Abstract
Avascular necrosis of the femoral head (ANFH) is a progressive, multifactorial, and challenging clinical condition that often leads to hip dysfunction and deterioration. The pathogenesis of ANFH is complex, and there is no foolproof treatment strategy. Although some pharmacologic and surgical treatments have been shown to improve ANFH, the associated side effects and poor prognosis are of concern. Therefore, there is an urgent need to explore therapeutic interventions with superior efficacy and safety to improve the quality of life of patients with ANFH. Salvia miltiorrhiza (SM), a traditional Chinese medicine with a long history, is widely used for the treatment of cardiovascular and musculoskeletal diseases due to its multiple pharmacological activities. However, the molecular mechanism of SM for the treatment of ANFH is still unclear. Therefore, this study aimed to explore the potential targets and mechanisms of SM for the treatment of ANFH using network pharmacology and molecular modeling techniques. By searching multiple databases, we screened 52 compounds and 42 common targets involved in ANFH therapy and identified dan-shexinkum d, cryptotanshinone, tanshinone iia, and dihydrotanshinlactone as key compounds. Based on the protein-protein interaction (PPI) network, TP53, AKT1, EGFR, STAT3, BCL2, IL6, and TNF were identified as core targets. Subsequent enrichment analysis revealed that these targets were mainly enriched in the AGE-RAGE, IL-17, and TNF pathways, which were mainly associated with inflammatory responses, apoptosis, and oxidative stress. In addition, molecular docking and 100 nanoseconds molecular dynamics (MD) simulations showed that the bioactive compounds of SM had excellent affinity and binding strength to the core targets. Among them, dan-shexinkum d possessed the lowest binding free energy (-215.874 kcal/mol and − 140.277 kcal/mol, respectively) for AKT1 and EGFR. These results demonstrated the multi-component, multi-target, and multi-pathway intervention mechanism of SM in the treatment of ANFH, which provided theoretical basis and clues for further experimental validation and development of anti-ANFH drugs.
Similar content being viewed by others
Introduction
Avascular necrosis of the femoral head (ANFH) is a progressive disease that manifests as bone death due to impaired subchondral bone supply and its delayed regeneration1. Most patients present with hip dysfunction 1–4 years after disease progression, and without timely intervention and treatment, it predisposes to permanent disability once the articular surface of the femur collapses2. ANFH can occur in both traumatic and non-traumatic backgrounds of ischemia3 and predominantly affects a population of middle-aged men in the most productive age group of 25–50 years4. To date, there have been no global epidemiological reports on the prevalence of ANFH. It has been estimated that approximately 10,000–20,000 cases of osteonecrosis are diagnosed annually in the United States, while the cumulative number of ANFH patients in China reaches 8.12 million5,6. The pathogenesis of ANFH is unknown, and common risk factors include corticosteroid use, fractures, hip dislocations, alcohol abuse, sickle cell disease, and coagulopathy7,8. Currently, commonly used medications for the treatment of early AVFH include anticoagulants, statins, vasodilators, and bisphosphonates; however, the efficacy of these medications is limited and associated with specific ad-verse effects9. In addition, some surgical strategies such as core decompression, vascularized bone grafts, osteotomies, and total hip arthroplasty (THA) play an important role in the treatment of ANFH, but these approaches have the disadvantages of being invasive, traumatic, and having a poor prognosis10,11,12. Therefore, there is an urgent need to investigate efficient, safe, and low-side-effect treatments to block the progression of early ANFH.
In recent years, traditional Chinese medicine (TCM) has attracted widespread attention due to its multi-component and multi-target properties13. Salvia miltiorrhiza (SM) is a perennial herb of the genus Sage in the family Labiatae, whose dried roots and rhizomes are commonly referred to as Danshen14. The medicinal history of SM can be traced back more than 2000 years, and it was first recorded in the ancient Chinese pharmacological monograph < Shennong Ben Cao Jing > 15. Studies have shown that SM is rich in diterpenoids and phenolic acids16 and possesses a variety of bio-logical activities, including anti-inflammatory, antioxidant, antitumor, antithrombotic, neuroprotective, and metabolic regulation functions17,18,19. Based on this, SM has shown remarkable efficacy in the treatment of various diseases, such as cardiovascular diseases20, musculoskeletal diseases21, liver diseases22, renal diseases23, and various cancers24. Recently, SM has been shown to treat ANFH by promoting bone regeneration and blood flow restoration25. Acupuncture point injection of SM can also effectively alleviate the pain level of patients with ANFH and improve hip joint mobility function and activities of daily living26. Although the role of SM in the treatment of ANFH has been confirmed, no pharmacological studies have been con-ducted to elucidate the molecular mechanisms involved.
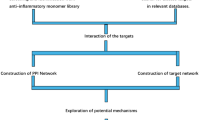
Based on the theory of systems biology, network pharmacology uses bioinformatics and network analysis methods to study the biological effects of small molecules from various natural resources27. This emerging technology has become an invaluable tool in the drug design process, especially helpful in identifying active ingredients and potential targets of TCM to provide newer therapeutic options for various diseases28. Molecular docking and molecular dynamics (MD) techniques allow in-depth characterization of intermolecular interactions and further validation of receptor-ligand affinity and binding stability. Therefore, this study aimed to explore the bioactive components, core targets, and key signaling pathways of SM for ANFH treatment through network pharmacology, molecular docking, and MD simulation to provide a theoretical basis for clinical treatment. The flow chart of this study is shown in Fig. 1.
Results
Acquisition of SM active compounds and targets
The initial screening of active compounds represents a pivotal initial step in the identification of promising botanical ingredients with the potential to exert therapeutic effects on ANFH. According to the criteria described in the Methods section, we obtained 202 active ingredients by searching the TCMSP database and screened 56 active compounds using OB ≥ 23% and DL ≥ 0.18 as criteria. The 56 SM compounds were subsequently evaluated by GI and drug similarity analysis using SwissADME, and it was found that they fulfilled the screening criteria. Subsequently, a search of the TCMSP database for the gene targets of the above active compounds revealed that four compounds had no target information and were therefore excluded from the study (Table S1). The results indicated that a total of 880 target genes were compiled by SM, with 132 remaining after the deletion of duplicate targets. The data were imported into Cytoscape 3.10.1 software for visualization, and a " herb-active ingredient-target " network with 185 nodes and 932 edges was con-structed (Fig. 2a).
Targets related to ANFH
Using " avascular necrosis of the femoral head,” we searched for disease targets in three databases: DisGeNET, OMIM, and GeneCards, which yielded 74, 477, and 797 targets, respectively. After the removal of duplicate values, a total of 1,285 ANFH-related targets were obtained. The 132 SM drug targets and 1285 ANFH targets were compared by the Venny 2.1.0 platform and plotted on a Venn diagram. Detailed information is provided in Supplementary Table S2. The 42 cross-targets derived were identified as potential targets for SM treatment of ANFH (Fig. 3). Furthermore, a " active ingredient-cross-target” network was constructed to identify the most crucial bioactive compounds (Fig. 2b). The results indicated that the top seven compounds, MOL000006 (luteolin), MOL007154 (tanshinone Iia), MOL007100 (dihydrotanshinlac-tone), MOL007088 (cryptotanshinone), MOL007093 (danshenshenxinkum d), MOL007049 (4-methylenemiltirone), and MOL007041 (2-isopropyl-8-methylphenanthrene-3,4-dione), were identified as corresponding to 28, 13, 8, 6, 6, 6, and 6 targets, respectively.
