Abstract
In yeast and mammals, the EXO70 subunit of the exocyst complex plays a key role in mediating the tethering of exocytic vesicles to the plasma membrane (PM). In plants, however, the role of EXO70 in regulating vesicle tethering during exocytosis remains unclear. In land plants, EXO70 has undergone significant evolutionary expansion, resulting in multiple EXO70 paralogues that may allow the exocyst to form various isoforms with specific functions. Previous research in Arabidopsis has shown that generally disrupting exocyst function leads to various defects in cellulose synthase (CESA) complex (CSC) trafficking. In this study, we utilized real-time imaging combined with genetic approaches to explore the role of EXO70A1, a member of the EXO70 family in Arabidopsis, in CSC trafficking. The exo70a1 mutant exhibited a decrease in crystalline cellulose content and a reduced density of functional CSCs in the PM. Moreover, the delivery of tdTomato-CESA6 from the cortex to the PM was compromised in the mutant, leading to the accumulation of CSC vesicles at the cell cortex. However, the velocity of tdTomato-CESA6 in the PM was unaffected in exo70a1. These findings suggest that EXO70A1 has a specific role in tethering CSCs to the PM.
Similar content being viewed by others
Introduction
Plant cell walls provide structural support and protection to plant cells and are a renewable source of biomass for humans1. The plant cell wall is a chemically complex structure composed mostly of polysaccharides: cellulose, hemicelluloses, and pectin. Hemicelluloses and pectin are produced in the Golgi apparatus, while cellulose is synthesized at the plasma membrane by cellulose synthase (CESA) complexes (CSCs)2. The most potentially functional CSC for primary cell wall formation consists of 18 individual CESA proteins organized into six lobes, each containing three CESA isoforms: CESA1, CESA3, and CESA6-like proteins (CESA2, CESA5, CESA6 and CESA9)3. CESAs are produced in the endoplasmic reticulum (ER), assembled into a complex in the ER/Golgi, and transported to the plasma membrane (PM) through the conventional secretory pathway for cellulose synthesis3,4,5,6. The post-Golgi trafficking of CSCs involved in several continuous processes: 1) the movement of CSCs from the sub-cortex to the cortex, which is dependent on actin, 2) subsequent translocation from the cortex to the PM facilitated by the cooperative action of actin and microtubules, and 3) the tethering and insertion of CSCs to the PM4,5,6,7,8,9. The trafficking of CSCs is developmentally regulated, hence playing crucial role in the dynamic organization of cell wall during plant growth and in response to environmental cues1,5,10.
Several key regulators have been identified in the regulation of CSCs trafficking. CSI1/POM2 interacts with cortical microtubules to designate the docking sites for CSCs secretion8. Nevertheless, the mutation of CSI1/POM2 does not compromise the efficiency of CESA delivery to the PM8, indicating that CSI1/POM2 is not responsible for tethering of CSC to the PM. Interestingly, PATROL1 (PTL1), which interacts with CSI1/POM2, plays a distinct role from CSI1/POM2 in the delivery of CSCs to the PM8. The absence of PTL1 does not impact the docking of CSCs vesicle to the cortical microtubules, but significantly reduces the delivery rate of CSCs to the PM, suggesting that PTL1 is probably involved in late step of CSC delivery8. Golgi-localized STELLO (STL) 1 and STL2 were reported to be engaged in the assembly and trafficking of CSCs11. Recently, STLs have been implicated in a TGN-independent CSC trafficking pathway. In stl-1 stl-2 mutants both the delivery of CESA to and its density in the PM were decreased7, possibly due to its compromised function in the formation of Golgi-derived SmaCCs/MASCs. Notably, myosin motors, which are important players in secretory vesicle trafficking in yeast and mammalian cells, were implicated in the delivery of CSCs to the PM in Arabidopsis10,12. In myosin XI-deficient cells, failed vesicle secretion events at PM and abnormal accumulation of CESA-containing compartments were observed, suggesting that myosin XI facilitates the tethering and fusion of CSC at the PM after its arrival at the insertion site defined by CSI1/POM210,12. Similar to myosin XI, the absence of TRANVIA(TVA), a plant-specific protein, also blocked the delivery of CSCs to the PM, suggested that TVA function may be associated with CSC insertion into the PM13. While these studies provide valuable insights into the trafficking regulation of CSCs to the PM, the tethering components involved in tethering CSC-vesicles to the PM have not been experimentally validated.
As a tethering complex, the exocyst complex is a hetero-octameric complex that is evolutionarily conserved14,15. Genetic and biochemical analysis has revealed that both yeast and mammalian exocyst complexes consist of eight subunits: SEC3, SEC5, SEC6, SEC8, SEC10, SEC15, EXO70, and EXO8416,17,18,19. Genetic analysis revealed that exocyst plays indispensable roles in various physiological activities by regulating exocytosis in animal and yeast cells. Mutations in exocyst subunits in budding yeast result in the accumulation of post-Golgi secretory vesicles at the budding site, likely due to a failure of vesicle tethering19,20. The orthologous genes encoding plant exocyst subunits were first identified in Arabidopsis thaliana21, and further biochemical studies demonstrated the existence of the exocyst as an octameric complex22. Interestingly, land plant genomes contain a large number of EXO70 paralogues (23 in Arabidopsis, 47 in rice), which is unique to land plants, as in other eukaryotes, EXO70 is encoded by a single gene. It has been suggested that the presence of multiple EXO70s enables the exocyst to form diverse isoforms involved in different cargo delivery processes in a cell-type-specific manner23. Consistently, genetic studies showed that different EXO70 paralogs participate in distinct physiological processes including cell growth, cell division, and stress responses23,24,25. So far however, a clear demonstration that such EXO70 subunit specificity exists within a cell has remained elusive26.
