Abstract
Adult myogenic cell lines are useful to study muscle development, repair and regeneration. In newts, which are known for their high regenerative capacity, myogenic cell lines have not been established in species other than the Eastern newt Notophthalmus viridescens. In this study, we established another myogenic cell line, named CpM01, from the skeletal muscle of the forearm of the adult Japanese fire-bellied newt Cynops pyrrhogaster. CpM01 maintained high proliferative ability even after numerous passages, and could be induced to differentiate into myotubes by changing the culture medium. CpM01 expressed myogenic regulatory factors (MRFs) such as Myf5, MRF4 and myogenin. Changes in the immunorectivities of MRFs during differentiation of CpM01 into myotubes were consistent with those during new muscle generation in limb regeneration. In newts, myogenic cells have two origins, muscle fibers or satellite cells. CpM01 expressed Pax7, suggesting the origin might be satellite cells. scRNA-seq analysis deeply characterized CpM01 and demonstrated that the expression patterns of myogenic genes (Pax3, Pax7, myocyte-specific enhancer factor 2 A, and genes encoding MRFs) in CpM01 are related to progress of the cell cycle. CpM01 can be a useful tool for future studies of limb muscle regeneration in adult newts.
Similar content being viewed by others
Myogenic cell lines created from skeletal muscles, such as rat L61, mouse C2C122, and various lines of humans3, which can literally be induced to differentiate into muscle in vitro, have been used extensively to study muscle development, repair and regeneration, as well as for drug discovery and victimless meat development4,5,6. These myogenic cells are presumed to originate from muscle stem cells, named satellite cells, that accompany muscle fibers and act in growth, metabolic renewal, or the repair and regeneration of muscles7,8,9.
Newts, a urodele amphibian belonging to the family Salamandridae, have an exceptionally high regenerative capacity among tetrapods, endowing them with the ability to regenerate various parts of their body, including parts of the limbs, tail, jaws as well as the heart, brain, and eyes (the retina and lens), even as adults adapted to a terrestrial environment10,11,12,13,14,15,16,17,18. Thus far, Eastern newts (Notophthalmus viridescens), Japanese fire-bellied newts (Cynops pyrrhogaster) and Iberian ribbed newts (Pleurodeles waltl) have mainly been used for studies on the regeneration of body parts19. However, nowadays, since inter-regional and inter-country transfer of amphibians is difficult due to problems such as chytridiomycosis20, researchers have no choice but to use sterile laboratory-reared individuals or regionally available species.
In N. viridescens, an adult myogenic cell line named A1 has been developed21. It has been applied to the study of muscle development and regeneration not only in newts but also in mammals22,23,24,25,26,27,28. However, for researchers using other newt species, it is still necessary to originally establish adult myogenic cell lines in their newts.
We have been studying the cellular processes of muscle generation in limb regeneration using adult C. pyrrhogaster15,18. In this model, it is evident that mature multinucleated muscle fibers undergo cellularization (i.e., dedifferentiation), giving rise to mononucleated cells, which migrate, proliferate, and redifferentiate into new muscle fibers, although the underlying molecular mechanisms remain to be studied. On the other hand, the contribution of satellite cells to muscle generation in limb regeneration is still under investigation. The acquisition of adult myogenic cell lines in this species is expected to create opportunities to elucidate the cellular properties of these two myogenic cell types derived from satellite cells and muscle fiber cells, and the differences in their capacities for muscle regeneration and in their medical applicability. Therefore, in this study, we established a myogenic cell line, named CpM01, from the forearm muscle of adult C. pyrrhogaster, and profiled its expressed genes by single cell mRNA sequencing (scRNA-seq).
Results and discussion
CpM01 cell line
We performed explant culture of forearm muscles of adult newts (Fig. 1a). In a total of 4-week culture, muscle explants attached to the basal surface of the culture dish had changed their morphology to a circular shape when viewed vertically, from which numerous mesenchymal cells migrated on the basal surface (Fig. 1b). After the 4-week culture period, the explants were removed from the dishes, and mesenchymal cells on the dishes underwent 10 passages every 6–10 days. The cells at passage 10 were uniformly fibroblast-like in shape, and these were named CpM01 (Fig. 1c). CpM01 cells were stored once in liquid nitrogen, then recovered and passaged to obtain master stocks, which were further amplified to obtain working stocks for subsequent experiments and their own replenishment (Fig. 1a). It must be noted that the CpM01 cell line was obtained on the first attempt.
CpM01 cell line. (a) Schematic diagram showing the process from explant culture of adult newt forearm muscles to the formation of CpM01. LN2 liquid nitrogen. See Methods for a detailed explanation. (b) Representative image of a 4-week cultured muscle explant. In this case, a piece of the flexor digitorum communis was cultured. Many mesenchymal cells had migrated from the explant. Scale bar, 1 mm. (c) Representative image of CpM01. The cells are on day 6 of culture at passage 3 from the working stock. Paired arrowheads indicate daughter cells of mitotic cells. Scale bar, 200 μm.
Proliferative capacity of CpM01
The proliferative capacity of CpM01 was evident from its ability to be passaged, as well as by the fact that many cells showed proliferating cell nuclear antigen (PCNA) immunoreactivity (Fig. 2a) and 5-bromo-2’-deoxyuridine (BrdU) incorporation (Fig. 2b). Moreover, the growth rate of CpM01 was unlikely to be affected by cell density until the stationary phase (Fig. 2c). Besides, cells from the working stock that had already undergone numerous passages could be passaged at least seven more times without losing their proliferative capacity, suggesting that CpM01 cells are likely immortal (Fig. 2d).
Proliferative capacity of CpM01. See Methods for a detailed explanation of procedures. (a) PCNA immunoreactivity. In this case, about 83% of cells showed PCNA immunoreactivity. Scale bar, 100 μm. (b) BrdU incorporation. In this condition, 52.6 ± 10.3% (range: 36.2–71.3%, n = 4) of cells were BrdU-positive. Scale bar, 100 μm. (c) Growth test. Schematic diagram shows the procedure. Graph shows growth curves for the initial cell densities. Symbols and vertical bars indicate means and standard errors. The total number of cells per dish increased gradually to about 3-fold in 8–10 days, regardless of the initial cell density: the fold change in cell density at day 10 compared to the initial cell density was 3.8 ± 0.0 (n = 3), 3.4 ± 0.4 (n = 3) and 3.1 ± 0.2 (n = 4) for 2 × 104, 4 × 104 and 6 × 104 densities, respectively. (d) Immortality test. Schematic diagram shows the procedure. Passages at an initial density of 6 × 104 cells/35 mm dish were repeated seven times every 8–10 days. The total number of cells per dish after culture was plotted at each passage number in the graph. Here, the results from four independent rounds of the test are shown in different colors. The total number of cells per dish after culture did not decline with an increasing number of passages.
Myogenic potency of CpM01
To examine the myogenic potency of CpM01, when the cells in a dish had grown to 80–90% confluence, the culture medium was changed from the growth medium to a differentiation medium (Fig. 3a), as is commonly applied to other adult myogenic cell lines to induce them to differentiate into myotubes27,29,30. As expected, the cells fused together to form a multinucleated tubular or spindle-like structure 6 days after induction and expressed myosin heavy chain (MHC) (Fig. 3b), suggesting that CpM01 has the ability to differentiate into myotubes depending on the extracellular environment.
Myogenic potency of CpM01. (a) Schematic diagram showing a procedure to induce differentiation. HS horse serum. See Methods for a detailed explanation. (b) Changes of cells in 6 days after induction. Upper panels show representative phase contrast images showing morphological changes (n = 5). Cells fused together to form multinucleated cells in tubular or spindle-like structures. Scale bars, 500 μm. Lower panels show representative images showing myosin heavy chain (MHC) immunoreactivity of cells before and 6 days after induction (n = 27). Green: MHC. Blue: DAPI nuclear stain. Scale bars, 200 μm.