Protein-protein interaction (PPI) network analysis and core target identification
The 42 cross-targets of SM and ANFH were submitted to the String database for PPI analysis. A PPI network with 42 nodes and 505 edges was constructed using Cytoscape 3.10.1. Network nodes represent target proteins, and lines between nodes represent interactions between proteins. The size of the nodes corresponds to the degree of protein targeting in the network. The core targets with the strongest interactions were filtered using criteria based on degree > 24.048, betweenness centrality > 17.476, and closeness centrality > 0.018 (Fig. 3). We obtained 15 core targets, and the top seven Degree ranked targets were: cellular tumor antigen p53 (TP53), RAC-alpha serine/threonine protein kinase (AKT1), epidermal growth factor receptor (EGFR), signal transducer and activator of transcription 3 (STAT3), bcl-2-like protein 1 (BCL2), interleukin-6 (IL6), and tumor necrosis factor (TNF) (Table 1).
Gene ontology (GO) and Kyoto Encyclopedia of genes and genomes (KEGG) enrichment analysis
To systematically investigate the potential mechanism of action of SM for the treatment of ANFH, we performed GO and KEGG enrichment analyses of 42 cross-targets using the Metascape database. The results showed that a total of 1122 GO terms were enriched (p < 0.01), including 1023 BP, 30 CC, and 69 MF terms. The top 15 enriched terms in each category were selected to plot the bar graph (Fig. 4). The five BP terms with the highest target enrichment were response to hormone, response to peptide, cellular response to nitrogen compound, cellular response to organonitrogen com-pound, and response to growth factor. The most affected CC terms are: transcription regulator complex, RNA polymerase II transcription regulator complex, protein kinase complex, transferring phosphorus-containing groups, and serine/threonine protein kinase complex. transferring phosphorus-containing groups, and serine/threonine protein kinase complex. The top MF terms were kinase binding, protein kinase binding, transcription factor binding, DNA-binding transcription factor binding, and RNA polymerase II-specific DNA-binding transcription factor binding.
In addition, the KEGG enrichment analysis yielded 159 highly enriched pathways29. The top 20 enriched entries were selected for bubble mapping (Fig. 5). The top 10 path-ways include pathways in cancer, fluid shear stress and atherosclerosis, lipid and atherosclerosis, hepatitis B, AGE-RAGE signaling pathway in diabetic complications, IL-17 signaling pathway, Epstein-Barr virus infection, TNF signaling pathway, Kaposi sarcoma-associated herpesvirus infection, and Chagas disease. Detailed information is provided in Supplementary Table S3. We focused on the IL-17 signaling pathway and the TNF signaling pathway based on the enrichment results of the core targets and pathways (Fig. 6)30.
Active ingredient-target-pathway (AI-TAR-PATH) network construction
The Cytoscape 3.10.1 software was utilized to construct the AI-TAR-PATH network. The network comprised 42 nodes (7 active compounds, 15 core targets, and 20 KEGG pathways) and 170 edges (Fig. 7). Among the identified targets, JUN, TNF, IL6, AKT1, CASP3, and PTGS2 were found to be associated with multiple pathways, including the AGE-RAGE signaling pathway in diabetic complications, the IL-17 signaling pathway, and the TNF signaling pathway. These findings indicate that the active compounds in SM may treat ANFH through multiple pathways and targets.
Molecular docking analysis
In accordance with the PPI network, the top 7 core targets (TP53, AKT1, EGFR, STAT3, BCL2, IL6, and TNF) were identified as receptors for molecular docking. The five components (luteolin, tanshinone Iia, dihydrotanshinlactone, cryptotanshinone, and dan-shexinkum d) with the highest median rankings in the “component-cross-targeting” network analysis were selected as the ligands. Finally, a total of 35 sets of molecular docking results for the receptor-ligands were obtained (Table 2), and the binding energy thermograms were plotted (Fig. 8a). It is generally accepted that a binding energy of less than − 5.0 kcal/mol indicates superior binding activity. Furthermore, the lower the binding energy, the more stable the binding interaction31. The docking results demonstrated that the binding energies of the SM targets and active compounds were all less than − 5 kcal/mol. Five moieties with the lowest binding energies were selected for the construction of molecular docking models (Fig. 8b-f). These included cryptotanshinone with AKT1, dan-shexinkum d with AKT1, dan-shexinkum d with EGFR, dihydro-tanshinlactone with AKT1, and tanshinone iia with AKT1. The results indicated that cryptotanshinone binds to the LYS-268 site of AKT1, dan-shexinkum d binds to the THR-211 site of AKT1, and dan-shexinkum d binds to the ARG-831 and ARG-832 sites of EGFR. Similarly, dihydrotanshinlactone binds to the LYS-268 site of AKT1, and tanshinone iia binds to the LYS-268 site of AKT1.
Heat maps and molecular docking results of SM’s biological compounds with core targets. (a) The binding energy value of molecular docking. A lower score indi-cates a stronger binding ability. (b) AKT1 docked with cryptotanshinone. (c) AKT1 docked with dan-shexinkum d. (d) EGFR docked with dan-shexinkum d. (e) AKT1 docked with dihydrotanshinlactone. (f) AKT1 docked with tanshinone iia.
MD simulation analysis
The score is based on the affinity of molecular docking, with lower negative values representing tighter binding of proteins and small molecules. To further analyze the molecular dynamics trajectories, we selected the three complexes (dan-shexinkum d with EGFR, dan-shexinkum d with AKT1, and cryptotanshinone with AKT1) with optimal binding activity to perform MD simulations for 100 ns, and provides the apo status for reference. The root mean square deviation (RMSD) curves reflect protein conformational fluctuations32. The average RMSD values of the three complexes were 0.229, 0.278, and 0.243 nm, respectively. The RMSD curves of all complexes exhibited smooth fluctuations (fluctuating values within 0.2 nm) during the 0-100 ns simulations (Fig. 9a). The distribution of the RMSD profiles remained stable throughout the simulation, indicating that the proteins were stable under strong lig-and binding strength. The root mean square fluctuation (RMSF) can be used to indicate the fluctuation of the complexes at the residue level, and if it has a low value, it indicates a more stable structure in that region33. The mean values of the RMSF for the three complexes were 0.092, 0.109, and 0.098 nm, respectively (Fig. 9b). Among the three complexes, Dan-shexinkum d exhibited the greatest residue flexibility in its interaction with the EGFR complex. In addition, we found that the RMSF values of the protein at ARG-831 and ARG-832 sites were significantly reduced after EGFR added small molecules at the binding site, and similarly, the RMSF values of AKT1 at THR-211 and LYS-268 sites were also reduced. This indicates that the binding of small molecules to the protein is stable and the small molecules do not affect the structure of the protein. The solvent-accessible surface area (SASA) of protein trajectories has been extensively utilized to investigate protein folding and unfolding, as well as structural stability. The results demonstrate that the solvent-accessible surface area of these complexes is remarkably stable, exhibiting fluctuations and amplitudes comparable to or even superior to those of the original proteins (Fig. 9c). The Rg is a crucial metric for evaluating changes in the densification of docked complexes34. A higher Rg value indicates greater stability, suggesting that the complex is more tightly bound. The figure indicates that the radius of gyration (Rg) values of dan-shexinkumd with EGFR and dan-shexinkumd with AKT1 treatment groups exhibited a decreasing trend, suggesting an increase in the tightness of the proteins. There was no significant change in cryptotanshinone with AKT1. Overall, all three simulations demonstrated stable binding (Fig. 9d). Hydrogen bonding is a strong, noncovalent interaction. The average number of hydrogen bonds for the three complexes throughout the run was 2.090, 0.329, and 0.030, respectively (Fig. 9e). The complex dan-shexinkum d exhibited the highest density and strength of hydrogen bonds with EGFR. Furthermore, the Gibbs free energy landscape map demonstrated the stability of the complexes (Fig. 9f-h). RMSD and Gyrate were selected as the constructors of the landscape map to investigate their equilibrium structures35. As illustrated in the accompanying figure, the dan-shexinkum d and EGFR complexes exhibited a relatively stable conformational state when the Rg values were 1.90–1.92 nm and the RMSD values were 0.22–0.26 nm. At a Rg value of 2.125–2.145 nm and an RMSD value of 0.27–0.31 nm, Dan-shexinkum d was stabilized in conjunction with AKT1. The free energy of cryptotanshinone with AKT1 was found to be lowest when the Rag value was 2.15–2.17 nm and the RMSD value was 0.224–0.267 nm.
