Abstract
Because a significant portion of oil remains in carbonate reservoirs, efficient techniques are essential to increase oil recovery from carbonate reservoirs. Wettability alteration is crucial for enhanced oil recovery (EOR) from oil-wet reservoirs. This study investigates the impact of different substances on the wettability of dolomite and calcite rocks. The substances include silicon dioxide (SiO2) and iron oxide (Fe3O4) nanofluids, gelatin biopolymer, surfactants (sodium dodecyl sulfate (SDS)), Fe3O4/SDS, seawater, and salt solutions (sodium chloride (NaCl) and calcium chloride (CaCl2)). Initially, water-wet rocks were exposed to crude oil for 22 days, resulting in significant contact angle changes. Dolomite and calcite contact angles increased from 56.50° and 50.70° to 107.70° and 104.00°, respectively, due to the presence of heavy and polar elements in the oil. The impact of aging time (7 and 11 days) on rock wettability was studied. Oil-wet rocks were treated with SiO2 and Fe3O4 nanofluids and SDS surfactants for 11 days. The contact angles of the treated rocks decreased significantly. For instance, the contact angles of dolomite and calcite treated with SDS surfactants decreased to 39.07° and 27.38°, respectively, indicating water-wet conditions. Dolomite and calcite surfaces aged with gelatin decreased the contact angles to 38.40° and 34.52°, respectively. Treatment with SiO2 nanofluid reduced the contact angles of dolomite and calcite to 54.27° and 53.17°, respectively, while treatment with Fe3O4 nanofluid decreased the contact angles to 46.08° and 51.16°, respectively. Using Fe3O4/gelatin nanocomposite resulted in contact angles of 26.00° for dolomite and 24.10° for calcite. The wettability alteration mechanism in nanofluids is attributed to structural disjoining pressure. Additionally, NaCl and CaCl2 solutions induced water-wet conditions, known as the salting-out effect, on dolomite and calcite specimens. Consequently, this study demonstrates the potential of various substances, such as nanofluids, surfactants, and salt solutions, to modify rock wettability and improve conditions for enhanced oil recovery.
Similar content being viewed by others
Introduction
The global demand for oil has increased in recent years, and oil production from existing oilfields is dropping due to reservoir depletion1,2. One of the major research areas of upstream industries in petroleum engineering is improving production and enhanced oil recovery (EOR) from oil reservoirs3.
Typically, there are three steps to the oil recovery process: primary, secondary, and tertiary, or enhanced oil recovery (EOR)4. Primary oil recovery is limited to hydrocarbons that are naturally produced from the existing displacement energy in a reservoir, such as solution-gas drive and natural water drive, etc5. In secondary production, the target formation is often re-pressurized by injecting gas or water, which leads to the movement of the remaining oil towards the production wells5. Tertiary recovery is related to oil production by some means of artificial stimulation after secondary recovery6. After application of primary and secondary oil recovery, two-third of the original oil in place (OOIP) remains in the reservoir4,7, which is trapped in place by surface and interfacial forces in the pore throats. The capillary forces that prevent the oil from moving in the porous medium can be reduced to produce residual oil8. Therefore, by altering the wettability of rock surfaces, capillary forces can be overcome9. The term “wettability” refers to a fluid’s propensity to stick or adsorb to a rock surface in the presence of another immiscible fluid. The position and distribution of reservoir fluids, which have an impact on the relative permeability of fluids and, consequently, on improved production, are determined by wettability. Therefore, the amount of oil that can be extracted from a reservoir depends significantly on wettability10,11,12. The wettability of a reservoir can be assessed by investigating its rock properties, fluid flow within the reservoir, variations in pressure and temperature, fluid production, and chemical substance injections used to improve oil recovery13,14,15. The main and most heavy part of crude oil is asphaltene16. The wettability of the reservoir rock alters due to the adsorption of the heavier and more polar ingredients of oil12. The various factors, including mineralogical structure and solution characteristics like salinity and pH, affect the wettability of minerals. In reality, a complete comprehension of all the intricate interactions and a thorough investigation of wettability offer a well-informed understanding of multiphase flows in oil reservoirs and lead to increased production in oil reservoirs17,18. To quantify the wettability alterations, any of the following methods can be utilized: measures of the surface image test, zeta potential, and contact angle4. Most research on wettability change measurements is performed using contact angle, which is defined by the point where the interface of the oil and water meets at the rock surface4. However, it is highly challenging to investigate contact angles in porous mediums, especially if it is a rock built up of a wide range of distinct minerals of different sizes19. After oil production, significant amounts of oil remain in reservoirs, necessitating the utilization of tertiary oil recovery methods, usually called EOR. Several techniques have been suggested for extracting residual oil17, including alkaline solution, polymer, miscible CO2, cyclic vapor, and bacteria20. Therefore, EOR methods can somewhat increase oil recovery21. The three primary categories of EOR procedures are typically chemical methods, gas injection methods, and thermal methods22. However, EOR methods are still economically, technically, and environmentally challenged because of high heat supply costs, excessive carbon dioxide (CO2) emissions, and costly post-treatment and maintenance23,24. Therefore, new nanomaterials can provide practical ways to overcome the disadvantages of EOR techniques20. One of the most prevalent EOR methods used in oil reservoirs includes water-based techniques like the infusion of NPs, polymers, salt solutions, or a mixture of them. High surface-to-volume ratios of nanoparticles cause more NPs to adsorb on the rock surface, which leads to a decrease in the contact angle25. Compared to the materials utilized in chemical EOR techniques, nanoparticles are more environmentally friendly. Furthermore, they can function more effectively in deep reservoirs that are subject to high temperatures and pressure26. Recently, there has been a lot of attention paid to the use of Fe3O4 nanoparticles for EOR applications27,28,29. Also, the performance of silica nanoparticles to improve the wettability of oil reservoir rocks has been studied30,31,32. Nanoparticles have recently found a specific place for modifying wettability in enhanced oil recovery as a result of progress achieved in the field of nanotechnology19. Polymers, among the EOR methods, have distinguished properties and high performance. Polymers improve the displacement of the oil that is trapped and, eventually, enhance oil recovery by reducing the ratio of the injection water’s mobility to that of the crude oil. Polymers could be generally divided into synthetic polymers and polymers that are natural. Natural polymers are polymers that are produced from natural plants. Hence, they are referred to as eco-friendly33,34.
Many studies showed that the surface modification of nanoparticles leads to improvement of the EOR mechanism, such as wettability alteration to water-wet state. Karimi et al.35 examined the effectiveness of nanofluids based on zirconium oxide (ZrO2) in modifying the wettability of carbonate reservoir rocks. It was demonstrated that developed ZrO2-based nanofluids could considerably change rock wettability from an oil-wet state to a water-wet state. Giraldo et al.36 performed studies on the performance of alumina (Al2O3)-based nanofluids on sandstones. It was shown that the presence of NPs can significantly improve the effectiveness of the surfactant and change the wettability of sandstone cores from an oil-wet state to a water-wet state. In another study, Roustaei and Bagherzadeh37 examined the effect of SiO2 NPs on the wettability of carbonate reservoirs. They declared that SiO2 NPs could alter the wettability of carbonate rocks from a strongly oil-wet condition to a strongly water-wet condition. Li et al.38 examined wettability modification with sandstone samples using nanoparticles. They found that silica-based nanofluid injection effectively enhanced oil recovery in sandstone reservoirs, causing wettability to alter to a neutrally wet condition.
Jang et al.39 wettability change tests on carbonate rocks were performed using silica NPs in reservoir conditions. The outcomes indicated that silica nanoparticles effectively improved dolomite and limestone wettability and reduced the contact angle. Mousavi et al.40 studied the effects of SDS surfactant and SiO2 NPs on the change in wettability of oil-wet carbonate reservoir rocks. The outcomes indicated that by increasing the aging time, the SiO2-SDS surfactant’s efficiency in wettability modification improves and the contact angle is substantially decreased. Hosseini et al.41 evaluated the effectiveness of TiO2 nanoparticles on the wettability change of carbonate reservoir rocks with high salinities and temperatures. The outcomes showed that nanofluids were highly stable at 20 w.t% salinity and 90 °C. Additionally, higher nanoparticle concentrations improve rock wettability.
Fe3O4@Chitosan nanocomposite was synthesized by Rezvani et al.25. According to their declaration, increasing the concentration of synthesized nanocomposite in seawater enhances oil recovery by altering the carbonate rock’s wettability to the water-wet state. Divandari et al.22 declared that citric acid-coated magnetite NPs have a higher efficiency in wettability change of the glass surface from oil-wet to water-wet compared to other nanoparticle solutions, which resulted in reducing the contact angle from 105° to 41°.
Numerous studies have been done recently on this subject due to the significance of wettability in the distribution and ultimately the motion of different fluids in the porous medium. Consequently, the purpose of this investigation is to look at the application of nanoparticles in the form of nanocomposite, the hybrid effect of NPs and SDS surfactant, biopolymer, various salts with various salinities, and seawater (Persian Gulf water) on the change of wettability. It should be noted that for the first time, the synthesized Fe3O4/gelatin nanocomposite and gelatin are investigated to alter the wettability on rocks of carbonate reservoirs (dolomite and calcite) for EOR applications.
Materials and methods
Figure 1 shows a diagram of the research process in this paper. According to Fig. 1, preparing the rock specimens, preparing the oil utilized in this study, and treating rocks with crude oil are demonstrated. Additionally, it illustrates the aging of the rock specimens using Fe3O4 nanofluid, SiO2 nanofluid, Fe3O4/gelatin nanocomposite, SDS-surfactant, Fe3O4-SDS, NaCl, CaCl2, Persian Gulf water, and gelatin. All of the tests were carried out a minimum of three times to ensure their precision.