Changes in the expression pattern of myogenic regulatory factors
Forearm regeneration
Myogenic regulatory factors (MRFs) including MyoD, Myf5, MRF4 and myogenin are known to govern the differentiation of myogenic cells during skeletal muscle development and regeneration31. Therefore, we first examined the changes in the expression pattern of myogenic factors (Myf5, MRF4 and myogenin) during the generation of new muscles in forearm regeneration, as a reference to evaluate the differentiation process of CpM01 into myotubes. Note that MyoD was not found in this study (see Methods). Transcriptome and PCR analyses predicted that Myf5, MRF4 and myogenin transcripts are all present in normal forearm muscle, but the amount of myogenin transcripts is much lower than that of the other myogenic factors, and when compared to normal forearm muscle, the relative amounts of Myf5 and MRF4 transcripts decreased in stage III forearm blastema32, while the relative amount of myogenin increased (Supplementary Fig. S1a–f). This stage of blastema contains mesenchymal cells, including myogenic cells derived from muscle fiber cells and potentially those from satellite cells, that have migrated from the area proximal to the amputation surface of the forearm15,18, and is simply referred to as “limb blastema” hereafter. Western blotting analysis suggested that Myf5 and MRF4 proteins are present in both normal muscle and limb blastema, and myogenin protein in limb blastema (Supplementary Figs. S1g, S2).
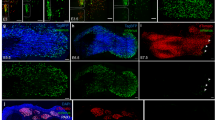
Consistent with these predictions, Myf5- and MRF4-immunoreactivity were observed in normal forearm muscles, while myogenin-immunoreactivity was hardly detectable (Fig. 4a). Of note, however, Myf5- and MRF4-immunoreactivity were mostly localized in perinuclear patterns. In the limb blastema, Myf5- and MRF4-immunoreactivity were observed in many cells inside the blastema (Fig. 4b). Of note, these reactivities were found inside the nucleus, unlike those in normal muscle, possibly reflecting the initiation of gene transcription by Myf5 and MRF4. In some cells, Myf5-immunoreactivity was also observed in the cytoplasm (Fig. 4b). Considering the predictions from transcriptome analysis (Supplementary Fig. S1a), such changes in the localization of immunoreactivity from perinuclear to intranuclear patterns might be due to changes in post-translational modifications of Myf5 and MRF4, rather than their excessive expression. On the other hand, consistent with the predictions from transcriptome and Western blotting analyses (Supplementary Fig. S1a,g), myogenin-immunoreactivity appeared in many cells in the limb blastema, although the intense immunoreactivity was characteristically confined to the perinuclear region (Fig. 4b). Such perinuclear localization of myogenin has also been reported in a mouse myogenic cell line C2C1233. At the time of hand and finger formation in the regenerating forearm, or the digit stage32, immunoreactivity for Myf5, MRF4 and myogenin were all observed in differentiating muscles surrounding regenerating cartilage, and all were localized both inside the nucleus and in the cytoplasm (Fig. 4c). However, the immunoreactivity of Myf5 in the nucleus had obviously decreased (Fig. 4c) compared to that in the blastema (Fig. 4b). On the other hand, the immunoreactivity of myogenin inside the nucleus and in the cytoplasm appeared to be higher than that of the other two factors (Fig. 4c), possibly reflecting active myogenin gene expression.
The process of muscle differentiation during limb development in tetrapods is conserved, with MyoD/Myf5 and MRF4 being involved in the commitment of the myogenic fate of somite-derived mesenchymal cells, while MRF4 and myogenin are involved in the terminal differentiation of myogenic cells31. Our results, showing intranuclear localization of Myf5- and MRF4-immunoreactivity in the blastema and elevated immunoreactivity for MRF4 and myogenin in differentiating muscle fibers during forearm regeneration, suggest that the conserved genetic program for myogenesis is also utilized during the generation of new muscle in limb regeneration, although MyoD involvement was not evident in this study.
Changes in the immunoreactivity of Myf5, MRF4 and myogenin during forearm regeneration. (a) Intact muscle of the forearm. Transverse sections of muscles are shown. Red: Myf5, MRF4 and myogenin. Arrowheads point to the immunoreactive area. Green: myosin heavy chain (MHC). Blue: DAPI nuclear stain. Scale bar, 50 μm. (b) Presumptive myogenic cells in blastema. Sections along the dorsoventral axis are shown. White line indicates the amputation plane. RFP: negative control. Scale bars, 500 μm (left); 100 μm (center); 50 μm (right). (c) Differentiating muscle cells in regenerating forearms at the digit stage. Sections along the dorsoventral axis are shown. Car: cartilage. Scale bars, 50 μm.
Differentiation of CpM01 into myotubes
We examined changes in the expression patterns of Myf5, MRF4 and myogenin during differentiation of CpM01 into myotubes (Fig. 5). The patterns of immunoreactivity for these factors in CpM01 (“Before” in Fig. 5a–c) was consistent with the patterns in mesenchymal cells in the limb blastema (Fig. 4b). For both Myf5 and MRF4, immunoreactivity was observed in the nuclei of almost all cells (Fig. 5a,b), although the intensity of immunoreactivity varied, with many nuclei having a relatively low signal (Fig. 5d,e). For Myf5, however, almost all the cells also had intense immunoreactivity in their cytoplasm (Fig. 5a). For myogenin, intense immunoreactivity was localized in the perinuclear region (Fig. 5c) as observed in the limb blastema (Fig. 4b). The ratio of cells with perinuclear immunoreacitivity was 46.8 ± 0.8% (n = 3), although intranuclear immunoreactivity with varying intensity was detected in almost all cells (Fig. 5f).
Upon induction of differentiation, immunoreactivity for Myf5, MRF4 and myogenin was observed in both the nucleus and cytoplasm of myotubes (“6 days after induction” in Fig. 5a–c), consistent with that in the forearm regeneration (Fig. 4c). For all three factors, nuclear immunoreactivity in myotubes was intense compared to that of mesenchymal cells surrounding the myotubes, which were not involved in myotube formation and exhibited varying intensities of signals (Fig. 5d–f). Semi-quantitative PCR was performed to examine gene expression in the samples before and 6 days after induction (n = 3 for each), but no statistically significant increase was observed in samples of 6 days after induction for any of the genes (Supplementary Fig. S3). Thus, although the semi-quantitative PCR results may be an underestimate due to the presence of a large number of cells not mobilized for myotube formation, the change in localization of the protein from the perinucler region to the nucleus was more evident than an increase in gene transcription. By contrast, nuclear immunoreactivity for Myf5 appeared to decline in the digit stage of forearm regeneration (Fig. 4c). This is probably because the differentiating muscle in the digit stage was more advanced than can be achieved in culture. Overall, the changes in the immunoreactivities of myogenic factors during differentiation of CpM01 into myotubes were in good agreement with those observed during muscle generation in forearm regeneration.
Changes in immunoreactivity for Myf5, MRF4 and myogenin during the differentiation of CpM01 into myotubes. Blue: DAPI nuclear stain. (a–c) Representative immunofluorescent images of cells before induction (left column; n = 3) and at 6 days after induction (right column; n = 3). Scale bars, 200 μm. (d–f) Immunofluorescence intensity of the nucleus before induction and at 6 days after induction. In each condition, the mean luminance of fluorescence in the nucleus was individually measured for all cells in six randomly captured images of rectangular (726 μm × 544 μm) fields in three dishes after immunostaining (see Methods). Note that perinuclear and cytoplasmic fluorescence were not measured. For all three factors, the distribution patterns of the mean luminance of a nucleus changed significantly within 6 days after induction (two-sample Kolmogorov-Smirnov test; Myf5, n = 1894 (before), n = 2108 (after), P < 0.001; MRF4, n = 1419 (before), n = 1968 (after), P < 0.001; myogenin, n = 1888 (before), n = 2876 (after), P < 0.001). Note that for all three factors, nuclei exhibiting saturation levels of high immunofluorescence at 6 days after induction were localized in myotubes.
