Abstract
The present investigation provides an easy and affordable strategy for fabrication of functional ceramics Bi0.5Na0.5TiO3-SrFe12O19 (BNT-SrF5) thick films on a flexible, inexpensive and electrically integrated substrate using electrophoretic deposition process (EPD). EPD is a widely accepted, environmentally friendly method for applying coatings from a colloidal suspension to conductive substrates. Lead-free ferroelectric BNT-SrF5 powder was synthesized by solid state method to fabricate bulk samples and thick films (30–160 μm) by EPD process. Thick films were deposited onto nickel substrate by applying EPD parameters, i.e. voltage (225–290 V) and coating time (30–180 s) to acetone based colloidal suspension without aid of any dispersing agent. In a comparative analysis, both thick films and bulk ceramics revealed significant densification with sintering temperature from 1025 to 1150 °C. Fourier transform Infrared (FTIR) and X-ray diffraction (XRD) analysis revealed presence of distorted perovskite structure following calcination and sintering processes. Scanning electron microscopy (SEM) provided the surface morphologies of BNT-SrF5 powder. The dielectric constant of film sample revealed more thermal stable response compared to the bulk ceramics. Impedance spectroscopy explained the electrically active regions and hopping conduction mechanism which witnessed NTCR behavior. The potential applications for the miniaturization of electronics are sensors, actuators and energy harvesting devices.
Similar content being viewed by others
Introduction
Ferroelectric materials have provided the most promising charge storage devices in the last few years, and are widely used in actuators, capacitors, transducers, medical devices, aerospace, communications and military applications. These materials store energy because of orientation of the polarized domains and confer a longer life span as compared to the conventional batteries and other electrochemical devices1,2. The energy storage capacity of these materials can be optimized if they are used in the form of thick films since they have high breakdown field and high dielectric properties and polarization3.
Currently several classes of ferroelectric materials have been developed but many of them have shortcomings like lead-based ferroelectric materials are deleterious to both health and environment. On the other hand, lead-free requires great attention to resolve ingrained problems like low Curie temperature, current leakage, high-cost substrate materials4,5. Apart from the advanced functionality and environmental issues, miniaturization and low costs are the basic need of current developments of electronic industries. The advanced energy devices i.e. piezo-nanogenerators, perovskite solar cells, tandem solar cells and structural health monitoring devices are being obtained by embedding metallic flexible substrates with potential lead-free materials6,7,8,9. As a result, thick films seem like a good option to supplant bulk ceramics10. To develop the mentioned devices multi-layered ceramics are utilized. Currently various techniques including sol-gel, co-precipitation, laser deposition, spray pyrolysis etc. have been developed for the preparation of thick films. However, electrophoretic deposition (EPD) is most effective of them because of its low-cost, adaptability, simple equipment, rapid and even deposition on complex shapes11. In this technique, an electric field is applied across the electrodes which are dipped in a colloidal suspension of nonconducting ceramic particles whose coating is required. Consequently, particles get partially charged and move through the organic medium and deposit on the metallic substrate. Various electrodes like copper, nickel, gold and platinum are being used for coating ferroelectric materials and their selection is based on the cost and sintering temperature of the composition to be coated12.
EPD coating of Ba0.77Ca0.23TiO3 (BCT23) on platinum electrode using different colloidal suspensions of BCT23 in isopropyl alcohol and 1:1 acetylacetone-ethanol mixture reports the crack free coatings using mixture of BCT23 with acetylacetone13, but the use of economical coating substrate demands attention. The effect of different dispersants e.g. iodine and sodium dodecyl sulfate (SDS) on colloidal suspensions of potassium sodium niobate (KNN) suggests the use of SDS for attaining densified film on platinum substrate10 nevertheless, quest of creating perfect suspension without using dispersant is under consideration. EPD of low sintering temperature Cu3TeO6 on platinized silicon substrate recommends that dielectric properties can be optimized by improving thick film density14. The comparison of 85BNT-15BT bulk and thick film sintered at 1125 °C reveals no change in composition, structure and electrical properties of conventional and EPD processed samples15 however, densification at respective sintering temperatures can alter the results. The EPD of strontium doped KNN in ethanol with PAM: BA dispersant reports that the deposition rate is inversely proportional to the conductivity of suspension which can be improved by varying electrodes size and distance between them. The optimized dielectric properties can also be achieved by improving film’s densification and reducing porosity16. The effect of graphene oxide coating on the corrosion rate of copper has been studied in literature using EPD with 2 mg/ml suspension. Defect free samples were obtained at 5 V for 10 s and coating revealed ~ 6 times reduction in corrosion rate as compared to bare Cu17.
