Abstract
Identifying dependable prognostic indicators is essential for the efficient management of metabolic dysfunction-associated steatotic liver disease (MASLD). The index of hemoglobin glycation (HGI) has been demonstrated to be closely linked to the onset and advancement of MASLD. Currently, no studies have investigated the relationship between HGI and mortality rates among MASLD patients. This study analyzed data from the National Health and Nutrition Examination Surveys (NHANES) covering 1999 to 2018, involving 8,257 adult patients diagnosed with MASLD. The HGI was determined using a linear regression model that correlated hemoglobin A1c (HbA1c) with fasting plasma glucose (FPG). The study employed Kaplan-Meier survival curves and weighted Cox proportional hazards models to evaluate the independent association between HGI and mortality risk. The study utilized restricted cubic splines (RCS) to visually depict the relationship between HGI and mortality risk. Over a median follow-up duration of 97.0 months, there were 1,352 recorded deaths, among which 386 were attributed to cardiovascular disease (CVD). Participants were classified into two groups based on their HGI values: the high HGI group (≥ 0.4605) and the low HGI group (< 0.4605). The results from the weighted Cox proportional hazards model indicated that individuals in the high HGI group faced a significantly higher risk of all-cause mortality (HR 1.47, 95% CI 1.19–1.82, P < 0.001). However, no significant increase in CVD mortality risk was observed (HR 1.38, 95% CI 0.95–1.99, P = 0.090). The RCS analysis identified a U-shaped association between HGI and both all-cause mortality and CVD mortality, with critical points at -0.0564 and − 0.0573, respectively. Below the critical points, HGI was negatively correlated with all-cause mortality (HR 0.82, 95% CI: 0.72–0.92, P < 0.001) and not significantly associated with CVD mortality (HR 0.78, 95% CI: 0.57–1.07, P = 0.126). Above the critical points, HGI was significantly positively correlated with both all-cause mortality (HR 1.36, 95% CI: 1.20–1.53, P < 0.001) and CVD mortality (HR 1.44, 95% CI: 1.11–1.88, P = 0.007). Further subgroup and interaction analyses corroborated the reliability of these findings. HGI could potentially function as a useful and dependable marker for evaluating all-cause mortality and cardiovascular mortality in MASLD patients.
Similar content being viewed by others
Introduction
Non-alcoholic fatty liver disease (NAFLD) stands as among the most prevalent chronic liver conditions, with its global prevalence increasing year by year. NAFLD manifests as hepatic steatosis, but its diagnosis should exclude significant alcohol consumption and other causes of secondary liver damage1. Each year, liver ailments such as viral hepatitis, alcohol-related liver disease (ALD), and NAFLD collectively contribute to more than 2 million deaths globally, comprising 4% of total mortality worldwide2. In recent years, there has been a perspective that the definition of NAFLD does not accurately reflect the connection between hepatic steatosis and systemic metabolic disorders. Therefore, the Delphi Consensus Statement of 2023 introduced a novel concept termed metabolic dysfunction-associated steatotic liver disease (MASLD)3. The global prevalence of MASLD among adults exceeds 25%, particularly in individuals with diabetes mellitus (DM), where it affects more than half4,5. MASLD is closely related to various cardiometabolic risk factors6,7. The 2019 Global Burden of Disease Study indicated that among 11 cardiometabolic conditions, MASLD exhibits the highest prevalence in low-income and lower-middle-income nations, totaling approximately 533.65 million cases, leading to increased MASLD burden and heightened cardiovascular risk in these populations8. To enhance MASLD surveillance and assess its mortality risk effectively, identifying a prognostic parameter that is simple to measure and cost-effective is imperative.
