Abstract
Although some studies have compared the treatment outcomes between modified endoscopic mucosal resection (m-EMR) and endoscopic submucosal dissection (ESD) for rectal neuroendocrine tumors (NETs), the results are based on the experience of experts from a single high-volume center. This multicenter study aimed to compare the outcomes between m-EMR and ESD for rectal NETs, with emphasis on the operator’s level. Data of patients with rectal NETs treated using m-EMR or ESD at seven institutions that included general hospitals in Japan were retrospectively reviewed. Patients treated using m-EMR and those treated using ESD were matched for age, sex, lesion size, lesion location, and operator level through propensity score matching. The treatment outcomes were compared between the two groups. In total 304 patients (m-EMR = 178, ESD = 126) were included, with 218 in the matched groups (m-EMR = 109, ESD = 109). The R0 resection rate was not significantly different between the two groups (90.0% vs. 82.3%, P = .221). However, the procedural time was significantly shorter for the m-EMR group than that for the ESD group (6 vs. 26 min, P < .001). No significant difference in adverse events was observed between the two groups (postprocedure bleeding rate: 5.5% vs. 2.8%, P = .335; perforation rate: 0.9% vs. 0.9%, P = 1.00). Subgroup analysis revealed that the R0 resection rate for the trainees was significantly higher in the m-EMR group than in the ESD group (87.9% vs. 64.5%, P = .017). m-EMR is the preferred technique for the treatment of rectal NETs and should be considered, particularly for the trainees.
Similar content being viewed by others
Introduction
In recent years, an increasing number of cases of rectal neuroendocrine tumors (NETs) has been reported, owing to the increased use of screening endoscopy for colorectal cancer1. Tumor size assessment is considered the simplest method to predict the behavior of rectal NETs. Current guidelines recommend local excision (including endoscopic resection [ER] and transanal endoscopic microsurgery) and radical resection with lymph node dissection for tumors < 10 mm and > 20 mm in size, respectively2,3,4,5. Contrarily, no consensus has been reached concerning the treatment strategy for rectal NETs sized between 10 and 20 mm. Several studies have reported that rectal NETs < 15 mm in size and confined to the submucosa behave similar to tumors < 10 mm in size2,6,7,8. Therefore, ER should be considered for patients with rectal NETs < 10 mm and can be considered for patients with rectal NETs < 15 mm in size who refuse to undergo surgery.
Because rectal NETs involve the submucosal or deeper layers of the rectal wall9, standard polypectomy or endoscopic mucosal resection (EMR) may not be suitable for achieving histologic complete resection. In a meta-analysis of 14 studies conducted on patients who had undergone polypectomy or EMR for rectal NETs, the complete resection rate was 59.1% (range: 17–90%)2. To achieve clear resection margins, more advanced techniques, such as endoscopic submucosal dissection (ESD) and modified EMR (m-EMR), are available for the ER of rectal NETs7,10,11. The application of ESD has gradually increased because of the high R0 resection rate in the treatment of rectal NETs; however, technical burden, prolonged procedural time, and related adverse events are considered disadvantages12. m-EMR includes endoscopic submucosal resection with a ligation device (ESMR-L) and cap-assisted EMR (EMR-C); both of these methods use suction and are easy to perform by beginners without a learning curve10,11.
Although some recent studies have verified the treatment outcomes of m-EMR and ESD, their results are based on the experience of experts from single high-volume centers13,14,15,16,17. Furthermore, only a few studies have examined the effect of the operator’s level on the treatment outcomes of m-EMR and ESD. Moreover, only tumors with a size of < 10 mm have been investigated13,14,15,16,18. Thus, in this multicenter study that included general hospitals, we aimed to evaluate and compare the efficacy and safety of ESD and m-EMR for the treatment of rectal NETs, including those > 10 mm, with a particular emphasis on the operator’s level.