Characterization of CpM01 by scRNA-seq analysis
Limb muscles of C. pyrrhogaster should have the capacity to give rise to two myogenic cells that originate from either contractile fibers or satellite stem cells15. Our previous muscle tracking studies with adult individuals demonstrated that dedifferentiated muscle fiber cells migrate into blastema that generate new muscle fibers as the patterned cartilage appears in the regenerating limb15,18, although the contribution of satellite cells to the blastema has not been determined. In this study, we found that the immunostaining patterns of MRFs in CpM01 resemble those in the limb blastema, raising doubt as to whether the cellular origin of CpM01 is muscle fibers or satellite cells. To obtain clues to resolve this doubt, we examined the expression of a transcription factor Pax7, which is a marker for satellite cells7,9. As a result, Pax7 expression was hardly detectable in CpM01 using current immunostaining protocols, although Pax7-immunoreactivity was evident in some mesenchymal cells that appeared around 4-week-old muscle explants, which were presumably derived from satellite cells (Fig. 6a). However, since there is a possibility that Pax7-immunoreactivity of satellite cells may be lost during their migration to the blastema in adult newt limb regeneration34, our result could not lead definitive conclusions. Therefore, we next conducted scRNA-seq of CpM01, and detected Pax7 expression in CpM01 at the transcriptional level (also see Supplementary Fig. S4 for the PCR result), suggesting that the origin of CpM01 is most likely satellite cells (see below).
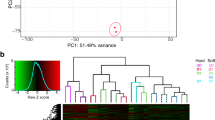
To characterize CpM01 more deeply, we compared CpM01 with clusters of certain erythroid and retinal cells. A t-Distributed Stochastic Neighbor Embedding (t-SNE) map revealed that CpM01 is obviously different from the other two cell types (Fig. 6b). Genes which were expressed at significantly higher levels in CpM01 than the other two cell types were selected and annotated (Fig. 6c; Supplementary Data). As anticipated, CpM01 characteristically expressed not only MRFs (Myf5, MRF4 and myogenin) but also Pax7 and Pax3, as well as myocyte-specific enhancer factor 2 A (MEF2A) (Fig. 6d). Both Pax7 and Pax3 are known to be involved in the specification of cell fate to muscle and in the quiescence of satellite cells7,9,31. MEF2A is known to be involved in the processes of growth and maturation of muscles in combination with MRFs35,36.
The CpM01 cluster was subdivided into five subclusters (Fig. 6e), which correspond to phases of the cell cycle (Fig. 6f). Clusters 2 and 3 appeared to be in the G1 phase and S phase, respectively, since both CDK4 and Cyclin D1 were expressed in both clusters, while increased expression of PCNA was detected in cluster 3. It is known that CDK4 and Cyclin D1 form a complex necessary for the transition from G1 to the S phase37, and PCNA is strongly expressed in the G1 − S phase, although its expression is detected across the entire cell cycle in C. pyrrhogaster11,17,38. Cluster 4 appeared to be in the G2 phase, since rapid and maximal expression of Cyclin B3 was detected. Cyclin B3 is involved in the transition from G2 to the M phase36. Then, cluster 1 could be in the G0 phase and cluster 5 in the M phase.
It is known that the expression of myogenic genes is regulated in relation to the cell cycle39. Therefore, we next examined the expression of myogenic genes in each subcluster (Fig. 6f). Transcripts of Pax7, Pax3 and MEF2A genes were all detected in cluster 1, i.e., the G0 phase. The amount of Pax7 and MEF2A transcripts decreased dramatically in cluster 2 (G1 phase), and the level of Pax7 expression recovered in cluster 3 (S phase), while the level of MEF2A expression increased considerably in cluster 4 (G2 phase), tended to increase in cluster 5 (M phase) and decreased in cluster 1 (G0 phase). The amount of Pax3 transcripts increased in cluster 3 (S phase) and was maintained over cluster 4 (G2 phase) and cluster 5 (M phase). Transcripts of Myf5, MRF4 and myogenin genes were also detected in cluster 1 (G0 phase), although their amounts were relatively lower than those of the other myogenic gene transcripts. The amount of Myf5, MRF4 and myogenin transcripts decreased in cluster 2 (G1 phase). Transcription of the Myf5 gene was detected again in cluster 4 (G2 phase), while transcription of the MRF4 gene was never detected in other clusters, or throughout the cell cycle. The amount of myogenin transcripts increased rapidly in cluster 3 (S phase), tended to increase over cluster 4 (G2 phase) and cluster 5 (M phase), and decreased sharply in cluster 1 (G0 phase). Taken together, the amount of transcripts of myogenic genes appeared to be regulated in association with the cell cycle.
In this study, we clearly detected the transcript of the Pax7 gene in CpM01 (Fig. 6d,f and Supplementary Fig. S4), while Pax7 immunoreactivity was difficult to detect (Fig. 6a). On the other hand, we detected distinct immunoreactivity for Myf5 and MRF4 in CpM01 (Fig. 5a,b), but only a small amount of transcripts for these genes (Fig. 6d,f). For myogenin, we detected both distinct expression of transcripts and immunoreactivity in CpM01 (Figs. 5c and 6d,f), but immunoreactivity showed perinuclear localization (Fig. 5c). Thus, the regulation of transcription and translation, as well as the modification and degradation of each product, is likely to be complex for each gene, also relating to the cell cycle39,40,41,42. The mechanisms underlying these regulations and modifications, and the physiological significance of myogenic factors in CpM01 itself, need to be investigated in the future.
Characterization of CpM01 by scRNA-seq analysis. (a) Representative images of Pax7 immunoreactivity. A satellite cell marker, Pax7, was detected in migrating cells around a 4-week-old muscle explant (left column; n = 4), but not in CpM01 (right column; n = 2). Blue: DAPI nuclear stain. Scale bars, 200 μm. (b) Comparison among CpM01, erythroid cells and retinal cells by principal component analysis with their scRNA-seq data (see Methods). These cell types were separated into different clusters on the t-SNE map. (c) A heat map visualized the top 20 genes expressed at significantly higher levels in each cell type. (d) Expression patterns of myogenic genes in these clusters. Transcripts of myogenic genes, including Pax7, were detected in CpM01. (e) Five subclusters of CpM01 visualized on the UMAP map. (d) Violin plots showing the expression patterns of cell cycle marker genes and myogenic genes in the subclusters.
Conclusions
In this study, we succeeded in obtaining an adult myogenic cell line, CpM01, from C. pyrrhogaster (Supplementary Fig. S5). The origin of the cells is assumed to be satellite cells, as in other vertebrate adult myogenic cell lines, because of the presence of Pax7 transcripts in these cells. However, to conclude this, it is necessary to prove the absence of Pax7 transcripts in the dedifferentiated muscle fiber cells and/or to obtain a molecular signature that can distinguish the dedifferentiated muscle fiber cells from satellite cell-derived myogenic cells. However, either way, CpM01 will be a useful tool in studies of muscle development, repair and regeneration.
