Abstract
Diabetes mellitus is a chronic disease characterized by metabolic defects, including insulin deficiency and resistance. Individuals with diabetes are at increased risk of developing cardiovascular complications, such as atherosclerosis, coronary artery disease, and hypertension. Conventional treatment methods, though effective, are often challenging, costly, and may lead to systemic side effects. This study explores the potential of nanomedicine applications, specifically Metal–Organic Frameworks (MOFs), as drug carriers to overcome these limitations. The Materials Institute Lavoisier-89 nanoparticles (nanoMIL-89) have previously demonstrated promise as a drug delivery vehicle for chronic diseases due to their anti-oxidant and cardio-protective properties. In this investigation, nanoMIL-89 was loaded with the anti-diabetic drug metformin (MET), creating MET@nanoMIL-89 formulation. We examined the drug release kinetics of MET@nanoMIL-89 over 96 h and assessed its impact on the viability of various endothelial cells. Furthermore, we investigated the nanoformulation effect on the inflammatory marker CXCL8 in these cells and explored its influence on phosphorylated eNOS, total eNOS, and AKT levels. Our findings indicate that nanoMIL-89 effectively released metformin over 96 h and caused a concentration-dependent reduction in CXCL8 release from endothelial cells. Notably, MET@nanoMIL-89 reduced dihydroethidium levels and increased phosphorylated eNOS, total eNOS, and AKT levels. Our results underscore the potential of nanoMIL-89 as a versatile potential drug delivery platform for anti-diabetic drugs, offering a prospective therapeutic approach for diabetic patients with associated cardiovascular complications.
Similar content being viewed by others
Introduction
Diabetes mellitus (DM) stands as one of the most prevalent chronic metabolic disorders globally, associated with decreased life expectancy and heightened mortality rates1. The escalating prevalence of DM is alarming, with a projected rise from approximately 424.9 million cases worldwide in 2019 to an anticipated 700 million cases by 20452. Notably, Type-2 DM constitutes approximately 90% of all DM cases globally3. Achieving glycemic control in type-2 DM involves lifestyle modifications alongside pharmacological interventions, including oral antidiabetic drugs such as biguanides (e.g., metformin), sulfonylureas, dipeptidyl peptidase IV (DPP-4) inhibitors (gliptins), glucagon-like peptide-1 (GLP-1) receptor agonists, sodium-glucose cotransporter (SGLT-2) inhibitors, alpha-glucosidase inhibitors, thiazolidinediones, and amylin analogs4. However, drug resistance, side effects, and individual variability responses may impede the effectiveness of these conventional therapies1. DM is frequently accompanied by complications such as vision, gastric, renal, and notably cardiovascular issues, with the latter being the most common, even with intensive glycemic control.5. Recent studies indicate that DM-associated cardiovascular complications may arise from dysfunctional endothelium, observable even in early DM stages, making endothelial dysfunction a potential early biomarker and risk factor for cardiovascular diseases (CVDs)6,7. Understanding the mechanisms through which DM induces endothelial cell dysfunction is crucial for preventing the onset of CVDs, such as atherosclerosis, hypertension, and stroke8.
Despite technological advancements in DM treatments, management remains suboptimal, marked by side effects and limitations4. Nanomedicine applications emerge as promising solutions to address these challenges. Nanotherapies tailored for DM management offer effective blood glucose regulation and gradual release of antidiabetic drugs1. This extension of drug duration reduces the likelihood of acute and chronic complications associated with antidiabetic drugs1. The exploration of various nanoparticles (NPs) loaded with antidiabetic drugs, including liposomes, niosomes, polymers, dendrimers, micelles, and metal-based NPs, has been ongoing9,10,11,12,13,14,15,16,17,18,19,20,21,22. However, safety, tolerance, and stability challenges have been encountered9,10,11,12,13,14,15,16,17,18,19,20,21,22. To overcome these limitations, we propose the utilization of iron oxide-based NPs, known for their anti-inflammatory and hypoglycemic properties23,24. They also possess antioxidant activities that can be easily detected via magnetic resonance imaging (MRI)25. Among these, the Materials Institute Lavoisier-89 nanoparticles (nanoMIL-89), a metal–organic framework (MOF), demonstrates suitability as a drug carrier. NanoMIL-89 has exhibited selective uptake by endothelial cells, passage to daughter cells, and reduced inflammatory markers in dysfunctional endothelial cells26. Additionally, it has proven to be a suitable carrier for the drug sildenafil, used to treat pulmonary arterial hypertension (PAH)27. Moreover, it has been demonstrated that nanoMIL-89 exhibits non-toxic effects both in vitro and in vivo, as evidenced by studies utilizing zebrafish embryos and rodent models24,26.
Our selection of nanoMIL-89 as a carrier for the antidiabetic drug metformin (MET) is grounded in its cardioprotective activity and ability to carry small drugs with a molecular weight under 500 Da27. Moreover, metformin’s positive effects on blood glucose levels, insulin sensitivity, antioxidant activity, and endothelial protection align with our goal of mitigating hyperglycemia’s impact on the endothelium28,29. Metformin’s association with a lower risk of CVDs in type-2 DM patients and its compatibility with nanoMIL-89 due to its size further reinforce our choice30.