MD simulation of receptor-ligand complexes. (a) RMSD curves of the com-plexes. (b) RMSF curves of the complexes. (c) SASA analysis of complexes. (d) Rg curves of the complexes. (e) Hydrogen bonding number and density analysis of the complexes. (f) Map of the free energy landscape of dan-shexinkum d with EGFR. (g) Map of the free energy landscape of dan-shexinkum d with AKT1. (h) Map of the free energy landscape of cryptotanshinone with AKT1.
MM‑PBSA binding free energy analysis
The binding energies were determined using the molecular mechanics Poisson-Boltzmann surface area (MM-PBSA) based on MD-simulated trajectories, selecting the intervals for energy calculations to come from the last 20 nanoseconds using 20 frames, and counting at 1-nanosecond intervals. This method enhances the precision of the binding interaction between reactive small molecules and target proteins. It is widely accepted that the free energy of a junction and summation is less than 0, indicating that the binding is stable. The smaller the value, the stronger the bond. The results demonstrated that the total binding free energies of dan-shexinkum d with EGFR, dan-shexinkum d with AKT1, and cryptotanshinone with AKT1 were − 140.277, -215.874, and − 119.668 KJ/mol, respectively (Table 3). This indicates that the protein-ligand complexes exhibit a notable binding affinity, with dan-shexinkum d exhibiting the lowest binding free energy and the strongest binding strength with AKT1.
Discussion
ANFH is a type of aseptic osteonecrosis that reduces the blood supply to the proximal femur, leading to osteoclastic death and progressive collapse of the articular surfaces, and consequently, hip dysfunction and deterioration36. The early clinical manifestations of ANFH are similar to those of cysts or lesions of the subchondral bone, vasculitis, and transient osteoporosis or osteoarthritis of the hip joint. This similarity makes it challenging to diagnose and treat the condition37. It has been proposed that three key pathways may be instrumental in the pathogenesis of ANFH, namely impaired angiogenesis, coagulation dysfunction, and endothelial dysfunction37. Currently, the treatment of ANFH is controversial and there are no foolproof therapeutic strategies, and most of these strategies depend on the severity of ANFH38. In recent years, re-search on TCM for musculoskeletal disorders has received considerable attention. SM, a traditional Chinese herb that promotes blood circulation and removes blood stasis, plays an important role in anti-inflammatory, antioxidant, antithrombotic, and im-proving vascular microcirculation39. In particular, SM has demonstrated therapeutic potential in the treatment of ANFH40. Nevertheless, the underlying mechanism of action remains to be elucidated. In this study, we employed network pharmacology, molecular docking, and MD simulations to elucidate the potential molecular mechanisms of SM in the treatment of ANFH.
Based on the results of the “active ingredient-cross-target” network, it was postulated that luteolin, tanshinone Iia, dihydrotanshinlactone, cryptotanshinone, and dan-shexinkum D are the principal active ingredients of SM for the treatment of ANFH. The inflammatory response, oxidative stress (OS), matrix destruction, and apoptosis play pivotal roles in the pathogenesis of ANFH. Modulation of these pathological processes can potentially retard disease progression41,42. Luteolins are flavonoids extracted from rhizomes that possess a variety of pharmacological activities, including anti-inflammatory, antioxidant, and antitumor effects43. It has been demonstrated that luteolin achieves vasoprotective and osteoprotective effects by protecting chondrocytes and human umbilical vein endothelial cells (HUVEC) from oxidative damage44,45. Furthermore, protein blotting experiments have demonstrated that luteolin alleviates endothelial damage and dysfunction in glucocorticoid-induced osteonecrosis of the femoral head by activating necrotic apoptosis through the RIPK1/RIPK3/MLKL pathway46. Tanshinone Iia is the most abundant diterpene quinone isolated from SM, which regulates FBXO11 expression, inhibits the PI3K/Akt and nuclear factor-κB (NF-κB) pathways, attenuates IL-1β, and modulates chondrocyte apoptosis and inflammation47. It is well established that bone marrow mesenchymal stem cell (BMSC) transplantation has been employed in the treatment of early acute non-traumatic ANFH48. It has been demonstrated that tanshinone Iia can facilitate the osteogenic differentiation of BMSCs by enhancing the activity of the bone morphogenetic protein (BMP) and Wnt signaling pathways49. In addition, tanshinone Iia has anticoagulant and endothelial protective effects. It inhibits adenosine diphosphate (ADP) and collagen-induced platelet aggregation by regulating the acetylation of microtubule proteins and inhibiting the phosphorylation of ERK2 50. Additionally, it enhances the expression of endothelial nitric oxide synthase (eNOS) and the synthesis of nitric oxide (NO) while inhibiting the production of endothelin-1 (ET-1), thereby protecting endothelial function51. Cryptotanshinone, the most potent water-soluble component of SM pharmacology, has been demonstrated to inhibit the activities of NF-κB, AP-1 inflammatory transcription factor, and cyclooxygenase-2 enzyme (COX2), exerting an anti-inflammatory effect in macrophages52. Yue et al.53 demonstrated that cryptotanshinone enhanced YY1-associated factor 2 (YAF2) expression and influenced chondrocyte apoptosis by modulating the methylation of miR-574-5p. ANFH is the result of an imbalance in bone remodeling involving osteoblasts, which are re-sponsible for bone formation, and osteoclasts, which are responsible for bone resorption. The activity of osteoblasts and osteoclasts is fine-tuned by the osteoprotegerin (OPG), receptor activator of NF-kB ligand (RANKL), and receptor activator of NF-kB (RANK) systems54. It is noteworthy that luteolin has been demonstrated to inhibit RANKL-induced osteoclastogenesis by suppressing the activation of activating tran-scription factor 2 (ATF2)55. Furthermore, it stimulates the proliferation and differentiation of osteoblasts by reducing oxidative stress and regulating the OPG/RANKL ratio while inducing various osteogenic markers56. In addition, tanshinone iia, cryptotanshinone, and dihydrotanshinlactone have been demonstrated to play a pivotal role in bone remodeling57. In conclusion, the aforementioned findings are in general agreement with our initial predictions, suggesting that the major active compounds of SM could exert potential therapeutic effects on ANFH by mediating processes such as inflammation, immunity, OS, and apoptosis.