Previous research has demonstrated that CESA6 biochemically interacts with exocyst subunits in Arabidopsis8. Consequently, dysfunction of SEC5 or SEC6 resulted in a decreased delivery rate of CESAs to the PM8. These findings indicate a crucial role of the exocyst in the trafficking of CSCs. Notably, aside from the impaired delivery rate, mutants of SEC5a SEC5b and SEC6 also display a range of abnormalities, including increased density and decreased velocity of PM-localized CSC8. Given that SEC6 and SEC5 are encoded by a single and two genes in the Arabidopsis genome, respectively, the various cellular defects are most likely due to general secretory defects caused by exocyst loss-of-function. Therefore, it is imperative to identify individual EXO70 isoforms that particularly regulate CSC trafficking. This could lay the groundwork for further research on CSC trafficking in plant cells.
In this study, we used genetic approaches combined with advanced live cell imaging to investigate the function of EXO70A1, a member of the EXO70 family in Arabidopsis, in the process of delivering CSCs to the PM. We demonstrated that the delivery of tdTomato-CESA6 from the cortex to the PM was disrupted in exo70a1 mutants, leading to the accumulation of CESA6 at the cell cortex and reduced density at the PM. However, the velocity of tdTomato-CESA6 in the PM were not affected in the mutant. Our results suggest that EXO70A1 is specifically implicated in tethering CSC to the PM.
Results
The exo70a1-1 plants exhibit decreased cellulose content
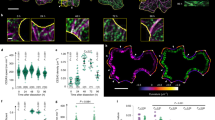
Recently, SEC5b and SEC6 have been proposed to play a role in CESAs delivery during cell wall formation8,10. In light of this, we sought to investigate the potential involvement possibility of EXO70A1, a key member of the EXO70 family in Arabidopsis, in the process of cellulose synthesis. To test this hypothesis, we performed a cellulose content analysis. Though exo70a1-1 was nearly sterile, the fertility defect can be overcome by grafting24,27, we were able to obtain sufficient mutant seeds from grafted plants for cellulose content analysis. The results revealed that the loss of EXO70A1 led to about a 23% reduction in crystalline cellulose content (Fig. 1A), suggesting that the EXO70A1 mutation impairs cellulose synthesis. The exo70a1-1, a null allele identified previously, displays a short-hypocotyl phenotype27 (Fig. 1B, C), which most likely caused by reduced cell elongation (Fig. 1D, E). To confirm that the observed phenotypic defects are indeed caused by mutation in the EXO70A1 locus, we generated transgenic plants expressing a C-terminal fusion of GFP to EXO70A1 under control of its native promoter in exo70a1-1. This construct (EXO70A1-GFP) successfully rescued the mutant phenotypes such as reduced cellulose content, short hypocotyl length, impaired cell elongation, plant dwarfism, and sterility (Fig. 1A-F, Supplementary Information Fig. S1A), demonstrating that mutating EXO70A1attenuates cellulose biogenesis. Furthermore, the EXO70A1-GFP signals are detected in different cell types (Supplementary Information Fig. S1B) and the PM-localized EXO70A1-GFP is developmentally regulated in the etiolated hypocotyl (Supplementary Information Fig. S2), similar as CESA5.
The phenotypes of exo70a1-1 hypocotyls suggest impaired cellulose synthesis. (A) Cellulose content measurement showing the reduced cellulose deposition in exo70a1-1, which can be restored by transgene of EXO70A1pro::EXO70A1-GFP. Ethanol-insoluble cell wall material (CWM) was prepared from 5-day-old etiolated hypocotyls of WT, exo70a1-1, and EXO70A1-GFP (The transgenic line of EXO70A1pro::EXO70A1-GFP in exo70a1-1 background), respectively. Values are means from three biological replicates, error bars represent SD. ANOVA was performed, letters [A-B] denote groups that show statistically significant differences with other groups, p < 0.01. (B) Representative figure showing the short-hypocotyl phenotype of 3-day-old dark grown exo70a1-1 mutant seedling was completely restored with transgene. Scale bar = 0.5 cm. (C) Quantification of hypocotyl length. Values are means from three biological replicates, error bars represent SD. The Student’s t-test was performed, letters [A-B] denote groups showing statistically significant differences with other groups, p < 0.01. (D) Morphology of elongated hypocotyl epidermal cells from 3-day-old seedlings. Epidermal cells were stained with Propidium iodide (PI) dye. Representative cells are highlighted and marked with arrows. Scale bar = 50 μm. (E) Quantification of cell length in (D). n = 35 cells for WT, n = 34 cells for exo70a1-1, n = 31 cells for EXO70A1-GFP from 10 seedlings for each group. Error bars represent SD. ANOVA was performed, letters [A-B] denote groups showing statistically significant differences with other groups, p < 0.01. (F) The developmental defects of exo70a1-1, such as dwarf and sterile plant, are restored in EXO70A1-GFP lines. Representative plants of 35-day-old are shown. Bar = 5 cm.
EXO70A1 is functionally associated with CESA6
The short hypocotyl phenotype is usually associated with primary cell wall defective mutants. In line with this, EXO70A1 has been biochemically identified to be copurified with CESA6, a subunit of CSC that functions in primary cell wall formation8. The direct interaction between EXO70A1 and CESA6 was verified by our luciferase complementation assay (Supplementary Information, Fig. S3A). To genetically corroborate the functional association between EXO70A1 and CESA6, a double mutant of EXO70A1 and CESA6 was generated by crossing. The cesa6prc1-1 (CESA6 mutant28) displayed a more pronounced short-hypocotyl phenotype compared to exo70a1-1, while the cesa6prc1-1 exo70a1-1 double mutant exhibited a hypocotyl length similar to that of the cesa6prc1-1 single mutant (Supplementary Information, Fig. S3B, C). These observations suggest a functional connection between EXO70A1 and CESA6, with EXO70A1 playing a regulatory role in hypocotyl growth that is dependent on CESA6.