Despite metformin’s low bioavailability and short half-life, loading it into nanoMIL-89 presents an opportunity to reduce dosage, improve patient compliance, and potentially minimize gastrointestinal side effects31,32,33. Therefore, our objective was to load metformin into nanoMIL-89 (MET@nanoMIL-89), investigate its release kinetics, and evaluate its impact on endothelial cell function and oxidative stress under hyperglycemic conditions, in vitro.
Results
Chemical characterization and pharmacokinetics of MET@nanoMIL-89
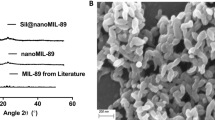
The synthesized nanoMIL-89 was characterized using Scanning Electron Microscope (SEM) and Powder X-ray Diffraction (PXRD) to validate the produced nanoparticles and compare their chemical characteristics with previously synthesized and published results. Results showed that the PXRD and SEM demonstrated similar results to the previously published results in the literature, showing a hexagonal structure. Furthermore, the pharmacokinetics of MET@nanoMIL-89 were investigated and compared to metformin alone. To ensure that metformin had been incorporated within the nanoMIL-89, the presence of metformin in the supernatant was measured, and results showed that ~ 3% of unincorporated metformin remained in the supernatant, suggesting that 97% of the drug was incorporated within the nanoMIL-89 to generate MET@nanoMIL-89 (Supplementary Fig. 1). Moreover, MET@nanoMIL-89 demonstrated a time-dependent release of metformin, persisting for up to 96 h with a cumulative release of approximately 96%, compared to a free metformin solution. Notably, during the initial time points (0.5, 1, 3, 6, and 24 h), a gradual release of metformin (~ 50%) was observed, reaching a peak of ~ 100% at 48 h and maintaining stability until the end of the experiment (Fig. 1). In contrast, the free metformin solution exhibited consistent concentrations of ~ 100% throughout the experiment, while nanoMIL-89 alone showed no detectable metformin release.
Characterization of nanoMIL-89 and MET@nanoMIL-89 using (A) Scanning Electron Microscopy (SEM), (B) Powder X-ray Diffraction (PXRD), and (C) percentage of metformin release from MET@nanoMIL-89 over 96 h, compared to nanoMIL-89 alone and a free metformin solution. Data are presented as mean ± SEM for n = 3.
Effects of MET@nanoMIL-89 on endothelial cell viability, cytotoxicity, and inflammatory state
The impact of nanoMIL-89 and MET@nanoMIL-89 on endothelial cell viability was assessed using different concentrations [0, 1, 3, 10, 30, and 100 µg/ml] of the formulation. The cell respiration rate, measured by the AlamarBlue® assay, indicated that nanoMIL-89 and MET@nanoMIL-89 had no significant effect on cell viability (respiration) at concentrations ≤ 30 μg/ml in all endothelial cells tested (Fig. 2A: HUVECs, 2D: PAECs, Fig. 3A: HAECs, and 3D: HAECs-Type-2 DM). The cytotoxicity of the nanoformulations used for antidiabetic drug delivery was investigated using the lactate dehydrogenase (LDH) assay. Results from the LDH release assay (Fig. 2B: HUVECs, 2E: PAECs; and Fig. 3B: HAECs, and 3E: HAECs-Type-2 DM) demonstrated that nanoMIL-89 and MET@nanoMIL-89 did not induce significant cytotoxic effects in any of the four cell lines. Furthermore, the anti-inflammatory effect of nanoMIL-89 and MET@nanoMIL-89 was examined by measuring the levels of CXCL8 in the conditioned media. Results (Fig. 2C: HUVECs, 2F: PAECs; and Fig. 3C: HAECs and 3F: HAECs-Type-2 DM) revealed that both nanoformulations exhibited a concentration-dependent decrease in CXCL8 release from endothelial cells. This effect was more pronounced in HUVECs and HAECs, where the reduction in CXCL8 release was more significant in the MET@nanoMIL-89 group compared to the group treated with nanoMIL-89 alone.
Effect of nanoMIL-89 and MET@nanoMIL-89 on cell viability, cell cytotoxicity, and CXCL8 release by HUVECs and PAECs in hyperglycemic media. Data are presented as mean ± SEM for n = 3. Statistical analysis comparing effects between different groups was conducted using two-way ANOVA, followed by Bonferroni post-tests, with #p < 0.05 considered significant. Each group was compared to the control using one-way ANOVA, followed by Dunnett’s Multiple Comparison Test, where *p < 0.05 was considered significant.
Effect of nanoMIL-89 and MET@nanoMIL-89 on cell viability, cell cytotoxicity, and CXCL8 release by HAECs and HAECs-Type 2 DM in hyperglycemic media. Data are presented as mean ± SEM for n = 3. Statistical analysis comparing effects between different groups was conducted using two-way ANOVA, followed by Bonferroni post-tests, with #p < 0.05 considered significant. Each group was compared to the control using one-way ANOVA, followed by Dunnett’s Multiple Comparison Tests, where *p < 0.05 was considered significant.