Subsequently, we constructed a PPI network and identified 15 core targets, with the top 7 including TP53, AKT1, EGFR, STAT3, BCL2, IL6, and TNF. These targets inter-acted more tightly in the protein network, suggesting that they may be critical for the treatment of ANFH58. TP53 is a member of the p53 gene family, a group of transcription factors that plays a pivotal role in regulating the cell cycle, including the processes of cellular senescence and apoptosis59. As a cell cycle factor, p53 inhibits the proliferation of BMSCs60. It has been demonstrated that p53 and Parkin act in con-cert to regulate mitochondrial autophagy in BMSCs, thereby promoting the repair of ANFH61. AKT1, a serine protein, is a principal mediator of angiogenic signaling and a distinctive signaling intermediate in osteoblasts. It regulates osteoblast and osteoclast differentiation and formation, thereby maintaining bone mass62,63. Furthermore, AKT1 plays a role in angiogenesis and ossification following end-stage injury to endochondral bone formation and may be a potential therapeutic target for the treatment of ANFH64. EGFR is a receptor tyrosine kinase that plays a role in regulating cell growth, differentiation, survival, and metastasis65. Studies have demonstrated that mice lacking EGFR exhibit growth retardation and severe bone defects66. Other studies have demonstrated that EGFR is a potent regulator of the bone progenitor cell pool, increasing the number of osteoblasts primarily through the MAPK/ERK pathway to promote cell proliferation and inhibit serum depletion-induced or TNFα-induced apoptosis67. STAT3, a member of the Janus kinase signal transducer and activator of transcription (JAK-STAT) family of proteins, is in-volved in regulating growth factors and a variety of cytokines. It has been demonstrated that STAT3 plays a pivotal role in osteoblast differentiation and bone formation. When STAT3 is absent in osteoblasts, it affects bone remodeling and reduces bone mass in adult mice68. Inflammation is involved in ANFH repair. Studies have identified IL-6 and tumor necrosis factor-α (TNF-α) as important inflammatory factors associated with ANFH69, suggesting that IL-6 and TNF are important targets of ANFH. Zheng et al.70 found that TNF-α regulates the development of ANFH through the p38 mitogen-activated protein kinase (MAPK)/NF-κB signaling pathway-mediated osteoblast autophagy and apoptosis, thereby regulating the early development of ANFH. Ren et al. found71 that anti-IL6 treatment reduced hip synovitis and osteoclastic bone resorption and in-creased new bone formation after ischemic osteonecrosis.
Furthermore, we elucidated the pathogenesis of SM treatment for ANFH by GO and KEGG enrichment analyses. Some of the BP and MF items in the GO analysis are mainly involved in cytokines, cell proliferation, cell differentiation, and immune regulation. KEGG analysis revealed key pathways for SM treatment of ANFH, including the AGE-RAGE signaling pathway in diabetic complications, the IL-17 signaling pathway, and the TNF signaling pathway. The IL-17 signaling pathway plays a pivotal role in the regulation of inflammatory responses, particularly in the promotion of synovial inflammation, cartilage destruction, bone erosion, and angiogenesis72,73. Studies have demonstrated that the activation of IL-17 enhances immune responses and promotes the proliferation of immune cells, particularly T cells and macrophages. These activated immune cells produce a plethora of inflammatory mediators, including chemokines, cytokines, growth factors, matrix metalloproteinases (MMP), prostaglandin E2 (PGE2), and NO, which collectively exacerbate joint inflammation and bone destruction74,75. Furthermore, local levels of IL-17 and Th17 have been demonstrated to be elevated in the synovium of patients with ANFH76. Th17 is a typical inflammatory cell type that mediates several downstream signals, including NF-κB and MAP3K (JNK, p38, and ERK), through the secretion of IL-17, ultimately promoting the gene expression of TNF-α, AP-1, and PTGS2/COX-2 77. These findings indicate that the inhibition of the IL-17 signaling pathway may contribute to the alleviation of ANFH progression. TNF-α is a pivotal cytokine in the regulation of bone homeostasis. It can promote osteoclast survival by binding to the phosphatidylinositol 3-kinase Akt and MEK/ERK pathways78. TNF-α, in conjunction with RANKL, also induces osteoclast differentiation, and the NF-κB pathway may play an important role in this process79. However, TNF in chondrocytes, osteoblasts, and endothelial cells can inhibit cell differentiation and proliferation and even promote apoptosis, thereby destroying bone tissue80,81. Furthermore, the TNF signaling pathway is associated with the immune response and inflammation through the activation of various receptor-mediated pathways, including MAPK and NF-κB signaling through the TNFR1 receptor and PI3K-Akt signaling through the TNFR2 receptor82. In recent years, the roles of the receptor for advanced glycosylation end products (RAGE) and its ligands (advanced glycosylation end products (AGEs)) in skeletal homeostasis and tissue repair have begun to be investigated in relation to the AGE-RAGE signaling pathway. Chen et al.83 demonstrated that AGEs can induce long-term bone apoptosis by binding to RAGE and activating the ERK1/2, p38, and STAT3 signaling pathways. Additionally, AGE-RAGE signaling has been shown to induce vascular endothelial cell damage and alter vascular permeability through the activation of ERK and p38 84. Moreover, AGEs regulate osteoblast proliferation and differentiation by stimulating OS in osteoblasts, increasing reactive oxy-gen species (ROS) production, and stimulating RAGE expression85. In conclusion, our predicted KEGG key pathways are consistent with the aforementioned findings, thereby confirming that SM may treat ANFH primarily through the AGE-RAGE signaling pathway and the TNF and IL-17 signaling pathways.
To continue to validate the active ingredient-target relationship, we performed molecular docking and MD simulations. In the molecular docking validation, the principal active compounds of SM demonstrated an exceptional capacity to bind with the core targets (TP53, AKT1, EGFR, STAT3, BCL2, IL6, and TNF). Among the compounds, cryptotanshinone and dan-shexinkum d exhibited the most robust binding affinity with the target protein AKT1. Subsequently, MD simulations were conducted to evaluate the stability of the protein-ligand complexes over 100 ns. The results demonstrat-ed that the principal active constituents of SM exhibited robust stability and binding strength to the primary target, particularly dan-shexinkum d with AKT1. This may further substantiate the potential of SM for the treatment of ANFH.
It should be noted, however, that the present study is not without limitations. The targets and mechanisms of SM and its bioactive compounds for the treatment of ANFH obtained in this study using network pharmacology and MD are merely rea-sonable predictions based on existing studies. Consequently, further clinical studies and experimental validation are required to substantiate the veracity of these predictions. Secondly, the construction of more comprehensive databases of traditional medicinal plants and target genes is necessary to enhance the precision of the outcomes of cyberpharmacological analyses, thus preventing discrepancies between the predicted and actual results. Furthermore, although we evaluated several pivotal active ingredients of SM, including cryptotanshinone and dan-shexinkum d, they remain inadequate for fully representing its pharmacological activity. Consequently, additional active ingredients must be validated in future studies. Moreover, the pharmacokinetics and toxicology of these active ingredients have not been fully elucidated, and further studies are necessary to evaluate the safety and efficacy of SM as a novel drug for the treatment of ANFH.
Methods
Identification of SM bioactive components and target genes
The active ingredients of SM were collected from the Traditional Chinese Medicine System Pharmacology Platform (TCMSP; https://tcmsp-e.com/, accessed April 18, 2024)86 and based on the 2 oral bioavailability (OB) ≥ 30% and drug-likeness (DL) ≥ 0.18 ADME attribute values for preliminary screening of active ingredients. These selected fractions were identified from the Pubchem database (https://pubchem.ncbi.nlm.nih.gov/, accessed April 18, 2024)87 using its Simplified Molecular Input Line Entry System (SMILES) form. These SMILES symbols were imported into the SwissADME database (www.swissadme.ch, accessed April 18, 2024)88 for further analysis. Those with a gastrointestinal (GI) absorption rating of “high” and a drug similarity with two or more items marked “yes” were selected as active ingredients. The TCMSP database was then used to retrieve the relevant targets for each compound, and all targets were converted to uniform gene symbols using the Uniprot protein database (https://www.uniprot.org, accessed April 18, 2024)89, using the organism “human” to standardize the species.