Methods
Animals
Sexually mature males of C. pyrrhogaster (total body length: 9–11 cm) were used. They were captured from Miyagi, Niigata, Fukui and Ishikawa prefectures by a supplier (Aqua Grace, Yokohama, Japan), and reared in plastic containers containing shallow water and a resting place (land) at 18–22 °C under natural light conditions until experiments were performed43. All experiments were carried out in accordance with the guidelines and regulations in the University of Tsukuba. All protocols with animals were approved by the Animal Care and Use Committee of the University of Tsukuba (approval number: 220125). Moreover, all methods were performed and reported in compliance with the ARRIVE guidelines 2.044.
Anesthesia
0.1% FA100 (4-allyl-2-methoxyphenol; LF28C054; DS Pharma Animal Health, Osaka, Japan) dissolved in water was used at room temperature (RT: 22 °C). Animals were placed in a sealed bottle containing the anesthetic solution (five or less individuals/300 mL) for 45 min before limb amputation11,15,17,18,32.
Limb amputation
After anesthesia, limb amputation was performed as described previously15,18,32,45,46. To obtain the normal forearm samples for muscle explant culture and biochemistry, the upper arm was amputated in the middle (at the mid-stylopod region) or near the level of the elbow. To induce forearm regeneration, the forearm was amputated in the middle (at the mid-zeugopod region). To obtain samples of regenerating forearms for histology, the upper arm was amputated in the middle, and for biochemistry, the regenerating forearm was amputated again at the level of the amputation plane. After the limb samples had been harvested, amputees were placed on dry paper towels until the bleeding stopped, transferred to moist containers with a lid containing air vents (up to three newts per container of length 200 mm × width 150 mm × height 55 mm) and allowed to recover. The moist container was always kept in a semi-dry condition in which the bottom was covered by a moist paper towel (Elleair Prowipe, Soft High Towel, Unbleached, 4P; Elleair Paper Business Support, Tokyou, Japan) that was tightly wrung. Paper towels were replaced with new ones every other day. The stages of limb regeneration were determined according to previous criteria32 otherwise noted.
Acquisition of the cell line
One male individual (total body length: 9 cm) was used for this purpose. After the right forelimb was amputated in the middle of the upper arm using a surgical blade (No. 19, Futaba, Tokyo, Japan) under a dissecting microscope (SZ61; Olympus/Evident, Tokyo, Japan), the sample was immersed in 70% ethanol (diluted in type1 MilliQ water, Merck Millipore, Merck, Tokyo, Japan) for 5 s, washed twice each with fresh sterile phosphate-buffered saline (PBS; pH 7.5), and then, under the dissecting microscope, immobilized on a silicon base (SILPOT 184 W/C; DuPont Toray Specialty Materials K.K., Tokyo, Japan) of a PBS-containing 35 mm plastic dish (353001; Falcon, Corning, Shizuoka, japan) with fine insect pins (20111; Shiga Konchu Fukyusha, Tokyo, Japan). In this condition, after the skin and bone of the forearm were carefully removed using fine forceps, two major muscles, the flexor digitorum communis (FDC) and extensor digitorum communis (EDC)47 without tendons were cut off from the rest. Both muscles were cut into 1 mm blocks along the longitudinal axis, and one block each of FDC and EDC was placed together on a collagen type I-coated glass base dish (27 mm; 4970-011; AGC Techno Glass, Shizuoka, Japan) filled with 1.5 mL of a Leibovitz’s L-15-based medium (72% Leibovitz’s L-15 medium (41300-039; Gibco, Thermo Fisher Scientific, Waltham, MA, USA), 10% FBS (SH30071.03, Hyclone, Cytive, Marlborough, MA, USA), 1% penicillin/streptomycin (15140-122; Gibco, Thermo Fisher Scientific)). A total of six dishes were prepared and incubated at 25 °C in a Parafilm-sealed condition for 3 weeks (Fig. 1a).
After culture for 3 weeks, four dishes were selected in which muscles were firmly attached to the bottom and mesenchymal cells appeared surrounding the muscles, the culture medium was replaced with “growth medium” (65% MEM (11090081; Gibco, Thermo Fisher Scientific), 10% FBS, 1% L-glutamine (25030081; Gibco, Thermo Fisher Scientific), 1% penicillin/streptomycin and 10 µg/mL insulin (I6634-100MG, Sigma-Aldrich, Merck, Tokyo, Japan)), and the dishes were incubated at 25 °C under a CO2 equilibration condition for an additional 1 week (Fig. 1a). During a total of 4 weeks of explant culture, half of the volume of the medium was replaced with fresh medium every 4 days. After 4 weeks of culture, muscle explants were removed from the dishes, and mesenchymal cells on the bottom of the dish were washed with 1 mL PBS three times, and incubated in 1 mL of 1/5x Trypsin-EDTA (0.25% Trypsin/0.53 mM EDTA in Hanks Balanced Salt Solution without calcium or magnesium, 30-2101; ATCC, Gaithersburg, MD, USA) diluted with PBS for 5 min at RT (trypsinization). In the meantime, the cells were separated from the bottom of the dish by tapping the dish lightly with a finger under the dissecting microscope. After 1 mL of growth medium was added to the dish to inactivate trypsin, the cell suspensions from four dishes were combined in one tube and centrifuged at 93 ɡ for 8 min (LC-120; TOMY, Tokyo, Japan). After the supernatant was removed, the cell pellet was resuspended in 1.5 mL of fresh growth medium and cultured in one 35 mm collagen type I-coated plastic dish (4000-010, IWAKI, Shizuoka, Japan) until the cells had grown to cover 80–90% of the bottom of the dish, i.e., 80–90% confluence (passage 1). The cells in the dish were collected in one tube as before, divided equally into two dishes, and cultured until each was 80–90% confluent (passage 2). The cells in the two dishes were collected in one tube, divided equally into four dishes, and cultured until each was 80–90% confluent (passage 3). The cells in the four dishes were collected in one tube, and a half volume of the cell suspension collected was divided equally into four dishes, and cultured until each was 80–90% confluent (passage 4). This step was repeated six more times until passage 10 to obtain CpM01 (Fig. 1a). The incubation period per passage was 6–10 days.
CpM01 cells in four dishes at passage 10 were collected by trypsinization, mixed in growth medium containing 8% dimethyl sulfoxide (DMSO; 4-X; ATCC, Manassas, VA, USA), transferred equally into two cryotubes (~ 2.0 × 105 cells/tube; 377267, Nunc™ Biobanking and Cell Culture Cryogenic Tubes; Thermo Fisher Scientific), and stored in LN2 (Fig. 1a). The cells in these two tubes were recovered in one dish (recovery rate was about 47%) and amplified through the following three passages (Supplementary Fig. S6a). Note that passages were performed so that cells from one dish were equally seeded into two dishes and cultured until each was 70–80% confluent. The cells after the culture of passage 3 (8 dishes) were mixed, transferred into three cryotubes (~ 2.0 × 105 cells/tube), and stored in LN2 (master stock). Remaining cells were used to check the proliferative activity of the cells for the master stock (Fig. 2a,b; see below). The cells in one tube of the master stock were amplified as before and stored in LN2 (working stock), and were further amplified to either replenish the working stock or use for experiments (Fig. 1a and Supplementary Fig. S6b).
Evaluation of proliferative capacity
To evaluate the proliferative capacity of CpM01, ~ 6.0 × 104 cells for the master stock (before freezing) were seeded into a collagen type I-coated glass base dish, cultured for 6 days until 80% confluence, and then examined for their PCNA immunoreactivity (Fig. 2a). This test was not repeated because of sample limitations. For the proliferative capacity of the working stock, cells amplified through 3–4 passages from the working stock were seeded at ~ 2.0 × 104 cells in a collagen type I-coated glass base dish and cultured for 2 days. The cells attached on the bottom were further cultured in the presence of 10 µM BrdU (B5002-100MG; Sigma-Aldrich, Merck) for 24 h, and then examined for their BrdU immunoreactivity (Fig. 2b).