The PM-localized CESA6 is attenuated in exo70a1-1
Given the biochemical and genetic interaction between EXO70A1 and CESA6, we hypothesized that the reduced cellulose content observed in exo70a1-1 may be due to a reduction in the number of functional CSC and/or a compromised function of CSCs in the PM. The PM-localized YFP-CESA6 could be visualized as discrete particles in expanding hypocotyl cells6. These particles exhibit iterative dynamics: they appear de novo at the PM, and after dwelling for ≈60 s, initiate a steady movement along linear trajectories for cellulose synthesis, and eventually being internalized6,10,29. Only those labeled particles exhibiting consistent velocities in the PM were considered as functional CSCs for further analysis of density and activity. Spinning disk confocal microscopy (SDCM) was employed to quantify the density and velocity of tdTomato-CESA6 in PM, as previously described in the literatures4,8. The abundance of PM-localized tdTomato-CESA6 was found to be significantly decreased by 14.7% in exo70a1-1 compared to the control (Fig. 2A, B; Supplemental movie S1). Additionally, we quantified the velocity of tdTomato-CESA6 particles at the PM, a common indicator of cellulose synthesis rate6,10,29. The average velocity of tdTomato-CESA6 particles in exo70a1-1 was 325.8 ± 120.3 nm·min−1, which was comparable to the wild type of control (330.4 ± 118.0 nm·min−1) (Fig. 3A-D). The reduced density and unaffected velocity of PM-localized tdTomato-CESA6 were also observed in endosidin2 (ES2, a small molecule was found to target the EXO70A1 in Arabidopsis and EXO70 in mammalian cells to inhibit exocytosis30) treatment (Fig. 2A, B; Supplemental movie S1; Fig. 3A-D). These results indicate that the decrease in abundance of PM-localized CSCs in the mutant may be responsible for the reduction in cellulose content. It is worth noting that our results differ from those of sec5a sec5b or sec68, where an increased density and a decrease in velocity of CSCs were observed. These discrepancies could be attributed to more extensive defects in the SEC5 or SEC6 mutant cells, where the general secretory pathways were impaired due to exocyst loss-of-function.
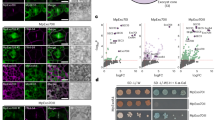
Mutation of EXO70A1 resulted in reduced density of PM-localized CESA. (A) The densities of tdTomato-CESA6 particles at the PM are reduced in exo70a1-1 cells and after Endosidin2 (ES2) treatment. The epidermal cells of 3-day-old etiolated hypocotyls were observed. Scale bar = 10 μm. (B) Quantifications of tdTomato-CESA6 density were analyzed in ROIs marked in (A). n = 57 cells for WT, n = 43 cells for DMSO, n = 47 cells for exo70a1-1, n = 32 cells for ES2 treated WT. Error bars represent SD. ANOVA was performed, letters [A-B] denote groups that show statistically significant differences with other groups, p < 0.01. Images of (A) were captured with SDCM.
Velocity of PM-localized CESA6 was not interfered by EXO70A1 mutation. (A) The 5 min-time projections show the trajectories of tdTomato-CESA6 at the PM. Time projections were generated by average intensity with 61 frames collected at 5-s intervals. The epidermal cells of 3-day-old etiolated hypocotyls were observed. Scale bar = 10 μm. (B) Representative kymographs show the linear movement of tdTomato-CESA6 during a 5-min interval in (A). (C, D) The motility of CSC particles at the PM is not compromised in EXO70A1-deficient cells. Quantitative analysis shows the distribution (C) and average velocities (D) of tdTomato-CESA6. n = 51 cells for WT, n = 35 cells for DMSO, n = 58 cells for exo70a1-1, n = 50 cells for ES2 treatment. Error bars represent SD. ANOVA was performed, “NS” is recognized as no significant difference comparing with WT, p > 0.05. Images in (A) were captured by SDCM.
The successful insertion of CSC in the PM is compromised in exo70a1-1
The reduced CSCs density could be attributed to a reduction in CSC secretion, an increase in CSC internalization, or a combination of both. As EXO70 is a subunit of the exocyst complex that is proposed to tether vesicles to the PM, we initially evaluated the delivery rate of CSCs to the PM using Fluorescent Recovery After Photobleaching (FRAP) analysis. After photobleaching a specified area of elongating hypocotyl epidermal cell, newly delivered tdTomato-CESA6 particles to the PM were monitored and quantified over a time-lapse movie. To analyze the successful insertion of CSCs, only those particles that showed a de novo appearance in the optical plane of the PM and steady movement in subsequent frames were quantified4,8. Our results showed that the delivery rate of CESA6 to the PM in exo70a1-1 was reduced by 55.1% compared to the control (Fig. 4A, B; Supplemental movie S2), indicative of compromised successful insertion of CSCs in the PM in exo70a1-1. Previous studies have demonstrated that the removal of PM-localized CESA is involved in the endocytic pathway31. FM4-64, a dye that stains the PM and is subsequently internalized through endocytotic vesicles, can be utilized to track endocytic processes32. To further explore whether the decreased CSC density is a result of enhanced endocytosis in exo70a1-1, seedlings were treated with FM4-64 for 20 min and the endocytic organelles with labeling were quantified. Surprisingly, our results revealed a reduction, rather than an elevation, in endocytic vesicles with labeling in mutant cells (Fig. 4C, D). Collectively, the reduced density of functional PM-localized CSCs in exo70a1-1 results from impaired insertion of CSCs into the PM.
