Abstract
Chronic complete spinal cord injury (SCI) is difficult to treat because of scar formation and cavitary lesions. While human iPS cell-derived neural stem/progenitor cell (hNS/PC) therapy shows promise, its efficacy is limited without the structural support needed to address cavitary lesions. Our study investigated a combined approach involving surgical scar resection, decellularized extracellular matrix (dECM) hydrogel as a scaffold, and hNS/PC transplantation. To mitigate risks such as prion disease associated with spinal cord-derived dECM, we used kidney-derived dECM hydrogel. This material was chosen for its biocompatibility and angiogenic potential. In vitro studies with dorsal root ganglia (DRG) confirmed its ability to support axonal growth. In a chronic SCI rat model, scar resection enhanced the local microenvironment by increasing neuroprotective microglia and macrophages, while reducing inhibitory factors that prevent axonal regeneration. The combination of scar resection and dECM hydrogel further promoted vascular endothelial cell migration. These changes improved the survival of transplanted hNS/PCs and facilitated host axon regeneration. Overall, the integrated approach of scar resection, dECM hydrogel scaffolding, and hNS/PC transplantation has been proven to be a more effective treatment strategy for chronic SCI. However, despite histological improvements, no functional recovery occurred and further research is needed to enhance functional outcomes.
Similar content being viewed by others
Introduction
Along with the significant advancements in the treatment strategies for spinal cord injury (SCI), several clinical studies have also progressed, particularly when the intervention occurs during the acute phase 1,2,3,4. We have initiated a clinical trial to evaluate the efficacy of human iPS cell-derived neural stem progenitor cell (hNS/PCs) transplantation for subacute SCI 5. However, the management of chronic SCI remains a challenge due to several complex pathological changes that occur over time, and it is difficult to progress to clinical trials in the chronic phase.
In chronic SCI, the formation of cavitary lesions following neuronal apoptosis 6,7, fibrous scar formation mediated by perivascular cells 8,9,10,11,12, and accumulation of reactive astrocytes leading to glial scar formation create a physical barrier that severely impedes axonal growth. Moreover, beyond these structural barriers, several inhibitors, such as chondroitin sulfate proteoglycan (CSPG) secreted by glial scars, semaphorin 3A (Sema3A), myelin-associated glycoprotein, oligodendrocyte myelin glycoprotein (Omgp), and Nogo-A (Nogo), tend to restrict the axonal extension 13,14,15. Moreover, neuroprotective markers, such as arginase-1 (Arg1), are reduced in the chronic phase compared with subacute SCI, providing a microenvironment of the spinal cord that is less conducive to cell-based therapy 16. Thus, cell transplantation alone is inadequate for treating chronic SCI, necessitating a multifaceted therapeutic approach 17,18.
In developing therapeutic strategies, it is essential to further categorize the pathology of chronic SCI into either incomplete or complete forms for a comprehensive understanding. In cases of incomplete injuries, while cavitary lesions and scar formation occur, residual axons remain at the epicenter. As a therapeutic strategy, transplanted cells can engraft into the remaining spinal cord tissue, which potentially allows for the achievement of effects through combination therapy. For example, several studies have reported functional improvements by combining cell transplantation as a base treatment with pharmacological therapies, such as treatments with chondroitinase ABC (C-ABC) 19,20 and Sema3A inhibitors 21. Moreover, some studies have reported functional recovery achieved by combining cell transplantation with rehabilitation 22,23. In contrast, in cases of complete injuries, there are no residual axons at the epicenter, and the engraftment of transplanted cells at the epicenter remains a challenge. Consequently, only a few reports have confirmed the therapeutic efficacy of treatments for complete injuries 24,25. In our previous study, the combination of scaffolds containing trophic factors and hNS/PC transplantation resulted in some improvement; however, these motor improvements were limited because of the presence of scars 25. Thus, considering scar formation as a factor in the treatment of chronic complete SCI, alternative approaches need to be developed.