The present study aims to provide a comparative analysis Bi0.5Na0.5TiO3-SrFe12O19 (BNT-SrF5) of the bulk ceramics and thick coating on flexible (Ni) substrate. The focus is to explore the temperature based sintering possibilities corresponding electrical properties. This work investigates the utilization of nickel substrate, a low-cost electrode material, high temperature stability, and excellent interface properties. The optimization is done with time, voltage, and temperature. This work presents an advancement in coating technology with an achieved coating thickness of approximately 160 μm, a Curie temperature near 400 °C, and enhanced dielectric properties (~ 150) supported by impedance studies, highlighting improvements over previous studies in these metrics. The potential applications are the miniaturization of electronics, e.g. sensors, actuators and energy harvesting devices.
Materials and methods
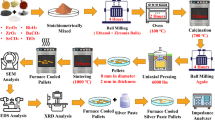
The solid-state reaction (SSR) was employed for the preparation of Bi0.5Na0.5TiO3-SrFe12O19 (BNT-SrF5) composite. The precursors taken for this composition were Bi2O3 (Sigma Aldrich ≥ s99%), Na2CO3 (Sigma Aldrich ≥ 99%), TiO2 (Merck ≥ 99%), SrCO3 (Sigma Aldrich ≥ 99%) and Fe3O4 (Riedel-de Haën ≥ 99.5%). Figure 1 Represents the basic mechanism for the preparation of BNT-SrF5 powder, thick film and bulk ceramics.
BNT-SrF5 powder preparation
The stoichiometric amounts of the constituents were taken with the help of the following balanced chemical equations as Eqs. 1 and 2
Both, Bismuth Sodium Titanate (BNT) and Strontium hexaferrite (SrF), were prepared separately, ball milled using a planetary ball mill (Fritsch Planetary Mill-Pulverisette6) in the acetone medium for 4 h with the help of zirconia balls at 200 rpm. The weight ratio of zirconia ball to powder was 1:10. After milling, it was sieved, dried in an oven and milled again to reduce the particle size. Furthermore, the BNT and SrF powders were mixed in the weight ratio 95:5 respectively and milled for 4 h to get BNT-SrF5 homogeneous powder. This powder was calcined in the VECSTAR box furnace at 850 °C with heating rate of 10 ºC/min for 30 min to convert the reactants into products for obtaining perovskite BNT-SrF5 ceramics. Following calcination, the powder was again ball milled to further reduce the BNT-SrF5 particle size, which in turn promotes the adherence during EPD process. Homogeneous and submicron particle size distribution is crucial for preparing the stable suspension, which significantly improves the quality of the resulting EPD films.
BNT-SrF5 thick films preparation
The thick coating was prepared with the help of the EPD technique. In this process, the BNT-SrF5 powder was dissolved in the acetone to make the EPD suspension of 1 g/100 ml. The suspension was stirred for 10 min on the magnetic plate and ultra-sonicated for 30 min to achieve a colloidal suspension for smooth EPD coating. Following that, two nickel electrodes, 20 cm apart, separated with the help of Teflon were dipped in the suspension. A voltage of 50–300 V was applied across the electrodes for a period of 30–180 s to deposit the BNT-SrF5 particles on one of the electrodes. As a result of the applied voltage, an anodic deposition of 30–160 μm was observed. After coating, the samples were dried in the oven to remove the acetone present in them.
The selection of electrode substrate was crucial for performing EPD. The sintering temperature of BNT-SrF5 film is 1025 ºC and it was mandatory for the substrate to have a melting point greater than sintering point and should be oxidation resistant. Previous research has revealed the use of platinum and gold substrates however they were expensive to use. Conductivity and flexibility of the substrate were also under observation. While considering various factors, nickel metal was found to be most suitable, fulfilling all the requirements and selected as an electrode substrate for accomplishing adhesive EPD. The surface dimensions taken for electrodes were 10 × 10 mm and well cleaned with acetone before coating.
BNT-SrF5 bulk ceramics preparation
The BNT-SrF5 pellets were prepared using uniaxial pressing by weighting the required amount of powder and placing it in a die of radius 5 mm. Pressure of 6000 Psi was applied to achieve a thick green pellet of 400 μm. The pellets and thick films were sintered in the box furnace at 1150 and 1025 °C for 15–60 min respectively to achieve the densified samples.
X-ray diffraction
Phase analysis and structure was studied through X-ray diffraction technique (PANalytical diffractometer) to confirm the formation of BNT-SrF5 composite. Functional group bond stretching, and vibrations were verified using Fourier transform infrared (FTIR-4100-typeA, Jasco Inc.).