Mutation of EXO70A1 displayed decreased both delivery rate of PM localized CESA6 and internalization of FM4-64. (A, B) Delivery rate of tdTomato-CESA6 to the PM is significantly reduced in exo70a1-1 cells. (A) Single-frame images displaying the tdTomato-CESA6 before, 5 s and 5 min after photobleaching. FRAP assays were performed in the WT and exo70a1-1. Yellow boxes mark the photobleached areas. Scale bar = 5 μm. (B) Quantifications of the delivery rate of tdTomato-CESA6 by FRAP assays descripted in (A). The delivery rate of tdTomato-CESA6 was calculated from the total number of new successfully delivered CESA6 during the initial 5 min of recovery divided by the measured area and time. n = 13 ROIs from 13 cells of WT, n = 17 ROIs from 17 cells of exo70a1-1. Error bars represent SD. The Student’s t-test was performed, p < 0.0001. (C, D) The exo70a1-1 exhibit defects in endocytosis of FM4-64. (C) Single-frame images displaying the intracellular FM4-64 puncta distribution after FM4-64 treatment 20 min, Scale bar = 5 μm. (D) Quantifications of the intracellular FM4-64 puncta density were analyzed. Error bars represent SD. The Student’s t-test was performed, p < 0.05. The epidermal cells of 3-day-old etiolated hypocotyls were observed. Images of (A) were captured with SDCM, Images of (C) were captured with LSCM (LEICA TCS SP8).
CESA6 accumulate beneath the PM in exo70a1-1
The accumulation of secretory vesicles beneath the PM is expected in the mutant when tethering was impaired, as previously revealed in yeast cells with an EXO70 mutation33. Therefore, we hypothesize that a similar phenomenon would be observed in exo70a1-1 cells. To test this, we further measured the densities of tdTomato-CESA6 positive structures at different focal planes beneath the PM. In the cortical region (0 to 0.6 μm below the plasma membrane), where the CSC is preparing for tethering10,12, we observed a significant increase in CSC vesicle density in mutant cells (Fig. 5). However, in the subcortical region (0.6 to 1 μm below the plasma membrane), where the CSC undergoes long-distance intracellular translocation10,12, this increase was not observed (Fig. 5). In conclusion, our findings suggest that EXO70A1 plays a specific role in tethering the CSC to the PM, rather than in long-distance translocation at subcortical regions.
Mutation of EXO70A1 resulted in increased density of cortical CESA compartments. (A) Representative single frames taken at cortical (−0.3 μm below PM) and subcortical (−0.6 μm below PM) focal planes in hypocotyl epidermal cells of WT and exo70a1-1. The epidermal cells of 3-day-old etiolated hypocotyls were observed. Scale bar = 5 μm. (B) Quantitative analysis shows that the number of CESA compartments was increased significantly in the cortical but not in the subcortical cytoplasm in exo70a1-1. n = 29 cells in WT, n = 27 cells in exo70a1-1, a total of 1482, 2019, 805, and 873 compartments were captured from total areas of 44,656, 39,623, 46,399, and 40,047 μm2 in WT cortex, exo70a1-1 cortex, WT subcortex, and exo70a1-1 subcortex, respectively. Error bars represent SD. ANOVA was performed, letters [A-C] denote groups that show statistically significant differences with other groups, p < 0.01. Images of (A) were captured with SDCM.
Discussion
EXO70A1 is the first member of the plant EXO70 family to be genetically characterized, with its knock-out mutants displaying various developmental defects, including compromised cell wall formation24,26,27,34. Although Zhang et al. mentioned the transient co-localization of EXO70A1 with CSC10, Zhu et al. investigated alterations in CSC trafficking in sec6 and sec5a sec5b mutants8; however, the specific role of the exocyst in CSC trafficking remains unclear. By using spinning disk confocal microscopy (SDCM) and variable-angle epifluorescence microscopy (VAEM), we investigated the dynamics of CSC in hypocotyl cells of exo70a1 seedlings. Our analysis revealed that disruption of EXO70A1 resulted in a decreased rate of CSC delivery to the PM, similar to what was observed in SEC6 or SEC5 mutant8,10, and a lower density of functional CSCs in the PM. However, the speed of CSC movement within the PM remained unaffected. Furthermore, we observed an accumulation of CSC vesicles beneath the PM in exo70a1. These results suggest that mutation in EXO70A1 impairs the trafficking of CSC vesicles, specifically during their journey from the cortex to the PM where they are tethered. Our findings indicate that EXO70A1 plays a crucial role in tethering CSC vesicles to the PM.
Previous research has found that in Arabidopsis, the exocyst, along with CSI1 and PTL1, plays a role in regulating the delivery of CSCs8. However, it is interesting to note that when the function of SEC5 or SEC6 is impaired, the density of PM-localized CSCs actually increases, rather than decreases8. Additionally, the speed at which CSCs move within the PM decreases, indicating a decrease in CSC activity. These discrepancies between past and present findings may be attributed to the different genetic materials used in the studies. The exocyst plays a crucial role in delivering various cargos to the PM, and comprehensively disrupting its function can lead to general cellular dysfunction. In Arabidopsis, SEC5 is encoded by two genes, SEC5a and SEC5b, while SEC6 is encoded by a single gene27,35,36. Knocking out both SEC5a and SEC5b, or SEC6, as done in previous research8, should result in general secretion problems due to the exocyst loss-of-functions. This interferes with the transportation of proteins to the PM, including regulators of CSCs like KOR and SHOU437,38. KOR is known to enhance CSC activity at the PM, while SHOU4 regulates the levels of CESA proteins at the PM37,38. Mutants lacking both SEC5a and SEC5b, or SEC6, would disrupt the transportation of KOR and SHOU4, resulting in a decrease in CSC activity (reflected by its speed) and an increase in CSC density, as previously observed8. Certainly, these possibilities need to be experimentally verified.