In 2002, Hempe proposed the Hemoglobin Glycation Index (HGI) as a metric to quantify the difference between an individual’s Hemoglobin A1c (HbA1c) and the average blood glucose level, thus indicating their glycation ability9,10. The HGI represents the difference between the actual HbA1c value and the estimated value derived from blood glucose levels, calculated using a regression equation that correlates HbA1c with fasting plasma glucose (FPG) in a reference population11. HGI can explain the inconsistency between the HbA1c criteria and the oral glucose tolerance test (OGTT) criteria during diabetes diagnosis, guide the selection of blood sugar-lowering treatments, and serve as a predictive indicator for macrovascular complications9,12,13,14,15. Recent studies have validated that a higher HGI correlates with increased incidence and mortality of macrovascular diseases, and individuals with high HGI have more severe atherosclerotic cardiovascular diseases (ASCVD)13. An analysis utilizing data from the U.S. National Health and Nutrition Examination Survey (NHANES) from 1999 to 2018, covering 7,607 individuals, emphasized a U-shaped association between increased HGI and all-cause mortality in hypertensive patients16. However, limited research has explored the correlation between HGI and mortality risk in patients with MASLD. It remains unknown whether a non-linear relationship also exists between HGI and mortality in patients with MASLD.
Therefore, this study aims to examine the association between HGI and mortality from all causes and CVD among MASLD patients, using a population survey reflecting the health status of American adults.
Methods
Study participants
The data for this study originated from the NHANES database, which gathers demographic details, pertinent health records, and laboratory test results from its participants. The NHANES study received approval from the ethics review committee, and all participants provided informed consent17. All methods were performed in accordance with the relevant guidelines and regulations.
In this study, we adopted the fatty liver index (FLI) as a measure of hepatic steatosis, primarily because ultrasound examinations were not conducted in most interview cycles. The FLI is a scientifically validated assessment tool that can indicate the presence of steatotic liver disease (SLD) with high reliability18,19. The calculation formula for FLI is derived from the research conducted by Bedogni et al.20. Prior research indicates that an FLI value below 60 generally signifies a lower risk of hepatic steatosis, whereas an FLI value of 60 or higher indicates an elevated risk20,21. Based on this, participants in this study with an FLI value less than 60 were excluded from the study population. In addition, individuals with liver conditions attributable to pathological factors like viral hepatitis, autoimmune or genetic liver diseases, drug-induced liver injury, alcoholic liver disease, and those exceeding recommended daily alcohol intake were also excluded from the study22. When diagnosing MASLD, participants must also meet at least one of the five conditions shown in Fig. 1. The details are as follows3: (1) BMI ≥ 25 kg/m2 or WC ≥ 94 cm for males and ≥ 80 cm for females; (2) FBG ≥ 100 mg/dL or 2-h post-load glucose levels ≥ 140 mg/dL or hemoglobin A1c ≥ 5.7% or DM or undergoing hypoglycemic therapy for DM; (3) blood pressure ≥ 130/85 mmHg or undergoing antihypertensive drug treatment; (4) fasting plasma triglycerides ≥ 150 mg/dL or undergoing lipid-lowering treatment; (5) plasma HDL-cholesterol < 40 mg/dL for males and < 50 mg/dL for females or undergoing lipid-lowering treatment.
This study excluded participants with incomplete follow-up data, those under the age of 18, and individuals missing HbA1c, FPG, and those who did not meet the diagnostic criteria for MASLD. Ultimately, the study included 8,257 eligible participants, with data sourced from the NHANES database between 1999 and 2018 (Fig. S1).
Measurement of HGI
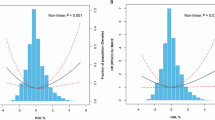
In this study, we constructed a linear regression model for all participating patients to explore the relationship between FPG and HbA1c. Based on this model, we derived the formula for predicting HbA1c: Predicted HbA1c = 0.0253 * FPG + 3.0336. Furthermore, we computed the difference between the measured HbA1c values and the anticipated values, known as the HGI23. Figure 2 illustrates the relationship between HGI and HbA1c graphically.
Determination of mortality
In this study, all-cause mortality refers to all recorded instances of death in the United States National Death Index until December 31, 2019. CVD fatalities are determined using the International Classification of Diseases, 10th Edition (ICD-10) codes I00-I09, I11, I13, and I20-I5124.