Patients and methods
Patients
This multicenter retrospective cohort study enrolled patients who had undergone ER for rectal NETs from November 2007 to December 2022. This study was conducted at seven Japanese facilities, including university hospitals, cancer centers, and general hospitals. We included patients who underwent m-EMR or ESD for rectal NETs < 15 mm in size. Patients who were treated other than by m-EMR or ESD, such as by standard EMR or precutting EMR, were excluded. Patients with multiple lesions were also excluded.
Written informed consent was obtained from all patients before endoscopic procedures. The ethics committee of Chiba Cancer Center approved the study protocol, which was displayed on the notification board for inpatients and outpatients (approval number: R02-190). This study was performed in accordance with the ethical principles stipulated in the Declaration of Helsinki.
Indications of ER for rectal NETs
In all patients, prior to the ER procedure, computed tomography was performed to exclude distant metastasis. In accordance with the guidelines, ER was indicated for rectal NETs < 10 mm in size5. In principle, surgery was recommended for patients having rectal NETs with a size between 10 and 14 mm. For those who refused to undergo surgery, ER was performed. Because rectal NETs < 10 mm in size are limited to the submucosal layer in most cases19, endoscopic ultrasonography (EUS) before ER was not routinely performed. For rectal NETs with a size between 10 and 14 mm, EUS was performed to exclude invasion of the rectal muscularis propria layer.
Endoscopic procedures
The ER method followed was at the discretion of the operator at each facility.
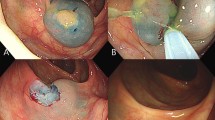
ESMR-L was conducted using a ligation device (Sumitomo Bakelite Co., Ltd., Tokyo, Japan) attached to the endoscope. After injecting 10% glycerin solution mixed with a small amount of indigo carmine into the submucosal layer, the lifted lesion was suctioned into the ligation device, and an elastic band was deployed10. The lesion was resected by snaring under the band. In the EMR-C procedure, the same solution used in ESMR-L was injected into the submucosal layer and a transparent cap for EMR-C was fitted to the scope. After a crescent-type snare was looped along the cap’s inner lip, the lesion was sequentially suctioned into the cap, grasped using the snare, and resected11. For the ESD procedure, 10% glycerin solution and/or 0.4% hyaluronic acid (MucoUp; Johnson & Johnson, Tokyo, Japan) were mixed with a small amount of indigo carmine and injected into the submucosal layer around the lesion. A Dual knife (Olympus) or an SB Knife Jr (Sumitomo Bakelite Co., Ltd.) was used for mucosal incision and submucosal resection. Hemostatic forceps were used to stop and prevent bleeding during the procedure.
Definition of efficacy: histopathological diagnosis
The tumor specimens resected via ER were routinely fixed with formalin and embedded in paraffin. All specimens were analyzed by two experienced pathologists at each institution and assessed microscopically for histopathological features, including the histopathological type, tumor size, invasion depth, and resection margin. The resection margin status was classified as positive, negative, or indeterminate based on the TNM classification.
Definition of safety and treatment outcomes
Procedural time was defined as the time from the initiation of submucosal injection to complete lesion removal. Postoperative bleeding was defined as any bleeding from the resection site that required endoscopic hemostasis. Perforation was defined as the defect of the whole rectal wall in which the surrounding tissues or organs could be seen through the hole during the procedure. “Experienced” endoscopists were defined as having an experience of performing EMR for at least 5 years or having performed 50 colonic ESDs during each treatment20,21.
Statistical analysis
No statistical sample size calculations were conducted. Propensity score matching was used to minimize differences in clinical characteristics between the m-EMR and ESD groups. A logistic regression model was used to calculate the propensity score of the case based on age, sex, lesion size and location, and operator level. One-to-one matching was performed between the m-EMR and ESD groups using the nearest-neighbor method with a.1 caliper width of the standard deviation of the propensity score logit (Supplementary Fig. 1).