In chronic SCI, for incomplete injuries, the presence of residual axons allows for the possibility of achieving beneficial effects by targeting the glial scars. Conversely, in cases of complete SCI, no residual axons are present in the central region, which is characterized by a cavitary lesion. Furthermore, fibrous scars are present along the cavitary lesion, with glial scars surrounding them 6,25. Consequently, interventions targeting both types of scars should be established. Several studies have documented positive outcomes from scar resection 26,27. In fact, scar resection reduces inflammation and improves conditions for axonal growth 28,29, providing a more favorable environment for cell transplantation. Therefore, scar resection could be used as a method to enhance the efficacy of hNS/PC transplantation in chronic complete SCI.
Regarding the scaffold for cavitary lesions, our previous study has proven the effectiveness of using a solid scaffold in treating complete SCI 25. However, this scaffold showed clinical limitations when considering the shape of the cavities formed in the spinal cord following the injury. Therefore, the present study focuses on evaluating the decellularized scaffolds as an alternative therapeutic strategy. A decellularized extracellular matrix (ECM) is produced by chemically and physically removing the cells from tissue- or organ-derived matrices via surfactants, leaving behind an ECM scaffold suitable for regenerative medicine applications 30,31. Considering their biological origin, these scaffolds possess high biocompatibility and a reduced risk of immune rejection following the removal of cellular components. Of the numerous studies that focused on evaluating the use of decellularized hydrogels for SCI, majority have used organ-specific neural hydrogels 32,33,34,35,36,37,38. However, concerns about the potential risk of prion diseases associated with animal-derived central nervous system tissues have been considered in clinical applications 39. A report has indicated that when culturing PC12 cells on decellularized neural scaffolds and decellularized kidney scaffolds, better axonal extension was achieved on the decellularized kidney scaffold 40. Based on these findings, we focused on the dECM hydrogel as a potential scaffold for SCI. A previous study has shown that placing dECM hydrogel in resected renal defects promotes host cell infiltration and angiogenesis 41. This study used the dECM hydrogel, considering the possibility that similar effects observed in organs with complex structures could be achieved when applied as a scaffold for SCI.
To address the challenges of scars and cavitary lesions in chronic complete SCI, this study aims to evaluate the therapeutic effects of scar resection combined with dECM hydrogel as a scaffold and hNS/PC transplantation.
Results
The dECM hydrogel facilitates neurite outgrowth
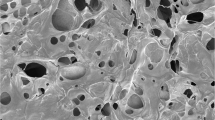
The use of decellularized extracellular matrix as a biomaterial has gained significant attention in tissue engineering and regenerative medicine due to its ability to accurately mimic the natural cellular environment. Based on our previous attempt to induce regeneration using kidney hydrogels, we were inclined to use them to produce a unique composition that may promote nerve regeneration. Initially, the kidney-derived dECM hydrogel was prepared with concentrations of 8 mg/ml and 16 mg/ml (Fig. 1a). The scanning electron microscope (SEM) images revealed that these hydrogels possessed a fibrous morphology with a microporous structure (Fig. 1b). This structure, as previously discussed 41, is conducive to cell infiltration and nutrient permeation. To evaluate the potential beneficial effects of dECM hydrogel as a scaffold in neuronal regeneration, we assessed their impact on neurite outgrowth using DRG (Fig. 1c). Significant axonal growth was observed on Day 5, as confirmed via immunohistochemical analyses using anti-β-III tubulin antibodies. DRG-derived β-III tubulin-positive axons showed considerable elongation on the 8 mg/ml dECM hydrogel compared with the 3 mg/ml collagen I and 16 mg/ml dECM hydrogel (p = 0.047) (Fig. 1d,e). Specifically, the average neurite length was 883.93 ± 61.01 μm on collagen I, 1107.40 ± 39.96 μm on the 8 mg/ml decellularized kidney hydrogel, and 963.80 ± 29.99 μm on the 16 mg/ml dECM hydrogel. Furthermore, these findings suggest that the 8 mg/ml dECM hydrogel provides an optimal microenvironment for promoting neurite extension, making it a promising scaffold for neural tissue engineering applications.
Preparation and characterization of dECM hydrogels and effect of dECM hydrogel substrates on DRG neurite growth. (a) Diagram of the preparation process for kidney-derived dECM hydrogel. (b) SEM images of decellularized kidney hydrogel (8 mg/ml). Scale bar: 5 μm. (c) Experimental design and images of DRG culture on dECM hydrogel. (d) Tuj1-positive DRG neurites visualized using fluorescently stained images on different hydrogels. Scale bar: 200 μm. (e) Quantification of the neurite length of DRG on collagen I (n = 3) and dECM hydrogel (8 mg/ml (n = 4) and 16 mg/ml (n = 3)). One-way ANOVA followed by the Tukey–Kramer test; N.S., not significant; *P < 0.05. Data are expressed as mean ± SEM.