Scanning electron microscopy
Microstructural analysis was done using scanning electron microscopy (FEG-SEM, TESCAN) to observe the homogeneity and densification of the coating. Coating thicknesses were measured via optical microscopy using ImageJ software (ImageJ® version 1.44p-USA, Java based image processing tool).
Electrical measurements
The electrical properties of ceramics bulk and thick film were studied using impedance microscopy (Tonghui™ LCR-TH-2829 C).
Results and discussion
Figure 2 presents both bulk and coated samples in green and sintered states. It illustrates the obtained pellets and thick films of BNT-SrF5 coated on nickel substrate using EPD at varying voltages (225–275 V) and deposition time (30–180 s). The optimization of voltage versus time was carried out to achieve the best BNT-SrF5 coating. The detachment of coating was observed when both voltage and deposition time were increased. Figure 2b depicts the best optimized results, showcasing a proper coating over the nickel substrate. This was due to the development of fine thin layer and halted the further deposition over it. In Fig. 2d, it can be clearly observed that the coating was either burned out or removed due to volatilization at specific sintering temperatures. This phenomenon occurred because of the lack of a backing layer at higher voltages when thermal energy was applied.
EPD samples at various stages of processing. (a) EPD samples deposited at different voltages (225–275 V) and times (30–180 s) before sintering, showing the effect of deposition parameters on coating morphology. (b) Optimized EPD sample obtained at 225 V for 180 s before sintering. (c) EPD samples after drying, ready for sintering, demonstrating the pre-sintering state. (d) EPD samples sintered at various temperatures, revealing the impact of thermal processing on coating integrity.
Figure 3a illustrates the coating thickness of the BNT-SrF5 with varying deposition time and voltage, measured using ImageJ software (ImageJ® version 1.44p-USA, Java based image processing tool) and corresponding values of coating thickness are provided in Table 1. It was clearly observed that increasing the voltage and time values accelerated the coating thickness on metallic nickel and the maximum measured coating thickness was 158 μm. Figure 3b displays the SEM image of lateral side of thick film obtained at 225 V and 180 s, revealing (i) BNT-SrF5 coating, (ii) interlayer of coating and substate, and (iii) the metallic shinny Ni substrate. Interestingly, the interlayer formation of metallic-mix ceramic composite was not observed indicating that the role of interface and interlayer was minimal in the current EPD. Figure 3c shows coating thickness as a function of voltages (225–275 V) and time (30–180 s) with linear fitted lines indicating an increasing trend. The EPD of pure BNT has also been studied in the literature at low voltage range (50–150 V) for comparative greater time (80–240 s) to obtain the best coating12.
Figure 4 shows the XRD pattern of pristine BNT, BNT-SrF5 thick film and bulk ceramics sintered at 1025 and 1150 ℃ respectively using Cu-Kα radiation. Peaks indexing was carried out according to JCPDS # (801197, 802377 and 36–0340)12,18. XRD analysis confirmed the presence of distorted perovskite structure. Two main phases α-(BNT) and β-(SrF) were identified. The α–phase at diffraction angle 2θ = 40.86° and 47.16° corresponding to plane (111) and (200) indicates the rhombohedral structure. In contrast, the peak intensities of β–phase at diffraction angle 2θ = 30.59°, 32.08° and 33.29° corresponding to planes (110), (107) and (114) respectively, suggest the hexagonal structure19,20.
Notably, the diffraction patterns of β phase show slight peak shift towards higher angles for the film samples compared with bulk sintered for 15 min. This may be attributed to the charge imbalance in the overall perovskite structure which induced strain. Furthermore, the intensity of peak at 2θ = 32.93° for bulk ceramics surged significantly in comparison to film likely due to high energy involvement and complete formation of SrFe12O19 as reported in previous studies21. Conversely, the peak at 2θ = 32.08° in the film vanished in the bulk indicating no traces of Fe2O3 due to proper sintering22. The highest peak (110) was observed at 2θ = 33.93° for BNT. Minimal distortion is evident in the obtained results, which are in good accord with values from literature.
The Crystallite size was measured from the observed peak of BNT-SrF5 film using the following Scherrer’s Eq.
Where D is the crystallite size, K is the Sherrer’s constant (0.94), λ is the X-rays wavelength, 1.5406 Å, β is the full width half maximum (0.493 Degree) and θ is the angle of diffraction (32.96). The calculated crystallite size was 17.5 nm, which is in line with the literature value (17.64 nm) measured for KNN film coated with EPD23.