Materials
Oil preparation
The crude oil employed in this investigation was obtained from an Iranian oil field. The characteristics of crude oil are displayed in Table 1.
Rock samples preparation
In this investigation, calcite (CaCO3) and dolomite (MgCa(CO3)2), two carbonate rocks, were used. The analysis of X-ray Fluorescence (XRF) of the calcite and dolomite rocks used in the present investigation is shown in Table 2. It is better to note that the XRF analysis was carried out using PW1410 (PHILIPS, NETHERLAND). After analyzing carbonate samples, small slabs of rock measuring 0.5 cm in height, 4 cm in width, and 4 cm in length were cut from rock samples to measure the wettability alteration. A smooth surface was also achieved by polishing the slices. The main purpose of this study is to investigate the effect of synthesized Fe3O4/gelatin nanocomposites and gelatin on wettability alteration in carbonate reservoir rocks. For this reason, we removed the rock’s roughness effect by polishing the rock’s surface to investigate precisely the effect of these substances on wettability alteration. In other words, all obtained results are affected by these substances. Therefore, the slabs were placed in Soxhlet’s washing cell, which included 400 ml of toluene (Merck, with a purity of 99 wt%), and washed for up to four hours. Following that, the specimens were kept in the oven for an hour at 40 °C. Finally, after washing the rock samples with distilled water for 10 h, we placed them in an oven at 40 °C for 12 h. To remove possible impurities, this technique was used to avoid errors in the evaluation of wettability and face scrubbing.
Chemical surfactant
In the current research, sodium dodecyl sulfate (SDS), an anionic surfactant, was utilized. Using the electrical conductivity measuring method, it was found that the critical micelle concentration (CMC) of SDS was almost 2450 ppm42. This substance was purchased from the Merck Company. Using a magnetic stirrer, surfactant solutions with a concentration of 2000 ppm were made in distilled water. The characteristics of the surfactant used in this research are illustrated in Table 3.
Salts
Monovalent and divalent cations are commonly present in brine solutions. and when rocks and oil are present, these cations may react differently during low-salinity water injection (LSWI)43. Subsequently, for this research, different salts, including calcium chloride (CaCl2) and sodium chloride (NaCl), were taken into consideration. NaCl (≥ 99.5%) and CaCl2 (≥ 99.5%) were bought from Merck Company and were employed to examine the impact of salinity on wettability alteration. Distilled water was used as the base fluid to make the brine solution, and various salt concentrations were added. The solutions were then combined for 40 min with a magnetic stirrer. Each solution was created and put to the test individually.
Persian Gulf water
Persian Gulf water was used to study how seawater salinity impacts the wettability of rocks. Therefore, the cut dolomite and calcite rocks were aged for 11 days in the water of the Persian Gulf. Finally, the desired experiments were performed on them. Table 4 indicates the water properties of the Persian Gulf.
Nanoparticles and nanofluids
In this research, Fe3O4 and SiO2 nanoparticles have been used. To create silica (SiO2) and iron oxide (Fe3O4) nanofluids, 0.752 gr of each material was added to 250 ml of distilled water separately. It was initially blended for one hour with the use of a magnetic stirrer to increase the stability of the suspension and achieve nanofluid homogeneity, and then it was put inside an ultrasonic apparatus. It should be noted that ultrasonication is advised for NPs44,45. To maintain the suspension stable for a longer period, the ultrasonic apparatus helps increase nanofluid stability by reducing the NPs clusters’ aggregation. To do this, a 3000 ppm solution of nanofluid was put inside an ultrasonic vibration device and subjected to ultrasonic vibration for 20 min at 100 W. Eventually, the carbonate rocks were aged in the prepared nanofluids for 11 days. The term “PPM” means grams of solute per million grams of solution46. In Table 5, the properties of Fe3O4 and SiO2 nanoparticles are listed.
Gelatin
In this study, gelatin (Sigma Aldrich) has been used as a biosurfactant to assess the wettability changes of oil-wet rock specimen surfaces. Therefore, 0.5 g of gelatin was added to 250 ml of distilled water and stirred with a magnetic stirrer for 1 h to create a 2000 ppm gelatin solution. After that, the oil-wet rock specimens were aged in gelatin solutions for 7 and 11 days.
Synthesis of Fe3O4/gelatin nanocomposite
Ferric chloride anhydrous (FeCl3) and ferrous chloride tetrahydrate (FeCl2.4H2O, both from Sigma-Aldrich) were prepared. Ammonium hydroxide (NH4OH at 25 w.t% in water (Merck)) was employed to alter the pH amount. Gelatin was used as a coating material.
In this investigation, the chemical co-precipitation technique was used to synthesize Fe3O4/gelatin nanocomposite. At first, a solution including 0.5 g of gelatin in 100 ml of distilled water was made for 5 h at 50 °C with constant stirring to prepare a gelatin solution. Then, the gelatin solution was chilled to 30 °C. Next, FeCl2.4H2O (Fe2+;1.5 g) and FeCl3 (Fe3+;3.0 g) were added to the gelatin solution. After that, it was stirred for an hour at 30 °C. Eventually, while the solution was constantly stirred, ammonium hydroxide was gradually added until the pH of the solution reached 10, and the resulting suspension was black. After 4 h of constant stirring at 30 °C, the suspension was centrifuged for 10 min at 3000 rpm. To eliminate excess chemical reagents, the precipitated particles were washed three times with distilled water and ethanol, after which they were dried for 12 h in an oven at 70 °C47.
Measurement of contact angle
Measuring the contact angle (i.e., the contact angle between the solid surface and liquid) is a significant technique for evaluating a rock’s wettability48. A stabilized wetness condition can be reached after 2–4 weeks of aging, according to Villard et al. (1993)49. In order to perform this research, the cut rocks were treated with crude oil at standard temperature and pressure for 5, 11, and 22 days. In this research, the contact angle was measured using the sessile drop method (Fig. 2). The technique included using a syringe pump with a needle and distilled water inside the syringe. Three drops of distilled water were poured on the rock surface, and then the contact angle between the solid surface and the distilled water drops was measured. Then the averaged data was registered. Ultimately, Image J software was utilized for analyzing the photos of the drops on the rock surface.
Specification of transmission electron microscopy (TEM) device and energy dispersive X-ray (EDX) device
TEM analysis is one of the most important methods for studying materials, especially nanostructured materials, due to its extremely high ability to image particles. Using TEM analysis, it is possible to study the microstructure of materials, observe nanoparticles, and examine their internal structure, such as their core and shell structures. Therefore, this study used EM208S 100KV (PHILIPS, NETHERLAND) for TEM analysis.
Rock specimens were identified in this investigation using energy dispersive X-ray (EDX) analysis (AMETEK, ELEMENT EDAX, USA).
Fourier transform infrared spectroscopy (FT-IR)
The apparatus used for measuring the FT-IR spectra of the solid samples in the range 400–4000 cm− 1 was a Brucker TENSOR 27 Spectroscopy. All spectra were acquired by averaging 32 scans at a resolution of 4 cm− 1. To perform the analysis, a dry, finely ground rock powder (fresh or aged) sample and an oil sample were directly mixed with KBr (potassium bromide, one of the most widely used alkali metal halides for FT-IR analysis) at a ratio of 100:1. The mixture was then compressed to make a pellet, which was placed in a desiccator to remove the moisture content of the samples50. The dried and treated samples were used for the FT-IR test.
Results and discussion
The current research investigated how oil affected the wettability of two dolomite and calcite rocks. First, the contact angle between the rock specimen surfaces (calcite and dolomite) and the drops of distilled water was measured. The measured contact angle between the surfaces of calcite and dolomite specimens with distilled water was 50.70° and 56.50°, respectively. This indicates that the rock specimens were water-wet before starting any experiment. Finally, after various aging times (5, 11, or 22 days), rock specimens were removed from crude oil, and the contact angles between the surfaces of oil-wet samples (i.e., calcite and dolomite) with distilled water were measured.
TEM and FT-IR of the synthesized Fe3O4/gelatin nanocomposite
TEM
When it is claimed that a core/shell nanocomposite is made, two characteristics must be proven: (1) the particles are nanosized, and (2) the formation of a core/shell nanocomposite. These two characteristics can be proved by using the TEM test. Therefore, Fig. 3 shows the TEM image of the synthesized Fe3O4/gelatin nanocomposite. As shown, the size range of synthesized nanocomposite was 30–80 nm, and gelatin was well coated on the external surface of Fe3O4 NPs with a nearly spherical shape. Eventually, we can validate the nanocomposites’ core/shell synthesis in Fig. 3. The spherical morphology of nanoparticles is suitable for injection into oil reservoirs due to high mobility and the easy separation of NPs22. Therefore, the nanocomposite synthesized in this research can be a good choice for injecting into oil reservoirs as an EOR agent. It should be noted that Fe3O4 and SiO2 NPs were purchased from the US Research Nanomaterials company (according to Table 5). All characteristics and TEM images of Fe3O4 and SiO2 NPs are available on the US Research Nanomaterials website. Therefore, a TEM analysis is not required for these NPs (i.e., SiO2 and Fe3O4 NPs). The Supplementary Information contains further details about SiO2 and Fe3O4 NPs. Also, other samples (i.e., NaCl, CaCl2, gelatin, and SDS surfactant) are not nanoparticles and are known substances. Therefore, a TEM test is not required for these substances.
