://doi.org/10.1200/jco.2015.62.6812 (2015).
Couñago, F. et al. Neoadjuvant treatment followed by surgery versus definitive chemoradiation in stage IIIA-N2 non-small-cell lung cancer: a multi-institutional study by the oncologic group for the study of lung cancer (Spanish Radiation Oncology Society). Lung Cancer. 118, 119–127. https://doi.org/10.1016/j.lungcan.2018.02.008 (2018).
Zheng, Y., Jaklitsch, M. T. & Bueno, R. Neoadjuvant therapy in Non-small Cell Lung Cancer. Surg. Oncol. Clin. N Am. 25, 567–584. https://doi.org/10.1016/j.soc.2016.02.010 (2016).
Uy, K. L. et al. Improved results of induction chemoradiation before surgical intervention for selected patients with stage IIIA-N2 non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 134, 188–193. https://doi.org/10.1016/j.jtcvs.2007.01.078 (2007).
Ginsberg, R. J. & Rubinstein, L. V. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann. Thorac. Surg. 60, 615–622. https://doi.org/10.1016/0003-4975(95)00537-u (1995). discussion 622 – 613.
Albain, K. S. et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet. 374, 379–386. https://doi.org/10.1016/s0140-6736(09)60737-6 (2009).
Arndt, A. T. et al. Redefining the risk of surgery for clinical stage IIIA (N2) non-small cell Lung Cancer: a pooled analysis of the STS GTSD and ESTS Registry. Lung. 199, 311–318. https://doi.org/10.1007/s00408-021-00447-5 (2021).
Sata, Y. et al. Keys to successful induction chemoradiotherapy followed by surgery for stage III/N2 non-small cell lung cancer. Surg. Today. 49, 547–555. https://doi.org/10.1007/s00595-019-1766-8 (2019).
Iranpour, N., Olive, J. K. & Antonoff, M. B. Management of stage IIIA non-small-cell lung cancer: role of surgery. Curr. Challenges Thorac. Surg. 3 (2020).
Balduyck, B., Hendriks, J., Lauwers, P. & Van Schil, P. Quality of life after lung cancer surgery: a prospective pilot study comparing bronchial sleeve lobectomy with pneumonectomy. J. Thorac. Oncol. 3, 604–608. https://doi.org/10.1097/JTO.0b013e318170fca4 (2008).
Gillaspie, E. A. & Wigle, D. A. Management of Stage IIIA (N2) non-small cell Lung Cancer. Thorac. Surg. Clin. 26, 271–285. https://doi.org/10.1016/j.thorsurg.2016.04.001 (2016).
Wang, S. et al. Lobectomy Versus Sublobectomy in Stage IIIA/N2 Non-small Cell Lung Cancer: a Population-based study. Front. Oncol. 11, 726811. https://doi.org/10.3389/fonc.2021.726811 (2021).
You, C. et al. ACTION++: improving semi-supervised Medical Image Segmentation with Adaptive Anatomical contrast. Med. Image Comput. Comput. Assist. Interv. 14223, 194–205. https://doi.org/10.1007/978-3-031-43901-8_19 (2023).
You, C., Zhou, Y., Zhao, R., Staib, L. & Duncan, J. S. SimCVD: simple contrastive voxel-wise representation distillation for Semi-supervised Medical Image Segmentation. IEEE Trans. Med. Imaging. 41, 2228–2237. https://doi.org/10.1109/tmi.2022.3161829 (2022).
Hofmann, H. S. et al. Multimodality therapy in subclassified stage IIIA-N2 non-small cell lung cancer patients according to the Robinson classification: heterogeneity and management. J. Thorac. Dis. 10, 3585–3594. https://doi.org/10.21037/jtd.2018.05.203 (2018).
Acknowledgements
No acknowledgment to make.
Funding
None.
Author information
Authors and Affiliations
Contributions
Bing-Yen Wang proposed the idea and concept. Pin-Ching Hu wrote the main manuscript text. Jing-Yang Huang and Ya-Fu Cheng prepared Tables 1, 2 and 3; Figs. 1, 2, 3 and 4. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
All authors have no conflicts of interest or financial ties to disclose.
IRB approval
Changhua Christian Hospital Institutional Review Board (IRB number:190410).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hu, PC., Huang, JY., Cheng, YF. et al. Trimodal therapy and surgical approaches in stage IIIA/N2 non-small cell lung cancer. Sci Rep 14, 29441 (2024). https://doi.org/10.1038/s41598-024-79158-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-79158-9