Scar formation prevents the engraftment and migration of transplanted cells
To further investigate its potential in a more complex and clinically relevant setting, we hypothesized that the decellularized kidney hydrogel can provide a conducive environment for the engraftment and migration of hNS/PCs within the injured spinal cord.
To assess the engraftment and migration of hNS/PCs in a chronic transected SCI model, we administered hNS/PCs at three locations within the lesion epicenter and surrounding tissue. Evaluations were performed on groups with cell TP only and those with cell TP + dECM hydrogel (8 mg/ml) injection. The dECM hydrogel was administered as a liquid (8 μl) to the epicenter, immediately followed by the three-point transplantation (Fig. 2a). Three months after SCI, we carefully monitored the engraftment and the migration of the grafted cells via immunohistochemistry. Our initial observation of tissue evaluation revealed that the transplanted cells did not survive in the epicenter in either group, and the STEM121 (detecting human-specific cytoplasmic antigen)-positive fibers derived from the transplanted cells did not migrate to the epicenter (Fig. 2b). The GFAP staining results revealed glial scars, whereas the picrosirius red staining results revealed fibrous scars, suggesting that the fibrous scar tissue particularly influenced the survival and migration of the transplanted cells (Fig. 2c). These observations suggest that hydrogel failed to mitigate the inhibitory effect of the fibrous scars, requiring additional intervention to provide a favorable microenvironment for cell survival and migration, particularly in the regions of the epicenter.
Therapeutic effects of dECM hydrogel injection and iPSC-NSPCs for chronic phase complete transected spinal cord. (a) Experimental design, images of the complete transection model, dECM hydrogel injection, and iPSC-NSPC transplantation in the chronic phase. TP, cell transplantation. (b) Representative STEM121-stained sagittal spinal cord sections at 12 weeks post-transplantation, showing non-viable transplanted cells in the epicenter. The arrows indicate the epicenter. Scale bar, 1000 μm. (c) Immunohistochemical analysis of the magnified boxed area from (b), illustrating the transplanted cells adhering to the cavity, glial scar (GFAP), and fibrous scar (picrosirius red stain). The arrows indicate the epicenter. Scale bar, 1000 μm.
Changes in the spinal cord microenvironment after scar resection
The significant hindrance caused by fibrous scar tissue prompted the inclusion of surgical scar resection in the treatment strategy. To determine the impact of surgical scar resection on the spinal cord microenvironment in the chronic stage of SCI, a series of immunohistochemical analyses were conducted 1 week post scar resection (Fig. 3a). Immunostaining for GFAP and CSPGs using the anti-CS56 antibody was conducted to observe the glial scar edges in both the control and scar resection group (Fig. S1a). Quantitative analysis revealed no significant difference in the CSPGs area, which is known to inhibit axonal regeneration in the CNS 19, between the two groups (Fig. S1b). However, a trend of decreased CSPGs level was observed at the glial scar margins following scar resection. These findings suggest that the scar resection procedure may alter the inhibitory environment at the glial scar termini. Immunostaining for Arg1, a marker associated with microglia and macrophages that exhibit a neuro-regenerative phenotype 16, indicated a significant increase in the Arg1–positive cells in the scar resection group (Fig. 3b). The results suggested that the scar resection procedure not only reduces the inhibitory factors but also enhances the presence of cells that may support regenerative processes. The quantitative data indicated a significant increase in the Arg1/Iba1 ratio in the scar resection group compared with the control group (Fig. 3c; control 0.39% ± 0.09%; scar resection group 8.00% ± 1.47%, p = 0.029).
Therapeutic effects of scar resection: immunohistochemical staining and RNA-seq analysis 7 days post-scar resection in the chronic transected SCI model. (a) Experimental design and images of scar resection in the chronic transected SCI model. (b) Representative midsagittal sections stained for Iba1 and Arg1 in the control and scar resection groups (enlarged images on the right). Scale bars, 1000 μm (left) and 20μm (right). (c) Percentages of the neuroprotective microglia and macrophages (Iba1 and Arg1) in the midsagittal sections (n = 4 each). Mann–Whitney U test; N.S., not significant; *P < 0.05. Data are expressed as mean ± SEM. (d) Heatmap of the axon inhibitor-associated and scar formation-related genes. Scale, z-score. (e) Enriched GO biological process terms increased in the scar resection group.