In the growth test (Fig. 2c), cells of the working stock were allowed to recover for 1–3 days and then passaged twice. When the culture of the second passage reached 70–80% confluence, the cells were collected and subcultured at an initial density of either 2.0, 4.0, or 6.0 × 104 cells/35 mm collagen type I-coated plastic dish for 10 days. The total number of cells per dish was counted every other day. Note that it always took 6–10 days for the cells to reach 70–80% confluence, when culturing cells from the working stock was started from a density of 6.0 × 104/35 mm dish.
In the immortality test (Fig. 2d), cells of the working stock were allowed to recover for 1–3 days and then passaged at an initial density of 6.0 × 104 cells/35 mm dish. Passages in the same manner were repeated six more times every 8–10 days.
Induction of differentiation
To induce the differentiation of CpM01 into myotubes, cells of the working stock were allowed to recover for 1–3 days and then passaged three times. As the cells of the third passage reached 80–90% confluence, the culture medium was changed from growth medium to “differentiation medium” (65% MEM, 1% horse serum (H1138-100ML; Sigma-Aldrich, Merck), 1% L-gultamine, 1% penicillin/streptomycin and 10 µg/mL insulin). The cells were cultured in this condition for 6 days. During this period, the medium was refreshed every other day.
Molecular cloning of C. pyrrhogaster MRFs
Contigs for the orthologs of Myf5, MRF4, and myogenin were found in a C. pyrrhogaster comprehensive transcriptome database, TOTAL (http://antler.is.utsunomiya-u.ac.jp/imori/)32 Supplementary Figs. S7–S11). Of note, however, contigs for MyoD were not found. The existence of these three transcripts in the limb muscle and blastema was validated by standard PCR-based molecular cloning (Supplementary Fig. S1b–e) and subsequent nucleotide sequencing by MinION (MIN-101B, Flow Cell (R10.4.1) FLO-MIN114, SQK-LSK114 sequencing kit, MinKNOW Stand-alone NC Windows version 23.11.5; Oxford Nanopore Technologies plc., Oxford, UK), using cDNAs which were constructed from normal forearm muscles and stage III forearm blastemas with Nucleospin RNA (Mini kit for RNA purification; Takara Bio Inc., Shiga, Japan) and the SuperScript IV First-Strand Synthesis System (Thermo Fisher Scientific)17,32. The nucleotide sequences determined here were deposited at GenBank (accession numbers: Myf5, PP934189; MRF4, PP934188; myogenin, PP934190). MEGA11 (version 11.0.013; https://www.megasoftware.net/) was used for molecular phylogenetic tree analysis with sequence data of 15 vertebrate species, and 500 bootstrap replicates (a maximum likelihood method based on the JTT matrix-based model) were performed. Amino acid (AA) sequences were obtained from the NCBI database (https://www.ncbi.nlm.nih.gov/) except for those of urodele amphibians, which were obtained from databases of Ambystoma mexicanum (AmexT_v47 - v.47; https://www.axolotl-omics.org/), P. waltl (iNewt; https://www.nibb.ac.jp/imori/main/), C. pyrrhogaster (TOTAL) and N. viridescence (RSNRP; http://sandberg.cmb.ki.se/redspottednewt/).
Antibodies
Antibodies used in this study are listed in Supplementary Fig. S12. Based on the nucleotide sequences, rabbit polyclonal antibodies against Myf5, MRF4, and myogenin of C. pyrrhogaster were generated (Eurofin genomics, Tokyo, Japan), and their specificity was confirmed by Western blotting (Supplementary Figs. S1g, S2).
Western blotting
Proteins were extracted from normal forearm muscles and stage III forearm blastemas, each of which were collected from four individuals (two males and two females) in a 1.5 mL protein low-binding tube (0030 108.116; Eppendolf, Hamburg, Germany) filled with 0.5 mL of chilled PBS. After centrifugation (93 ɡ, 1 min), PBS was carefully removed from the tube, and the weight of the sample was measured. The sample was added with lysis buffer (25 mM Tris, 150 mM NaCl, 1 mM EDTA-2Na, 1% Igepal CA-630, 1% sodium deoxycholate, 0.1% SDS) containing 1% proteinase inhibitor cocktail (P8340; Sigma-Aldrich, Merck) in the volume equivalent to the same weight of water, and mashed (Biomasher, 893062; Nippi, Tokyo, Japan). To make the lysate smooth, it was frozen with LN2 and sonicated (46 kH, VS-70U; Iuchi/AS ONE, Osaka, Japan) in chilled water for 5 min. This step was repeated five times. After spinning down the content (15,000 ɡ, 10 min, 4 °C), the supernatant was transferred to a fresh 1.5 mL protein low-binding tube. It was mixed with the same volume of 2x Laemmli Sample Buffer (161–0737; Bio-Rad, Hercules, CA, USA), heat-denatured by placing it in boiling water for 5 min, and stored at − 80 °C until use. Western blotting was performed as described previously11,32,46. Briefly, protein samples and the molecular weight marker proteins (All Blue Prestained Protein Standards, 1610373; Bio-Rad, Tokyo, Japan) were separated on a 4–15% gradient gel (4561083, Mini-PROTEAN TGX Precast Gels; Bio-Rad) by SDS-PAGE, and transferred to an activated Immun-Blot® PVDF membrane (1620–174; Bio-Rad).
Tissue preparation
Tissue sections for immunostaining were prepared as described previously11,15,17,18,32. Normal and regenerating limb samples were fixed in a modified Zambony’s fixative (2% paraformaldehyde and 0.2% picric acid dissolved in PBS) for 5 h at 4 °C. Exceptionally, for normal forearm muscle, the skin was removed from the limb samples before fixation. Fixed samples were thoroughly washed with chilled PBS and immersed in 30% sucrose-PBS solution overnight (15 h) at 4 °C. They were embedded in Tissue-Tek O.C.T. Compound (4583; Sakura Finetek USA, Inc., Torrance, CA, USA), frozen at − 20 °C in a cryostat (CM1860; Leica Biosystems, Tokyo, Japan), and then stored at − 80 °C until use. The samples were sectioned at between − 30 and − 20 °C by the cryostat: for normal muscle, transverse sections of limbs were made at a thickness of 12 μm (30–40 slices/sample); for regenerating limbs, sections along the dorsoventral axis were made at a thickness of 20 μm (20–30 slices/sample). Tissue sections were placed on gelatin-coated coverslips, air-dried, and then stored at − 20 °C until use.
Culture preparation
Cultures for immunostaining were fixed in 2% paraformaldehyde in PBS solution (pH 7.5) for 20 min at 4 °C and rinsed gently (PBS, 0.2% TritonX-100 in PBS (PBST), PBS; each for 15 min). In the case of BrdU staining, they were incubated in 2 N HCl (080-01066; Wako; Fujifilm, Osaka, Japan) diluted in type1 MilliQ water for 30 min at RT, and then rinsed in PBS before immunostaining.
Immunostaining
For each immunoblotting, a Western-blotted membrane from a gel electrophoresed with the same amount of protein in adjacent lanes was cut into two pieces prior to immunostaining for the test and control antibodies (Supplementary Figs. S1g, S2). Protein bands on the membranes were immunostained as described previously11,32,46. Signals were visualized with an ABC (Vectastain ABC Elite kit, PK-6100; Vector Laboratories, Newark, CA 94560)–DAB (SK-4100; Vector Laboratories) system.
Fixed tissue sections were immunostained as described previously11,15,17,18,32. Signals were visualized with fluorescent secondary antibodies.