FT-IR
Figure 4 depicts the FT-IR test of the Fe3O4 NPs, gelatin, and Fe3O4/gelatin nanocomposite. In the gelatin spectrum, the characteristic band at 3425.4 cm− 1 is related to the stretching vibrations of the N-H band51. Also, the characteristic peaks at 1644.3 and 1552.6 cm− 1 are assigned to the amide I and amide II bands, respectively51. In the Fe3O4 NPs spectrum, the Fe-O band is found in the 574.3 and 445.5 cm− 1 peaks52. The characteristic peaks of Fe3O4 NPs and gelatin can be found in the spectrum of Fe3O4/gelatin nanocomposite, which confirms the successful coating of gelatin onto the surface of Fe3O4 NPs. Moreover, in the spectrum of Fe3O4/gelatin nanocomposite, shifting of the N-H peak to a lower wave number and increasing broadness of its band indicate effective interaction between Fe3O4 NPs and gelatin by electrostatic attraction53.
Wettability change
Contact angles between 0° and 80°, 80° and 100°, and 100° and 160° are grouped as water-wet, neutral, and oil-wet, respectively54. All of the stated contact angles between water droplets (distilled water) and rock surfaces were measured in the tests.
Effect of oil on wettability
In this part, we examined the influence of the oil and its polar ingredients on rock specimens. Rock specimens (dolomite and calcite) were aged in oil for 5, 11, and 22 days. Figure 5 illustrates the contact angle characteristics of water droplets on rock specimen surfaces both before and after treatment in oil during a 22-day contact with oil period. It must be noted that all the tests in this study were done at ambient temperature and pressure. The contact angles of calcite and dolomite after 22 days of aging with oil were 104.00° and 107.70°, respectively. One of the factors impacting wettability change is the surface charge of carbonate rocks. Dolomite rocks show more change in wettability than calcite rocks. Compared to calcite, dolomite rocks have a greater positive surface charge40. Consequently, according to the results, the wettability change in dolomite is greater than in calcite. The wettability of rock surfaces is significantly affected by aging time55. According to Fig. 6, as the aging time of the rocks increases, the contact angle of the rock samples increases, and the two rocks, calcite and dolomite, become oil-wet. At pH values below 8–9.5, carbonate surfaces are often positively charged56,57 and act as feeble bases. Therefore, the acidic ingredients in the oil could adsorb onto the carbonate rock’s surfaces and change the rock’s initial surface wettability characteristics58. According to the results of numerous investigations, carboxylic acids irreversibly adsorb onto the surface of calcite59,60. Surface wettability may change due to the presence of polar functional groups in oil and other polar ingredients, such as asphaltene. Also, it is possible to change wettability with phenols and crude oil amides61,62.
Analysis of EDX
The results of the EDX investigation of the rock surfaces before treatment (pure calcite and dolomite rocks) and after aging with oil are displayed in Fig. 7. It should be noted that EDX analysis was performed on rock samples that had been aged in oil for 22 days. According to the analysis, the calcite is mainly composed of O (52.45 wt%), Ca (30.96 wt%), C (12.99 wt%), Br (1.41 wt%), and Si (1.19 wt%). And Fe (1.01 wt%) that are present in the composition of untreated calcite. Additionally, for dolomite rock, the percentage of elements identified in the EDX spectrum was O (46.55 wt%), Ca (33.59 wt%), Mg (9.69 wt%), C (7.41 wt%), Fe (2.37 wt%), and Si (0.39 wt%). Calcite and dolomite are the two types of rock samples determined by the amounts of Ca, O, C, and Mg components63. To comprehend more about the interaction of oil with the rock specimen’s surfaces, EDX tests of the surfaces were taken after aging with oil. Table 6 indicates components observed by EDX analysis on the surfaces of rock specimens before and after treatment with crude oil.
The results show that before aging, the carbon content in calcite rock and dolomite rock was 12.99 and 7.41 wt%, respectively, while it was approximately 83.45 and 49.18 wt% in treated rocks with oil. Therefore, the rise in carbon atoms is the outcome of oil absorption on the rock’s surface, which causes the wettability change. Therefore, the oil adsorption on the rock samples altered the wettability of dolomite and calcite rocks from a water-wet to an oil-wet condition.
FT-IR analysis
A useful analytical method that provides thorough insights into the chemical bonds and functional groups of both organic and inorganic compounds is infrared spectroscopy50,64,65. Oscillators are reflected in the infrared spectrum as a result of molecular vibration and spin50. To identify molecules, their chemical structures, and how they interact with their surroundings, infrared spectroscopy can be used50,65,66. Bond length or bond angle can change as a result of vibrations, leading to bending or stretching. Bond stretching can occur symmetrically or asymmetrically, in or out of phase67. Figure 8 illustrates the FT-IR spectra of pure calcite, pure dolomite, and calcite and dolomite aged with oil in the range of 4000 –400 cm− 1. The adsorption peaks of the carbonate group are displayed in Fig. 8, which is a spectrum of pure calcite. The carbonate anion bond relates to a peak of 1800.23 cm− 1 64. C-O stretching, out-of-plane bending, and in-plane bending vibrations are all attributable to the band at 1435.90 cm− 1, the band at 875.26 cm− 1, and the peak at 710.85 cm− 1, respectively. The results obtained are very similar to the opinions of other studies50, 64,66,68,69,70. The carbonate group relates to the C-O stretching vibration at an area of 1435.90 cm− 1. The vibrations of adsorbed water’s O-H stretching are attributed to the band between 3405.96 and 3548.91 cm[− 1 50, 64.
Band-symmetric deviations in the spectrum are visible after the calcite in oil has aged. An asymmetrical band indicates a mixture. After aging calcite with oil, this can be an indication of sample alteration67. The spectra of the oil-treated calcite illustrate more absorption at 2515.98 cm− 1, which indicates the concentration of hydrogen carbonate after treatment was increased71. An alkyl C-H bond can be seen in the peak at 2971.03 cm− 172. Because of the asymmetric stretch vibrations of the C-O bonds, adsorbing carboxylate groups formed a tiny absorption band of approximately 1798.80 cm− 1 64. Methyl asymmetric C-H stretching can be attributed to the band at 2869.36 cm− 1 67. These findings match the results that have been reported in research40,67,71. The FT-IR analysis results of pure dolomite and dolomite treated with oil are shown in Table 7.
Effects of seawater, SDS surfactant and Fe3O4/SDS on the wettability
For wettability modification, seawater can be used as a fluid. Thus seawater can enhance oil recovery through spontaneous absorption74,75. The attendance of Mg2+, SO42−, and Ca2+ ions in seawater is the agent of the wettability alteration40. Strong bonds are formed between the carboxylic groups and the positively charged calcium on the rock surfaces. On the surfaces of rocks, Mg2+ could substitute for Ca2+. Or, in other words, Ca2+ bound to the carboxylic group can be displaced by Mg2+. Therefore, Mg2+ can play a role in the mechanism of wettability change in seawater because it is a reactive ion towards the surfaces of rocks. A repelling force between carboxyl groups and SO42− occurs through the absorption of SO42− ions on the surfaces of calcite and dolomite. Therefore, the wettability of dolomite and calcite rocks alters when carboxyl groups are displaced from the rock surfaces40,75.
Oil-wet dolomite and calcite rocks were aged for 7 and 11 days in seawater. After placing the rocks in the oven and cooling them, their contact angle was measured. The contact angle of oil-wet dolomite rock decreased from 107.70° to 99.38° after aging for 7 days in Persian Gulf water. Also, the contact angle of oil-wet calcite rock decreased from 104.00° to 98.00° after 7 days of aging in Persian Gulf water. With rising treatment times, the contact angles of oil-wet dolomite and calcite rocks decreased from 107.70° to 92.09° and from 104.00° to 91.59°, respectively. The experimental observations indicate that seawater can only slightly alter the contact angle of rock specimens. The high salinity of seawater may be the cause of the slight contact angle reduction it causes. Based on the process of the salting-out effect, fewer carboxylic compounds can dissolve in the aqueous phase55.
Different surfactants have been evaluated for their effectiveness in oil recovery through laboratory testing and field research. According to the type of hydrophilic head group, they could be generally classified as anionic, non-ionic, cationic, and zwitterionic surfactants4. As a result, in this study, we evaluated wettability modification using SDS, an anionic surfactant. This type of surfactant contains a surface-active part with a negative charge4. To assess wettability alterations caused by surfactant, dolomite and calcite rocks aged with oil (rocks aged for 22 days) were chosen. The initial contact angles of dolomite and calcite rocks after aging in oil for 22 days were 107.70° and 104.00°, respectively. It is obvious that the rocks are oil-wet from the calculated contact angles. So, for 7 and 11 days, calcite and dolomite rocks were submerged in surfactant at a 2000 ppm concentration. The rocks were subsequently placed in a 45 °C oven for 40 min, and their contact angle was measured. After being treated with surfactants, the measured angles of the rocks, calcite, and dolomite, considerably decreased.
The dolomite contact angle was reduced from 107.70° to 44.12° and calcite from 104.00° to 33.16° after 7 days of SDS surfactant treatment. Dolomite rock’s contact angle decreased from 107.70° to 39.07° and calcite rocks from 104.00° to 27.38° after 11 days of aging in SDS surfactant. Therefore, according to Fig. 9, with increased aging time, the wettability of the rock changes to a water-wet state. The outcomes indicated that SDS surfactant is highly effective at modifying the wettability of rock surfaces. This is related to the fact that surfactants have charges on their surfaces; after forming micelles in the solution, SDS produces positive and negative charges, respectively. Since carbonates are positively charged, SDS functional groups adsorb to them, causing a surface to become more water-wet76. The molecules of surfactants contain both hydrophilic and hydrophobic parts. They can be absorbed in very low concentrations. The wettability of the minerals and, consequently, the movement of the oil are influenced by surfactant adsorption on the minerals and the position the surfactant selects17.