To gain a better understanding of the molecular changes induced by scar resection, RNA sequencing on the spinal cord tissue samples from both the scar resection and control groups was performed. The PCA of the RNA sequencing data revealed a distinct clustering of samples from the scar resection and control groups, suggesting that scar resection led to significant changes in the gene expression profiles (Fig. S2a). This clear separation in the PCA plot emphasizes the significant influence of scar resection on the spinal cord microenvironment. The DEG analysis identified a total of 71 upregulated and 313 downregulated genes in the scar resection group compared with the control group based on the criteria of raw p of < 0.05 and FC of ≥ 2 or FC ≤ 0.5 (Fig. S2b). Of note, several genes associated with axonal growth inhibition were significantly downregulated in the scar resection group, including Semaphorin3b (Sema3b) (raw p = 0.00022, FC = 0.36), Ephrin-B3 (Efnb3) (raw p = 0.00045, FC = 0.38), roundabout guidance receptor 1 (Robo1) (raw p = 0.002, FC = 0.41), and reticulon 4 receptor (Rtn4r) (raw p = 0.03, FC = 0.41) (Fig. 3d). These genes are known to have critical roles in inhibiting axonal growth, and their downregulation suggests that scar resection may reduce some of the molecular barriers to neural regeneration. Conversely, the genes that were associated with neuroprotection and neural repair were significantly upregulated in the scar resection group. Of these, Arg1 (raw p = 0.00026, FC = 5.23) and NGF (raw p = 0.026, FC = 2.04) showed substantial increases in expression. Arg1 is involved in the anti-inflammatory response and tissue repair, whereas NGF is a critical factor for the survival and growth of neurons 42. The upregulation of these genes further supports the fact that scar resection creates a more favorable microenvironment for cell transplantation. To further investigate the biological processes influenced by scar resection, a GO analysis on the DEGs was conducted and revealed that immune-related biological processes were prominently enriched among the top 10 GO terms (Fig. 3e). These findings are consistent with our immunohistochemical data, suggesting that scar resection not only reduces the inhibitory factors but also enhances the immune responses that are conducive to neural repair.
Scar resection combined with scaffold promotes angiogenesis
While scar resection facilitates the improvement of the spinal cord microenvironment, the excision of scar tissue alone creates a gap within the spinal cord, necessitating an appropriate scaffold for cell transplantation. Consequently, a dECM hydrogel was used as a scaffold to bridge the gap formed following the scar resection. To evaluate the changes in the spinal cord microenvironment one week after scar resection and scaffold placement, a histological assessment was performed (Fig. 4a). Considering that the presence of endothelial cells, which indicates angiogenesis, is important for the engraftment of transplanted cells 43, we evaluated the expression of CD31, a marker for endothelial cells. The CD31 staining results revealed significant increases in blood vascular endothelial cells in the scar resection + scaffold group, suggesting the potential for neovascularization (Fig. 4c). A quantitative analysis was conducted and revealed the following results: control, 0.055 ± 0.005 mm2; scar resection group, 0.077 ± 0.009 mm2; and scar resection + scaffold group, 0.114 ± 0.011 mm2, indicating a statistically significant difference (p = 0.008) (Fig. 4d). These results suggest that the use of decellularized hydrogel after scar resection creates an effective scaffold for cell transplantation. To further evaluate the molecular changes induced by the placement of decellularized hydrogel following scar resection, RNA sequencing on the spinal cord tissue samples from the scar resection + scaffold group was conducted. The RNA sequencing-based PCA analysis results revealed limited clustering between the scar resection group and the scar resection + scaffold group (Fig. S3a), with few DEGs identified (Fig. S3b). Although no significant changes in the expression of the genes associated with angiogenesis were observed, the GO analysis results revealed an increased expression of genes related to angiogenesis in the comparison that included the scar resection + scaffold group (Fig. 4b). These RNA sequencing results support the histological findings and indicate that scar resection with scaffold therapy enhances angiogenesis. However, the lower limb motor function did not improve after scar resection and scaffold treatment (Fig. S4a).