Ability of MET@nanoMIL-89 to reduce reactive oxygen species production by endothelial cells
Hyperglycemic conditions are known to induce endothelial dysfunction, reducing nitric oxide (NO) production and increasing reactive oxygen species (ROS) release and oxidative stress. As metformin is acknowledged for its in vitro antioxidant effects independently34, this experiment explored whether MET@nanoMIL-89 could sustain or amplify these antioxidant properties. Results demonstrated that hyperglycemia increased ROS levels, as evidenced by the higher dihydroethidium (DHE) fluorescence intensity in cells cultured in hyperglycemic media in the control groups. The use of nanoMIL-89 and MET@nanoMIL-89 induced a concentration-dependent reduction in DHE intensity, starting at concentrations ≥ 1 µg/ml in the hyperglycemic environment (Fig. 4). This indicates that the nanoformulation possesses antioxidant activity, reducing ROS release under hyperglycemic conditions.
Effect of nanoMIL-89 and MET@nanoMIL-89 on ROS levels, measured by DHE fluorescence intensity, in HUVECs cultured in hyperglycemic media. Data are presented as mean ± SEM for n = 3. Statistical analysis comparing effects between different groups was conducted using two-way ANOVA, followed by Bonferroni post-tests, with #p < 0.05 considered significant. Each group was compared to the control using one-way ANOVA, followed by Dunnett’s Multiple Comparison Tests, where *p < 0.05 was considered significant.
Effect of MET@nanoMIL-89 on eNOS and AKT levels in endothelial cells
Given that hyperglycemic conditions are known to reduce eNOS phosphorylation levels in endothelial cells, we aimed to test whether our MET@nanoMIL-89 conjugate could increase these levels. Both nanoMIL-89 and MET@nanoMIL-89 demonstrated the ability to increase eNOS levels, with MET@nanoMIL-89 inducing a more pronounced increase (Fig. 5A and B). This effect can be attributed to the individual contributions of the nanoparticle and metformin in elevating eNOS levels. When combined, MET@nanoMIL-89 further enhanced the efficacy of metformin, thereby promoting greater eNOS activity.
Effect of nanoMIL-89 and MET@nanoMIL-89 on (A) Phosphorylated eNOS and (B) Total eNOS, (C) Phosphorylated AKT, and (D) total AKT expression in HUVECs treated under hyperglycemic conditions. Data are presented as mean ± SEM for n = 3. Statistical analysis using one-way ANOVA, followed by Dunnett’s Multiple Comparison Tests, was performed to compare the effects of each condition to the relevant control, with *p < 0.05 considered significant.
Additionally, we measured AKT levels, as hyperglycemic conditions typically reduce AKT levels in endothelial cells. Under hyperglycemic conditions, nanoMIL-89 increased phospho-AKT levels, while MET@nanoMIL-89 elevated both phospho-AKT and total AKT levels compared to the control. Furthermore, MET@nanoMIL-89 induced a more substantial increase in AKT levels compared to nanoMIL-89 alone (Fig. 5C and D). This aligns with the observation that both nanoMIL-89 and metformin exhibit antioxidant activity and their conjugation enhances the overall efficacy of the treatment.
Discussion
Cardiovascular issues related to diabetes mellitus contribute significantly to increased mortality35. Hyperglycemia-induced endothelial dysfunction is a key contributor to these issues, as evidenced by decreased eNOS and Akt phosphorylation, both of which are required for vascular function. Metformin has been demonstrated to prevent these effects by increasing eNOS and Akt phosphorylation and decreasing oxidative stress29,34. Given its potential to improve endothelial function, current research aims to explore nanomedicine-based strategies to deliver metformin more effectively. Encapsulating metformin in nanoparticles provided a focused method to enhance its therapeutic benefits, perhaps giving higher protection against cardiovascular problems in DM. In this study, we loaded metformin into the iron-based nanoparticle (nanoMIL-89) to explore its potential as a carrier for metformin. We investigated the release kinetics of metformin and further explored the impact of metformin-loaded nanoparticles on endothelial cell viability, membrane integrity, and CXCL8 release. Additionally, we assessed their effects on eNOS/AKT phosphorylation and reactive oxygen species (ROS) generation under hyperglycemic conditions.