Two-sample t or Wilcoxon rank-sum tests were used to compare continuous variables between the m-EMR and ESD groups, and Pearson chi-square or Fisher’s exact test was used to compare categorical variables. Multivariate logistic regression analysis was performed to evaluate the factors associated with histologic incomplete resection in the total cohort. All tests were two-tailed, and a P value of < 0.05 was considered significant. All statistical analyses were conducted using R version 3.6.2 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics and treatment outcomes before propensity score matching
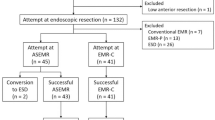
The study flowchart is shown in Fig. 1. We initially enrolled 327 patients with 330 rectal NETs < 15 mm in size treated via ER. Among them, we excluded 20 patients who had undergone ER via a method other than m-EMR or ESD (n = 15 standard EMR; n = 5 precutting EMR). We also excluded three patients with multiple NET lesions resected in a single procedure. Altogether, 304 patients (m-EMR, n = 178; ESD, n = 126) were included in the final cohort.
The baseline characteristics of the enrolled patients are shown in Table 1. The median tumor size was larger in the ESD group than that in the m-EMR group (m-EMR vs. ESD, 51,2,3,4,5,6,7,8,9,10,11 mm vs. 52,3,4,5,6,7,8,9,10,11,12,13 mm; P < .001). The proportion of experts was significantly greater in the ESD group than that in the m-EMR group (73.8% vs. 62.4%, P = .047). In the m-EMR group, 170 lesions were treated with ESMR-L and the remaining 8 lesions were treated with EMR-C.
The treatment outcomes between m-EMR and ESD before matching are shown in Table 2. The median procedural time was significantly shorter in the m-EMR group than that in the ESD group based on analyses before matching (m-EMR vs. ESD, 62,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17 min vs. 26 [7–120] min; P < .001). The en bloc resection rate was 100% in both groups. The histologic complete resection rate was significantly higher in the m-EMR group than that in the ESD group (91.0% vs. 81.7%, P = .038). Postprocedural bleeding occurred in 6.7% (n = 12) and 3.2% (n = 4) of the patients in the m-EMR and ESD groups, respectively (P = .201). Perforation occurred in 0.6% (n = 1) and 1.6% (n = 2) of the patients in the m-EMR and ESD groups, respectively (P = .572). All bleeding or perforation episodes were successfully treated using endoscopic procedures, and no patient required surgical treatment.
Treatment outcomes between the m-EMR and ESD groups after propensity score matching
The matching factors and treatment outcomes between the m-EMR and ESD groups after propensity score matching are shown in Table 3. In total, 109 pairs were matched.
Regarding the treatment outcomes, the procedural time was significantly shorter for the m-EMR group than that for the ESD group (62,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17 min vs. 26 [7–120] min; P < .001).The m-EMR group had a higher R0 resection rate than the ESD group, although it was not statistically significant (90.0% vs. 82.3%, P = .221).
Postprocedure bleeding occurred in 5.5% (n = 6) and 2.8% (n = 3) of the patients in the m-EMR and ESD groups, respectively (P = .335). Perforation occurred in 0.9% (n = 1) and 0.9% (n = 1) of the patients in the m-EMR and ESD groups, respectively (P = 1.00).
Subgroup analysis of treatment outcomes after propensity score matching
We performed post-hoc subgroup analysis, and the subgroup analysis results of treatment outcomes based on the operator level is shown in Table 4. For the experts, the median procedural time was significantly shorter in the m-EMR group than that in the ESD group (5.53,4,5,6,7,8,9,10,11,12,13,14,15,16,17 min vs. 22 [7–97] min; P < .001). However, no significant difference in the histologic complete resection rate was observed between the m-EMR and ESD groups. For the trainees, the median procedural time was significantly shorter in the m-EMR group than that in the ESD group (62,3,4,5,6,7,8,9,10,11,12,13 min vs. 40 [20–120] min; P < .001). In addition, the histologic complete resection rate was significantly higher in the m-EMR group than that in the ESD group (87.9% vs. 64.5%, P = .017).