Covariate assessment
This study analyzed all-cause mortality and CVD mortality, incorporating a set of common covariates to enhance the accuracy and comprehensiveness of the analysis. The study subjects’ characteristics included various important indicators such as age, gender, race, education level, marital status, poverty income ratio (PIR), smoking and drinking habits, body mass index (BMI), DM, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), total cholesterol (TC) levels, and serum creatinine (Scr) concentration.
Statistical analysis
This study carefully considered the intricate sample design of NHANES and performed analyses using corresponding sample weights, stratification, and clustering techniques. The NHANES dataset provides a sophisticated sampling design that includes stratification, clustering, and weighting. We used survey year-specific weights provided by NHANES (e.g., wtmec4 year and wtmec2 year). The analysis incorporated sample weights for the years 1999–2002, derived by dividing the 4-year MEC weights (wtmec4 year) of the fasting sample, and for 2003–2018, calculated from the 2-year MEC weights (wtmec2 year) of the fasting sample divided. We utilized survey-weighted chi-square tests to compare categorical variables, and survey-weighted linear regression for comparing continuous variables. For variables where missing values were less than 20% (Table S1), random forest multiple imputation was employed to address the missing data. The random forest method of filling missing data is applicable to different types of data, both continuous and discrete, and is suitable for large data, while also accommodating nonlinear data. It makes the filled data more random and reliable by multiple interpolation.
Utilizing the “maxstat” software package, we employed the maximum selection rank statistical method to determine the optimal cutoff value for HGI, thereby stratifying the study subjects into low and high HGI groups. To investigate the potential non-linear relationship between HGI and mortality in MASLD patients, Restricted Cubic Splines (RCS) were employed. Knots were selected at the 5th, 35th, 65th, and 95th percentiles to construct the RCS model. In cases where a nonlinear relationship was detected, segmented linear regression models, were employed to model each segment and determine either threshold or saturation phenomena. A likelihood ratio test was then utilized to evaluate the single linear model against the segmented linear regression model, thereby assessing whether a threshold effect was present.
Survival analysis employed the Kaplan-Meier method to evaluate survival probabilities among MASLD patients at various HGI levels, with comparisons conducted using the log-rank test. We used survey-weighted Cox proportional hazards models to assess the independent relationship between HGI and both all-cause mortality and CVD mortality in MASLD patients. To address the challenge of multiple testing and evaluate multicollinearity in the multivariate Cox model, we computed the variance inflation factor (VIF) and tolerance. A VIF < 10 and tolerance ≥ 0.2 suggest minimal risk of multicollinearity influencing the estimation results25.
In addition, variables such as age, gender, race, PIR, CVD, and hypertension were included for stratified and interaction analyses. Additional sensitivity analyses were performed to confirm the reliability of the findings. We conducted all statistical analyses using R and EmpowerStats software, considering a two-sided P-value less than 0.05 as indicative of statistical significance.
Results
Characteristics of the study participants
This study included a cohort of 8,257 patients diagnosed with MASLD (Fig. S1). Applying the maximum selection rank statistical method, we determined the optimal cutoff value of HGI (0.4605) that had the most significant impact on survival. Based on this cutoff, participants were stratified into two groups: those with higher HGI (≥ 0.4605, n = 1,164) and those with lower HGI (< 0.4605, n = 7,093) (Fig. 3). In contrast to the lower HGI group, participants in the higher HGI group tended to be elderly women, non-Hispanic White, overweight, and smokers. They also exhibited a lower prevalence of drinkers, lower levels of education and income, higher rates of CVD, hypertension, and DM, elevated levels of HbA1c, FPG, and Scr, and decreased levels of TC and LDL. Table 1 provides comprehensive details regarding the participants’ characteristics. Additionally, participants were stratified into four groups (Q1 to Q4) according to the quartiles of HGI (Table S2). The findings showed a progressive increase in the proportion of elderly, women, overweight individuals, smokers, and those with higher education beyond high school gradually increased, and the number of individuals with CVD, DM, and hypertension also increased, with FPG and HDL levels rising accordingly.