Figure 5 shows the Fourier transform infrared spectroscopy of BNT-SrF5 bulk ceramics and thick film sintered for 15 min. The room temperature measurements show the range from 4000 –400 cm− 1. Both functional group region and molecular orbital region were seen from the graphs (inset). It was confirmed that no specific change was identified in the spectra region between 4000 –1000 cm− 1. The strong bands visible, in bulk and film, at 415 cm− 1, 444 cm− 1, 453 cm− 1 and 505 cm− 1 indicate the metal oxide vibrations (O-Ti-O, Ba-O, Na-O). The TiO6 octahedra is confirmed by the intrinsic bands 646 cm− 1, 679 cm− 1 and 697 cm− 1. The bending and stretching vibrations of Ti-O are positioned at 527 cm− 1, 756 cm− 1 and 785 cm− 1. The characteristic peaks between 900 and 1000 cm− 1 represent the C-O residual organic compounds24,25,26,27,28.
Figure 6 shows real time samples, SEM images and grain size distribution of BNT-SrF5 bulk and film sintered at 1150 and 1025 ○C for 15 min respectively. A comparative analysis indicates that the bulk pellet reveals needle-like microstructure with an average grain size of 2–4 μm however BNT-SrF5 film shows acicular-round shapes29,30 with an average grain size of 0.5–2 μm. The bulk ceramics undergo agglomeration of particles during uniaxial pressing and sintering31.
Figure 7 shows the morphology of BNT-SrF5 bulk ceramics and thick film sintered for 15, 30 and 60 min, giving the porosity / voids comparison. It is agreed from the images that there are no specific open pores visible in the bulk because of proper sintering and grain growth, with no observed shrinkage. However, in film surface energy is more with a thermal mis-match at the metal-ceramic interface which is the reason of overall porosity22,32. Films were sintered for 15, 30 and 60 min to analyze the sintering mechanism and densification above 90% was achieved33.
Electrical measurements
Complex impedance spectroscopy (CIS) is a tool to predict the electrical properties of ceramics. Impedance analyzer (Tonghui™ LCR-TH-2829 C) was used and the response of the ceramics towards varying frequency (100 Hz-1 MHz) with change in temperature from room temperature to higher degree was recorded and plotted.
Figure 8 shows frequency dependent real impedance graphs of BNT-SrF5 bulk ceramics and thick film sintered for 15 min at 1150 °C and 1025 °C respectively. The samples were characterized electrically within the frequency range of 100 Hz-1 MHz. At lower temperature, a straight line is obtained for both bulk and film, revealing the higher value of resistances. This might be due to the gathering of space charges at the interface, attributing the existence of vacancies/defects in samples34,35,36,37. As the frequency and temperature increased (205–310 ℃), the impedance descends to low points indicating the space charge release and minimum polarization. The amalgamation of the impedance values at high temperature (~ 400 ℃) showed the nullification of polarization. The impedance barrier at maximum temperature for bulk is Z’~ 3 kΩ and for film is ~ 750 Ω. Overall behavior is like semiconductors showing negative temperature coefficient of resistance38,39.
Figure 9 shows the variation in the imaginary component of impedance (Z”) with frequency for BNT-SrF5 bulk ceramics and thick film sintered for 15 min. At 205 °C, a strong peak is observed around 400 kHz (relaxation frequency) and peak shifted toward higher frequency with the increase in temperature and converge due to no charge polarization, depicting the relaxation behavior of samples and thermally activated pores/defects34,40,41. The broadening of peak with the increase of temperature assigns temperature dependent relaxation.
Figure 10 shows the graph between Real (Z’) and Imaginary (Z’’) part of impedance, Cole-Cole or Nyquist plot for BNT-SrF5 bulk and thick film at 205–310 °C. The conduction mechanism, electrical contribution of grain, grain boundaries and non-Debye relaxation is explained by this plot42,43. For bulk ceramics, merged semicircles were observed depicting the contribution of both, grain and grain boundaries44,45,46. The higher the temperature the more depressed semicircles predicting the behavior to be negative temperature coefficient of resistance (NTCR)47,48. For film, the Cole-Cole plot revealed the interrupted semicircles indicating no response of grain bulk and the contribution was only from the grain boundaries49,50. SEM analysis also supported the presence of more grain boundaries than grains in the film, due to their smaller grain size observed in the microstructure.