Target genes of ANFH
To obtain disease-related target genes, three public databases were searched using the keyword " avascular necrosis of the femoral head”: the DisGeNet database (www.disgenet.org, accessed April 18, 2024)90, the OMIM database (https://www.omim.org/, accessed April 18, 2024)91, and the GeneCards database (www.genecards.org, accessed April 20, 2024)92. These databases contain rich and up-to-date disease-related targets. The retrieved results are then summarized and de-duplicated to create a library of disease target genes.
PPI network construction and core target screen-ing
The following network and pathway analyses were performed for overlapping targets between ANFH-related genes and SM target genes. Venn diagrams were drawn using venny 2.1 (https://bioinfogp.cnb.csic.es/tools/venny/). The overlapping targets were imported into the STRING platform (https://string-db.org/, accessed April 24, 2024)93 and configured with the following biological attributes: ‘Homo sapiens’, ‘highest confidence’ (> 0.4), and the rest of the settings were set by default. Next, the PPI network was edited using Cytoscape 3.10.1 software, and core target screening was performed using Centiscape 2.2 and NetworkAnalyzer tools.
GO and KEGG pathway enrichment analysis
GO analysis was used to define target activity, including evaluation of biological processes (BP), cellular components (CC), and molecular functions (MF), and KEGG enrichment analysis elucidated potential pathways associated with the final targets of SM and ANFH. The obtained core targets were imported into Metascape (https://metascape.org)94 for GO and KEGG analysis. The significance criteria for enrichment terms were set as a p-value less than 0.01, a minimum number of 3, and an enrichment factor greater than 1.5. False positive rate (FPR) analyses were eliminated using the Benjamini-Hochberg method with a q-value of 0.05 or less. These enrichment terms were then visualized using a bioinformatics platform (https://www.bioinformatics.com.cn), and bar and bubble plots were generated.
AI-TAR-PATH network construction
Based on the PPI network and KEGG analysis, the relationship between SM key bioactive compounds, core gene targets, and the top 20 KEGG pathways (AI-TAR-PATH) was constructed using Cytoscape 3.10.1. In the constructed network, nodes represent bioactive compounds, targets, and pathways, and edges represent interactions among the three.
Receptor-ligand molecular docking simulations
Complex interactions between receptors (core targets) and ligands (bioactive components) were analyzed using advanced molecular docking methods. The 3D crystal structure of the core target was downloaded from the Protein Data Bank (PDB) (https://www.rcsb.org)95 and saved in PDB format. The molecules were dehydrated and desolvated using PyMOL 2.4.0 software. The pdb format file was then imported into the AutoDock Tools 1.5.7 software for hydrogenation of the target protein and exported as a qdbqt format file. The mol2 structure files of the bioactive components were downloaded from TCMSP and converted to qdbqt format files using AutoDock Tools 1.5.7. Finally, molecular semi-flexible docking was performed using AutoDock software, and the conformation with the best binding energy was selected as the final conformation based on the principle of low energy and reasonable conformation and imaged using Pymol software.
MD simulation
MD simulations were performed using the GROMACS 2020.3 software. The amber 99sb-ildn force field was used to generate protein topology files, while the general amber force field (GAFF) was used to generate ligand topology files. The protein-ligand complexes were placed in a cubic tank of water and solvated. To neutralize the system charge, appropriate amounts of Cl- and Na + were added to balance the system charge. The steepest descent method was used for 5.0 × 104 steps to achieve energy minimization with a 1.4 nm cutoff for coulombic and van der Waals interactions. A first-phase equilibrium was then performed with the NVT system integration at 300 K for 100 ps to stabilize the temperature of the system. The second-phase equilibrium was simulated with the NPT integration at 1 bar and 100 ps. All MD simulations were performed under isothermal and isostatic conditions at 300 K and 1 atmosphere with a simulation time of 100 nanoseconds (ns). Finally, general MD simulation parameters such as RMSD, Rg, and RMSF were evaluated for each complex. These parameters provide important information about the stability, conformational changes, and flexibility of the complexes during the simulations.
Binding free energy calculations
Binding free energy calculations can verify the strength of intermolecular interactions in receptor-ligand complexes96. We performed binding-free energy calculations using the MM-PBSA method. The gmx_MMPBSA module was used to analyze the energy contribution parameters, and the specific equations used in this study are as follows:
Conclusions
The objective of this study was to investigate the molecular mechanism of SM for ANFH by integrating network pharmacology, molecular docking, and MD simulation. Our findings indicate that SM-containing bioactive compounds, including dan-shexinkum d, cryptotanshinone, tanshinone Iia, and dihydrotanshinlactone, may be effective in treating ANFH by modulating the inflammatory response, apoptosis, OS, bone remodeling, and promoting vasoprotection. The major involved mechanisms include AGE-RAGE, IL-17, and TNF signaling pathways. Moreover, SM primarily tar-gets TP53, AKT1, EGFR, STAT3, BCL2, IL6, and TNF, which in turn modulate the aforementioned pathways and biological processes. These findings comprehensively elucidate the multi-component, multi-target, multi-pathway intervention mechanism of SM for the treatment of ANFH. They are expected to provide rationale and new in-sights for the further identification and characterization of potential drug candidates. Nevertheless, due to the intrinsic constraints of this study, it is imperative to substantiate and expand our findings through additional in vivo and in vitro investigations.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article (and/or) its Supplementary Materials.
Abbreviations
- TCM:
-
Traditional Chinese medicine
- MD:
-
Molecular dynamics
- PPI:
-
Protein–protein interaction
- GO:
-
Gene ontology
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- MM-PBSA:
-
Molecular Mechanics Poisson-Boltzmann Surface Area
- TP53:
-
Tumor antigen p53
- AKT1:
-
RAC-alpha serine/threonine protein kinase
- EGFR:
-
Epidermal growth factor receptor
- STAT3:
-
Signal transducer and activator of transcription 3
- BCL2:
-
Bcl-2-like protein 1
- IL6:
-
Interleukin-6
- TNF:
-
Tumor necrosis factor
- OS:
-
Oxidative stress
- BMSC:
-
Bone marrow mesenchymal stem cell
References
Narayanan, A. et al. Avascular necrosis of femoral head: a metabolomic, Biophysical, biochemical, Electron Microscopic and histopathological characterization. Sci. Rep. 7, 10721. https://doi.org/10.1038/s41598-017-10817-w (2017).
Rajpura, A., Wright, A. C. & Board, T. N. Medical management of osteonecrosis of the hip: a review. Hip Int. 21, 385–392. https://doi.org/10.5301/hip.2011.8538 (2011).
Petek, D., Hannouche, D. & Suva, D. Osteonecrosis of the femoral head: pathophysiology and current concepts of treatment. EFORT Open. Rev. 4, 85–97. https://doi.org/10.1302/2058-5241.4.180036 (2019).
Kamal, D. et al. A case of bilateral aseptic necrosis of the femoral head. Curr. Health Sci. J. 40, 289–292. https://doi.org/10.12865/chsj.40.04.12 (2014).
Zhao, D. et al. Guidelines for clinical diagnosis and treatment of osteonecrosis of the femoral head in adults (2019 version). J. Orthop. Translat. 21, 100–110. https://doi.org/10.1016/j.jot.2019.12.004 (2020).
Zhao, D. W. et al. Prevalence of nontraumatic osteonecrosis of the femoral head and its Associated Risk factors in the Chinese Population: results from a nationally Representative Survey. Chin. Med. J. (Engl). 128, 2843–2850. https://doi.org/10.4103/0366-6999.168017 (2015).
Tripathy, S. K., Goyal, T. & Sen, R. K. Management of femoral head osteonecrosis: current concepts. Indian J. Orthop. 49, 28–45. https://doi.org/10.4103/0019-5413.143911 (2015).