The correlation between HGI and mortality among MASLD patients
Over a median follow-up of 97 months (Interquartile range (IQR): 51.00 to 146.00 months), among 8,257 MASLD patients, 1,352 (16.4%) deceased, including 386 (4.7%) attributed to CVD. Kaplan-Meier survival analysis curves were employed to compare major outcome incidences between the two groups using the binary HGI criteria (Figs. 4A and 4B). The findings indicated that the survival rate was notably reduced in the group with elevated HGI when juxtaposed with the group exhibiting a reduced HGI (P < 0.001). After adjusting for multiple variables, Cox regression analysis demonstrated a significantly higher risk of all-cause mortality in the higher HGI group compared to the lower HGI group (HR 1.47, 95% CI: 1.19–1.82, P < 0.001) (Table 2). No substantial correlation was found in the continuous HGI analysis (P = 0.736). Similarly, for CVD mortality, no significant associations were found in either the continuous or binary HGI analyses (P > 0.05). To investigate the correlation between HGI and mortality further, an RCS analysis was performed. The RCS analysis disclosed a U-shaped correlation between the HGI and both all-cause and CVD mortality (P for non-linearity < 0.05) (Figs. 5A and 5B). Within the analysis pertaining to the threshold effect, our investigation identified the critical values of the HGI associated with all-cause and CVD mortality, which were − 0.0564 and − 0.0573 (Table 3). Through the assessment of collinearity diagnostics, the VIFs for the potential confounders within the multivariate Cox proportional hazards model are consistently below the threshold of 4, signifying the absence of multicollinearity among these covariates (Tables S3-5).
Subgroup analysis and sensitivity analysis
We conducted stratified analyses of HGI risk across various subgroups, including age, gender, race, PIR, CVD, and hypertension (Table 4). Upon controlling for all potential confounders, the findings revealed substantial interactions between HGI and all-cause mortality and CVD mortality within the age subgroup (P for interaction < 0.001). No noteworthy associations were detected among gender, race, PIR, CVD, hypertension, and the HGI (P for interaction > 0.05). Specifically, we found that higher HGI levels were associated with an increased risk of all-cause mortality, particularly in males, Non-Hispanic Black, and Mexican American subgroups. Furthermore, RCS analysis was utilized to investigate the relationship between HGI and mortality across various subgroups of MASLD patients, including those of different ages and genders, with DM, CVD, and hypertension. The analysis revealed a nonlinear relationship between HGI and all-cause mortality across all subgroups (Figs. S2-6). Additionally, multiple sensitivity analyses were conducted to validate the robustness and reliability of the primary findings. First, the results remained consistent when analyzed using the original dataset without imputation (Table S6). Second, the results based on the quartiles of HGI were consistent with the main findings (Table S7). Third, following the exclusion of participants who passed away during the initial two-year period of the investigation, the HGI continued to demonstrate a stable correlation with overall mortality and CVD mortality (Table S8). Finally, even accounting for caloric and protein consumption from diet, as well as the use of dietary supplements, the HGI remained a substantial correlation with mortality risk among patients with MASLD (Table S9).
Discussion
This research extensively investigated the correlation of the HGI with both overall mortality and CVD mortality within the MASLD demographic, employing a range of analytical techniques. By analyzing the physical examination data of 8,257 MASLD participants, we identified a U-shaped correlation between the HGI and the risks of overall and cardiovascular mortality. Additionally, to consider the possible impact of population characteristic differences on this U-shaped relationship, we conducted RCS analysis across different subgroups. The analytical findings demonstrated that this U-shaped correlation persists consistently among various subgroups. These results are consistent across various sensitivity analyses, indicating that our main findings are robust and not dependent on the influence of early deaths, suggesting that HGI may be an independent risk factor for patients with MASLD.