The surfactant absorbs to the surface through a hydrophobic reaction with hydrocarbon layers that have been absorbed on the surfaces of the dolomite and calcite rocks. The surfactants are absorbed as a monolayer of molecules of surfactant40,77. There has been a lot of investigation performed on the mixture of NPs with surfactants for EOR techniques. Their combination alters the characteristics of the reservoir by modifying wettability and adsorption at the oil-water interface due to surface-active groups78. When surfactant is injected alone in EOR methods, its amount decreases due to the surfactant’s high absorption on the rock surfaces79,80,81. The composition of chemicals and characteristics of the rock surfaces affect the surfactant’s absorption in the oil reservoirs. Also, high salinity, regardless of the concentration of the surfactant, causes high surfactant absorption4,82. Nevertheless, because nanoparticles aggregate quickly, using them alone has restrictions for the injection processes. Integrating nanoparticles with surfactants can result in stronger characteristics than utilizing these two substances separately due to their synergistic effects83. Through a phenomenon known as competitive adsorption, the surfactant’s interaction with nanoparticle surfaces reduces the quantity of surfactant that is absorbed into rock pores84,85.
Therefore, in the next portion of the experiment, the wettability of oil-wet rock specimens was investigated using a combination of SDS surfactant and Fe3O4 nanoparticles. Accordingly, dolomite and calcite rocks that had been treated with oil for 22 days were immersed in nano-surfactant (Fe3O4/SDS surfactant) for 7 and 11 days, respectively. The concentrations of Fe3O4 NPs and SDS surfactant used in the nano-surfactant solution were 3000 ppm and 2000 ppm as the optimum concentrations, respectively. To maintain the same conditions throughout the experiment, the base fluid employed was distilled water.
Dolomite and calcite rocks aged in nano-surfactant were put into the oven at 45 °C for 45 min. Afterwards, after the rocks cooled down, their contact angle was measured.
Dolomite and calcite were aged in nano-surfactant for 7 days, and the contact angles were measured at 34.65° and 29.46°, respectively. Also, the measured contact angles of dolomite and calcite after being immersed in nano-surfactant fluid for 11 days were 29.67° and 25.03°, respectively. For comparison and better understanding, the contact angle changes of dolomite and calcite samples due to seawater, SDS surfactant, and Fe3O4/SDS can be seen in Fig. 9.
Due to the outcomes obtained, the contact angles of the rocks decreased significantly with increasing the aging time of the rocks for 11 days in nano-surfactant. It indicates that the nano-surfactant used in this research was efficient in modifying the wettability of rock surfaces.
Wettability change by Fe3O4 and SiO2 NPs
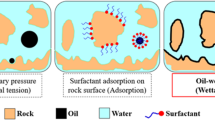
The impact of Fe3O4 and SiO2 NPs on the modification of rocks’ wettability was investigated in the next step of the experiment. The wettability change mechanism in nanofluids is called “structural disjoining pressure86. In the beginning, the ordering of the nanoparticles causes a wedge-shaped structure to form between the solid and oil substrates (as illustrated in Fig. 10)87. The wedge tip’s NPs spreads due to the rising structural disjoining pressure. Thus, the possibility of NPs dispersion increases88. Ultimately, the oil adhered to the surface would be removed by the water film containing NPs by applying force and lowering the system’s free energy89. Brownian motion and the electrostatic repelling force between NPs are important factors in the creation of structural disjoining pressure90. The oil drop desorption can be helped by the nanoparticles’ ability to destabilize oil layers on oil-wet surfaces. Oil droplets exiting the surface cause the wettability to shift into a water-wet state91. One of the other possible mechanisms can be the absorption of NPs on the rock surface, which leads to a wettability change on the rock surface by increasing the disjoining pressure35. physisorption of the NPs on the rock can be named as the primary identified wettability alteration mechanism during nanofluid treatment92. During NPs adsorption, layer(s) of NPs are formed over the rock (or oleic phase) to change the surface wettability92,93. NPs are small-sized materials with an extraordinarily high surface area, they can absorb chemicals on their surface, based on their surface energy and the structure of adjacent molecules94,95. Besides, adsorption of the NPs can impact the substrate roughness and thus can shift the system headed for a more water-wet depending on NPs and surface wettability35,96,97.

Modified from98.
Mechanisms: (a) wettability change by NPs and (b) structural disjoining pressure by NPs.
Oil-wet rock specimens were submerged in Fe3O4 and SiO2 nanofluids for 7 and 11 days, respectively. The optimal concentration for both nanofluids was determined to be 3000 ppm. After placing the rocks in the oven and cooling them in the ambient air, their contact angle was measured. Figure 11 indicates the contact angle changes caused by SiO2 and Fe3O4 nanofluids.
As can be observed, after 7 days, the contact angle of rocks aged in SiO2 nanofluid became water-wet. Therefore, the contact angle of dolomite decreased from 107.70° to 66.16°, and the contact angle of calcite decreased from 104.00° to 64.13°. With the increase in the immersion of rocks for 11 days, the contact angle of dolomite decreased from 107.70° to 54.27°, and that of calcite decreased from 104.00° to 53.17°. Thus, it can be concluded that nanoparticles like surfactants alter the wettability of rock surfaces99.
The contact angles of dolomite and calcite rocks were then measured after being immersed in Fe3O4 nanofluid for 7 and 11 days, respectively. According to Fig. 11, after the treatment for 7 days with Fe3O4 nanofluid, the contact angle of dolomite rock decreased from 107.70° to 53.38° and that of oil-wet calcite rock decreased from 104.00° to 56.21°. With the aging period of the rocks increasing (11 days), the measured contact angles decreased significantly. Thus, the contact angle of dolomite rock decreased from 107.70° to 46.08°, and that of oil-wet calcite rock decreased from 104.00° to 51.16°. This may be caused by a change in surface free energy due to nanoparticles adsorption onto the solid surface35.
EDX analysis
EDX analysis was performed for calcite and dolomite rocks after immersion in SiO2 and Fe3O4 nanofluids. According to Table 8, the silica amount of calcite rock after treatment by SiO2 nanofluid was 17.20 wt%. Before aging, no silica was identified in dolomite rock. While the amount of silica after aging of dolomite rock in SiO2 nanofluid became 15.17 wt%. Also, no iron was observed in the calcite and dolomite rocks before treatment, whereas iron amounts were 32.18 wt% and 32.35 wt%, respectively, after treatment. As a result, it can be observed from the outcomes of the EDX test and measurement of contact angle that Fe3O4 and SiO2 NPs can decrease the contact angle of rock specimens. The EDX test of rock specimens after treatment with SiO2 and Fe3O4 nanofluids is illustrated in Fig. 12.
The impacts of NaCl and CaCl2 on the wettability
In this section, we examine the individual impacts of ions on rock surfaces. In this way, solutions with different concentrations were created as follows to evaluate the impact of NaCl and CaCl2 salts on the modification of the wettability of rock surfaces:
Aqueous solutions were made with NaCl and CaCl2 salts at concentrations of 1000, 5,000, 10,000, 25,000, 50,000, 100,000, and 200,000 ppm. Distilled water was used to make the solution. Then, calcite and dolomite rocks were treated for 11 days at different concentrations in an aqueous NaCl solution. Also, the aqueous solution was made with CaCl2 salt at similar concentrations. After aging rock specimens in CaCl2 and NaCl solutions, their contact angles were examined. The contact angles of dolomite and calcite rocks after treatment with NaCl and CaCl2 solutions are illustrated in Fig. 13.
As seen in Fig. 13(a) and (b), at first, when the NaCl solution concentration rises from 1000 to 10,000 ppm, the contact angle of calcite rocks and oil-wet dolomite decreases from 104.00° and 107.70° to 70.10° and 73.10°, respectively. After reaching the optimal salinity, the contact angle of rock specimens increased when the concentration of NaCl increased. Therefore, with the rise in concentration of NaCl from 10,000 to 200,000 ppm, the contact angles of calcite and dolomite specimens rise from 70.10° and 73.10° to 83.00° and 97.00°, respectively. Likewise, according to Fig. 13(c) and (d), at first, as the CaCl2 concentration increased, the rock specimens’ contact angles quickly decreased in the solution. After reaching the optimum point, an increase in the contact angle with an increasing concentration of CaCl2 was observed. Thus, the contact angle of oil-wet calcite and dolomite rocks decreased from 104.00° to 64.20° and from 107.70° to 66.00°, respectively, with increasing concentrations of CaCl2 solution (from 1000 to 25000 ppm). The contact angle of calcite and dolomite rocks increased from 64.20° to 85.90° and from 66.00° to 89.60° with rising concentrations of CaCl2 solution (from 25,000 to 200,000 ppm).
The results obtained demonstrate that alterations in the contact angle of the rocks are a function of the concentration of solutions of NaCl and CaCl2. As seen in Fig. 13, the wettability change of oil-wet rock specimens is not possible in NaCl and CaCl2 solutions with high salinity. The process of the salting-out effect and double-layer expansion for wettability change in low-salinity states was proposed by Lashkarbolooki et al. (2016)100.