The results of the subgroup analysis of treatment outcomes based on lesion size are shown in Table 5. For rectal NETs < 5 and ≥ 5 mm in size, the median procedural times were significantly shorter in the m-EMR group than those in the ESD group (63,4,5,6,7,8,9,10,11,12,13,14 min vs. 21.5 [9–73] min; P < .001 and 62,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17 min vs. 27 [7–120] min; P < .001). Although not statistically significant, the m-EMR group had a higher R0 resection rate than the ESD group for both rectal NETs < 5 and ≥ 5 mm in size (97.2% vs. 86.8%, P = .189; 86.3% vs. 80.3%, P = .529). While comparing rectal NETs of ≥ 7 mm in size, a similar tendency was observed in the m-EMR and ESD groups (80.6% vs. 71.9%).
There were seven lesions each in the m-EMR and the ESD groups for rectal NETs ≥ 10 mm. The R0 resection rate was 85.7% and 71.4% in the m-EMR and the ESD groups, respectively.
Factors associated with histologic incomplete resection
The results of the multivariate logistic regression analysis for incomplete histologic resection are shown in Table 6. In the total cohort, the lesion size ≥ 5 mm (odds ratio [OR], 1.25; 95% confidence interval [CI], 1.05–1.40; P = .008) and ESD (OR, 2.08; 95% CI, 1.01–4.35; P = .048) were strongly associated with histologic incomplete resection.
Discussion
In this study, we performed a propensity score-matched analysis of data from a multicenter database to compare the efficacy and safety of m-EMR and ESD for the treatment of rectal NETs. We found significantly shorter procedural time with m-EMR than that with ESD. Furthermore, high curability and safety were achieved with m-EMR, with en bloc and complete resection rates of 100% and 89.9%, respectively; however, the postprocedural bleeding and perforation rates were 5.5% and 0.9%, respectively, which were not significantly different compared with those of ESD.
ESD was significantly associated with incomplete resection in the multivariate analysis of the total cohort. In the present study, the R0 resection rate of ESD was lower than that of expert resection reported previously7. Considering the possibility that the operator level affects the resection rate, we performed a subgroup analysis of propensity score matching by operator level and found no significant difference in the R0 resection rate with the expert resection group; however, the R0 resection rate with ESD was significantly reduced to 64.5% in the cases resected by trainees. We speculated that the low R0 resection rate in the trainee group had a significant impact on overall ESD outcomes. ESD is easier to perform on rectal NETs than on colonic lesions because of their smaller lesion size and lower perforation risk as they are mostly located in the lower rectum. However, to secure a deep margin, it is necessary to dissect just above the muscle layer with minimal electrocautery coagulation, and the procedure necessitates skill. Therefore, rectal NETs are not considered suitable lesions for trainees to initiate ESD.
Previous studies compared the therapeutic outcomes of ESMR-L and ESD for rectal NETs < 10 mm in size and found that ESMR-L and ESD had similar R0 resection rates; however, ESMR-L was associated with a significantly shorter procedural time than ESD13,15,16,18. A meta-analysis reported that ESMR-L is a more suitable method for lesions with a size of < 10 mm than ESD22. A recent single-center randomized controlled trial comparing EMR-C and ESD for rectal NETs ≤ 10 mm in size also reported that EMR-C was noninferior to ESD, with a similar complete resection rate and shorter procedural time23. Contrarily, no consensus has been reached regarding an optimal treatment strategy for rectal NETs > 10 mm in size. One study reported no difference in the R0 resection rates between the m-EMR and ESD groups for lesions < 20 mm in size17. ESD might offer higher complete resection rates for the treatment of lesions with a size between 7 and 16 mm than EMR-C24. ESD might also be better than EMR-C for lesions > 8 mm in size11. In Japan, since 2018, ESD for rectal NETs ≥ 5 mm in size has been covered under health insurance. Therefore, we performed a subgroup analysis of propensity score matching by lesion size and found that m-EMR had a higher R0 resection rate for both lesions < 5 mm and ≥ 5 mm in size than ESD. A subgroup analysis was also performed for lesions > 7 mm in size, and a similar trend was observed. These results suggest that m-EMR is more suitable than ESD for treating not only lesions < 5 mm in size but also those between 5 and 14 mm. Furthermore, the operation cost of m-EMR is lower than that of ESD ($333 vs. $1,469). m-EMR can be performed as an outpatient procedure, and its hospitalization costs are less than those for ESD.