In contrast, the presence of numerous EXO70 subunit genes in land plant genomes leads to the formation of diverse exocyst complexes in different cell types25,26,39. This speculation is strongly supported by previous research indicating the unique role of individual EXO70 subunits in different cellular processes23,26,40. The current findings demonstrate that the EXO70A1 mutant disrupts the function of the EXO70A1-type exocyst, specifically affecting CSC trafficking. Consistent with this speculation, mutations in sec5a sec5b, or sec6 result in more severe developmental defects compared to exo70a1 mutation8,27,41. Additionally, the hypocotyl elongation of EXO70A1 mutant is less affected when compared to SEC6 and SEC5a SEC5b mutants. The significance of multiple EXO70 paralogues in land plants is not yet fully understood. Both previous and current studies suggest that different EXO70 subunits may regulate vesicle tethering in a cell-type-specific and cargo-specific manner25,26. The multiplication of EXO70 subunit genes may represent a unique solution for plants to increase the complexity of their secretory trafficking pathways. However, it is worth noting that, despite the deletion of EXO70A1, some successful CSC insertion events are still observed. This implies that there may be other EXO70 paralogs that serve as substitutes for EXO70A1 within the cells. Identifying these additional members in future research will be of great interest. Alternatively, it is possible that EXO70A1 promotes, rather than being necessary for, the tethering of CSC to the PM.
A successful CSC insertion event usually involves a three-step process at the cell periphery: first, a transient erratic phase, representing a vesicle locating the docking site; next, a pause phase, likely involving the docking at cMT, tethering to the PM, fusion, and activation of the CSC in the PM; and lastly, a steady movement phase, showing an active CSC producing cellulose along a linear trajectory in the PM4,8,10,12. Several proteins have been shown to be involved in the delivery of CSCs at the cell periphery. PTL1 is involved in the late step of CSC delivery, while the PTL1 mutation did not affect the dynamics of the exocyst, suggesting that the exocyst complex may function prior to PTL18. Myosin XI, an interacting protein of the exocyst, is involved in the docking of CSCs at insertion site, which is defined by CSI1/POM2 and microtubules, facilitating CSC insertion into the PM10,12. Myosin XI associates with secretory vesicles before the exocyst and is required for the efficient delivery and proper behavior of the exocyst complex at the tethering site10. Based on these findings, we can propose that EXO70A1 acts downstream of Myosin XI and upstream of PTL1 in regulating CSC tethering (Fig. 6). In yeast and mammalian cells, EXO70 is known to recruit exocyst to the PM through binding to PtdIns(4,5)P242,43. Recent studies show that EXO70A1-phospholipid interactions are critical for exocyst recruitment to the PM in plants44. In Arabidopsis root endodermal cells, PtdIns(4,5)P2 accumulation was found to be specifically localized at the future Casparian strip, coinciding with EXO70A1 recruitment26. These results suggest that plant EXO70A1 shares similar biochemical properties with its yeast and mammalian counterparts. Besides phospholipids, Rho GTPases have been identified to interact with EXO70 and regulate polarized exocytosis in yeast33,42,45. However, the interaction between EXO70A1 and GTPases remains unexplored in plants. Identifying proteins that interact with EXO70A1 is crucial for understanding the regulatory mechanism of targeted vesicle tethering in plant cells.
A proposed model illustrating the role of EXO70A1 in tethering cortical CSC vesicles to the PM. A successful insertion of CSC typically involves a series of sequential steps in wild-type cells, including (1) delivery of CSC vesicles from the sub-cortex to the cortex, (2) docking of vesicles at the cortical microtubule, (3) attachment to the PM for tethering, and (4) finally integration into the PM via membrane fusion. Several proteins have been identified to be involved in the delivery of CSCs at the cell periphery. EXO70A1, acting after CSI1/POM2 /Myosin XI and before PTL1, specifically regulates the tethering step. In cells lacking EXO70A1, such as in exo70a1 mutants, CSC vesicles can reach the cell cortex, but are unable to tether to the PM, leading to reduced CSCs in the PM and an accumulation of CSC vesicles beneath the PM.
The identification of EXO70A1 as an essential regulator in tethering CSC vesicles to the PM provides an opportunity to deepen and expand our understanding of CSC trafficking. Despite the identification of multiple proteins that interact with the exocyst, the mechanisms by which they coordinate in CSC tethering at the plasma membrane remain unclear. This requires combined efforts in cell biology, genetics, and biochemistry. Moreover, to elucidate the fundamental mechanism of exocyst function in CSC trafficking, it is essential to know the assembly process of the exocyst complex and how this assembly is regulated. Given the involvement of EXO70 paralogs in various plant biological processes, further investigation into the regulation of EXO70s in different physiological contexts at the cellular level is warranted, offering insights into the diversification of EXO70 genes in terrestrial plants.
Materials and methods
Plant materials and growth conditions
All Arabidopsis lines are Columbia ecotype (Col-0). The exo70a1-1 mutant (SALK_014826) was characterized as previously reported and exo70a1-1 homozygous seeds were propagated by grafting with stock of wild type and scion of mutant plant24. Preparation of cesa6prc1-1 seeds were described previously28. The tdTomato-CESA6 transgenic line has been described previously9. To create the exo70a1-1 cesa6prc1-1 double mutant line, exo70a1-1 was crossed into cesa6prc1-1. Homozygous of exo70a1-1 was identified by PCR genotyping (Supplemental Data S1). Homozygous cesa6prc1-1 mutant was selected by the method of dCAPs with EcoRI digestion (Supplemental Data S1).
The lines of tdTomato-CESA6 in exo70a1-1 were identified in F2 population by PCR-based genotyping (Supplemental Data S1).