Long-term hyperglycemia is closely linked to both the incidence and advancement of microvascular diseases among individuals afflicted with DM26. Hyperglycemia accelerates the non-enzymatic glycation of crucial proteins, resulting in the formation of glycated proteins. In addition to hemoglobin, other structural proteins are also susceptible to non-enzymatic glycation, resulting in the formation of advanced glycation end-products (AGEs)27,28. AGEs are believed to trigger inflammatory signaling, enhance oxidative stress, and ultimately contribute to atherosclerosis by harming arterial endothelial cells and speeding up lipid oxidation29. Research indicates that people with high HGI elevated levels of inflammatory indicators, including thrombocyte and leukocyte counts, and this phenomenon is independent of other confounding factors30. AGEs can alter the structure of extracellular proteins, potentially leading to protein dysfunction31. Furthermore, AGEs can trigger a series of biological reactions by binding to specific cell surface receptors, including chemotaxis, oxidative stress, inflammatory responses, cellular dysfunction, fibrosis, and apoptosis, which may ultimately lead to organ damage32,33. Recent research has also highlighted inflammation’s pivotal role in the pathogenesis and persistence of MASLD, exacerbating liver damage by inducing tissue inflammation and oxidative stress34. In addition, HGI has been proven to have predictive power for the severity and mortality of DM, hypertension, coronary artery disease, and chronic kidney disease16,35,36,37,38,39. The aforementioned evidence indicates that HGI could serve as a prognostic marker for disease progression and mortality risk across various populations. Nevertheless, the role of HGI in the MASLD population remains uncertain.
Our findings align with those reported by Xing et al.34, showing that the prevalence of MASLD patients exhibiting characteristics such as advanced age, female gender, high BMI, elevated FPG, and increased HDL levels correlated with higher levels of HGI. These findings have significant implications for the adjustment of clinical diagnostic and treatment strategies, especially in how to formulate personalized treatment plans for patient groups with these characteristics. We conducted RCS analysis on different subgroup populations to explore the differences in response to the rise in HGI. The study results show that although there is a U-shaped relationship between HGI and all-cause mortality risk in all subgroups, the HGI thresholds for each subgroup are not the same. Threshold effect analysis indicates that when HGI is below − 0.0564, all-cause mortality decreases as HGI increases; whereas when HGI is above − 0.0564, all-cause mortality increases with the rise in HGI. A U-shaped association between HGI and cardiovascular mortality as well as all-cause mortality has been previously observed in patients with hypertension and cardiovascular diseases16,37. This suggests that patients face different mortality risks at different levels of HGI, and different patient groups may require different HGI management strategies. Our study additionally revealed that patients with elevated HGI exhibit higher FPG levels compared to those with lower HGI. Furthermore, Liver steatosis shows a strong correlation with elevated blood glucose levels, indicating the significant role of HGI in the onset and progression of MASLD. These findings align with the research outcomes reported by Yoo et al.40.
Currently, research on HGI as a risk factor for MASLD is insufficient, and its underlying mechanisms remain incompletely understood. This study reveals a U-shaped association between HGI and the risk of death. Previous studies have shown that low HGI values are associated with an increased risk of all-cause mortality in patients with severe coronary heart disease37. This correlation may be related to the phenomenon of stress-induced hyperglycemia41, which can cause elevated FPG levels, leading to a decrease in HGI. However, this hypothesis still requires further scientific research for validation. In the high HGI group of this study, the proportion of diabetic patients was as high as 69.70%. In individuals with diabetes, FPG levels can fluctuate under the influence of drug therapy. There is evidence that insulin therapy can significantly reduce FPG, resulting in higher HGI values in patients receiving such treatment, even with individual differences in glycation levels23. In groups with higher HGI, hypoglycemia and postprandial hyperglycemia are more common; such glucose fluctuations may lead to glucose homeostasis imbalance, exacerbate vascular endothelial damage, and promote the development of atherosclerosis. Additionally, hypoglycemia may increase the risk of cardiovascular mortality through non-atherosclerotic pathways, such as inducing arrhythmias42,43. On the other hand, an increase in HGI may indirectly affect the risk of cardiovascular mortality by promoting inflammatory responses and increasing oxidative stress, which can impact atherosclerosis44. Apart from the aforementioned factors, drug use, disease status, and iron metabolism may also influence HGI levels. To fully understand the complex interactions between these factors, future research needs to delve deeper to clarify their relationship with HGI.