Fixed cultures were immunostained as follows: they were incubated with blocking solution (PBST containing 5% serum of the same species as that in which secondary antibody was produced) at RT for 30 min, and then incubated with primary antibody diluted in the blocking solution at 4 °C for 15 h. After washing, they were incubated in secondary antibody diluted in the blocking solution at RT for 30 min. After washing, cell nuclei were counter stained with 4,6-diaminodino-2-phenylindole (DAPI, 1: 50,000; D1306; Thermo Fisher Scientific). In these experiments, normal donkey serum (017-000-001; Jackson ImmunoResearch, West Grove, PA, USA) or normal goat serum (S-1000; Vector Laboratories) was used for the blocking solution.
Image acquisition
Images of living cultures were captured by a single-lens reflex camera system (EOS Kiss X7; EOS Utility, Version 2.13.30.0; Cannon, Tokyo, Japan) mounted on an inverted phase contrast microscope (IMT-2, Olympus/Evident). Images of immunostained cultures were acquired by an all-in-one fluorescence inverted microscope system (BZ-X800; KEYENCE, Osaka, Japan) with filter sets for EGFP (OP-87763; exciter: 470/40 nm; emitter: 525/50 nm), TRITC (OP-87764; exciter: 545/25 nm; emitter: 605/70 nm), and DAPI (OP-87762; exciter: 360/40 nm; emitter: 460/50 nm). Images of immunostained tissue sections were acquired through a confocal microscope system (LSM700; ZEN 2009, ver. 6.0.0.303; Carl Zeiss, Oberkochen, Germany) with filter sets for rhodamine (Diode 555 Laser; emitter: BP 575–640 nm), Alexa Fluor 488 (Diode 488 Laser; emitter: BP 515–565 nm) and DAPI (Diode 405-5 Laser; emitter: BP 445/50 nm).
Single cell mRNA sequencing
CpM01 cells were harvested from three dishes at the third passage from a master stock, as usual (Supplementary Fig. S6b), except for the time of culture (8 days), cell density (80–90% confluence), and trypsinization conditions (1x Trypsin-EDTA, 28 °C for 1 h). The cells were stored in LN2 as for the master stock and transported to the Life Science Data Research Center (LiSDaC) in Tokyo University (Kashiwa, Japan), the site for the cDNA library construction and sequencing (see below), in a frozen condition.
For erythroid cells, blood/mesenchymal cells were collected from regenerating parts of the right forelimbs of 30 adult males (mid bud stage (or stage III31), 20; late bud stage, 1; palette stage, 6; early digit stage, 3)48 as follows: the regenerate samples were harvested in 100 µL drops (4–5 samples/drop) of 30 mM tetrasodium 1,2-bis(2-aminophenoxy)ethane-N, N,N’,N’-tetraacetate (BAPTA-Na4) dissolved in PBS (T2844; Tokyo Chemical Industry, Tokyo, Japan), which were placed on the bottom of a 90 mm plastic dish (01–013; Sansei Medical, Kyoto, Japan) on ice. The regenerates were minced by a blade (FA-10; FEATHER Safety Razor, Osaka, Japan), and released cells were carefully transferred into a 1.5 mL protein low-binding tube with cell saver micropipettes (200 µL, PT-004; 1000 µL, PT-004; INA OPTIKA, Osaka, Japan). The cells were washed twice in PBS by centrifuging at 1800 ɡ for 10–20 s, placed on ice, and brought to LiSDaC within 1 h.
For retinal cells, retinal pigment epithelium cells were collected from 20 normal retina-less eye-cups (a posterior half of eyeball from which the neural retina was removed) prepared from adult females and males (5 each) as described previously49. Briefly, the animals were anesthetized as for limb amputation, except that they were placed in the anesthetic solution for 2 h in dark50. After decapitation, normal eyeballs were enucleated from the head under the dissecting microscope, and placed cornea side up on a membrane filter (0.45 μm MF-Millipore MCE Membrane, HAWP01300; Merck) in a 35 mm Petri dish (Nunc™ Cell Culture/Petri Dishes 171099; Thermo Fisher Scientific) (one eyeball/dish). PBS was poured into the dish so that the eyeball was immersed. A portion of the eyeball anterior to the cornea-sclera boundary that includes the ciliary marginal zone was excised, and the posterior portion was used as an eye-cup (this process was repeated for all eyeballs). Then, the neural retina was carefully removed from the eye-cup in PBS, making a retina-less eye-cup (this process was repeated for all eye-cups). The retina-less eye-cups on the membrane filter were transferred into 180 µL drops of newt normal saline (in mM: NaCl, 115; KCl, 3.7; CaCl2, 3; MgCl2, 1; D-glucose, 18; HEPES, 5; pH 7.5 adjusted with 0.3 N NaOH) (one sample/drop) placed on the bottom of a 90 mm plastic dish (01–013; Sansei Medical), and incubated at RT for 1 min. These samples were transferred into 180 µL drops of 0.2 mM collagenase I (SCR103; Sigma-Aldrich, Merck) in the newt normal saline (one sample/drop) placed in another 90 mm plastic dish, and incubated at 28 °C for 90 min. The collagenase I solution on the sample was replaced with PBS, the volume of PBS was carefully reduced as much as possible, and then the samples were immersed in 180 µL of 1x Trypsin-EDTA solution and further incubated at 28 °C for 45 min. The sample was transferred into PBS in a 40 μm cell strainer (pluriStrainer, 45-50040-03; pluriSelect Life Science, Deutscher Platz 5c, 04103 Leipzig, Germany) placed in a well of a 6-well plate (Nunc™ Non-Treated Multidishes 6-well, 150239; Thermo Fisher Scientific), the membrane filter was removed, and then cells on the surface of the sample, including RPE cells, were dissociated by pipetting with cell saver micropipettes under a dissecting microscope. This process was repeated for all the samples on the same strainer in the same well. The cells collected in the well were transferred into PBS in a 5 μm cell strainer (pluriStrainer, 43-50005-13; pluriSelect Life Science) placed in a plastic Tupperware (length 200 mm × width 150 mm × height 55 mm) to remove small debris and pigment granules. Cells remaining on the mesh of the strainer were transferred into several 1.5 mL protein low-binding tubes with cell saver micropipettes, and integrated into one tube after centrifugation at 80 ɡ for 1–2 min. After the supernatant was removed, cells were mixed with growth medium containing 8% DMSO, as was done for CpM01, and stored at − 80 °C. The cell-containing tube was transported to LiSDaC in a frozen condition.
At LiSDaC, 3’ scRNA-seq was carried out with Chromium Next GEM Single Cell 3ʹ Kit v3.1 (16 rxns, PN-1000268; 10x Genomics, Pleasanton, CA, USA) on Chromium iX (10x Genomics) and NovaSeq 6000 (Illumina, San Diego, CA, USA) according to manufacturers’ instructions. Read data are available in NCBI (BioProject accession number: PRJNA1138950; SRA accession number: for CpM01, SRR29978956; for erythroid cells, SRR29978954; for retinal cells, SRR29978918).
Data analysis
The total number of cells in a dish was estimated per passage by counting cells in cell suspension for the next passage using C-Chip (DHC-N01-M5; AR BROWN, Tokyo, Japan). Images were analyzed with software for image acquisition systems and by Adobe Photoshop 2024 (Adobe Systems, San Jose, CA, USA). In investigations of the expression of MRFs in cultures, the mean luminance of fluorescence in the nucleus was measured in Adobe Photoshop 2024.