George, G. & Lane, J. M. Osteonecrosis of the femoral head. J. Am. Acad. Orthop. Surg. Glob Res. Rev. 6 https://doi.org/10.5435/JAAOSGlobal-D-21-00176 (2022).
Sen, R. K. Management of avascular necrosis of femoral head at pre-collapse stage. Indian J. Orthop. 43, 6–16. https://doi.org/10.4103/0019-5413.45318 (2009).
Marker, D. R., Seyler, T. M., Ulrich, S. D., Srivastava, S. & Mont, M. A. Do modern techniques improve core decompression outcomes for hip osteonecrosis? Clin. Orthop. Relat. Res. 466, 1093–1103. https://doi.org/10.1007/s11999-008-0184-9 (2008).
Moya-Angeler, J., Gianakos, A. L., Villa, J. C., Ni, A. & Lane, J. M. Current concepts on osteonecrosis of the femoral head. World J. Orthop. 6, 590–601. https://doi.org/10.5312/wjo.v6.i8.590 (2015).
Lespasio, M. J., Sodhi, N. & Mont, M. A. Osteonecrosis of the hip: a primer. Perm J. 23 https://doi.org/10.7812/tpp/18-100 (2019).
Gu, H. et al. Exploring the mechanism of Jinlida granules against type 2 diabetes mellitus by an integrative pharmacology strategy. Sci. Rep. 14, 10286. https://doi.org/10.1038/s41598-024-61011-8 (2024).
Liu, Y., Wang, K., Yan, Z. Y., Shen, X. & Yang, X. Prediction of active ingredients in Salvia miltiorrhiza Bunge. based on soil elements and artificial neural network. PeerJ 10, e12726. https://doi.org/10.7717/peerj.12726 (2022).
Guo, R. et al. Pharmacological activity and mechanism of Tanshinone IIA in Related diseases. Drug Des. Devel Ther. 14, 4735–4748. https://doi.org/10.2147/dddt.S266911 (2020).
XD, M. E. et al. Danshen: a phytochemical and pharmacological overview. Chin. J. Nat. Med. 17, 59–80. https://doi.org/10.1016/s1875-5364(19)30010-x (2019).
Zou, L. F. et al. Salvianolic acids from Salvia miltiorrhiza Bunge and their anti-inflammatory effects through the activation of α7nAchR signaling. J. Ethnopharmacol. 317, 116743. https://doi.org/10.1016/j.jep.2023.116743 (2023).
Wang, X., Yang, Y., Liu, X. & Gao, X. Pharmacological properties of tanshinones, the natural products from Salvia miltiorrhiza. Adv. Pharmacol. 87, 43–70. https://doi.org/10.1016/bs.apha.2019.10.001 (2020).
Zhou, L., Zuo, Z. & Chow, M. S. Danshen: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J. Clin. Pharmacol. 45, 1345–1359. https://doi.org/10.1177/0091270005282630 (2005).
Chen, W. & Chen, G. Danshen (Salvia Miltiorrhiza Bunge): a prospective Healing Sage for Cardiovascular diseases. Curr. Pharm. Des. 23, 5125–5135. https://doi.org/10.2174/1381612823666170822101112 (2017).
Ye, Z. et al. Expanding the therapeutic potential of Salvia miltiorrhiza: a review of its pharmacological applications in musculoskeletal diseases. Front. Pharmacol. 14, 1276038. https://doi.org/10.3389/fphar.2023.1276038 (2023).
Tao, S. et al. Tanshinone I activates the Nrf2-dependent antioxidant response and protects against as(III)-induced lung inflammation in vitro and in vivo. Antioxid. Redox Signal. 19, 1647–1661. https://doi.org/10.1089/ars.2012.5117 (2013).
Zhang, X. W. et al. Lipophilic extract and Tanshinone IIA derived from Salvia Miltiorrhiza Attenuate Uric Acid Nephropathy through suppressing oxidative stress-activated MAPK pathways. Am. J. Chin. Med. 48, 1455–1473. https://doi.org/10.1142/s0192415x20500718 (2020).
Jia, Y. et al. Salvia Miltiorrhiza Bunge (Danshen) based nano-delivery systems for anticancer therapeutics. Phytomedicine. 128, 155521. https://doi.org/10.1016/j.phymed.2024.155521 (2024).
Wu, Y., Zhang, C., Wu, J., Han, Y. & Wu, C. Angiogenesis and bone regeneration by mesenchymal stem cell transplantation with danshen in a rabbit model of avascular necrotic femoral head. Exp. Ther. Med. 18, 163–171. https://doi.org/10.3892/etm.2019.7556 (2019).
Zhang, H. F., Zhang, S. Y. & Liu, J. M. [Effect of point injection of red-sage-root on the hip joint function in ischemic femoral head necrosis patients]. Zhen Ci Yan Jiu. 34, 57–60 (2009).
Noor, F. et al. Network Pharmacology Approach for Medicinal Plants: Review and Assessment. Pharmaceuticals (Basel). 15 https://doi.org/10.3390/ph15050572 (2022).
Jiashuo, W. U., Fangqing, Z., Zhuangzhuang, L. I., Weiyi, J. & Yue, S. Integration strategy of network pharmacology in traditional Chinese medicine: a narrative review. J. Tradit Chin. Med. 42, 479–486. https://doi.org/10.19852/j.cnki.jtcm.20220408.003 (2022).
Kanehisa, M. & Goto, S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30. https://doi.org/10.1093/nar/28.1.27 (2000).
Kanehisa, M., Furumichi, M., Sato, Y., Kawashima, M. & Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 51, D587–d592. https://doi.org/10.1093/nar/gkac963 (2023).
Gaillard, T. Evaluation of AutoDock and AutoDock Vina on the CASF-2013 Benchmark. J. Chem. Inf. Model. 58, 1697–1706. https://doi.org/10.1021/acs.jcim.8b00312 (2018).
Brüschweiler, R. Efficient RMSD measures for the comparison of two molecular ensembles. Root-mean-square deviation. Proteins. 50, 26–34. https://doi.org/10.1002/prot.10250 (2003).
Baskin, L. S. Electric conductance and pH measurements of isoionic salt-free bovine mercaptalbumin solutions. An evaluation of root-mean-square proton fluctuations. J. Phys. Chem. 72, 2958–2962. https://doi.org/10.1021/j100854a047 (1968).
Lobanov, M., Bogatyreva, N. S. & Galzitskaia, O. V. [Radius of gyration is indicator of compactness of protein structure]. Mol. Biol. (Mosk). 42, 701–706 (2008).
Bharatiy, S. K. et al. In Silico Designing of an industrially sustainable carbonic anhydrase using Molecular Dynamics Simulation. ACS Omega. 1, 1081–1103. https://doi.org/10.1021/acsomega.6b00041 (2016).
Mont, M. A., Zywiel, M. G., Marker, D. R., McGrath, M. S. & Delanois, R. E. The natural history of untreated asymptomatic osteonecrosis of the femoral head: a systematic literature review. J. Bone Joint Surg. Am. 92, 2165–2170. https://doi.org/10.2106/jbjs.I.00575 (2010).
Singh, M. et al. A molecular Troika of Angiogenesis, Coagulopathy and endothelial dysfunction in the Pathology of Avascular necrosis of femoral head: a Comprehensive Review. Cells 12 https://doi.org/10.3390/cells12182278 (2023).
Konarski, W. et al. Avascular necrosis of femoral head-overview and current state of the art. Int. J. Environ. Res. Public. Health 19 https://doi.org/10.3390/ijerph19127348 (2022).
Wei, B. et al. Bioactive components and molecular mechanisms of Salvia miltiorrhiza Bunge in promoting blood circulation to remove blood stasis. J. Ethnopharmacol. 317, 116697. https://doi.org/10.1016/j.jep.2023.116697 (2023).