This research possesses some merits. Firstly, our study is the pioneering investigation to establish a link between HGI and mortality risk among MASLD patients. Although this study emphasizes the potential of HGI in MASLD patients, future research needs to further explore the comparative advantages of HGI over traditional non-invasive tests (such as the FIB-4 index, NAFLD fibrosis score). Future studies could compare the prognostic value of HGI with other commonly used non-invasive tests (NITs) in different disease courses and clinical subgroups through large-scale prospective cohort studies, thereby helping to determine whether HGI can replace or supplement these traditional methods in clinical practice. Secondly, NHANES applied a stratified multistage sampling approach, thereby improving the applicability of study findings to the broader non-institutionalized population. Moreover, the substantial sample size guarantees the reliability and accuracy of the findings.
However, this research exhibits certain constraints. Firstly, the NHANES database lacks detailed information on ultrasound and magnetic resonance imaging, which limits a more nuanced and intuitive diagnosis of MASLD. Secondly, due to the temporal limitations of data collection, our study results may not fully reflect the current situation. Lastly, the research sample comprises MASLD patients from the US; therefore, additional research is necessary to validate the applicability of these findings to MASLD patients in other countries.
Conclusion
Our study findings suggest a U-shaped association between HGI and all-cause and CVD mortality risk among MASLD patients in the US. This implies that mortality rates may increase at both lower and higher HGI levels. These findings indicate that the HGI could function as an accessible instrument for detecting individuals at elevated risk and directing specific preventative strategies. Future prospective studies are required to corroborate these findings and elucidate the underlying mechanisms.
Data availability
The National Health and Nutrition Examination Survey (NHANES) data are publicly available at https://www.cdc.gov/nchs/nhanes/.
References
Manikat, R., Ahmed, A. & Kim, D. Up-to-date global epidemiology of nonalcoholic fatty liver disease. Hepatobiliary Surg. Nutr. 12(6), 956–959 (2023).
Asrani, S. K., Devarbhavi, H., Eaton, J. & Kamath, P. S. Burden of liver diseases in the world. J. Hepatol. 70(1), 151–171 (2019).
Rinella, M. E. et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 79(6), 1542–1556 (2023).
Younossi, Z. M. et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64(1), 73–84 (2016).
Eskridge, W. et al. Metabolic dysfunction-associated steatotic liver disease and metabolic dysfunction-associated steatohepatitis: The patient and physician perspective. J. Clin. Med. 12(19), 6216 (2023).
Chalasani, N. et al. American Association for the Study of Liver Diseases. The diagnosis and management of non-alcoholic fatty liver disease: Practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology 142(7), 1592–1609 (2012).
Balta, S. Atherosclerosis and non-alcoholic fatty liver disease. Angiology 73(8), 701–711 (2022).
Danpanichkul, P. et al. Disparities in metabolic dysfunction-associated steatotic liver disease and cardiometabolic conditions in low and lower middle-income countries: A systematic analysis from the global burden of disease study 2019. Metabolism 158, 155958 (2024).
Hempe, J. M., Gomez, R., McCarter, R. J. Jr & Chalew, S. A. High and low hemoglobin glycation phenotypes in type 1 diabetes: A challenge for interpretation of glycemic control. J. Diabetes Complications. 16(5), 313–320 (2002).
Hsia, D. S. et al. Implications of the hemoglobin glycation index on the diagnosis of prediabetes and diabetes. J. Clin. Endocrinol. Metab. 105(3), e130–e138 (2020).
Hempe, J. M. & Hsia, D. S. Variation in the hemoglobin glycation index. J. Diabetes Complications 36(7), 108223 (2022).
van Steen, S. C. et al. Haemoglobin glycation index and risk for diabetes-related complications in the action in diabetes and vascular disease: Preterax and Diamicron Modified Release Controlled evaluation (ADVANCE) trial. Diabetologia 61(4), 780–789 (2018).