Therapeutic effects of scar resection and dECM hydrogel (scaffold). (a) Experimental design and images of scar resection and dECM hydrogel (scaffold) in the chronic transected SCI model. (b) Heatmap of the angiogenesis-associated genes in the scar resection and scar resection + scaffold groups. Scale, 0 < − log 10|adjusted P| < 1.00. (c) Examination of neovascularization at the epicenter using immunohistochemical staining for CD31. Scale bar, 1000 μm. (d) Quantification of the CD31+ area in the 1000-μm-wide regions at the epicenter (n = 4 each). One-way ANOVA followed by the Tukey–Kramer test; N.S., not significant; *P < 0.05. Data are expressed as mean ± SEM.
Combination of hNS/PC transplantation and scar resection with scaffold
The findings indicating that the improvement of lower limb motor function was not achieved with scar resection + scaffold therapy alone prompted the decision to combine these treatments with hNS/PC transplantation. One week after the initial treatments of scar resection + scaffold therapy, which improved the microenvironment following chronic SCI, hNS/PCs were transplanted to evaluate their potential beneficial effects (Fig. 5a). It was found that the combination of TP with scaffold after scar resection (TP + scaffold) significantly improved the cell survival rates in the lesion center compared with TP alone (Fig. 5b,c, rostral; TP group, 0.160 ± 0.027 mm3; TP + scaffold group, 0.157 ± 0.030 mm3; N.S, epicenter; TP group, 0.010 ± 0.004 mm3; TP + scaffold group, 0.093 ± 0.029 mm3; p = 0.040, caudal; TP group, 0.146 ± 0.020 mm3; TP + scaffold group, 0.197 ± 0.019 mm3, N.S). These observations suggested that combining scar resection and the use of scaffolds can result in successful cell engraftment at the epicenter in chronic complete SCI. Furthermore, the cells engrafted at the epicenter showed differentiation into neurons, astrocytes, and oligodendrocytes (Fig. 5d,e).
Therapeutic effects of iPSC-NSPC transplantation after scar resection and scaffold. (a) Experimental design and images of iPSC-NSPC transplantation after scar resection and scaffold in the chronic phase. (b) Representative immunohistochemical HNA staining of the midsagittal sections. Scale bar, 1000 μm. (c) Quantification of the grafted cell volume in the transplantation and transplantation + scaffold groups (n = 9 each). Mann–Whitney U test; N.S., not significant; *P < 0.05. Data are expressed as mean ± SEM. (d, e) Representative images of the neural cells differentiated from the graft cells (HNA+), showing co-staining with ELAVL3/4 (neurons), GFAP (astrocytes), and APC (oligodendrocytes). Scale bars, 20 μm.
Combined therapy induces host axonal regeneration
This study aims to determine whether axonal regeneration at the epicenter could be achieved by combining scar resection + scaffold therapy with hNS/PC transplantation. To investigate the host axon regeneration, immunohistochemical analyses using anti-NF-H antibodies were conducted. The results revealed an increase in the NF-H–positive neuronal fibers at the lesion center in the TP + scaffold group (Fig. 6a). A quantitative analysis indicated that NF-H–positive fibers were more prominent in the TP + scaffold group after scar resection than in the control and scar resection groups (Fig. 6b; control group, 0.0073 ± 0.0009 mm2; scar resection group, 0.0073 ± 0.0014 mm2; scar resection + scaffold group, 0.0108 ± 0.0027 mm2; TP group, 0.0098 ± 0.0027 mm2; TP + scaffold group, 0.0186 ± 0.0026 mm2; control vs. TP + scaffold, p = 0.047; scar resection vs. TP + scaffold, p = 0.047). The immunohistochemical analyses using the STEM121 and TUJ1 antibodies revealed that the axons, which were presumed to be of host origin, extended along the transplanted cells (Fig. 6c). It can be implied that these results may be attributed to the trophic factors provided by the transplanted cells, as indicated in previous studies 44,45. However, STEM121-positive fibers, which indicate axons derived from transplanted cells, were observed in the rostral spinal cord tissue but not in the lesion center. This suggests that further refinement of the treatment with scar resection + scaffold therapy may be necessary to allow transplanted cells to extend their axons. Furthermore, the BBB scores did not indicate a significant recovery during the observation period (Sup Fig. 4a, b).