For the construction of EXO70A1pro:EXO70A1-eGFP transgenic line (EXO70A1-GFP), eGFP was amplified from donor vector (pBGWSF7) with primers: P1-F and P1-R, which contained SacI and XhoI sites, respectively. We inserted three glycine codons upstream of SacI site as a link with the target protein. The fragment of EXO70A1pro-EXO70A1 was amplified by PCR from Col-0 genomic DNA with primers: P2-F and P2-R, contained kpnI and SacI sites. The 3'UTR of EXO70A1 was amplified from Col-0 genomic DNA with primer: P3-F and P3-R, containing XhoI and PstI sites. eGFP and 3'UTR of EXO70A1 were introduced in pEASY-Blunt vector, respectively, for sequencing. EXO70A1pro-EXO70A1 fragment was introduced in pEASY-T1 for sequencing. Three fragments of EXO70A1pro-EXO70A1, eGFP and 3'UTR were released from cloning vectors by digestions with restriction enzymes, and introduced in pCAMBIA1300 vector one by one, resulted pCAMBIA1300-EXO70A1-GFP-3'UTR. pCAMBIA1300-EXO70A1-GFP-3'UTR was introduced in exo70a1-1 plants by Agrobacterium tumefaciens-mediated floral dip, the transgenic lines were screened with hygromycin, and named as EXO70A1-GFP.
Seeds were surface sterilized and sown on the media containing 0.5 × MS (Murashige and Skoog salts, Duchefa), 1% sucrose, and 1% agar, pH 5.8–6.0. Seeds were cold treated at 4 °C for 72 h. For light growth, plants were grown under long-day lighting conditions (16 h light/8 h dark) at 22 °C. For dark growth, seeds were exposed to light for 5 h and then grown vertically for 60–72 h in continuous darkness at 22 °C.
Length measurements of hypocotyl and hypocotyl epidermal cell
For hypocotyl length measurement, seedlings were grown in darkness for 72 h and scanned by scanner (MICROTEK, MRS-9600TFU2L). The lengths of about 30 hypocotyls of each group were measured with Fiji.
For the measurement of epidermal cell length, the seedlings grown in darkness for three days were stained with 10 μg/ml Propidium Iodide (PI) dye solution in 0.6 kPa for 10 min. The OLYMPUS FV1200 (on the OLMPUS IX83 microscope with a PMT GaAsP Spectrum Detector) confocal laser scanning microscope was used for imaging under a 20 × objective of 0.75-numerical aperture (NA). PI was excited at 559 nm. The length of epidermal cells was measured using FV10-ASW software.
Cellulose content measurement
The crystalline cellulose content was determined as modified from Updegraff46. Hypocotyls from 5-day-old etiolated seedlings of exo70a1-1, wild type and EXO70A1-GFP were cutted, freeze dried and milled to powder. The dry powder was washed with 70% alcohol twice and chloroform/methanol solution (methanol:chloroform = 1:1; v/v ) three times, then completely freeze dried. About 1 mg dry material was hydrolyzed with 1.5 ml Updegraff reagent (acetic acid: nitric acid: deionized water = 8:1:2) at 98℃ for 30 min, the insoluble material was collected after centrifuged at 15000 g for 15 min and washed with acetone for 4 times. The crystalline cellulose was hydrolyzed with 72% concentrated sulfuric acid. The solution then was incubated in anthrone reagent (18% hydrochloric acid as the stock solution, 0.04% anthrone in final incubated solution) with boiling water bath for 5 min for fully color producing reaction. Absorbance of the color solution was determined at 620 nm using a spectrophotometer. Measurements were performed in 3 duplicates. Cellulose content was quantified based on the standard curve of glucose as follows:
X: crystalline cellulose content (μg/mg); A: content of glucose at standard curve (μg/ml); B: weight of dry cell wall materials (mg); V: the solution volume after 72% concentrated sulfuric acid hydrolyzing (ml); C: the dilution times of solution after sulfuric acid hydrolyzing.
Drug treatments
3-day-old etiolated seedlings were cultured in drug or control solutions in darkness before live-cell imaging. Treatments were performed as follows: 80 μM ES2 for 4 h. For the internalization, 3-day-old etiolated seedlings were treated with 5 μM FM4-64 for 20 min.
Live-cell imaging
For live-cell imaging, seedlings were grown vertically in petri dishes with 0.5 × MS for 3 days in dark. Images were taken on epidermal cells of etiolated hypocotyls at 2 mm below apical hook unless otherwise stated.
Imaging was performed by using: 1) A spinning disk scanner unit (Andor dragonfly 505) and a TIRF illuminator on a LEICA DMi8 microscope, equipped with a 100 × 1.47-numerical aperture (NA) UPlanSApo oil objective (LEICA), two SONA 2.0B-11 sCMOS cameras (Andor); 2) LEICA TCS SP8 on a LEICA DMi8 microscope, equipped with a 100 × 1.40-numerical aperture (NA), two HyD GaAsP Spectrum Detector (LEICA).
GFP was excited at 488 nm, and emission was collected at 502–540 nm. tdTomato and FM4-64 were excited at 561 nm, and emissions were collected at 572.5–615.5 nm.
For the imaging of EXO70A1-GFP, at the PM, time-lapse images were collected with a 2-s interval for 60 frames by TIRF illuminator. And the PM-localized tdTomato-CESA6 imaging was collected with 5-s interval for 61 frames by SDCM.
For cortical and subcortical located tdTomato-CESA6 imaging, z-series at 0.3 μm step sizes plus time lapse with 1.6-s intervals for 35 frames were collected by SDCM.
For FRAP experiments, photo-bleaching was performed with a 405-nm laser line at 100% power and a 100 μm2 region was photobleached. The tdTomato-CESA6 imaging was performed using SDCM. For imaging of tdTomato-CESA6, time-lapse images were collected at the PM with a 5-s interval for 63 frames.