Kim, M. K. et al. Hemoglobin glycation index predicts cardiovascular disease in people with type 2 diabetes mellitus: A 10-year longitudinal cohort study. J. Diabetes Complications. 32(10), 906–910 (2018).
Ostergaard, H. B. et al. Limited benefit of haemoglobin glycation index as risk factor for cardiovascular disease in type 2 diabetes patients. Diabetes Metab. 45(3), 254–260 (2019).
Alberti, K. G. & Zimmet, P. Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 15(7), 539–553 (1998).
Shangguan, Q., Yang, J., Li, B., Chen, H. & Yang, L. Association of the hemoglobin glycation index with cardiovascular and all-cause mortality in individuals with hypertension: Findings from NHANES 1999–2018. Front. Endocrinol. 15, 1401317 (2024).
Duan, M. et al. Metabolic score for insulin resistance (METS-IR) predicts all-cause and cardiovascular mortality in the general population: Evidence from NHANES 2001–2018. Cardiovasc. Diabetol. 23(1), 243 (2024).
Park, J. et al. The associations of hepatic steatosis and fibrosis using fatty liver index and BARD score with cardiovascular outcomes and mortality in patients with new-onset type 2 diabetes: A nationwide cohort study. Cardiovasc. Diabetol. 21(1), 53 (2022).
Park, J. et al. The associations between changes in hepatic steatosis and heart failure and mortality: A nationwide cohort study. Cardiovasc. Diabetol. 21(1), 287 (2022).
Bedogni, G. et al. The fatty liver index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 6, 33 (2006).
Choi, W. et al. Combined impact of prediabetes and hepatic steatosis on cardiometabolic outcomes in young adults. Cardiovasc. Diabetol. 23(1), 422 (2024).
Min, Y. et al. Prognostic effect of triglyceride glucose-related parameters on all-cause and cardiovascular mortality in the United States adults with metabolic dysfunction-associated steatotic liver disease. Cardiovasc. Diabetol. 23(1), 188 (2024).
Hempe, J. M. et al. The hemoglobin glycation index identifies subpopulations with harms or benefits from intensive treatment in the ACCORD trial. Diabetes Care 38(6), 1067–1074 (2015).
Yang, F., Wang, M., Chen, Y., Wu, J. & Li, Y. Association of cardio-renal biomarkers and mortality in the U.S.: A prospective cohort study. Cardiovasc. Diabetol. 22(1), 265 (2023).
Kutner, M. H., Nachtsheim, C. J., Neter, J. & Wasserman, W. Applied Linear Regression Models (McGraw-Hill/Irwin, 2004).
Diabetes, C. et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 329(14), 977–986 (1993).
Ahmed, N. & Thornalley, P. J. Advanced glycation endproducts: What is their relevance to diabetic complications? Diabetes Obes. Metab. 9(3), 233–245 (2007).
Monnier, V. M. et al. The role of the amadori product in the complications of diabetes. Ann. N. Y. Acad. Sci. 1126, 81–88 (2008).
Yang, P., Feng, J., Peng, Q., Liu, X. & Fan, Z. Advanced Glycation End products: potential mechanism and therapeutic target in Cardiovascular complications under Diabetes. Oxid. Med. Cell. Longev. 2019, 9570616 (2019).
Hu, D. S. et al. Association between Hemoglobin Glycation Index and NAFLD in Chinese nondiabetic individuals. Can. J. Gastroenterol. Hepatol. 2019, 8748459 (2019).
Matafome, P., Rodrigues, T., Sena, C. & Seica, R. Methylglyoxal in metabolic disorders: Facts, myths, and promises. Med. Res. Rev. 37(2), 368–403 (2017).
Thornalley, P. J. Cell activation by glycated proteins. AGE receptors, receptor recognition factors and functional classification of AGEs. Cell. Mol. Biol. 44(7), 1013–1023 (1998).