Figure 11 presents the dielectric studies of BNT-SrF5 bulk and film ceramics by varying frequency and temperature. Dielectric constant and dielectric loss was measured by using the following formula
Where C is the capacitance of BNT-SrF5 obtained from LCR meter, εo is the permittivity of free space, εr is the relative permittivity, A is the area of sample in contact, D is the thickness of the sample, Tan δ is the dielectric loss and ε′ is σ / ω εo. The literature values of dielectric constant and dielectric loss for pure BNT bulk ceramics are in the range of 200–800 and 0.001-0.2 at room temperature and maximum dielectric constant temperature, with and without dopant, is in range of 260–400 ℃ respectively51,52,53,54. The dielectric constant for BNT-SrF5 bulk ceramics was measured ~ 320. The dispersion of frequency lines at elevated temperatures exhibits a broad peak, corresponding to the maximum dielectric constant temperature Tm around 410 ℃. Relaxation of frequency was due to the presence of hexaferrite at the boundaries of the BNT bulk ceramics. Furthermore, the dielectric loss for BNT-SrF5 bulk ceramics is ≤ 0.1 which is comparable. This is in clear agreement for bulk ceramics to utilize it for applications where high temperature and low factor of energy dissipation is required. Similarly, the BNT-SrF5 film-based ceramics showed a diffused peak for Tm as observed in bulk. The profound peak measured was at ~ 400 ℃ and permittivity around 150 at room temperature. Notably, the dielectric constant of film sample revealed more thermal stable response compared to the bulk ceramics55. Conversely, the loss factor for film is around ~ 0.2 which is relatively more than bulk ceramics that might be due to the presence of porosity and other defects10,22,56.
Figure 12 represents the frequency dependent conductivity comparison for BNT-SrF5 bulk and film at different temperatures from 205 to 310 °C. The trend of conductivity vs. frequency in bulk ceramics is almost stable at low frequency and increases relative to frequency, however in film, it has a nearly direct relation57. As the temperature increased, the boundaries of high and low frequencies, known as hopping frequency, shifted towards high values for both bulk ceramics and thick film58. Furthermore, the conductivity values of bulk are much higher than film due to large grain size and less grain boundaries.
The movement of electron-hole charges from valance band to conduction band and conduction mechanism is corelated with the defects present in the sample40,59. The activation energy was measured by using the classical Arrhenius equation as follows.
Where k is the Boltzmann constant (8.617 × 10− 5 eVK− 1), Ea is activation energy of the charges, R is resistance and T is the temperature. Figure 13 represent the graph between inverse of resistivity (1/σ) and temperature (1000/T) for bulk and film BNT-SrF5 at different frequencies i.e. 1 kHz, 10 kHz, 100 kHz starting from 205 to 310 °C temperature. The straight line observed in all the sintered compositions showed that the bulk ceramics are more stable than film-based ceramics as represented in Table 2. It is clear from the plots that material shows the behavior of negative temperature coefficient of resistance (NTCR) which is attributed to the oxygen vacancies present23,60.
In this work, a comparative analysis between ferroelectric BNT-SrF5 bulk ceramics and thick film was done. Conventional solid-state method was used to fabricate bulk ceramics whereas electrophoretic deposition technique was used to make thick films (30–160 μm). The films were deposited on economical metallic substrate by applying the voltage (225–290 V) and coating time (30–180 s). The analysis revealed more than 90% densification at 1025 °C for thick films and 1150 °C for bulk ceramics. FTIR revealed no change in fingerprint region for both cases after sintering. The XRD analysis confirmed the peaks of BNT-SrF5 composition. Needle-like microstructure for bulk and acicular-round shape for thick film samples was observed by SEM. CIS analysis showed the merged semicircles for bulk ceramics which confirmed the electrically active grain and grain boundary regions. The dielectric constant and dielectric loss values at room temperature were around 320 and ≤ 0.1 for bulk ceramics and 150 and ≤ 0.2 for thick films respectively. The maximum dielectric constant temperature (Tm) was 410 °C for bulk and 400 °C for film. The conduction mechanism represented an increase in conductivity with the increase in temperature and frequency for both ceramics. Overall, it can be concluded that thick film coatings obtained by electrophoretic deposition technique can be optimized to make energy storage devices.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Shkuratov, S. I. & Lynch, C. S. A review of ferroelectric materials for high power devices. J. Materiomics. 8 (4), 739–752 (2022).
Ramana, E. V. et al. Insights into improved ferroelectric and electrocaloric performance of Ba0.85Ca0.15Ti0.9Zr0.1O3 thick films grown by the electrophoretic deposition. Surf. Interfaces. 33, 102257 (2022).
Chen, J. et al. Relaxor behavior and dielectric properties of lead magnesium niobate–lead titanate thick films prepared by electrophoresis deposition. J. Alloys Compd. 478 (1), 853–857 (2009).
Maqbool, A. et al. Evolution of phase structure and giant strain at low driving fields in bi-based lead-free incipient piezoelectrics. Mater. Sci. Engineering: B. 199, 105–112 (2015).
Hussain, A. et al. Texture performance of lead-free Bi1/2Na1/2TiO3–BaZrO3 ceramics. Ceram. Int. 49 (6), 10073–10082 (2023).