The evaluation of metformin release from nanoMIL-89 at different time points revealed a successful and controlled release, reaching approximately 100 µg/ml at 96 h36,37. This gradual release pattern is crucial for evading premature drug deactivation by the immune system, preventing rapid immune tolerance, and ensuring sustained therapeutic efficacy38. These findings align with a previous study on nanoMIL-89, which demonstrated its ability to sustain drug release in a human plasma matrix in vitro for up to 4 days27, potentially addressing the short plasma half-life of metformin31,32. Metformin has a half-life between 4–5 h when administered orally, with plasma peak levels of 20 μM. The bioavailability of metformin ranges between 40 and 60%, with absorption completed within 6 h of ingestion39. Due to its pharmacokinetics, metformin requires high doses and frequent administration, increasing the risk of side effects and reducing patient compliance40. However, a controlled-release formulation could significantly improve bioavailability and reduce the need for multiple doses, thereby enhancing patient compliance. Further confirmation of the pharmacokinetics of MET@nanoMIL-89 should be investigated in vivo to assess the appropriate dose, frequency, and route of administration of this formulation for optimal therapeutic levels. An oral delivery would be more appropriate to improve patient compliance. This will require protection of the NP in the gastrointestinal tract from pre-mature biodegradation. Further surface modifications might be required to ensure the stability of the formulation and optimal distribution41,42. One strategy to overcome that is using hydrogel-–MOF hybrids, which have previously shown enhanced protection of MOF payload in the gastric and early small intestine43. The absorption and distribution of the NPs from the gastrointestinal tract and to the circulation should also be validated in an animal model. Other routes of administration are possible for these formulations, such as parenteral or inhalation44,45.
It is well-documented that DM and hyperglycemia result in endothelial cell dysfunction. To that end, we sought to investigate the effect of the developed formulation on these cells and started by investigating effects on viability, cytotoxicity, and cytokine release. Results from this study, utilizing endothelial cells from various sources, revealed no detectable cytotoxic effects of MET@nanoMIL-89 or nanoMIL-89. Metabolic dysfunction, rather than toxicity from the nanoformulation, was responsible for the decreased cell viability. The results of the cell cytotoxicity assays indicate that LDH release was insignificant in hyperglycemic media. This demonstrates that the different MOF concentrations did not show any cytotoxicity effects, and the decrease in cell viability was due to metabolic dysfunction and reduced cellular respiration rather than MOF toxicity. Therefore, using MOF concentrations up to 10 µg/ml does not affect the viability or membrane integrity of endothelial cells. Furthermore, the anti-inflammatory effects of the nanoformulations were investigated by measuring cytokine release from endothelial cells seeded in hyperglycemic media. As previously discussed, hyperglycemic conditions affect diabetic cell function, inducing the secretion of high levels of inflammatory markers such as CXCL8. The treatment with a range of concentrations of both nanoMIL-89 and MET@nanoMIL-89 demonstrated a progressive and consistent decrease in CXCL8 release. Notably, the highest concentration of the nanoformulation resulted in the most substantial reduction in cytokine levels, further emphasizing the dose-dependent nature of the response. This significant decrease strongly suggests that metformin possesses noteworthy anti-inflammatory properties, contributing to its therapeutic potential in managing inflammatory processes associated with DM37. Previous studies have shown that nanoMIL-89 lowered CXCL8 and endothelin-1 release from endothelial cells24, which may indicate that both nanoMIL-89 and MET@nanoMIL-89 have anti-inflammatory effects26.
Hyperglycemia-induced dysfunction is characterized by reduced eNOS phosphorylation, altered total eNOS function, decreased Akt phosphorylation29, and increased oxidative stress, represented by elevated ROS levels34. Loading metformin into nanoMIL-89 effectively countered hyperglycemia-induced oxidative stress, reducing ROS levels. This reduction in oxidative stress is critical in managing diabetes-induced vascular dysfunction, as oxidative damage plays a significant role in the progression of diabetic vascular complications46. Additionally, MET@nanoMIL-89 elevated total and phosphorylated eNOS and Akt levels in endothelial cells under hyperglycemic conditions, consistent with metformin’s endothelial protective action in diabetic conditions, which is mediated via eNOS47. Enhancing eNOS and Akt activity is vital for maintaining endothelial function and vascular health. This positive effect of this formulation mitigates endothelial dysfunction, thus possibly reducing the vascular complications commonly associated with DM. This action of reducing oxidative stress and improving endothelial function highlights the therapeutic potential of MET@nanoMIL-89 in managing diabetes vascular complications. These findings imply that nanoMIL-89 may possess cardioprotective activity by mitigating endothelial cell dysfunction, a property often associated with metallic NPs like iron oxide NPs3. Further validation of the effects of this formulation on other prime metabolic targets of metformin, such as hepatic and skeletal muscle cells, is crucial to comprehensively understand the effectiveness of this formulation on the management of metformin action.
Methodology
NPs synthesis, characterizations, and metformin loading
NanoMIL-89 was synthesized according to previously published procedures24,48. In brief, 10 mmol of iron (III) chloride hexahydrate (FeCl3.6H2O) and 10 mmol of trans–trans muconic acid were dissolved in 100 ml of absolute ethanol (99.8%). After subjecting the reaction mixture to sonication for 15 min, the subsequent step involved adding 20 ml of glacial acetic acid (99.8%) before heating it at 90 °C for 24 h within a Parr reactor. Precipitated nanoparticles were recovered by centrifugation at 7000 rpm for 15 min, followed by several washes in distilled water. The precipitate was then vacuum-dried to obtain nanoMIL-89, which presented as a brown precipitate (100–200 mg/reaction).