Because of the greater ionic strength of CaCl2 than NaCl, the optimal salinity in CaCl2 solutions has a more pronounced effect on contact angle. The reduction in the solubility of organic compounds due to increased salinity and the salting-out effect is another factor related to the contact angle rising in these two salts’ presence55. As a result, carboxylic components have more solubility in the aqueous solution when the salt concentration is reduced. Finally, Breaking the bond between the positively charged rock surfaces and the negatively charged acidic groups in the oil, Causes the contact angle to decrease. The process of double-layer expansion will be activated by lowering the NaCl and CaCl2 salt concentrations55,101.
Finally, Fig. 13 indicates the CaCl2 solution’s contact angle reduction is higher than that of the NaCl solution. For reducing contact angles, divalent ions are superior to monovalent ions40.
The impacts of gelatin and Fe3O4/gelatin nanocomposite on the wettability
Gelatin is an ideal candidate for surface modification of NPs to use in nano-flooding because of its special characteristics, like biocompatibility, non-toxicity, surface activity, and low cost102. Collagen from animal skin and bones is thermally denatured or partially hydrolyzed to produce this natural polymer103,104. Gelatin is a mix of amino acids joined by amide bonds in a long molecular chain105. This substance has hydrophilic amino acids and hydrophobic amino acids. Table 9 shows the hydropathy index and other information used for gelatin. Therefore, gelatin can act as a biosurfactant due to its hydrophilic and hydrophobic amino acids. The process for wettability change of the rock specimen surfaces by gelatin is to create capillary forces via hydrophobic interaction between its hydrophobic amino acids and the oleic phase. As a result, this force removes the oil coating from the rock surface and causes the rock surface to become water-wet.
The mechanism of change in wettability by gelatin is shown in Fig. 14. In this research, gelatin was used as a natural polymer to assess the wettability of samples of oil-wet rock. Therefore, a 2000 ppm gelatin solution was created, and rock specimens were aged in the gelatin solution for 7 and 11 days, respectively, followed by a measurement of their contact angle. The contact angle of dolomite and calcite rocks decreased from 107.70° and 104.00° to 44.00° and 41.00° in 7 days. By increasing the contact time of rock specimens in gelatin solution, the contact angles of dolomite and calcite rocks decreased from 107.70° and 104.00° to 38.40° and 34.52°, respectively. According to the test findings, gelatin, as a natural polymer, can play an effective role in reducing the contact angle.
Some experiments have shown the modified behavior of Fe3O4 nanoparticles25. Although the usage of gelatin hasn’t been extensively researched in recent years as a polymer in EOR techniques, this could be an opportunity to assess this polymer’s potential for EOR applications. Hence, this study tries to examine the combination and synergistic impacts of Fe3O4 NPs and gelatin in the form of a nanocomposite. Figure 15 depicts the wettability alteration of dolomite and calcite rocks with gelatin and Fe3O4/gelatin nanocomposite. Therefore, the contact angle of dolomite and calcite samples aged in nanocomposite solution for 7 days decreased from 107.70° and 104.00° to 32.00° and 29.00°, respectively. With rising aging time (11 days), the contact angle of dolomite and calcite rocks decreased from 107.70° and 104.00° to 26.00° and 24.10°, respectively.
Nanocomposites can resist the force of adhesion between oil droplets and rock surfaces and separate the oil droplets from the surfaces by increasing the disjoining pressure on the rock surface. Also, nanocomposites lead to coatings on rock surfaces. Therefore, this coating creates a hydrophilic layer on the rock surface107,108. Consequently, the wedge layer’s disjoining pressure rises due to the presence of nanocomposites. Electrostatic repulsion forces between NPs have an impact on this mechanism. Disjoining pressure depends on various factors, like injection fluid salinity, temperature, and particle size8. According to the test results, the synergistic effect of Fe3O4/gelatin nanocomposite has better efficiency and performance compared to Fe3O4 nanoparticles and gelatin separately in reducing the contact angle.
The comparison of all the experiments performed in this study to evaluate the wettability of calcite and dolomite rocks in the presence of different salts, seawater, nanofluids, gelatin, nanocomposite, and SDS is shown in Fig. 16.
Conclusion
The purpose of this research was to investigate the ability of the synthesized Fe3O4/gelatin nanocomposite to change the wettability of carbonate reservoir rocks and compare it with traditional nanoparticles (Fe3O4 and SiO2), SDS surfactant, seawater, different salts with different salinities, biopolymers, the effect of the combination Fe3O4/SDS surfactant, and the effect of aging time (7 and 11 days). The important results obtained in this research are:
-
1.
1. By using crude oil, the surface of water-wet carbonate rocks became oil-wet during the aging process for 22 days. The contact angles of dolomite and calcite rocks were 107.70° and 104.00°, respectively. The reason for this wettability change may be due to the existence of heavy and polar elements in crude oil.
-
2.
2. Due to the synergistic effects of Fe3O4/SDS surfactant, a significant decrease in the reduction of the contact angle of oil-wet rock samples was observed. The contact angle of oil-wet dolomite and calcite samples decreased from 107.70° and 104.00° to 29.67° and 25.03°, respectively.
-
3.
3. With the increasing aging time, the wettability change to the water-wet state increases.
-
4.
4. This study used a chemical co-precipitation technique to synthesize the Fe3O4/gelatin nanocomposite, which was assessed utilizing FT-IR and TEM analyses.
-
5.
5. The synthesized Fe3O4/gelatin nanocomposite in terms of spherical shape and nano size is appropriate for injection into oil reservoirs.
-
6.
6. Seawater showed the least decrease in contact angle, and the synthesized nanocomposite showed the highest decrease in contact angle. Consequently, after 11 days of rock specimens aging in nanocomposite solution, the contact angle of oil-wet dolomite and calcite specimens decreased from 107.70° and 104.00° to 26.00° and 24.10°, which can be the cause of this reduction in contact angle due to the disjoining pressure of nanofluids.
-
7.
7. Due to the modified type of nanocomposites, which have two important properties: (1) wettability change from oil-wet condition to water-wet condition and (2) stability in reservoirs under different conditions, the use of this nanocomposite in harsh conditions in oil reservoirs as an EOR agent is considered a new and innovative study.
Data availability
Data avalibilityThe dataset generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Izadi, N. and Bahram Nasernejad. Newly engineered alumina quantum dot-based nanofluid in enhanced oil recovery at reservoir conditions. Scientific Reports 12. Nature Publishing Group UK London: 9505. (2022). https://doi.org/10.1038/s41598-022-12387-y
Ahmadi, Y., Mansouri, M. & Pourafshary, P. Enhanced oil recovery by using modified ZnO nanocomposites in sandstone oil reservoirs. Scientific Reports 14. Nature Publishing Group UK London: 2766. (2024). https://doi.org/10.1038/s41598-024-53138-5
Mansouri Zadeh, Morteza, F., Amiri, S., Hosseni & Ghamarpoor, R. Synthesis of colloidal silica nanofluid and assessment of its impact on interfacial tension (IFT) and wettability for enhanced oil recovery (EOR). Scientific Reports 14. Nature Publishing Group UK London: 325. (2024). https://doi.org/10.1038/s41598-023-51038-8
Gbadamosi, A. O., Radzuan Junin, M. A., Manan, A., Agi & Adeyinka, S. Y. An overview of chemical enhanced oil recovery: recent advances and prospects. International Nano Letters 9. Springer: 171–202. (2019). https://doi.org/10.1007/s40089-019-0272-8
Adil, M., Lee, K., Zaid, H. M., Noor Rasyada Ahmad Latiff, and & Mohamad Sahban, A. Experimental study on electromagnetic-assisted ZnO nanofluid flooding for enhanced oil recovery (EOR). Edited by Yogendra Kumar Mishra. PLOS ONE 13. Public Library of Science San Francisco, CA USA: e0193518. (2018). https://doi.org/10.1371/journal.pone.0193518
Roebuck, I. F. Jr The Future Outlook for Tertiary Recovery. Journal of Petroleum Technology 13. SPE: 416–418. (1961).
Gbadamosi, A. O., Kiwalabye, J., Junin, R. & Augustine, A. A review of gas enhanced oil recovery schemes used in the North Sea. Journal of Petroleum Exploration and Production Technology 8. Springer: 1373–1387. (2018). https://doi.org/10.1007/s13202-018-0451-6
Kazemzadeh, Y., Sharifi, M., Riazi, M., Rezvani, H. & Tabaei, M. Potential effects of metal oxide/SiO2 nanocomposites in EOR processes at different pressures. Colloids and Surfaces A: Physicochemical and Engineering Aspects 559. Elsevier: 372–384. (2018). https://doi.org/10.1016/j.colsurfa.2018.09.068
Kumar, A. and Ajay Mandal. Characterization of rock-fluid and fluid-fluid interactions in presence of a family of synthesized zwitterionic surfactants for application in enhanced oil recovery. Colloids and Surfaces A: Physicochemical and Engineering Aspects 549. Elsevier: 1–12. (2018). https://doi.org/10.1016/j.colsurfa.2018.04.001
Donaldson, E. C., Rex, D., Thomas & Philip, B. L. Wettability Determination and Its Effect on Recovery Efficiency. Society of Petroleum Engineers Journal 9. SPE: 13–20. (1969). https://doi.org/10.2118/2338-PA
Lorenz, P. B., Erle, C., Donaldson & Rex, D. T. The Use of Centrifugal Measurements of Wettability to Predict Oil RecoveryVol. 7873 (US Department of the Interior, Bureau of Mines, 1974).