Bößl, F. et al. Piezocatalytic degradation of pollutants in water: importance of catalyst size, poling and excitation mode. Chem. Eng. J. Adv. 7, 100133 (2021).
Abdullah, A. M. et al. KNN based piezo-triboelectric lead-free hybrid energy films. Nano Energy. 86, 106133 (2021).
Zhang, H. Y. et al. Two-Dimensional Hybrid Perovskite Ferroelectric Induced by Perfluorinated Substitution. J. Am. Chem. Soc. 142 (47), 20208–20215 (2020).
Zhang, A. et al. Vibration catalysis of eco-friendly Na0.5K0.5NbO3-based piezoelectric: an efficient phase boundary catalyst. Appl. Catal. B. 279, 119353 (2020).
Mahajan, A. et al. Unleashing the full sustainable potential of Thick films of lead-free Potassium Sodium Niobate (K0.5Na0.5NbO3) by Aqueous Electrophoretic Deposition. Langmuir 32 (21), 5241–5249 (2016).
Jian, G. et al. Cobalt ferrite dispersion in organic solvents for electrophoretic deposition: influence of suspension parameters on the film microstructure. Mater. Chem. Phys. 143 (2), 653–660 (2014).
Rafiq, M. A. et al. A facile and cost-effective approach for the fabrication Bi0.5Na0.5TiO3 thick films on flexible substrate for energy storage capacitor applications. Ceram. Int., 46(16, Part A): 25113–25121 (2020).
Antonelli, E. et al. Electrophoretic deposition of Ba0.77Ca0.23TiO3 nanopowders. J. Mater. Process. Technol. 203 (1), 526–531 (2008).
Zhu, X. L., Wang, Z. H. & Vilarinho, P. M. Cu3TeO6 Dielectric Thick films: Processing by Electrophoretic Deposition and Electrical characterization. Key Eng. Mater. 654, 114–121 (2015).
Kim, M. et al. Fabrication of lead-free piezoelectric (Bi0.5Na0.5)TiO3–BaTiO3 ceramics using electrophoretic deposition. J. Mater. Sci. 53 (4), 2396–2404 (2018).
Mercier, H. et al. Electrophoretic deposition and properties of strontium-doped sodium potassium niobate thick films. J. Eur. Ceram. Soc. 37 (16), 5305–5313 (2017).
Raza, M. A. et al. Corrosion study of electrophoretically deposited graphene oxide coatings on copper metal. Thin Solid Films. 620, 150–159 (2016).
Hessien, M. M. et al. Synthesis and magnetic properties of strontium hexaferrite from celestite ore. J. Alloys Compd. 476 (1), 373–378 (2009).
Cong-ju, L. & Guo-rong, X. Template preparation of strontium hexaferrite (SrFe12O19) micro/nanostructures: characterization, synthesis mechanism and magnetic properties. Mater. Res. Bull. 46 (1), 119–123 (2011).
Ramírez-Ayala, M. F. et al. Study of neodymium addition on the magnetic and structural properties of strontium hexaferrite synthesized by the Pechini method. J. Magn. Magn. Mater. 582, 170985 (2023).
Için, K. et al. Investigation of nano-crystaline strontium hexaferrite magnet powder from mill scale waste by the mechanochemical synthesis: Effect of the annealing temperature. Mater. Chem. Phys. 290, 126513 (2022).
Marjeghal, M. A., Sedghi, A. & Baghshahi, S. The effect of the citric acid to metal nitrates molar ratio on the structural and magnetic properties of strontium hexaferrite nanoparticles synthesized by the sol-gel combustion method. J. Alloys Compd. 968, 171765 (2023).
Habib, M. S. et al. Experimental determination of electrophoretic deposition parameters and electrical characterization for K 0.5 na 0.5 NbO3 perovskite thick films for energy harvesting applications. Mater. Chem. Phys. 316, 129074 (2024).
Rhimi, N. et al. Structural, morphological, optical and dielectric properties of sodium bismuth titanate ceramics. Inorg. Chem. Commun. 146, 110119 (2022).
Supriya, S. et al. Analysis of single and binary phases in Cerium Doped Sodium Bismuth Titanate -Na0.5Bi0.5TiO3 materials. Energy Procedia. 84, 190–196 (2015).
Jayasri, S., Elorika, P. & Anwar, S. Effect of sintering time on electrical, ferroelectric, and piezoelectric performances of microwave synthesized sodium bismuth titanate ceramics. Mater. Sci. Eng.: B. 298, 116823 (2023).
Jyotsna, K., Chanu, L. P. & Phanjoubam, S. Effect of Fe substitution on the structural, microstructural and dielectric properties of sodium bismuth titanate. Mater. Today: Proc.. 65: 2781–2787 (2022).