Subsequently, nanoMIL-89 was loaded with metformin as follows: A solution of 1000 µg/ml metformin in Phosphate Buffer Saline (PBS; pH 7.4) was prepared. Then, 5 ml of metformin concentration was taken, and 200 mg of nanoMIL-89 was added. The mixture was incubated overnight (16–18 h.) at room temperature, and metformin-loaded nanoMIL-89 was harvested by centrifugation at 7000 rpm for 15 min at room temperature. The harvested metformin-loaded nanoMIL-89 was characterized by Infrared Imaging (IR), Powder X-ray Diffraction (PXRD), and Scanning Electron Microscope (SEM), with results compared to the unloaded nanoMIL-89. Finally, the metformin nanoMIL-89 prototype was utilized for pharmacokinetic and in vitro experiments.
Metformin release pharmacokinetics
As described above, nanoMIL-89 was loaded with metformin, and the release kinetics were measured over 96 h. NanoMIL-89 loaded with metformin was precipitated at 7000 rpm at room temperature for 15 min. The resulting precipitate was dissolved in 5 ml of PBS and incubated at room temperature. At various time points (0, 0.5, 1, 3, 6, 24, 48, 72, and 96 h), 1.5 ml of the supernatant was collected. The amount of released metformin into PBS was determined using a previously described colorimetric method49, which relies on the interaction of metformin with ninhydrin reagent at pH 5.6, resulting in a red color. A metformin standard curve was incorporated. Each collected 1.5 ml aliquot was mixed with 10.0 ml of Phthalate buffer (pH = 5.6) and 5.0 ml of 0.1% Ninhydrin. The mixture underwent heating in a water bath for 1 h at 90 ºC. Subsequently, the solution was cooled to room temperature and topped up with distilled water to a final volume of 25 ml, and the optical density was measured at 567 nm. Readouts were corrected for a blank, and concentrations were estimated using the metformin standard curve49.
Effects of MET@nanoMIL-89 on endothelial cell viability, cytotoxicity, and inflammatory state
Cell culture
Standard cell culture techniques were used for seeding and maintaining the following endothelial cell lines: Human Pulmonary Artery Endothelial Cells (PAECs; Cell Biologics, Chicago, IL)50 and Human Umbilical Vein Endothelial Cells (HUVECs; Cell Biologics, Chicago, IL)51. The responses of healthy Human Primary Aortic Endothelial Cells (HAECs; Cell Biologics, Chicago, IL), cultured under hyperglycemic conditions, were compared to those of a diabetic endothelial cell line: Human Primary Diabetic Aortic Endothelial Cells (HAECs, Type-2 Diabetes; Cell Biologics, Chicago, IL). All cell lines were cultured according to the manufacturer’s instructions52.
Cell viability, cytotoxicity, and cytokine release
Endothelial cells were plated in 96-well plates at a seeding density of 10,000 cells/well under hyperglycemic conditions (25 mM glucose). After overnight incubation (16–18 h) in humidified conditions (37 ºC, 5% CO2), cells were treated with either nanoMIL-89 alone (0, 1, 3, 10, 30, and 100 µg/ml), metformin alone (50 µM), or MET@nanoMIL-89 (0, 1, 3, 10, 30, and 100 µg/ml) for 24 h under the same conditions. Adherent cells were used to determine the effects of different treatments on cell viability using the AlamarBlue® assay following the manufacturer’s instructions. Conditioned media were collected to assess cell cytotoxicity using the LDH Kit (Abcam, UK) and anti-inflammatory effects using CXCL8-ELISA Kits (R&D systems, USA). AlamarBlue, LDH, and ELISA assays were performed following manufacturer instructions.
Effect of MET@ nanoMIL-89 on reactive oxygen species, eNOS, and AKT levels
Endothelial cells were seeded into a black 96-well plate and treated as described above. After 24 h in humidified conditions (37 ºC, 5% CO2), the Dihydroethidium (DHE, Abcam, UK) assay kit was used to measure Reactive Oxygen Species (ROS) release according to the manufacturer’s instructions. For Endothelial Nitric Oxide Synthase (eNOS) and Phospho-AKT, cells were seeded into 6-well plates at a density of 100,000 cells/well and treated with 10 μg/ml of nanoMIL-89 and MET@nanoMIL-89 for 24 h. Following treatment, proteins were extracted, and the levels of phosphorylated eNOS and total eNOS were measured using the Human Phospho-eNOS and Total eNOS ELISA kit (RayBio®, USA) as instructed. Additionally, the total and phosphorylated AKT expression was measured using the Phospho-AKT (RayBio®, USA) ELISA kit following the manufacturer’s instructions.
Statistical analysis
Results from drug release studies and in vitro culture studies were analyzed and presented as mean ± SEM for n experiments, with well-defined figure legends. Statistical tests were performed using GraphPad Prism v5. Two-way ANOVA, followed by Bonferroni post-tests, was used to compare the effects of nanoformulation concentrations between different groups, with #p < 0.05 considered significant. One-way ANOVA, followed by Dunnett’s Multiple Comparison Tests, was used to compare the effects of each condition (i.e., metformin-loaded nanoMIL-89, metformin alone, and unloaded nanoMIL-89) compared to the relevant control, where *p < 0.05 was considered significant.