For cell clustering with the scRNA-seq data, a SuperTranscript database (918,436 nucleotide sequences) was used as a reference51. It was constructed by Trinity_gene_splice_modeler.py in Trinity v.2.15.152, using 1,336,820 contigs (mean length: 714.7 base) constructed by Trinity v.2.15.1 itself with all the read data for TOTAL, the comprehensive transcriptome database of C. pyrrogaster32. Using this reference database, the scRNA-seq data were analyzed by CellRanger (pipeline version: cellranger-7.1.0; 10x Genomics) and Loupe Browser 8.0.0 (10x Genomics). In the CpM01 sample, estimated number of cells: 6,413; mean reads per cell: 71,686; median genes per cell: 323. In the sample for erythroid cells, estimated number of cells: 6,426; mean reads per cell: 74,596; median genes per cell: 676. In the sample for retinal cells, estimated number of cells: 7,490; mean reads per cell: 67,213; median genes per cell: 499. In preliminary examinations of gene expression profiling of cell clusters on the Loupe Browser, cross contamination was suspected in some transcripts that are abundantly expressed in certain types of cells, such as hemoglobin of erythrocytes. Therefore, in this study, raw_feature_bc_matrix.h5 files deduced by CellRanger were further filtered by CellBender53 (version 0.3.0 for CpM01 and erythroid cells; version 0.2.2 for retinal cells), and using the filtered files re-clustering was made by Seurat54 (for CpM01 and erythroid cells, version 5.0.1; for retinal cells, version 4.4.0) as follows.
The filtered file was loaded on the ‘CreateSeuratObject’ function of Seurat (for CpM01 and erythroid cells, min.cells = 1, min.feature = 200; for retinal cells, min.cells = 3, min.feature = 200), the count data was normalized by the ‘NormalizeData’ function, 2,000 differentially expressed genes were selected by the ‘FindVariableFeature’ function (nfeature = 2000), scaling of the gene expression levels was made by the ‘ScaleData’ function, the dimension reduction was made by the functions ‘RunPCA’, and then clustering was made by the functions ‘FindNeighbors’ and ‘FindClusters’. Exceptionally, for CpM01, after the filtered data was loaded on the ‘CreateSeuratObject’ function, the number of cells that had a Unique Molecular Identifier (UMI) between 13,246 and 164,705 and the number of expressed genes between 690 and 18,202 were selected by the ‘subset’ function (nCount_RNA > 13245 & nCount_RNA < 164706 & nFeature_RNA > 689 & nFeature_RNA < 18203). Clustering was made by the ‘FindCluster’ function with a low resolution parameter (= 0.1) so that all the cells belonged to the same cluster. Finally, for CpM01, erythroid cells and retinal cells, a total of 1 (#0), 22 (#0–#21) and 13 (#0–#12) clusters, respectively were deduced.
The results of clustering (Seurat objects) for CpM01, erythroid cells and retinal cells were merged by the ‘merge’ function, cluster #0 of CpM01, #13 of erythroid cells, and #6 of retinal cells were isolated and integrated into one object by the ‘JoinLayer’ function, 2,000 differentially expressed genes were selected by the ‘FindVariableFeature’ function (nfeature = 2000), scaling of the gene expression levels of the three clusters was made by the ‘ScaleData’ function, and then the dimension reduction was made by the functions ‘RunPCA’ for principal component analysis (PCA), ‘RunUMAP’ for Uniform Manifold Approximation and Projection (UMAP), and ‘RunTSNE’ for t-SNE. The result of t-SNE is shown in Fig. 6b.
To find differentially expressed genes among the three clusters, after the gene expression levels of the three clusters were scaled, the FindAllMarker function was applied to select those genes whose expression levels were significantly higher in a given cluster. For CpM01, the genes were functionally annotated via Blast2GO 6.0.355 and the results were exported to a csv file and analyzed by Microsoft Excel (Supplementary Data). The top 20 genes for each cluster are shown as a heatmap by the ‘DoHeatmap’ function (Fig. 6c).
Loupe Browser was applied to visualize and analyze Seurat data, producing UMAP and violin plots that show the expression patterns of featured genes.
Figures were prepared using Adobe Photoshop 2024. Image brightness, contrast and sharpness were adjusted according to the journal’s guidelines. Statistical analysis was made using BellCurve for Excel (version 4.07, Social Survey Research Information, Tokyo, Japan). Data in the text are presented as the mean ± SE.
Conference presentation
N/A.
Data availability
Sequence data that support the findings of this study have been deposited in the National Center for Biotechnology Information with the primary accession code PRJNA1138950.
References
Richler, C. & Yaffe, D. The in vitro cultivation and differentiation capacities of myogenic cell lines. Dev. Biol. 23, 1–22 (1970).
Yaffe, D. & Saxel, O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature 270, 725–727 (1977).
Blau, H. M. & Webster, C. Isolation and characterization of human muscle cells. Proc. Natl. Acad. Sci. U. S. A. 78, 5623–5627 (1981).
Wong, C. Y., Al-Salami, H. & Dass, C. R. C2C12 cell model: its role in understanding of insulin resistance at the molecular level and pharmaceutical development at the preclinical stage. J. Pharm. Pharmacol. 72, 1667–1693 (2020).
Lathuiliere, A. et al. Immortalized human myoblast cell lines for the delivery of therapeutic proteins using encapsulated cell technology. Mol. Ther. Methods Clin. Dev. 26, 441–458 (2022).
Schätzlein, E. & Blaeser, A. Recent trends in bioartificial muscle engineering and their applications in cultured meat, biorobotic systems and biohybrid implants. Commun. Biol. 5, 737 (2022).
Relaix, F. et al. Perspectives on skeletal muscle stem cells. Nat. Commun. 12, 692 (2021).
Sousa-Victor, P., García-Prat, L. & Muñoz-Cánoves, P. Control of satellite cell function in muscle regeneration and its disruption in aging. Nat. Rev. Mol. Cell. Biol. 23, 204–226 (2022).
Yeh, C. J., Sattler, K. M. & Lepper, C. Molecular regulation of satellite cells via intercellular signaling. Gene 858, 147172 (2023).
Brockes, J. P. & Kumar, A. Plasticity and reprogramming of differentiated cells in amphibian regeneration. Nat. Rev. Mol. Cell. Biol. 3, 566–574 (2002).
Chiba, C. et al. Visual cycle protein RPE65 persists in new retinal cells during retinal regeneration of adult newt. J. Comp. Neurol. 495, 391–407 (2006).
Kurosaka, H., Takano-Yamamoto, T., Yamashiro, T. & Agata, K. Comparison of molecular and cellular events during lower jaw regeneration of newt (Cynops pyrrhogaster) and west African clawed frog (Xenopus tropicalis). Dev. Dyn. 237, 354–365 (2008).
Eguchi, G. et al. Regenerative capacity in newts is not altered by repeated regeneration and ageing. Nat. Commun. 2, 384 (2011).
Witman, N., Murtuza, B., Davis, B., Arner, A. & Morrison, J. I. Recapitulation of developmental cardiogenesis governs the morphological and functional regeneration of adult newt hearts following injury. Dev. Biol. 354, 67–76 (2011).
Tanaka, H. V. et al. A developmentally regulated switch from stem cells to dedifferentiation for limb muscle regeneration in newts. Nat. Commun. 7, 11069 (2016).
Urata, Y., Yamashita, W., Inoue, T. & Agata, K. Spatio-temporal neural stem cell behavior leads to both perfect and imperfect structural brain regeneration in adult newts. Biol. Open 7, bio033142 (2018).
Ishii, T. et al. Skin wound healing of the adult newt, Cynops pyrrhogaster: a unique re-epithelialization and scarless model. Biomedicines 9, 1892 (2021).
Yu, Z. Y. et al. The latent dedifferentiation capacity of newt limb muscles is unleashed by a combination of metamorphosis and body growth. Sci. Rep. 12, 11653 (2022).
Joven, A., Elewa, A. & Simon, A. Model systems for regeneration: salamanders. Development 146, dev167700 (2019).