Singh, M. et al. Fabrication of nanostructured lead-free bismuth sodium titanatethin film and its liquefied petroleum gas sensing. Sens. Actuators A: Phys. 301, 111765 (2020).
Masoudpanah, S. M., Seyyed, S. A. & Ebrahimi Synthesis and characterization of nanostructured strontium hexaferrite thin films by the sol–gel method. J. Magn. Magn. Mater. 324 (14), 2239–2244 (2012).
Muhammed, K. R. et al. Physical properties and sinterability of pure and iron-doped bismuth sodium titanate ceramics. J. Aust. Ceram. Soc. 56 (4), 1441–1449 (2020).
Gao, C. et al. Comparative investigation of Li-modified Ni0.6Zn0.4O thin films and the bulk counterparts in the application of thermistors. Surf. Interfaces. 46, 104068 (2024).
Jia, Y. et al. Giant electro-induced strain in lead-free relaxor ferroelectrics via defect engineering. J. Eur. Ceram. Soc. 43 (3), 947–956 (2023).
Zhu, L. F. et al. Enhanced piezoelectric and ferroelectric properties of BiFeO3-BaTiO3 lead-free ceramics by optimizing the sintering temperature and dwell time. J. Eur. Ceram. Soc. 38 (10), 3463–3471 (2018).
Koval, V. et al. Dielectric relaxation and conductivity phenomena in ferroelectric ceramics at high temperatures. J. Eur. Ceram. Soc. 44 (5), 2886–2902 (2024).
Meher, R., Padhee, R. & Parida, S. K. Cerium modified (BiFeO3)0.5 – (MgTiO3)0.5 ceramics: structural, microstructural, dielectric, transport and optical properties. J. Mol. Struct. 1276, 134765 (2023).
Arora, M. et al. Evidence of non-debye behavior of Pb0.76Sm0.24Ti0.76Fe0.24O3 ceramics by complex impedance spectroscopy. Mater. Today: Proc. 80, 1079–1085 (2023).
Hashim, M. et al. Structural, magnetic and electrical properties of Al3 + substituted Ni–Zn ferrite nanoparticles. J. Alloys Compd. 511 (1), 107–114 (2012).
Behera, B., Nayak, P. & Choudhary, R. N. P. Impedance spectroscopy study of NaBa2V5O15 ceramic. J. Alloys Compd. 436 (1), 226–232 (2007).
Mishra, S., Choudhary, R. N. P. & Parida, S. K. Structural, dielectric, electrical and optical properties of a double perovskite: BaNaFeWO6 for some device applications. J. Mol. Struct. 1265, 133353 (2022).
Hajra, S. et al. Structural, dielectric and impedance characteristics of (Bi0.5Na0.5)TiO3-BaTiO3 electronic system. J. Alloys Compd. 750, 507–514 (2018).
Sahoo, S. et al. Influence of (0.19HfO2-0.81ZrO2) ceramics filler content on structural and dielectric properties of PVDF polymer. Synth. Met. 287, 117097 (2022).
Rehman, M. U. et al. Structural, dielectric and complex impedance analysis of Pb-free BaTiO3-Bi(Mg0.5Ce0.5)O3 ceramics. J. Alloys Compd. 947, 169575 (2023).
Shi, J. et al. Dielectric, Impedance, and Ferroelectric Performances with Structural Heterogeneity of Sr0.7Bi0.2TiO3 Modified lead-free Bismuth ferrite-based Ceramics (Ceramics International, 2024).
Ali, A. M. et al. Impedance spectroscopy, ferroelectric and ferromagnetic properties of 60Bi2O3–10SrO–30Fe2O3 glass-ceramic nano-composites for energy storage applications. Ceram. Int. 50 (10), 17499–17512 (2024).
Zhao, J. et al. Microstructure, dielectric response and impedance spectroscopy of SrCu3Ti4O12 ceramics prepared by a sol-gel technique. Mater. Chem. Phys. 291, 126701 (2022).
Chen, Y. et al. Gd3 + substitution effects, electrical and AC impedance characterizations of Gd/Cr co-doped SrBi4Ti4O15 aurivillius-phase ceramics. Ceram. Int. 50 (10), 16909–16920 (2024).
Mesrar, M. et al. Microstructural and high-temperature dielectric, piezoelectric and complex impedance spectroscopic properties of K0.5Bi0.5TiO3 modified NBT-BT lead-free ferroelectric ceramics. Heliyon 9 (4), e14761 (2023).
Monika Aniz, I. et al. Frequency dependence of dielectric and impedance properties of ZnO and TiO2 added K0.5Na0.5NbO3 ceramics. Materials Today: Proceedings, (2023).