Sun, K. et al. Tanshinone I alleviates steroid-induced osteonecrosis of femoral heads and promotes angiogenesis: in vivo and in vitro studies. J. Orthop. Surg. Res. 18, 474. https://doi.org/10.1186/s13018-023-03934-y (2023).
Chen, R. et al. Exploring the potential mechanism of Taohong Siwu decoction in the treatment of avascular necrosis of the femoral head based on network pharmacology and molecular docking. Med. (Baltim). 102, e35312. https://doi.org/10.1097/md.0000000000035312 (2023).
Shi, W. et al. Identification of hub genes and pathways Associated with oxidative stress of cartilage in osteonecrosis of femoral Head using Bioinformatics Analysis. Cartilage. 13, 19476035221074000. https://doi.org/10.1177/19476035221074000 (2022).
Imran, M. et al. Luteolin, a flavonoid, as an anticancer agent: a review. Biomed. Pharmacother. 112, 108612. https://doi.org/10.1016/j.biopha.2019.108612 (2019).
Zhou, Z. et al. Luteolin protects chondrocytes from H(2)O(2)-Induced oxidative Injury and attenuates osteoarthritis progression by activating AMPK-Nrf2 signaling. Oxid. Med. Cell. Longev. 2022, 5635797. https://doi.org/10.1155/2022/5635797 (2022).
Ou, H. C. et al. Luteolin: a natural flavonoid enhances the survival of HUVECs against oxidative stress by modulating AMPK/PKC pathway. Am. J. Chin. Med. 47, 541–557. https://doi.org/10.1142/s0192415x19500289 (2019).
Xu, X. et al. Luteolin ameliorates necroptosis in glucocorticoid-induced osteonecrosis of the femoral head via RIPK1/RIPK3/MLKL pathway based on network pharmacology analysis. Biochem. Biophys. Res. Commun. 661, 108–118. https://doi.org/10.1016/j.bbrc.2023.04.023 (2023).
Xu, J., Zhi, X., Zhang, Y. & Ding, R. Tanshinone IIA alleviates IL-1β-induced chondrocyte apoptosis and inflammation by regulating FBXO11 expression. Clin. (Sao Paulo). 79, 100365. https://doi.org/10.1016/j.clinsp.2024.100365 (2024).
Liao, H. et al. Bone mesenchymal stem cells co-expressing VEGF and BMP-6 genes to combat avascular necrosis of the femoral head. Exp. Ther. Med. 15, 954–962. https://doi.org/10.3892/etm.2017.5455 (2018).
Qian, K., Xu, H., Dai, T. & Shi, K. Effects of Tanshinone IIA on osteogenic differentiation of mouse bone marrow mesenchymal stem cells. Naunyn Schmiedebergs Arch. Pharmacol. 388, 1201–1209. https://doi.org/10.1007/s00210-015-1154-x (2015).
Maione, F. et al. Tanshinone IIA, a major component of Salvia milthorriza Bunge, inhibits platelet activation via Erk-2 signaling pathway. J. Ethnopharmacol. 155, 1236–1242. https://doi.org/10.1016/j.jep.2014.07.010 (2014).
Chen, L. et al. Tanshinone IIA ameliorated endothelial dysfunction in rats with chronic intermittent hypoxia. Cardiovasc. Pathol. 31, 47–53. https://doi.org/10.1016/j.carpath.2017.06.008 (2017).
Jeon, S. J., Son, K. H., Kim, Y. S., Choi, Y. H. & Kim, H. P. Inhibition of prostaglandin and nitric oxide production in lipopolysaccharide-treated RAW 264.7 cells by tanshinones from the roots of Salvia miltiorrhiza bunge. Arch. Pharm. Res. 31, 758–763. https://doi.org/10.1007/s12272-001-1223-4 (2008).
Yue, S., Su, X., Teng, J., Wang, J. & Guo, M. Cryptotanshinone interferes with chondrocyte apoptosis in osteoarthritis by inhibiting the expression of miR–574–5p. Mol. Med. Rep. 23 https://doi.org/10.3892/mmr.2021.12063 (2021).
Vezzani, G. et al. Hyperbaric oxygen therapy modulates serum OPG/RANKL in femoral head necrosis patients. J. Enzyme Inhib. Med. Chem. 32, 707–711. https://doi.org/10.1080/14756366.2017.1302440 (2017).
Lee, J. W. et al. Inhibitory effect of luteolin on osteoclast differentiation and function. Cytotechnology. 61, 125–134. https://doi.org/10.1007/s10616-010-9253-5 (2009).
Jing, Z. et al. Luteolin attenuates glucocorticoid-induced osteoporosis by regulating ERK/Lrp-5/GSK-3β signaling pathway in vivo and in vitro. J. Cell. Physiol. 234, 4472–4490. https://doi.org/10.1002/jcp.27252 (2019).
Ekeuku, S. O., Pang, K. L. & Chin, K. Y. The skeletal effects of Tanshinones: a review. Molecules. 26 https://doi.org/10.3390/molecules26082319 (2021).
Tong, A. H. et al. A combined experimental and computational strategy to define protein interaction networks for peptide recognition modules. Science. 295, 321–324. https://doi.org/10.1126/science.1064987 (2002).
Bieging, K. T., Mello, S. S. & Attardi, L. D. Unravelling mechanisms of p53-mediated tumour suppression. Nat. Rev. Cancer. 14, 359–370. https://doi.org/10.1038/nrc3711 (2014).
Gu, Z. et al. p53/p21 Pathway involved in mediating cellular senescence of bone marrow-derived mesenchymal stem cells from systemic lupus erythematosus patients. Clin Dev Immunol 2013, 134243. https://doi.org/10.1155/2013/134243 (2013).
Zhang, F. et al. P53 and Parkin co-regulate mitophagy in bone marrow mesenchymal stem cells to promote the repair of early steroid-induced osteonecrosis of the femoral head. Cell. Death Dis. 11, 42. https://doi.org/10.1038/s41419-020-2238-1 (2020).
Vandoorne, K. et al. Bone vascularization and trabecular bone formation are mediated by PKB alpha/Akt1 in a gene-dosage-dependent manner: in vivo and ex vivo MRI. Magn. Reson. Med. 64, 54–64. https://doi.org/10.1002/mrm.22395 (2010).
Mukherjee, A. & Rotwein, P. Selective signaling by Akt1 controls osteoblast differentiation and osteoblast-mediated osteoclast development. Mol. Cell. Biol. 32, 490–500. https://doi.org/10.1128/mcb.06361-11 (2012).
Ulici, V. et al. The role of Akt1 in terminal stages of endochondral bone formation: angiogenesis and ossification. Bone. 45, 1133–1145. https://doi.org/10.1016/j.bone.2009.08.003 (2009).
Papanastasiou, A. D. et al. RANK and EGFR in invasive breast carcinoma. Cancer Genet. 216–217, 61–66. https://doi.org/10.1016/j.cancergen.2017.07.004 (2017).
Linder, M. et al. EGFR controls bone development by negatively regulating mTOR-signaling during osteoblast differentiation. Cell. Death Differ. 25, 1094–1106. https://doi.org/10.1038/s41418-017-0054-7 (2018).
Chandra, A., Lan, S., Zhu, J., Siclari, V. A. & Qin, L. Epidermal growth factor receptor (EGFR) signaling promotes proliferation and survival in osteoprogenitors by increasing early growth response 2 (EGR2) expression. J. Biol. Chem. 288, 20488–20498. https://doi.org/10.1074/jbc.M112.447250 (2013).