Regarding adverse events, postprocedural bleeding was observed in 5.5% and 2.8% of the patients in the m-EMR and ESD groups, respectively. The postprocedural bleeding rate of the ESD group in this study was similar to that previously reported in a multicenter study on ESD for colorectal neoplasms (2.0%)21. Contrarily, the postprocedural bleeding rate of the m-EMR group was higher than that of small polyp reported previously25. We speculate that since m-EMR resects a deeper layer of the submucosa than the conventional EMR, a thicker blood vessel is resected, which is presumed to lead to postprocedural bleeding.
This study had some limitations. First, this was a retrospective study, and the choice of the ER method used depended on the operator at each facility. Therefore, selection biases related to the study design cannot be denied. However, we tried to overcome this limitation using propensity score matching. Second, most patients in the m-EMR group had undergone ESMR-L. This study could not compare the treatment outcomes between ESMR-L and EMR-C. It was also not possible to perform a three-group comparison among ESMR-L, EMR-C, and ESD. Finally, this study included relatively few lesions > 10 mm because ER was performed for rectal NETs with a size between 10 and 14 mm in refusal of surgery.
In conclusion, based on our findings, m-EMR has a higher R0 resection rate than ESD, although the difference is insignificant; however, m-EMR is associated with a significantly shorter procedural time than ESD. In addition, subgroup analysis revealed that the R0 resection rate for the trainees was significantly higher in the m-EMR group than that in the ESD group. Therefore, m-EMR is the preferred technique for the treatment of rectal NETs and should be considered, particularly for the trainees.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Scherubl, H. Rectal carcinoids are on the rise: early detection by screening endoscopy. Endoscopy 41, 162–165 (2009).
de Mestier, L., Brixi, H., Gincul, R., Ponchon, T. & Cadiot, G. Updating the management of patients with rectal neuroendocrine tumors. Endoscopy 45, 1039–1046 (2013).
Wood, D. E. National Comprehensive Cancer Network (NCCN) clinical practice guidelines for lung cancer screening. Thorac. Surg. Clin. 25, 185–197 (2015).
Delle Fave, G. et al. (2016) ENETS consensus guidelines update for gastroduodenal neuroendocrine neoplasms. Neuroendocrinology 103, 119–124 (2016).
Japanese Neuroendocrine Society. Guidelines, ed 2 [in Japanese]. Tokyo, Kanehara Shuppan (2019).
Park, C. H. et al. Criteria for decision making after endoscopic resection of well-differentiated rectal carcinoids with regard to potential lymphatic spread. Endoscopy 43, 790–795 (2011).
Park, H. W. et al. Endoscopic submucosal dissection for treatment of rectal carcinoid tumors. Gastrointest. Endosc. 72, 143–149 (2010).
Kwaan, M. R., Goldberg, J. E. & Bleday, R. Rectal carcinoid tumors: review of results after endoscopic and surgical therapy. Arch. Surg. 143, 471–475 (2008).
Soga, J. Carcinoids of the rectum: an evaluation of 1271 reported cases. Surg. Today 27, 112–119 (1997).
Mashimo, Y. et al. Endoscopic submucosal resection with a ligation device is an effective and safe treatment for carcinoid tumors in the lower rectum. J. Gastroenterol. Hepatol. 23, 218–221 (2008).