Chaudhary, S., Devi, S. & Jindal, S. Enhancing structural, optical, magnetic, dielectric and impedance properties of Ba0.95Sr0.05Ca5Ti2-xFexNb8O30 tungsten bronze ferroelectric ceramic through Fe/Ti ratio on optimization for the advanced device applications. Mater. Chem. Phys.. 312, 128580 (2024).
Zhao, N. et al. Enhanced energy storage properties of sm(Mg0.5Ti0.5)O3 modified (Bi0.5Na0.5)0.7Sr0.3TiO3 relaxor ferroelectric ceramics. Ceram. Int. 50 (5), 8325–8331 (2024).
Yang, H. et al. High energy storage density over a broad temperature range in sodium bismuth titanate-based lead-free ceramics. Sci. Rep. 7 (1), 8726 (2017).
Seifert, D. U. et al. Processing and properties of translucent bismuth sodium titanate ceramics. J. Eur. Ceram. Soc. 41 (2), 1221–1229 (2021).
Truong-Tho, N. & Vuong, L. D. Effect of sintering temperature on the dielectric, ferroelectric and energy storage properties of SnO2-doped Bi0.5(Na0.8K0.2)0.5TiO3 lead-free ceramics. J. Adv. Dielectr. 10 (04), 2050011 (2020).
Fan, Y. et al. Pb, Bi, and rare earth free X6R barium titanate–sodium niobate ceramics for high voltage capacitor applications. Appl. Phys. Lett. 122 (14), 143901 (2023).
Xu, P. et al. Enhancing grain boundary contributions to improve the dielectric properties of (In0.5Nb0.5)0.05Ti0.95O2 ceramics by Bi aided sintering. J. Phys. Chem. Solids. 187, 111881 (2024).
Zhang, L., Zhai, J. & Yao, X. Dielectric properties of barium strontium titanate thick films prepared by electrophoretic deposition. Mater. Res. Bull. 44 (5), 1058–1061 (2009).
Sahoo, S. et al. Structural evolution and enhanced dielectric properties of CeO2 modified lead-free (Bi0.5Na0.5TiO3)-(BaTiO3) solid solutions. Appl. Phys. A. 128(9), 843 (2022).
Xu, Q. et al. Structure and electrical properties of lead-free Bi0.5Na0.5TiO3-based ceramics for energy-storage applications. RSC Adv. 6 (64), 59280–59291 (2016).
Yang, F. et al. Enhanced energy storage properties and large electrostrictive effect of Bi0.35Na0.35Ba0.09Sr0.21Ti(1-x)(Al0.5Nb0.5)xO3 relaxor ceramics. Ceram. Int.. 49(4), 6068–6076 (2023).
Rafiq, M. A. et al. Impedance spectroscopic study on microwave sintered (1 – x) Na0.5Bi0.5TiO3–x BaTiO3 ceramics. Ceram. Int. 46(2), 2489–2499 (2019).
Acknowledgements
The authors are grateful to Metallurgical & Materials Engineering Department, UET, Lahore, Pakistan for providing materials for the research facilities in the Electronic Materials and Devices Lab. This article has been produced with the financial support of the European Union under the REFRESH - Research Excellence For Region Sustainability and High-tech Industries project number CZ.10.03.01/00/22_003/0000048 via the Operational Programme Just Transition and paper was supported by the following project TN02000025 National Centre for Energy II. The authors also extend their appreciation to Taif University, Taif Saudi Arabia, for supporting this work through project number (TUDSPP-2024-25).
Funding
This research was funded by Taif University, Saudi Arabia, Project No. TU-DSPP-2024-25.
Author information
Authors and Affiliations
Contributions
I.H.K, M.S.H., A.M., and M.A.R. conceived the project. I.H.K, and M.S.H. prepared specimens and analyzed their microstructures and dielectric properties. A.A., and K.N. discussed the results. A.M., A.A., A.I., and N. interpreted the experimental results. I.H.K, M.S.H., A.M., and M.A.R. wrote the paper. V.B. and S.M. critically reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Khan, I.H., Habib, M.S., Maqbool, A. et al. Comparative analysis of bulk ceramics and thick film coatings for optimized energy storage technologies. Sci Rep 14, 31800 (2024). https://doi.org/10.1038/s41598-024-82067-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-82067-6
Keywords
This article is cited by
-
Enhanced structural and electrical properties of furnace-cooled SrZrO3-modified BFBT lead-free piezoceramics for high-temperature applications
Scientific Reports (2025)
-
Exploring thermally stable dielectric and energy storage response of Bi-based ceramics for renewable energy storage applications
Journal of Electroceramics (2025)