Crocker, M. E. & Marchin, L. M. Wettability and Adsorption Characteristics of Crude-Oil Asphaltene and Polar Fractions. Journal of Petroleum Technology 40. SPE: 470–474. (1988). https://doi.org/10.2118/14885-PA
Bartell, F. E. & Niederhauser, D. O. Fundamental Research on Occurrence and Recovery of Petroleum57 (API, 1949).
Dodd, C. G., John, W., Moore & Milton, O. D. Metalliferous Substances Adsorbed at Crude Petroleum—Water interfaces. Industrial Eng. Chem. 44 ACS Publications. 2585–2590. https://doi.org/10.1021/ie50515a034 (1952).
Seifert, W. K. & Glenn Howells, W. Interfacially active acids in a California crude oil. Isolation of carboxylic acids and phenols. Analytical Chemistry 41. ACS Publications: 554–562. (1969). https://doi.org/10.1021/ac60273a002
Ahmadi, M., Ali, M., Galedarzadeh & Seyed Reza, S. Wettability Alteration in Carbonate Rocks by Implementing New Derived Natural Surfactant: Enhanced Oil Recovery Applications. Transport in Porous Media 106. Springer: 645–667. (2015). https://doi.org/10.1007/s11242-014-0418-0
Somasundaran, P. & Zhang, L. Adsorption of surfactants on minerals for wettability control in improved oil recovery processes. Journal of Petroleum Science and Engineering 52. Elsevier: 198–212. (2006). https://doi.org/10.1016/j.petrol.2006.03.022
Maghzi, A., Mohammadi, S., Ghazanfari, M. H., Kharrat, R. & Masihi, M. Monitoring wettability alteration by silica nanoparticles during water flooding to heavy oils in five-spot systems: A pore-level investigation. Experimental Thermal and Fluid Science 40. Elsevier: 168–176. (2012). https://doi.org/10.1016/j.expthermflusci.2012.03.004
Taber, J. J. Research on enhanced oil recovery: past, present and future. Pure and Applied Chemistry 52. De Gruyter: 1323–1347. (1980). https://doi.org/10.1351/pac198052051323
Cheng, Y. et al. Water-Dispersible Reactive Nanosilica and Poly(2-acrylamido-2-methyl-1-propanesulfonic acid sodium) Nanohybrid as Potential Oil Displacement Agent for Enhanced Oil Recovery. Energy & Fuels 31. ACS Publications: 6345–6351. (2017). https://doi.org/10.1021/acs.energyfuels.7b00743
Olajire, A. A. Review of ASP EOR (alkaline surfactant polymer enhanced oil recovery) technology in the petroleum industry: Prospects and challenges. Energy 77. Elsevier: 963–982. (2014). https://doi.org/10.1016/j.energy.2014.09.005
Divandari, H., Hemmati-Sarapardeh, A., Schaffie, M. & Ranjbar, M. Integrating synthesized citric acid-coated magnetite nanoparticles with magnetic fields for enhanced oil recovery: Experimental study and mechanistic understanding. Journal of Petroleum Science and Engineering 174. Elsevier: 425–436. (2019). https://doi.org/10.1016/j.petrol.2018.11.037
Guo, K. & Li, H. and Zhixin Yu. In-situ heavy and extra-heavy oil recovery: A review. Fuel 185. Elsevier: 886–902. (2016). https://doi.org/10.1016/j.fuel.2016.08.047
Saboorian-Jooybari, H., Dejam, M. & Chen, Z. Heavy oil polymer flooding from laboratory core floods to pilot tests and field applications: Half-century studies. Journal of Petroleum Science and Engineering 142. Elsevier: 85–100. (2016). https://doi.org/10.1016/j.petrol.2016.01.023
Rezvani, H., Riazi, M., Tabaei, M., Kazemzadeh, Y. & Sharifi, M. Experimental investigation of interfacial properties in the EOR mechanisms by the novel synthesized Fe3O4@Chitosan nanocomposites. Colloids and Surfaces A: Physicochemical and Engineering Aspects 544. Elsevier: 15–27. (2018). https://doi.org/10.1016/j.colsurfa.2018.02.012
Krishnamoorti, R. Technology Tomorrow: Extracting the Benefits of Nanotechnology for the Oil Industry. Journal of Petroleum Technology 58. SPE: 24–26. (2006). https://doi.org/10.2118/1106-0024-JPT
Yu, H. et al. and Chun Huh. Transport and Retention of Aqueous Dispersions of Paramagnetic Nanoparticles in Reservoir Rocks. In All Days. SPE. (2010). https://doi.org/10.2118/129887-MS
Kazemzadeh, Y., Malayeri, M. R., Riazi, M. & Parsaei, R. Impact of Fe3O4 nanoparticles on asphaltene precipitation during CO2 injection. Journal of Natural Gas Science and Engineering 22. Elsevier: 227–234. (2015). https://doi.org/10.1016/j.jngse.2014.11.033
Setoodeh, N., Darvishi, P. & Lashanizadegan, A. A comparative study to evaluate the performance of coated Fe3O4 nanoparticles for adsorption of asphaltene from crude oil in bench scale. Journal of Dispersion Science and Technology 39. Taylor & Francis: 711–720. (2018). https://doi.org/10.1080/01932691.2017.1386111
Ju, B. & Ma, M. Tailiang Fan, and Enhanced oil recovery by flooding with hydrophilic nanoparticles. China Particuology 4. Elsevier: 41–46. (2006). https://doi.org/10.1016/S1672-2515(07)60232-2
Le, N. Y., Thi, D. K., Pham, K. H., Le & Phuong Tung, N. Design and screening of synergistic blends of SiO2 nanoparticles and surfactants for enhanced oil recovery in high-temperature reservoirs. Advances in Natural Sciences: Nanoscience and Nanotechnology 2. IOP Publishing: 035013. (2011). https://doi.org/10.1088/2043-6262/2/3/035013
Taheri-Shakib, J. et al. Experimental and mathematical model evaluation of asphaltene fractionation based on adsorption in porous media: Part 1. calcite reservoir rock. Journal of Petroleum Science and Engineering 177. Elsevier: 24–40. (2019). https://doi.org/10.1016/j.petrol.2019.02.032
Couto, M. R. et al. The biopolymer produced by Rhizobium viscosum CECT 908 is a promising agent for application in microbial enhanced oil recovery. New Biotechnology 49. Elsevier: 144–150. (2019). https://doi.org/10.1016/j.nbt.2018.11.002
Gbadamosi, A. et al. and Jeffrey Oseh. Application of Polymers for Chemical Enhanced Oil Recovery: A Review. Polymers 14. MDPI: 1433. (2022). https://doi.org/10.3390/polym14071433
Karimi, A. et al. and. Wettability Alteration in Carbonates using Zirconium Oxide Nanofluids: EOR Implications. Energy & Fuels 26. ACS Publications: 1028–1036. (2012). https://doi.org/10.1021/ef201475u
Giraldo, J. et al. Wettability Alteration of Sandstone Cores by Alumina-Based Nanofluids. Energy & Fuels 27. ACS Publications: 3659–3665. (2013). https://doi.org/10.1021/ef4002956
Roustaei, A. & Bagherzadeh, H. Experimental investigation of SiO2 nanoparticles on enhanced oil recovery of carbonate reservoirs. Journal of Petroleum Exploration and Production Technology 5. Springer: 27–33. (2015). https://doi.org/10.1007/s13202-014-0120-3
Li, R. et al. Experimental Investigation of Silica-Based Nanofluid Enhanced Oil Recovery: The Effect of Wettability Alteration. Energy & Fuels 31. ACS Publications: 188–197. (2017). https://doi.org/10.1021/acs.energyfuels.6b02001
Jang, H., Lee, W. & Lee, J. Nanoparticle dispersion with surface-modified silica nanoparticles and its effect on the wettability alteration of carbonate rocks. Colloids and Surfaces A: Physicochemical and Engineering Aspects 554. Elsevier: 261–271. (2018). https://doi.org/10.1016/j.colsurfa.2018.06.045
Mousavi, S. P. et al. and. Toward mechanistic understanding of wettability alteration in calcite and dolomite rocks: The effects of resin, asphaltene, anionic surfactant, and hydrophilic nano particles. Journal of Molecular Liquids 321. Elsevier: 114672. (2021). https://doi.org/10.1016/j.molliq.2020.114672
Hosseini, M., Sadat, M., Khazaei, M., Misaghi & Mohammad Hossein, K. Improving the stability of nanofluids via surface-modified titanium dioxide nanoparticles for wettability alteration of oil-wet carbonate reservoirs. Mater. Res. Express. 9, 035005. https://doi.org/10.1088/2053-1591/ac4fdf (2022). IOP Publishing.