Conclusion
This study highlights nanoMIL-89 as a biocompatible nanoparticle endowed with cardioprotective properties, as evidenced by its antioxidant and anti-inflammatory effects, along with its capacity to enhance the nitric oxide pathway. The observed potential suggests that this nanoparticle could have versatile applications in diverse diseases, such as cardiovascular diseases and diabetes mellitus. The experimental findings underscore nanoMIL-89 as a promising carrier for delivering antidiabetic drugs, specifically metformin. However, to gain a comprehensive understanding of its mechanism of action, pharmacodynamics, and long-term biological effects, further in vivo studies utilizing diabetic models are imperative. The success of such studies could propel this nanoparticle into pre-clinical stages, unveiling promising therapeutic opportunities not only for diabetes but also for other challenging diseases, including cancer. The ongoing exploration of nanoMIL-89’s capabilities holds great potential for advancing our understanding of nanomedicine applications and their broader impact on medical treatments.
Data availability
The datasets generated during and/or analyzed during the current study are publicly available from the corresponding author on reasonable request.
References
Kesharwani, P. et al. Nanotechnology based approaches for antidiabetic drugs delivery. Diabetes Res. Clin. Pract. 136, 52–77. https://doi.org/10.1016/j.diabres.2017.11.018 (2018).
Saeedi, P. et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 157, 107843. https://doi.org/10.1016/j.diabres.2019.107843 (2019) (Epub 2019 Sep 10).
Lushchak, O., Zayachkivska, A. & Vaiserman, A. Metallic nanoantioxidants as potential therapeutics for type 2 diabetes: a hypothetical background and translational perspectives. Oxid. Med. Cell Longev. 2018, 3407375. https://doi.org/10.1155/2018/3407375 (2018).
Chaudhury, A. et al. Clinical review of antidiabetic drugs: implications for type 2 diabetes mellitus management. Front. Endocrinol. 8, 6. https://doi.org/10.3389/fendo.2017.00006 (2017).
Moral-Sanz, J. et al. Different patterns of pulmonary vascular disease induced by type 1 diabetes and moderate hypoxia in rats. Exp. Physiol. 97, 676–686. https://doi.org/10.1113/expphysiol.2011.062257 (2012).
Hadi, A. R. & Hadi, J. A. S. Endothelial dysfunction in diabetes mellitus. Vasc. Health Risk Manag. 3, 853–876 (2007).
Tabit, C. E., Chung, W. B., Hamburg, N. M. & Vita, J. A. Endothelial dysfunction in diabetes mellitus: molecular mechanisms and clinical implications. Rev. Endocr. Metab. Disord. 11, 61–74. https://doi.org/10.1007/s11154-010-9134-4 (2010).
Abernethy, A. D. et al. Impact of diabetes in patients with pulmonary hypertension. Pulm. Circ. 5, 117–123. https://doi.org/10.1086/679705 (2015).
Wu, Z. H., Ping, Q. N., Wei, Y. & Lai, J. M. Hypoglycemic efficacy of chitosan-coated insulin liposomes after oral administration in mice. Acta Pharmacol. Sin. 25, 966–972 (2004).
Manconi, M. et al. Improving oral bioavailability and pharmacokinetics of liposomal metformin by glycerolphosphate-chitosan microcomplexation. AAPS PharmSciTech. 14, 485–496. https://doi.org/10.1208/s12249-013-9926-4 (2013).
Hanato, J. et al. Liposomal formulations of glucagon-like peptide-1: improved bioavailability and anti-diabetic effect. Int. J. Pharm. 382, 111–116. https://doi.org/10.1016/j.ijpharm.2009.08.013 (2009).
Moghassemi, S. et al. Uptake and transport of insulin across intestinal membrane model using trimethyl chitosan coated insulin niosomes. Mater. Sci. Eng. C Mater. Biol. Appl. 46, 333–340. https://doi.org/10.1016/j.msec.2014.10.070 (2015).
Sankhyan, A. & Pawar, P. K. Metformin loaded non-ionic surfactant vesicles: optimization of formulation, effect of process variables and characterization. Daru 21, 7. https://doi.org/10.1186/2008-2231-21-7 (2013).
Namdev, S., Kishore, G., Mandlik, S. & Jamkar, P. Preparation and in vivo characterization of niosomal carriers of the antidiabetic drug repaglinide. Int. J. Pharm. Sci. Nanotechnol. 8, 2756–2767 (2015).
Haider, M. F. et al. Pioglitazone loaded vesicular carriers for antidiabetic activity: development and optimization as per central composite design. J. Pharm. Sci. Pharmacol. 2, 11–20 (2015).
Rathi, J. C., Tamizharasi, S., Dubey, A. & Rathi, V. Development and characterization of niosomal drug delivery of gliclazide. J. Young Pharm. 1, 205 (2009).
Elsayed, A. et al. Chitosan-sodium lauryl sulfate nanoparticles as a carrier system for the in vivo delivery of oral insulin. AAPS PharmSciTech 12, 958–964. https://doi.org/10.1208/s12249-011-9647-5 (2011).