Yang, D. H. et al. Cap-assisted EMR for rectal neuroendocrine tumors: comparisons with conventional EMR and endoscopic submucosal dissection (with videos). Gastrointest. Endosc. 83, 1015–1022 (2016).
Cao, Y. et al. Meta-analysis of endoscopic submucosal dissection versus endoscopic mucosal resection for tumors of the gastrointestinal tract. Endoscopy 41, 751–757 (2009).
Bang, B. W. et al. Endoscopic resection for small rectal neuroendocrine tumors: comparison of endoscopic submucosal resection with band ligation and endoscopic submucosal dissection. Gastroenterol. Res. Pract. 2016, 6198927 (2016).
Choi, C. W. et al. The clinical outcomes and risk factors associated with incomplete endoscopic resection of rectal carcinoid tumor. Surg. Endosc. 31, 5006–5011 (2017).
Harada, H. et al. Endoscopic submucosal dissection for small submucosal tumors of the rectum compared with endoscopic submucosal resection with a ligation device. World J. Gastrointest. Endosc. 9, 70–76 (2017).
Kamigaichi, Y. et al. Clinical outcomes of endoscopic resection for rectal neuroendocrine tumors: advantages of endoscopic submucosal resection with a ligation device compared to conventional EMR and ESD. DEN Open. 2, e35 (2022).
Wang, X. Y. et al. The outcomes of modified endoscopic mucosal resection and endoscopic submucosal dissection for the treatment of rectal neuroendocrine tumors and the value of endoscopic morphology classification in endoscopic resection. BMC Gastroenterol. 20, 1–2 (2020).
Matsuno, K. et al. Comparison of endoscopic submucosal resection with ligation and endoscopic submucosal dissection for small rectal neuroendocrine tumors: a multicenter retrospective study. DEN Open. 3, e163 (2023).
Scherübl, H., Jensen, R. T., Cadiot, G., Stölzel, U. & Klöppel, G. Management of early gastrointestinal neuroendocrine neoplasms. World J. Gastrointest. Endosc. 3, 133–139 (2011).
Takeuchi, Y. et al. Factors associated with technical difficulties and adverse events of colorectal endoscopic submucosal dissection: retrospective exploratory factor analysis of a multicenter prospective cohort. Int. J. Colorectal Dis. 29, 1275–1284 (2014).
Kobayashi, N. et al. Outcomes of endoscopic submucosal dissection for colorectal neoplasms: prospective, multicenter, cohort trial. Dig. Endosc. 34, 1042–1051 (2022).
Hong, S. M. & Baek, D. H. Endoscopic treatment for rectal neuroendocrine tumor: which method is better? Clin. Endosc. 55, 496–506 (2022).
Gao, X. et al. Modified cap-assisted endoscopic mucosal resection versus endoscopic submucosal dissection for the treatment of rectal neuroendocrine tumors ≤ 10 mm: a randomized noninferiority trial. Am. J. Gastroenterol. 117, 1982–1989 (2022).
Wang, X. et al. Endoscopic submucosal dissection for the treatment of rectal carcinoid tumors 7–16 mm in diameter. Int. J. Colorectal Dis. 30, 375–380 (2015).
Kim, H. S. et al. Risk factors for immediate postpolypectomy bleeding of the colon: a multicenter study. Am. J. Gastroenterol. 101, 1333–1341 (2006).
Acknowledgements
We thank all members of the CAM-united group, for helping with this study.
Funding
The present study received no funding.
Author information
Authors and Affiliations
Contributions
Y.K. did study design and manuscript writing. T.S., A.M., K.O., T.M., T.S., H.I., T.M., H.T., H.S., and N.K. analyzed and interpreted the data.All the authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kitagawa, Y., Suzuki, T., Miyakawa, A. et al. Comparison of endoscopic submucosal dissection and modified endoscopic mucosal resection for rectal neuroendocrine tumors. Sci Rep 15, 5424 (2025). https://doi.org/10.1038/s41598-024-82082-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-82082-7