Hosseini, H., Apourvari, S. N. & Schaffie, M. Wettability alteration of carbonate rocks via magnetic fields application. Journal of Petroleum Science and Engineering 172. Elsevier: 280–287. (2019). https://doi.org/10.1016/j.petrol.2018.08.022
Safari, M., Rahimi, A., Gholami, R., Permana, A. & Wee Siaw, K. Underlying mechanisms of shale wettability alteration by low salinity water injection (LSWI). Journal of Dispersion Science and Technology 43. Taylor & Francis: 33–41. (2022). https://doi.org/10.1080/01932691.2020.1813156
Ivanova, A. A., Chi, P., Barifcani, A., Iglauer, S. & Alexey, N. C. Effect of Nanoparticles on Viscosity and Interfacial Tension of Aqueous Surfactant Solutions at High Salinity and High Temperature. Journal of Surfactants and Detergents 23. Wiley Online Library: 327–338. (2020). https://doi.org/10.1002/jsde.12371
Ivanova, A. A., Cheremisin, A. N. & Spasennykh, M. Y. Application of Nanoparticles in Chemical EOR. In IOR 2017-19th European Symposium on Improved Oil Recovery, 2017:1–10. EAGE Publications BV. (2017). https://doi.org/10.3997/2214-4609.201700247
Moeini, F., Ghazanfari, A. H. S. M. H., Masihi, M. & Shahab Ayatollahi. and. Toward mechanistic understanding of heavy crude oil/brine interfacial tension: The roles of salinity, temperature and pressure. Fluid Phase Equilibria 375. Elsevier: 191–200. (2014). https://doi.org/10.1016/j.fluid.2014.04.017
Sirivat, A. and Nophawan Paradee. Facile synthesis of gelatin-coated Fe3O4 nanoparticle: Effect of pH in single-step co-precipitation for cancer drug loading. Materials & Design 181. Elsevier: 107942. (2019). https://doi.org/10.1016/j.matdes.2019.107942
Rezvani, H., Khalilnezhad, A., Ganji, P. & Kazemzadeh, Y. How ZrO2 nanoparticles improve the oil recovery by affecting the interfacial phenomena in the reservoir conditions? Journal of Molecular Liquids 252. Elsevier: 158–168. (2018). https://doi.org/10.1016/j.molliq.2017.12.138
Morrow, N. R. Wettability and its Effect on Oil Recovery. J. Petrol. Technol. 42, 1476–1484. https://doi.org/10.2118/21621-PA (1990).
Stuart, B. H. Infrared Spectroscopy: Fundamentals and Applications. Analytical Techniques in the Sciences (Wiley, 2004). https://doi.org/10.1002/0470011149
GAIHRE, B. & M KHIL, D. L. E. E. and H KIM. Gelatin-coated magnetic iron oxide nanoparticles as carrier system: Drug loading and in vitro drug release study. International Journal of Pharmaceutics 365. Elsevier: 180–189. (2009). https://doi.org/10.1016/j.ijpharm.2008.08.020
Belikov, V. G., Kuregyan, A. G. & Ismailova, G. K. Standardization of magnetite. Pharmaceutical Chemistry Journal 36. Springer: 333–336. (2002). https://doi.org/10.1023/A:1020845110683
Dorniani, D. et al. Preparation of Fe3O4 magnetic nanoparticles coated with gallic acid for drug delivery. Int. J. Nanomed. Taylor Francis. 5745. https://doi.org/10.2147/IJN.S35746 (2012).
Chilingar, G. V. & Yen, T. F. Some Notes on Wettability and Relative Permeabilities of Carbonate Reservoir Rocks, II. Energy Sources 7. Taylor & Francis: 67–75. (1983). https://doi.org/10.1080/00908318308908076
Rahimi, A., Honarvar, B. & Safari, M. The role of salinity and aging time on carbonate reservoir in low salinity seawater and smart seawater flooding. Journal of Petroleum Science and Engineering 187. Elsevier: 106739. (2020). https://doi.org/10.1016/j.petrol.2019.106739
Somasundaran, P. & Agar, G. E. The zero point of charge of calcite. Journal of Colloid and Interface Science 24. Elsevier: 433–440. (1967). https://doi.org/10.1016/0021-9797(67)90241-X
Anderson, W. G. Wettability Literature Survey- Part 1: Rock/Oil/Brine Interactions and the Effects of Core Handling on Wettability. Journal of Petroleum Technology 38. SPE: 1125–1144. (1986). https://doi.org/10.2118/13932-PA
Tiab, D. & Erle, C. D. Petrophysics: Theory and Practice of Measuring Reservoir rock and Fluid Transport Properties (Gulf professional publishing, 2015).
Lagerge, S., Rousset, P., Zoungrana, T., Douillard, J. M. & Partyka, S. Adsorption of benzoic acid from organic solvents on calcite and dolomite: Influence of water. Colloids and Surfaces A: Physicochemical and Engineering Aspects 80. Elsevier: 261–272. (1993). https://doi.org/10.1016/0927-7757(93)80207-U
Thomas, M., Moisio, J. A., Clouse & John, M. L. Adsorption of organic compounds on carbonate minerals. Chemical Geology 109. Elsevier: 201–213. (1993). https://doi.org/10.1016/0009-2541(93)90070-Y
Thomas, M., Moisio, J. A., Clouse & John, M. L. Adsorption of organic compounds on carbonate minerals. Chemical Geology 109. Elsevier: 227–237. (1993). https://doi.org/10.1016/0009-2541(93)90072-Q
Sayyouh, M. H., Hemeida, A. M., Al-Blehed, M. S. & Desouky, S. M. Role of polar compounds in crude oils on rock wettability. J. Petrol. Sci. Eng. 6, 225–233. https://doi.org/10.1016/0920-4105(91)90015-F (1991).
Tennant, C. B. & Berger, R. W. X-ray determination of dolomite-calcite ratio of a carbonate rock. American Mineralogist: Journal of Earth and Planetary Materials 42. Mineralogical Society of America: 23–29. (1957).
Derrick, M. R., Dusan, Stulik, James, M. & Landry Infrared Spectroscopy in Conservation Science (Getty, 2000).
Dumitru, M., Daniel, D., Miljkovic, R. I., Scorei & Rotaru, P. FT-IR and Raman spectroscopic analysis of a calcium fructoborate sample. Phys. AUC. 20, 113–119 (2010).
Fernández-Carrasco, Lucia, D., Torrens-Martín, L. M., Morales & Sagrario Martínez-Ramírez Infrared spectroscopy in the analysis of building and construction materials. Infrared spectroscopy–Materials science, engineering and technology 510. InTech, Rijeka, Croatia. (2012).
Karimi, M., Al-Maamari, R. S., Ayatollahi, S., Nasir & Mehranbod Mechanistic study of wettability alteration of oil-wet calcite: The effect of magnesium ions in the presence and absence of cationic surfactant. Colloids and Surfaces A: Physicochemical and Engineering Aspects 482. Elsevier: 403–415. (2015). https://doi.org/10.1016/j.colsurfa.2015.07.001
Andersen, F. A. et al. Infrared spectra of amorphous and crystalline calcium carbonate. Acta Chem. Scand. 45, 1018–1024 (1991).
Tanur, A. E. et al. Insights into the composition, morphology, and formation of the calcareous shell of the serpulid Hydroides dianthus. Journal of Structural Biology 169. Elsevier: 145–160. (2010). https://doi.org/10.1016/j.jsb.2009.09.008
Long, Xia, M. J., Nasse, Y., Ma & Qi, L. From synthetic to biogenic Mg-containing calcites: a comparative study using FTIR microspectroscopy. Phys. Chem. Chem. Phys. 14, 2255. https://doi.org/10.1039/c2cp22453d (2012). Royal Society of Chemistry.
Osman, M. A., Ulrich, W. & Suter Surface treatment of calcite with fatty acids: structure and Properties of the Organic monolayer. Chem. Mater. 14 ACS Publications. 4408–4415. https://doi.org/10.1021/cm021222u (2002).