Image analysis and quantification
For the density of PM-localized tdTomato-CESA6, an ROI avoiding Golgi signal-masked area was selected using the ‘Freehand selections’ tool of Fiji. The area of the ROI was determined by the ‘Measure’ function, and the CESA particles (those showed a transient stationary motion before moving with constant velocities) within the ROI were detected using the ‘find maxima’ tool as previously reported8, specifically, in the windows of ‘find maxima’ tool, the ‘Prominence > 2’ was set, while the option for ‘Light background’ was deselected. For the density of PM-localized EXO70A1-GFP, the number of EXO70A1-GFP particles was counted by the TrackMate plug-in with Difference of Gaussians algorithm. EXO70A1-GFP particle density was calculated as the number of detected particles divided by the area of ROI, as previously reported12. The cortical and subcortical tdTomato-CESA6 densities were measured as previously reported12. The criteria for the measurement were determined manually based on their size (smaller than a Golgi or TGN compartment) and dynamic behavior4.
Velocities of PM-localized tdTomato-CESA6 particles were measured automatically using Imaris (version number 9.7.2).
For FRAP assays, a smaller area (8 × 8 μm2) within the bleached region (10 × 10 μm2) was used for analysis9. The newly appeared tdTomato-CESA6 florescence during the initial 5 min of recovery was manually counted, and the criteria of newly delivered tdTomato-CESA6 were defined as described previously4,6. The tdTomato-CESA6 delivery rate was calculated as the number of delivery events divided by the measured area and time.
Luciferase complementation imaging assay
The split-luciferase complementation assay was performed as described previously47. The combination and control were co-infiltrated into 4-week-old Nicotiana benthamiana leaves. Interaction was determined based on the fluorescent signal intensity collected by Taon (5200 Multi).
Data availability
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).
References
Zhu, Y. & McFarlane, H. E. Regulation of cellulose synthesis via exocytosis and endocytosis. Curr. Opin. Plant Biol. 69, 102273 (2022).
Gu, Y. & Rasmussen, C. G. Cell biology of primary cell wall synthesis in plants. Plant Cell. 34, 103–128 (2022).
Polko, J. K. & Kieber, J. J. The regulation of cellulose biosynthesis in plants. Plant Cell. 31, 282–296 (2019).
Gutierrez, R., Lindeboom, J. J., Paredez, A. R., Emons, A. M. & Ehrhardt, D. W. Arabidopsis cortical microtubules position cellulose synthase delivery to the plasma membrane and interact with cellulose synthase trafficking compartments. Nat. Cell Biol. 11, 797–806 (2009).
Crowell, E. F. et al. Pausing of Golgi bodies on microtubules regulates secretion of cellulose synthase complexes in Arabidopsis. Plant Cell. 21, 1141–1154 (2009).
Paredez, A. R., Somerville, C. R. & Ehrhardt, D. W. Visualization of cellulose synthase demonstrates functional association with microtubules. Science. 312, 1491–1495 (2006).
Liu, L. et al. Actomyosin and CSI1/POM2 cooperate to deliver cellulose synthase from Golgi to cortical microtubules in Arabidopsis. Nat. Commun. 14, 7442 (2023).
Zhu, X., Li, S., Pan, S., Xin, X. & Gu, Y. CSI1, patrol1, and exocyst complex cooperate in delivery of cellulose synthase complexes to the plasma membrane. Proc. Natl. Acad. Sci. U. S. A. 115, E3578–E3587 (2018).
Sampathkumar, A. et al. Patterning and lifetime of plasma membrane-localized cellulose synthase is dependent on actin organization in Arabidopsis interphase cells. Plant Physiol. 162, 675–688 (2013).
Zhang, W., Huang, L., Zhang, C. & Staiger, C. J. Arabidopsis myosin XIK interacts with the exocyst complex to facilitate vesicle tethering during exocytosis. Plant Cell. 33, 2454–2478 (2021).
Zhang, Y. et al. Golgi-localized stello proteins regulate the assembly and trafficking of cellulose synthase complexes in Arabidopsis. Nat. Commun. 7, 11656 (2016).
Zhang, W., Cai, C. & Staiger, C. J. Myosins XI are involved in exocytosis of cellulose synthase complexes. Plant Physiol. 179, 1537–1555 (2019).
Vellosillo, T., Dinneny, J. R., Somerville, C. R. & Ehrhardt, D. W. Tranvia (Tva) facilitates cellulose synthase trafficking and delivery to the plasma membrane. Proc. Natl. Acad. Sci. U. S. A. https://doi.org/10.1073/pnas.2021790118 (2021).
Mei, K. & Guo, W. Exocytosis: A new exocyst movie. Curr. Biol. 29, R30–R32 (2019).
Heider, M. R. & Munson, M. Exorcising the exocyst complex. Traffic. 13, 898–907 (2012).
TerBush, D. R., Maurice, T., Roth, D. & Novick, P. The exocyst is a multiprotein complex required for exocytosis in Saccharomyces Cerevisiae. Embo. J. 15, 6483–6494 (1996).
Hsu, S. C. et al. The mammalian brain rsec6/8 complex. Neuron 17, 1209–1219 (1996).
TerBush, D. R. & Novick, P. Sec6, sec8, and sec15 are components of a multisubunit complex which localizes to small bud tips in Saccharomyces Cerevisiae. J. Cell Biol. 130, 299–312 (1995).
Novick, P., Field, C. & Schekman, R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell 21, 205–215 (1980).
Finger, F. P. & Novick, P. Sec3P is involved in secretion and morphogenesis in Saccharomyces Cerevisiae. Mol. Biol. Cell 8, 647–662 (1997).
Elias, M. et al. The exocyst complex in plants. Cell Biol. Int. 27, 199–201 (2003).
Hala, M. et al. An exocyst complex functions in plant cell growth in Arabidopsis and Tobacco. Plant Cell. 20, 1330–1345 (2008).
Zarsky, V. Exocyst functions in plants: secretion and autophagy. Febs. Lett. 596, 2324–2334 (2022).
Li, S. et al. EXO70A1-mediated vesicle trafficking is critical for tracheary element development in Arabidopsis. Plant Cell. 25, 1774–1786 (2013).