Connelly, P. J., Ross, N., Stringham, O. C. & Eskew, E. A. United States amphibian imports pose a disease risk to salamanders despite lacey act regulations. Commun. Earth Environ. 4, 351 (2023).
Brockes, J. P. Introduction of a retinoid reporter gene into the urodele limb blastema. Proc. Natl. Acad. Sci. U. S. A. 89, 11386–11390 (1992).
Tanaka, E. M., Gann, A. A. F., Gates, P. B. & Brockes, J. P. Newt myotubes reenter the cell cycle by phosphorylation of the retinoblastoma protein. J. Cell. Biol. 136, 155–165 (1997).
Tanaka, E. M., Drechsel, D. N. & Brockes, J. P. Thrombin regulates S-phase re-entry by cultured newt myotubes. Curr. Biol. 9, 792–799 (1999).
McGann, C. J., Odelberg, S. J. & Keating, M. T. Mammalian myotube dedifferentiation induced by newt regeneration extract. Proc. Natl. Acad. Sci. U. S. A. 98, 13699–13704 (2001).
Velloso, C. P., Simon, A. & Brockes, J. P. Mammalian postmitotic nuclei reenter the cell cycle after serum stimulation in newt/mouse hybrid myotubes. Curr. Biol. 11, 855–858 (2001).
Yun, M. H., Gates, P. B. & Brockes, J. P. Sustained ERK activation underlies reprogramming in regeneration-competent salamander cells and distinguishes them from their mammalian counterparts. Stem Cell. Rep. 3, 15–23 (2014).
Wang, H. et al. Turning terminally differentiated skeletal muscle cells into regenerative progenitors. Nat. Commun. 6, 7916 (2015).
Middleton, R. C. et al. Newt A1 cell-derived extracellular vesicles promote mammalian nerve growth. Sci. Rep. 13, 11829 (2023).
Blau, H. M., Chiu, C. P. & Webster, C. Cytoplasmic activation of human nuclear genes in stable heterocaryons. Cell 32, 1171–1180 (1983).
Kawesa, A., Vanstone, J. & Tsilfidis, C. A differential response to newt regeneration extract by C2C12 and primary mammalian muscle cells. Skelet. Muscle 5, 19 (2015).
Asfour, H. A., Allouh, M. Z. & Said, R. S. Myogenic regulatory factors: the orchestrators of myogenesis after 30 years of discovery. Exp. Biol. Med. 243, 118–128 (2018).
Casco-Robles, R. M. et al. Novel erythrocyte clumps revealed by an orphan gene Newtic1 in circulating blood and regenerating limbs of the adult newt. Sci. Rep. 8, 7455 (2018).
Ferri, P. et al. Expression and subcellular localization of myogenic regulatory factors during the differentiation of skeletal muscle C2C12 myoblasts. J. Cell. Biochem. 108, 1302–1317 (2009).
Wang, H. & Simon, A. Skeletal muscle dedifferentiation during salamander limb regeneration. Curr. Opin. Genet. Dev. 40, 108–112 (2016).
Naya, F. J. & Olson, E. MEF2: a transcriptional target for signaling pathways controlling skeletal muscle growth and differentiation. Curr. Opin. Cell. Biol. 11, 683–688 (1999).
Mohammadabadi, M., Bordbar, F., Jensen, J., Du, M. & Guo, W. Key genes regulating skeletal muscle development and growth in farm animals. Animals 11, 853 (2021).
Otto, T. & Sicinski, P. Cell cycle proteins as promising targets in cancer therapy. Nat. Rev. Cancer 17, 93–115 (2017).
Sakami, S., Etter, P. & Reh, T. A. Activin signaling limits the competence for retinal regeneration from the pigmented epithelium. Mech. Dev. 125, 106–116 (2008).
Wu, J. & Yue, B. Regulation of myogenic cell proliferation and differentiation during mammalian skeletal myogenesis. Biomed. Pharmacother. 174, 116563 (2024).
Luo, H. et al. Functional non-coding RNA during embryonic myogenesis and postnatal muscle development and disease. Front. Cell. Dev. Biol. 9, 628339 (2021).
Shi, D. L. & Grifone, R. RNA-binding proteins in the post-transcriptional control of skeletal muscle development, regeneration and disease. Front. Cell. Dev. Biol. 9, 738978 (2021).
Vicente-García, C., Hernández-Camacho, J. D. & Carvajal, J. J. Regulation of myogenic gene expression. Exp. Cell. Res. 419, 113299 (2022).
Casco-Robles, M. M. et al. Expressing exogenous genes in newts by transgenesis. Nat. Protoc. 6, 600–608 (2011).
du Sert, N. P. et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. PLoS Biol. 18, e3000410 (2020).
Casco-Robles, M. M., Yasuda, K., Yahata, K., Maruo, F. & Chiba, C. Reviewing the effects of skin manipulations on adult newt limb regeneration: implications for the subcutaneous origin of axial pattern formation. Biomedicines 9, 1426 (2021).
Chen, X. et al. Newtic1 is a component of globular structures that accumulate along the marginal band of erythrocytes in the limb blastema of adult newt, Cynops pyrrhogaster. Biomedicines 10, 2772 (2022).
Walthall, J. C. & Ashley-Ross, M. A. Postcranial myology of the California newt, Taricha torosa. Anat. Rec. Discov. Mol. Cell. Evol. Biol. 288, 446–457 (2006).
Iten, L. E. & Bryant, S. V. Forelimb regeneration from different levels of amputation in the newt, Notophthalmus viridescens: length, rate, and stages. Wilhelm Roux Arch. 173, 263–282 (1973).
Yoshikawa, T. et al. MEK-ERK and heparin-susceptible signaling pathways are involved in cell-cycle entry of the wound edge retinal pigment epithelium cells in the adult newt. Pigment Cell. Melanoma Res. 25, 66–82 (2012).
Nakamura, K. et al. A transcriptome for the study of early processes of retinal regeneration in the adult newt, Cynops pyrrhogaster. PLoS ONE 9, e109831 (2014).
Davidson, N. M., Hawkins, A. D. K. & Oshlack, A. SuperTranscripts: a data driven reference for analysis and visualisation of transcriptomes. Genome Biol. 18, 148 (2017).
Grabherr, M. G. et al. Full-length transcriptome assembly from RNA-seq data without a reference genome. Nat. Biotechnol. 29, 644–652 (2011).
Fleming, S. J. et al. Unsupervised removal of systematic background noise from droplet-based single-cell experiments using CellBender. Nat. Methods 20, 1323–1335 (2023).
Hao, Y. et al. Dictionary learning for integrative, multimodal and scalable single-cell analysis. Nat. Biotechnol. 42, 293–304 (2024).
Conesa, S. et al. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 21, 3674–3676 (2005).
Acknowledgements
This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology in Japan (221S0002) and the Japan Society for the Promotion of Science (18H04061; 23H05483) to C. C. We would like to thank all members of the Japan Newt Research Community (JNRC) and the Newtic Organ Regeneration Forum for their valuable comments and discussion, and the citizens of Toride City for helping us with daily maintenance of Toride-Imori in the field of Imori-no-Sato.
Author information
Authors and Affiliations
Contributions
S.S., F.T and C.C. conceived the idea and evaluated the data. S.S., Y.M., Z.W., R.A., M.M.C.-R., F.M., F.T. and C.C. designed and performed all experiments, and co-wrote the paper. F.T. designed and performed in silico bioinformatics analysis for scRNA-seq data, and evaluated the data. F.M. contributed to wild-type newts, and evaluated the data.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shiga, S., Murakami, Y., Wang, Z. et al. An adult myogenic cell line of the Japanese fire-bellied newt Cynops pyrrhogaster. Sci Rep 14, 30041 (2024). https://doi.org/10.1038/s41598-024-81899-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-81899-6