Sadowska-Bartosz, I. & Bartosz, G. Effect of glycation inhibitors on aging and age-related diseases. Mech. Ageing Dev. 160, 1–18 (2016).
Xing, Y., Zhen, Y., Yang, L., Huo, L. & Ma, H. Association between hemoglobin glycation index and non-alcoholic fatty liver disease. Front. Endocrinol. 14, 1094101 (2023).
Ibarra-Salce, R. et al. Correlation between Hemoglobin Glycation Index measured by continuous glucose monitoring with complications in type 1 diabetes. Endocr. Pract. 29(3), 162–167 (2023).
Wu, J. D. et al. Association between hemoglobin glycation index and risk of cardiovascular disease and all cause mortality in type 2 diabetic patients: A Meta-analysis. Front. Cardiovasc. Med. 8, 690689 (2021).
Wei, X. et al. Risk analysis of the association between different hemoglobin glycation index and poor prognosis in critical patients with coronary heart disease-A study based on the MIMIC-IV database. Cardiovasc. Diabetol. 23(1), 113 (2024).
Cheng, M. D. et al. Association of hemoglobin glycation index with prognosis of coronary artery disease after percutaneous coronary intervention: A retrospective cohort study. Diab Vasc Dis. Res. 20(4), 14791641231193306 (2023).
Lin, L. et al. Predictive value of hemoglobin glycation index for chronic kidney disease. Zhonghua Nei Ke Za Zhi 61(12), 1310–1317 (2022).
Yoo, J. H. et al. The haemoglobin glycation index is associated with nonalcoholic fatty liver disease in healthy subjects. Clin. Endocrinol. 91(2), 271–277 (2019).
Cui, K. et al. Stress hyperglycemia ratio and long-term mortality after acute myocardial infarction in patients with and without diabetes: A prospective, nationwide, and multicentre registry. Diabetes Metab. Res. Rev. 38(7), e3562 (2022).
Akhaury, K., Wanjari, A., Sinha, A. H. & Kumar, M. Hypoglycemia and cardiovascular disease: Exploring the connections. Cureus 15(10), e47784 (2023).
International Hypoglycaemia Study Group. Hypoglycaemia, cardiovascular disease, and mortality in diabetes: Epidemiology, pathogenesis, and management. Lancet Diabetes Endocrinol. 7(5), 385–396 (2019).
Song, J. et al. Elevated glycosylated hemoglobin levels and their interactive effects on hypertension risk in nondiabetic Chinese population: A cross-sectional survey. BMC Cardiovasc. Disord 20(1), 218 (2020).
Funding
This research was funded by the Hospital Capability Enhancement Project of Xiyuan Hospital, China Academy of Chinese Medical Sciences (NO. XYZX0404-10) and the Beijing Research Ward Demonstration Construction - Phase I Ward Construction (NO. BCRW202108).
Author information
Authors and Affiliations
Contributions
MJ.Z., N.S., and YR.C.; data collection, methodology, software, statistical analysis, writing—original draft preparation; WT.Z. and JJ.J.: visualization, writing—review and editing: QY.L., F.L., and WL.W.; conceptualization, writing—review and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The research received clearance from the National Center for Health Statistics (NCHS) Research Ethics Review Committee. Each participant provided written consent prior to engagement in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhao, M., Sun, N., Cheng, Y. et al. Hemoglobin glycation index and mortality risk in metabolic dysfunction-associated steatotic liver disease patients: a novel U-shaped association. Sci Rep 15, 1465 (2025). https://doi.org/10.1038/s41598-024-82034-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-82034-1
Keywords
This article is cited by
-
Relationship between hemoglobin glycation index and all-cause mortality in patients with acute myocardial infarction: based on MIMIC-IV database
Diabetology & Metabolic Syndrome (2025)
-
Association of hemoglobin glycation index with all-cause mortality in critically ill patients with atrial fibrillation: a retrospective analysis from MIMIC-IV
BMC Cardiovascular Disorders (2025)