Ebrahimi, H. A., Javadzadeh, Y., Hamidi, M. & Jalali, M. B. Repaglinide-loaded solid lipid nanoparticles: effect of using different surfactants/stabilizers on physicochemical properties of nanoparticles. Daru 23, 46. https://doi.org/10.1186/s40199-015-0128-3 (2015).
Pereira, A. et al. Metformin hydrochloride-loaded PLGA nanoparticle in periodontal disease experimental model using diabetic rats. Int. J. Mol. Sci. https://doi.org/10.3390/ijms19113488 (2018).
Nowacka, O. et al. Generation-dependent effect of PAMAM dendrimers on human insulin fibrillation and thermal stability. Int. J. Biol. Macromol. 82, 54–60. https://doi.org/10.1016/j.ijbiomac.2015.10.029 (2016).
Alai, M. S., Lin, W. J. & Pingale, S. S. Application of polymeric nanoparticles and micelles in insulin oral delivery. J. Food Drug Anal. 23, 351–358. https://doi.org/10.1016/j.jfda.2015.01.007 (2015).
Joshi, H. M., Bhumkar, D. R., Joshi, K., Pokharkar, V. & Sastry, M. Gold nanoparticles as carriers for efficient transmucosal insulin delivery. Langmuir 22, 300–305. https://doi.org/10.1021/la051982u (2006).
Neupane, B. P. et al. Himalayan honey loaded iron oxide nanoparticles: synthesis, characterization and study of antioxidant and antimicrobial activities. Int. J. Nanomedicine 14, 3533–3541. https://doi.org/10.2147/ijn.s196671 (2019).
Mohamed, N. A. et al. Chemical and biological assessment of metal organic frameworks (MOFs) in pulmonary cells and in an acute in vivo model: relevance to pulmonary arterial hypertension therapy. Pulm. Circ. 7, 643–653. https://doi.org/10.1177/2045893217710224 (2017).
Estelrich, J., Sanchez-Martin, M. J. & Busquets, M. A. Busquets, MA Nanoparticles in magnetic resonance imaging: from simple to dual contrast agents. Int. J. Nanomedicine 10, 1727–1741. https://doi.org/10.2147/ijn.s76501 (2015).
Al-Ansari, D. E. et al. Internalization of metal-organic framework nanoparticles in human vascular cells: implications for cardiovascular disease therapy. Nanomaterials 10(6), 1028 (2020).
Mohamed, N. A. et al. Studies on metal-organic framework (MOF) nanomedicine preparations of sildenafil for the future treatment of pulmonary arterial hypertension. Sci. Rep. 11, 4336. https://doi.org/10.1038/s41598-021-83423-6 (2021).
Koffert, J. P. et al. Metformin treatment significantly enhances intestinal glucose uptake in patients with type 2 diabetes: Results from a randomized clinical trial. Diabetes Res. Clin. Pract. 131, 208–216. https://doi.org/10.1016/j.diabres.2017.07.015 (2017).
Ghosh, S. et al. Metformin improves endothelial function in aortic tissue and microvascular endothelial cells subjected to diabetic hyperglycaemic conditions. Biochem. Pharmacol. 98, 412–421. https://doi.org/10.1016/j.bcp.2015.10.008 (2015).
Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 352, 854–865 (1998).
Tucker, G. T. et al. Metformin kinetics in healthy subjects and in patients with diabetes mellitus. Br. J. Clin. Pharmacol. 12, 235–246. https://doi.org/10.1111/j.1365-2125.1981.tb01206.x (1981).
Padwal, R. S. et al. Effect of gastric bypass surgery on the absorption and bioavailability of metformin. Diabetes Care 34, 1295–1300. https://doi.org/10.2337/dc10-2140 (2011).
Cetin, M. & Sahin, S. Microparticulate and nanoparticulate drug delivery systems for metformin hydrochloride. Drug Deliv. 23, 2796–2805. https://doi.org/10.3109/10717544.2015.1089957 (2016).
Ouslimani, N. et al. Metformin decreases intracellular production of reactive oxygen species in aortic endothelial cells. Metabolism 54, 829–834. https://doi.org/10.1016/j.metabol.2005.01.029 (2005).
Leon, B. M. & Maddox, T. M. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World J. Diabetes 6(13), 1246–1258. https://doi.org/10.4239/wjd.v6.i13.1246 (2015).
Graham, G. G. et al. Clinical pharmacokinetics of metformin. Clin. Pharmacokinet. 50(2), 81–98. https://doi.org/10.2165/11534750-000000000-00000 (2011).
Triggle, C. R. et al. Repurposing metformin for vascular disease. Curr. Med. Chem. 30(35), 3955–3978. https://doi.org/10.2174/0929867329666220729154615 (2023).
Li, H., Yang, Y. G. & Sun, T. Nanoparticle-based drug delivery systems for induction of tolerance and treatment of autoimmune diseases. Front. Bioeng. Biotechnol. 10, 889291. https://doi.org/10.3389/fbioe.2022.889291 (2022).
Scheen, A. J. Clinical pharmacokinetics of metformin. Clin. Pharmacokinetics 30(5), 359–371. https://doi.org/10.2165/00003088-199630050-00003 (1996).