Meléndez, L. V., Lache, A., Orrego-Ruiz, J. A., Pachón, Z. & Enrique Mejía-Ospino. and. Prediction of the SARA analysis of Colombian crude oils using ATR–FTIR spectroscopy and chemometric methods. Journal of Petroleum Science and Engineering 90–91. Elsevier: 56–60. (2012). https://doi.org/10.1016/j.petrol.2012.04.016
Jarrahian, K., Seiedi, O., Sheykhan, M., Vafaie Sefti, M. & Ayatollahi, S. Wettability alteration of carbonate rocks by surfactants: A mechanistic study. Colloids and Surfaces A: Physicochemical and Engineering Aspects 410. Elsevier: 1–10. (2012). https://doi.org/10.1016/j.colsurfa.2012.06.007
Austad, T., Strand, S., Høgnesen, E. J. & Zhang, P. Seawater as IOR Fluid in Fractured Chalk. In All Days. SPE. (2005). https://doi.org/10.2118/93000-MS
Zhang, P. & Tweheyo, M. T. and Tor Austad. Wettability alteration and improved oil recovery by spontaneous imbibition of seawater into chalk: Impact of the potential determining ions Ca2+, Mg2+, and SO42–. Colloids and Surfaces A: Physicochemical and Engineering Aspects 301. Elsevier: 199–208. (2007). https://doi.org/10.1016/j.colsurfa.2006.12.058
Tajikmansori, A., Dehaghani, A. H. S. & Haghighi, M. Improving chemical composition of smart water by investigating performance of active cations for injection in carbonate Reservoirs: A mechanistic study. Journal of Molecular Liquids 348. Elsevier: 118043. (2022). https://doi.org/10.1016/j.molliq.2021.118043
Salehi, M., Johnson, S. J. & Jenn-Tai, L. Mechanistic Study of Wettability Alteration Using Surfactants with Applications in Naturally Fractured Reservoirs. Langmuir 24. ACS Publications: 14099–14107. (2008). https://doi.org/10.1021/la802464u
Almahfood, M. & Bai, B. The synergistic effects of nanoparticle-surfactant nanofluids in EOR applications. Journal of Petroleum Science and Engineering 171. Elsevier: 196–210. (2018). https://doi.org/10.1016/j.petrol.2018.07.030
Ma, K. et al. Adsorption of cationic and anionic surfactants on natural and synthetic carbonate materials. Journal of Colloid and Interface Science 408. Elsevier: 164–172. (2013). https://doi.org/10.1016/j.jcis.2013.07.006
Amirianshoja, T., Junin, R., Idris, A. K. & Rahmani, O. A comparative study of surfactant adsorption by clay minerals. Journal of Petroleum Science and Engineering 101. Elsevier: 21–27. (2013). https://doi.org/10.1016/j.petrol.2012.10.002
Bera, A., Kumar, T., Ojha, K. & Mandal, A. Adsorption of surfactants on sand surface in enhanced oil recovery: Isotherms, kinetics and thermodynamic studies. Applied Surface Science 284. Elsevier: 87–99. (2013). https://doi.org/10.1016/j.apsusc.2013.07.029
Yekeen, N., Manan, M. A. & Idris, A. K. and Ali Mohamed Samin. Influence of surfactant and electrolyte concentrations on surfactant Adsorption and foaming characteristics. Journal of Petroleum Science and Engineering 149. Elsevier: 612–622. (2017). https://doi.org/10.1016/j.petrol.2016.11.018
Shafiei, M., Kazemzadeh, Y., Martyushev, D. A., Dai, Z. & Riazi, M. Effect of chemicals on the phase and viscosity behavior of water in oil emulsions. Scientific Reports 13. Nature Publishing Group UK London: 4100. (2023). https://doi.org/10.1038/s41598-023-31379-0
Suresh, R., Kuznetsov, O., Agrawal, D., Darugar, Q. & Khabashesku, V. Reduction of Surfactant Adsorption in Porous Media Using Silica Nanoparticles. In Day 1 Mon, April 30, 2018. OTC. (2018). https://doi.org/10.4043/28879-MS
Wu, Y. et al. and Baolei Jiao. Reducing surfactant adsorption on rock by silica nanoparticles for enhanced oil recovery. Journal of Petroleum Science and Engineering 153. Elsevier: 283–287. (2017). https://doi.org/10.1016/j.petrol.2017.04.015
Wasan, D. T. & Alex, D. N. Spreading of nanofluids on solids. Nature 423. Nature Publishing Group UK London: 156–159. (2003). https://doi.org/10.1038/nature01591
Wasan, D., Nikolov, A. & Kondiparty, K. The wetting and spreading of nanofluids on solids: Role of the structural disjoining pressure. Current Opinion in Colloid & Interface Science 16. Elsevier: 344–349. (2011). https://doi.org/10.1016/j.cocis.2011.02.001
Kondiparty, K., Nikolov, A., Wu, S. & Wasan, D. Wetting and Spreading of Nanofluids on Solid Surfaces Driven by the Structural Disjoining Pressure: Statics Analysis and Experiments. Langmuir 27. ACS Publications: 3324–3335. (2011). https://doi.org/10.1021/la104204b
Noruzi, Y. et al. The State-of-the-Art of wettability alteration in sandstones and Carbonates: A mechanistic review. Fuel 356. Elsevier: 129570. (2024). https://doi.org/10.1016/j.fuel.2023.129570
Ahmadi, R., Farmani, Z., Osfouri, S. & Azin, R. Condensate blockage remediation in a gas reservoir through wettability alteration using natural CaCO3 nanoparticles. Colloids and Surfaces A: Physicochemical and Engineering Aspects 579. Elsevier: 123702. (2019). https://doi.org/10.1016/j.colsurfa.2019.123702
Shirin, S., Bila, A. & Torsæter, O. Experimental Investigation of the Effect of Silica Nanoparticles on Interfacial Tension and Wettability during Low Salinity Water Flooding: A Micromodel Study. Journal of Modern Nanotechnology 2. Innovation Forever Publishing Corporation. (2022). https://doi.org/10.53964/jmn.2022002
Keykhosravi, A., Bedrikovetsky, P. & Simjoo, M. Experimental insight into the silica nanoparticle transport in dolomite rocks: Spotlight on DLVO theory and permeability impairment. Journal of Petroleum Science and Engineering 209. Elsevier: 109830. (2022). https://doi.org/10.1016/j.petrol.2021.109830
Lim, S., Horiuchi, H., Nikolov, A. D. & Wasan, D. Nanofluids Alter the Surface Wettability of Solids. Langmuir 31. ACS Publications: 5827–5835. (2015). https://doi.org/10.1021/acs.langmuir.5b00799
Mousazadeh, B., Mohammadi, N. & Hamoule, T. Removal of phosphate from the aqueous environment using iron oxide/activated carbon composites: activated carbon derived from Ziziphus nuts as a new precursor. Iran. J. Chem. Eng. (IJChE) 18 Iran. Association Chem. Eng. (IAChE. 52–62. https://doi.org/10.22034/ijche.2022.315429.1415 (2021).
Mohammadi, N., Mousazadeh, B. & Hamoule, T. Synthesis and characterization of NH2-SiO2@Cu-MOF as a high-performance adsorbent for Pb ion removal from water environment. Environment, Development and Sustainability 23. Springer: 1688–1705. (2021). https://doi.org/10.1007/s10668-020-00646-9
Hsieh, C. T., Chen, J. M., Kuo, R. R., Lin, T. S. & Chu-Fu, W. Influence of surface roughness on water- and oil-repellent surfaces coated with nanoparticles. Applied Surface Science 240. Elsevier: 318–326. (2005). https://doi.org/10.1016/j.apsusc.2004.07.016
He, L., Lin, F., Li, X., Sui, H. & Xu, Z. Interfacial sciences in unconventional petroleum production: from fundamentals to applications. Chem. Soc. Rev. 44, 5446–5494. https://doi.org/10.1039/C5CS00102A (2015). Royal Society of Chemistry.
Afolabi, R. O. Enhanced oil recovery for emergent energy demand: challenges and prospects for a nanotechnology paradigm shift. International Nano Letters 9. Springer: 1–15. (2019). https://doi.org/10.1007/s40089-018-0248-0
Mousavi Moghadam, Asefe & Mahsa Baghban, S. Enhancing hydrocarbon productivity via wettability alteration: a review on the application of nanoparticles. Reviews in Chemical Engineering 35. De Gruyter: 531–563. (2019). https://doi.org/10.1515/revce-2017-0105
Lashkarbolooki, M., Riazi, M., Hajibagheri, F. & Ayatollahi, S. Low salinity injection into asphaltenic-carbonate oil reservoir, mechanistical study. Journal of Molecular Liquids 216. Elsevier: 377–386. (2016). https://doi.org/10.1016/j.molliq.2016.01.051
Lashkarbolooki, M., Ayatollahi, S. & Riazi, M. Mechanistical study of effect of ions in smart water injection into carbonate oil reservoir. Process Safety and Environmental Protection 105. Elsevier: 361–372. (2017). https://doi.org/10.1016/j.psep.2016.11.022
Yılmaz, H. & Senay Hamarat, S. Preparation of magnetic gelatin nanoparticles and investigating the possible use as chemotherapeutic agent. Artificial cells, nanomedicine, and biotechnology 41. Taylor & Francis: 69–77. (2013).
Wang, H., Ding, F., Ma, L. & Zhang, Y. Edible films from chitosan-gelatin: Physical properties and food packaging application. Food Bioscience 40. Elsevier: 100871. (2021).
Feng, X. et al. Properties of Pickering emulsion stabilized by food-grade gelatin nanoparticles: Influence of the nanoparticles concentration. Colloids and Surfaces B: Biointerfaces 196. Elsevier: 111294. (2020).
Xing, Q. et al. Increasing mechanical strength of gelatin hydrogels by divalent metal ion removal. Scientific reports 4. Nature Publishing Group UK London: 4706. (2014).
Kyte, J., Russell, F. & Doolittle A simple method for displaying the hydropathic character of a protein. Journal of Molecular Biology 157. Elsevier: 105–132. (1982). https://doi.org/10.1016/0022-2836(82)90515-0
Hendraningrat, L., Li, S., Ole & Torsæter A coreflood investigation of nanofluid enhanced oil recovery. Journal of Petroleum Science and Engineering 111. Elsevier: 128–138. (2013).
Li, S., Ole & Torsæter Experimental investigation of the influence of nanoparticles adsorption and transport on wettability alteration for oil wet berea sandstone. In SPE Middle East Oil and Gas Show and Conference, SPE-172539. SPE. (2015).
Author information
Authors and Affiliations
Contributions
Mohammad Ebrahimi : Investigation, Data curation, Writing original draft, Hossein Ghalenavi : Investigation, Methodology, Writing original draft, Mahin Schaffie : Validation, Methodology, Writing-Review & Editing, Mohammad Ranjbar : Validation, Visualization, Writing-Review & Editing, Abdolhossein Hemmati-Sarapardeh : Supervision, Conceptualization, Validation, Visualization,
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ebrahimi, M., Ghalenavi, H., Schaffie, M. et al. Toward mechanistic understanding of wettability alteration in carbonate rocks in the presence of nanoparticles, gelatin biopolymer, and core-shell nanocomposite of Fe3O4@gelatin. Sci Rep 14, 31679 (2024). https://doi.org/10.1038/s41598-024-80893-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-80893-2
Keywords
This article is cited by
-
Derivation of explicit mathematical equations for gypsum solubility in aqueous electrolyte solutions using GP, GEP, and GMDH techniques
Scientific Reports (2025)
-
Experimental investigation of wettability alteration in sandstone rock by nanoparticles, gelatin biopolymer, salt ions, and synthesized Fe3O4/gelatin nanocomposite for EOR applications
Scientific Reports (2025)