Li, S. et al. Expression and functional analyses of EXO70 genes in Arabidopsis implicate their roles in regulating cell type-specific exocytosis. Plant Physiol. 154, 1819–1830 (2010).
Kalmbach, L. et al. Transient cell-specific EXO70A1 activity in the CASP domain and casparian strip localization. Nat. Plants. 3, 17058 (2017).
Synek, L. et al. AtEXO70A1, a member of a family of putative exocyst subunits specifically expanded in land plants, is important for polar growth and plant development. Plant J. 48, 54–72 (2006).
Fagard, M. et al. PROCUSTE1 encodes a cellulose synthase required for normal cell elongation specifically in roots and dark-grown hypocotyls of Arabidopsis. Plant Cell 12, 2409–2423 (2000).
Allen, H., Zhu, X., Li, S. & Gu, Y. The TRAPPIII subunit, Trs85, has a dual role in the trafficking of cellulose synthase complexes in Arabidopsis. Plant J. https://doi.org/10.1111/tpj.16688 (2024).
Zhang, C. et al. Endosidin2 Targets conserved exocyst complex subunit EXO70 to inhibit exocytosis. Proc. Natl. Acad. Sci. U. S. A. 113, E41–E50 (2016).
Bashline, L., Lei, L., Li, S. & Gu, Y. Cell wall, cytoskeleton, and cell expansion in higher plants. Mol. Plant. 7, 586–600 (2014).
Rigal, A., Doyle, S. M. & Robert, S. Live cell imaging of FM4-64, a tool for tracing the endocytic pathways in Arabidopsis root cells. Methods Mol. Biol. 1242, 93–103 (2015).
Wu, H., Turner, C., Gardner, J., Temple, B. & Brennwald, P. The Exo70 subunit of the exocyst is an effector for both Cdc42 and Rho3 function in polarized exocytosis. Mol. Biol. Cell 21, 430–442 (2010).
Vukasinovic, N. et al. Microtubule-dependent targeting of the exocyst complex is necessary for xylem development in Arabidopsis. New Phytol. 213, 1052–1067 (2017).
Cvrckova, F. et al. Evolution of the land plant exocyst complexes. Front. Plant Sci. 3, 159 (2012).
Zhang, Y., Liu, C. M., Emons, A. M. & Ketelaar, T. The plant exocyst. J. Integr. Plant Biol. 52, 138–146 (2010).
Polko, J. K. et al. SHOU4 proteins regulate trafficking of cellulose synthase complexes to the plasma membrane. Curr. Biol. 28, 3174–3182 (2018).
Lane, D. R. et al. Temperature-sensitive alleles of RSW2 link the KORRIGAN endo-1,4-β-glucanase to cellulose synthesis and cytokinesis in Arabidopsis. Plant Physiol. 126, 278–288 (2001).
Zarsky, V., Sekeres, J., Kubatova, Z., Pecenkova, T. & Cvrckova, F. Three subfamilies of exocyst EXO70 family subunits in land plants: early divergence and ongoing functional specialization. J. Exp. Bot. 71, 49–62 (2020).
Kulich, I. et al. Exocyst subunit EXO70H4 has a specific role in callose synthase secretion and silica accumulation. Plant Physiol. 176, 2040–2051 (2018).
Wu, J. et al. Regulation of cytokinesis by exocyst subunit SEC6 and KEULE in Arabidopsis thaliana. Mol. Plant. 6, 1863–1876 (2013).
He, B., Xi, F., Zhang, X., Zhang, J. & Guo, W. Exo70 interacts with phospholipids and mediates the targeting of the exocyst to the plasma membrane. Embo. J. 26, 5167 (2007).
Liu, J., Zuo, X., Yue, P. & Guo, W. Phosphatidylinositol 4,5-bisphosphate mediates the targeting of the exocyst to the plasma membrane for exocytosis in mammalian cells. Mol. Biol. Cell 18, 4483–4492 (2007).
Synek, L. et al. Plasma membrane phospholipid signature recruits the plant exocyst complex via the EXO70A1 subunit. Proc. Natl. Acad. Sci. U. S. A. 118, e2105287118 (2021).
Robinson, N. G. et al. Rho3 of Saccharomyces Cerevisiae, which regulates the actin cytoskeleton and exocytosis, is a GTPase which interacts with Myo2 and Exo70. Mol. Cell. Biol. 19, 3580–3587 (1999).
Updegraff, D. M. Semimicro determination of cellulose in biological materials. Anal. Biochem. 32, 420–424 (1969).
Chen, H. et al. Firefly luciferase complementation imaging assay for protein-protein interactions in plants. Plant Physiol. 146, 368–376 (2008).
Acknowledgements
We thank Prof. Chaowen Xiao (Sichuan University, China) for providing the cesa6prc1-1 and tdTomato-CESA6 transgenic lines. This work was supported by National Natural Science Foundation of China (31571467 and 31800234), and by the Shandong Natural Science Foundation (ZR2020MC067).
Author information
Authors and Affiliations
Contributions
Su Jiang, Zhendong Liu, and Shipeng Li designed research; Su Jiang, Zhendong Liu, Shuju Zhao, Juan Li, Tonghui Li, and Dali Yu performed research; Su Jiang, Zhendong Liu, and Can Bu have analyzed data; Shan Gao, Xiaonan Liu, Guangyou Duan and Dayong Cui, contributed Arabidopsis lines/analytic tools, and revised the paper; Su Jiang, Zhendong Liu, and Shipeng Li wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Video 1.
Supplementary Video 2.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jiang, S., Liu, Z., Zhao, S. et al. Tethering of cellulose synthase complex to the plasma membrane relies on the isoform of EXO70A1 in Arabidopsis. Sci Rep 14, 31245 (2024). https://doi.org/10.1038/s41598-024-82606-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-82606-1