Kenechukwu, F. C., Isaac, G. T., Nnamani, D. O., Momoh, M. A. & Attama, A. A. Enhanced circulation longevity and pharmacodynamics of metformin from surface-modified nanostructured lipid carriers based on solidified reverse micellar solutions. Heliyon 8(3), e09100. https://doi.org/10.1016/j.heliyon.2022.e09100 (2022).
Wang, W. et al. Adapted nano-carriers for gastrointestinal defence components: surface strategies and challenges. Nanomed. Nanotechnol. Biol. Med. https://doi.org/10.1016/j.nano.2020.102277 (2020).
Aun, R. & Wei, W. Metal-organic frameworks in oral drug delivery. Asian J. Pharm. Sci. https://doi.org/10.1016/j.ajps.2024.100951 (2024).
Gao, M. et al. Hydrogel-metal-organic-framework hybrids mediated efficient oral delivery of siRNA for the treatment of ulcerative colitis. J. Nanobiotechnol. 20(1), 404. https://doi.org/10.1186/s12951-022-01603-6 (2022).
Benny, A., Sunaja Devi, K. R., Pinheiro, D. & Chundattu, S. J. Metal organic frameworks in biomedicine: Innovations in drug delivery. Results Chem. 7, 101414–101414. https://doi.org/10.1016/j.rechem.2024.101414 (2024).
Chenthamara, D. et al. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 23, 20. https://doi.org/10.1186/s40824-019-0166-x (2019).
Asmat, U., Abad, K. & Ismail, K. Diabetes mellitus and oxidative stress-A concise review. Saudi Pharm. J. 24(5), 547–553. https://doi.org/10.1016/j.jsps.2015.03.013 (2016).
Ghosh, S. et al. Metformin improves endothelial function in aortic tissue and microvascular endothelial cells subjected to diabetic hyperglycaemic conditions. Biochem. Pharmacol. 98(3), 412–421. https://doi.org/10.1016/j.bcp.2015.10.008 (2015).
Horcajada, P. et al. Porous metal-organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat. Mater. 9, 172–178. https://doi.org/10.1038/nmat2608 (2010).
Singh, V. V., GT, A. K. & Kulkarni, R. H. Estimation and validation of metformin by colorimetry method. Anal. Chem. 9(1), 145–150 (2010).
Sakao, S., Tatsumi, K. & Voelkel, N. F. Endothelial cells and pulmonary arterial hypertension: apoptosis, proliferation, interaction and transdifferentiation. Respir. Res. 10, 95. https://doi.org/10.1186/1465-9921-10-95 (2009).
Bala, K., Ambwani, K. & Gohil, N. K. Effect of different mitogens and serum concentration on HUVEC morphology and characteristics: implication on use of higher passage cells. Tissue Cell 43, 216–222. https://doi.org/10.1016/j.tice.2011.03.004 (2011).
Cutiongco, M. F. A., Chua, B. M. X., Neo, D. J. H., Rizwan, M. & Yim, E. K. F. Functional differences between healthy and diabetic endothelial cells on topographical cues. Biomaterials 153, 70–84. https://doi.org/10.1016/j.biomaterials.2017.10.037 (2018).
Acknowledgements
We sincerely thank the Qatar National Research Fund for providing financial support for this research through grant #UREP26-019-3-006, awarded to H.A.S. We also extend our gratitude to Qatar University and the Grant Office for covering the article processing fees. Additionally, we acknowledge the British Pharmacological Society, United Kingdom, for their generous assistance through the Pickford Award granted to N.A.M. This support played a crucial role in facilitating the development of the nanoparticles employed in this study. The graphical abstract utilized in this research was generated using BioRender.com.
Funding
This research was supported by Qatar National Research Fund under the Undergraduate Research experience Program Grant no. [UREP26-019-3-006] (to H.A.S.), and the Pickford Award from the British Pharmacological Society (to N.A.M).
Author information
Authors and Affiliations
Contributions
Conceptualization: [N.A.M., I.M., H.A.S.], Methodology: [N.A.M., I.M., H.A.S.], Formal analysis and investigation: [N.A.M., I.M., H.A.S., H.A.M., S.S.M., H.K.B., A.M.A.]; Writing—original draft preparation: [H.A.M, N.A.M.]; Writing—review and editing: [H.A.S., S.C., N.A.M., I.M., C.R.T., H.D.]; Funding acquisition: [H.A.S., N.A.M., I.M.]; Resources: [N.A.M., I.M., H.A.S.]; Supervision: [N.A.M., H.A.S.]. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mohamed, H.A., Mohamed, N.A., Macasa, S.S. et al. Metformin-loaded nanoparticles reduce hyperglycemia-associated oxidative stress and induce eNOS phosphorylation in vascular endothelial cells. Sci Rep 14, 30870 (2024). https://doi.org/10.1038/s41598-024-81427-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-81427-6
Keywords
This article is cited by
-
The impact of metformin on myocardial hypertrophy: an updated systematic review, meta-analysis, and meta-regression of randomized controlled trials
Diabetology & Metabolic Syndrome (2025)