Zhou, S. et al. STAT3 is critical for skeletal development and bone homeostasis by regulating osteogenesis. Nat. Commun. 12, 6891. https://doi.org/10.1038/s41467-021-27273-w (2021).
Samara, S. et al. Predictive role of cytokine gene polymorphisms for the development of femoral head osteonecrosis. Dis. Markers. 33, 215–221. https://doi.org/10.3233/dma-2012-0928 (2012).
Zheng, L. W. et al. TNF-α regulates the early development of avascular necrosis of the femoral head by mediating osteoblast autophagy and apoptosis via the p38 MAPK/NF-κB signaling pathway. Cell. Biol. Int. 44, 1881–1889. https://doi.org/10.1002/cbin.11394 (2020).
Ren, Y. et al. Anti-interleukin-6 therapy decreases hip synovitis and bone resorption and increases bone formation following ischemic osteonecrosis of the femoral head. J. Bone Min. Res. 36, 357–368. https://doi.org/10.1002/jbmr.4191 (2021).
Beringer, A. & Miossec, P. Systemic effects of IL-17 in inflammatory arthritis. Nat. Rev. Rheumatol. 15, 491–501. https://doi.org/10.1038/s41584-019-0243-5 (2019).
Geng, W., Zhang, W. & Ma, J. IL-9 exhibits elevated expression in osteonecrosis of femoral head patients and promotes cartilage degradation through activation of JAK-STAT signaling in vitro. Int. Immunopharmacol. 60, 228–234. https://doi.org/10.1016/j.intimp.2018.05.005 (2018).
Miljkovic, D. & Trajkovic, V. Inducible nitric oxide synthase activation by interleukin-17. Cytokine Growth Factor. Rev. 15, 21–32. https://doi.org/10.1016/j.cytogfr.2003.10.003 (2004).
Zhang, Y. et al. IL-17A synergizes with IFN-γ to upregulate iNOS and NO production and inhibit chlamydial growth. PLoS One. 7, e39214. https://doi.org/10.1371/journal.pone.0039214 (2012).
Zou, D. et al. Th17 and IL-17 exhibit higher levels in osteonecrosis of the femoral head and have a positive correlation with severity of pain. Endokrynol Pol. 69, 283–290. https://doi.org/10.5603/EP.a2018.0031 (2018).
Ren, G. W. et al. Network-based pharmacology and bioinformatics study on the mechanism of action of gujiansan in the treatment of steroid-induced avascular necrosis of the femoral head. Biomed Res Int 2022, 8080679. https://doi.org/10.1155/2022/8080679 (2022).
Lee, S. E. et al. Tumor necrosis factor-alpha supports the survival of osteoclasts through the activation of akt and ERK. J. Biol. Chem. 276, 49343–49349. https://doi.org/10.1074/jbc.M103642200 (2001).
Luo, G., Li, F., Li, X., Wang, Z. G. & Zhang, B. TNF–α and RANKL promote osteoclastogenesis by upregulating RANK via the NF–κB pathway. Mol. Med. Rep. 17, 6605–6611. https://doi.org/10.3892/mmr.2018.8698 (2018).
Wang, L. M. et al. Tumor necrosis factor-alpha inhibits osteogenic differentiation of pre-osteoblasts by downregulation of EphB4 signaling via activated nuclear factor-kappab signaling pathway. J. Periodontal Res. 53, 66–72. https://doi.org/10.1111/jre.12488 (2018).
Madge, L. A. & Pober, J. S. TNF signaling in vascular endothelial cells. Exp. Mol. Pathol. 70, 317–325. https://doi.org/10.1006/exmp.2001.2368 (2001).
Chen, G. & Goeddel, D. V. TNF-R1 signaling: a beautiful pathway. Science. 296, 1634–1635. https://doi.org/10.1126/science.1071924 (2002).
Chen, H. et al. Advanced glycation end products induced IL-6 and VEGF-A production and apoptosis in osteocyte-like MLO-Y4 cells by activating RAGE and ERK1/2, P38 and STAT3 signalling pathways. Int. Immunopharmacol. 52, 143–149. https://doi.org/10.1016/j.intimp.2017.09.004 (2017).
Advanced glycation end products. Induce actin rearrangement and subsequent hyperpermeability of endothelial cells. Apmis. 117, 549. https://doi.org/10.1111/j.1600-0463.2009.02464.x (2009).
McCarthy, A. D. et al. Non-enzymatic glycosylation of a type I collagen matrix: effects on osteoblastic development and oxidative stress. BMC Cell. Biol. 2, 16. https://doi.org/10.1186/1471-2121-2-16 (2001).
Ru, J. et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 6, 13. https://doi.org/10.1186/1758-2946-6-13 (2014).
Kim, S. et al. PubChem 2023 update. Nucleic Acids Res. 51, D1373–d1380. https://doi.org/10.1093/nar/gkac956 (2023).
Daina, A., Michielin, O. & Zoete, V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 7, 42717. https://doi.org/10.1038/srep42717 (2017).
UniProt. The Universal protein knowledgebase in 2023. Nucleic Acids Res. 51, D523–d531. https://doi.org/10.1093/nar/gkac1052 (2023).
Piñero, J., Saüch, J., Sanz, F. & Furlong, L. I. The DisGeNET cytoscape app: exploring and visualizing disease genomics data. Comput. Struct. Biotechnol. J. 19, 2960–2967. https://doi.org/10.1016/j.csbj.2021.05.015 (2021).
Amberger, J. S. & Hamosh, A. Searching online mendelian inheritance in man (OMIM): a knowledgebase of human genes and Genetic Phenotypes. Curr. Protoc. Bioinf. 58, 1.2.1–1.2.12. https://doi.org/10.1002/cpbi.27 (2017).
Stelzer, G. et al. The GeneCards suite: from Gene Data Mining to Disease Genome sequence analyses. Curr. Protoc. Bioinf. 54, 1.30.31–31.30.33. https://doi.org/10.1002/cpbi.5 (2016).
Szklarczyk, D. et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 47, D607–d613. https://doi.org/10.1093/nar/gky1131 (2019).
Zhou, Y. et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 10, 1523. https://doi.org/10.1038/s41467-019-09234-6 (2019).
Burley, S. K. et al. RCSB Protein Data Bank: powerful new tools for exploring 3D structures of biological macromolecules for basic and applied research and education in fundamental biology, biomedicine, biotechnology, bioengineering and energy sciences. Nucleic Acids Res. 49, D437–d451. https://doi.org/10.1093/nar/gkaa1038 (2021).
Liu, L. et al. Network Pharmacology, Molecular Docking and Molecular Dynamics to explore the potential immunomodulatory mechanisms of deer Antler. Int. J. Mol. Sci. 24 https://doi.org/10.3390/ijms241210370 (2023).
Funding
This study was supported by the Natural Science Foundation of Sichuan Province, China (2022ZYD104, 2023NSFSC1806).
Author information
Authors and Affiliations
Contributions
Conceptualization, X.W. and L.W.; methodology, X.W., L.W., and D.L.; software, L.H., X.W., and H.W.; validation, L.H. and H.W.; formal analysis, L.W.; investigation, D.L.; resources, X.W. and B.P.; data curation, L.W.; Writing—Original Draft: X.W.; Writing—Review & Editing: X.W., L.W., D.L., L.H., H.W.; supervision, B.P.; project administration, B.P.; funding acquisition, B.P. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, X., Wu, L., Luo, D. et al. Mechanism of action of Salvia miltiorrhiza on avascular necrosis of the femoral head determined by integrated network pharmacology and molecular dynamics simulation. Sci Rep 14, 28479 (2024). https://doi.org/10.1038/s41598-024-79532-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-79532-7