Combined therapy promoted the regeneration of neuronal fibers. (a) Examination of the host neurons around the lesion epicenter via immunohistochemical staining for rodent-specific NF-H. Scale bar, 1000 μm. (b) Quantification of the NF-H + area: control group (n = 4), scar resection + PBS group (n = 4), scar resection + scaffold group (n = 4), TP group (n = 5), and TP + scaffold group (n = 5). One-way ANOVA followed by the Tukey–Kramer test; N.S., not significant; *P < 0.05. Data are expressed as mean ± SEM. (c) Immunohistochemical analysis of the magnified arrow area from (b). Representative staining for Tuj and STEM121 in the midsagittal sections. The arrows indicate Tuj1-positive fiber. Scale bar, 200 μm.
The results of the present study indicate that although the combined therapy resulted in improved cell engraftment and host axon regeneration in the severe environment of chronic complete SCI, further improvements in research design are still needed.
Discussion
This study determined the feasibility of integrating scar resection, kidney-derived dECM hydrogel, and hNS/PC transplantation in treating chronic complete SCI. Axonal growth was assessed in vitro to determine the optimal concentration of hydrogel. For chronic complete SCI, dECM hydrogel was used as a scaffold within the cavitary lesion, and cell transplantation was applied. The presence of scar tissue prevented the successful engraftment of the transplanted cells at the injury epicenter. To address this, the surgical removal of these scars reduced the axonal growth inhibitors and promoted the neuroprotective microglia and macrophages, thereby creating an environment conducive to cell transplantation. The improvement in the spinal cord microenvironment, combined with the use of dECM hydrogel that supported angiogenesis, resulted in successful cell engraftment. In particular, the transplanted cells at the lesion epicenter facilitated the host axonal regeneration. Thus, the combination of scar resection and hNS/PC transplantation is considered a promising treatment approach for chronic complete SCI.
The kidney-derived dECM hydrogel used as a scaffold for SCI contains ECM components, such as collagen IV and heparan sulfate proteoglycans 41, which are known to promote axonal growth. Heparan sulfate proteoglycans have been found to initiate axon extension signaling by binding to the PTPσ receptors that also bind to CSPGs, which are known inhibitors of axonal growth 46,47. Thus, they could be considered important components as a scaffold material for chronic SCI. The presence of these components likely contributed to the significant axonal outgrowth observed with the dECM hydrogel compared with collagen I in our in vitro experiments. Moreover, a previous report suggested that axonal extension was significantly enhanced in the decellularized kidney scaffolds, which is consistent with our findings 40. Therefore, our results indicate that kidney-derived dECM hydrogel can be a potential scaffold material for SCI. Compared with previous studies using solid scaffolds 25, the demonstrated advantages of liquid, biocompatible dECM hydrogels, which carry lower clinical risks, suggest that these hydrogels may offer a more practical and clinically feasible alternative for scaffold applications.
The presence of glial and fibrotic scars is considered a contributing factor to the failure of cell engraftment without scar resection. Of note, fibrotic scars impede both the engraftment and migration of transplanted cells. While several treatment options, such as C-ABC, have been shown to be effective in improving glial scars, fibrotic scars cannot be dissolved in the same manner 6,7. Therefore, surgical resection focused on fibrous scars was performed in the present study. Since the complete removal of glial scars can negatively affect axonal growth 48, our focus was on fibrous scars, while performing the partial removal of the glial scars. The RNA sequencing results following scar resection revealed a significant decrease in the expression of genes associated with scar formation and axonal growth inhibitors, such as Robo1, Efnb3, Sema3b, and Rtn4r 6,49. Moreover, a significant upregulation of the neuroprotective markers, including Arg1 and NGF, was observed 42. Consistent with the current findings, previous reports have shown that scar resection effectively alters the distribution of microglia/macrophages, thereby providing a more conducive environment for spinal cord repair 28. Consequently, the scar resection approach has been shown to improve the chronic spinal cord microenvironment and provide a more favorable condition for transplanted cells. Based on the findings of the present study, scar resection is regarded as a promising therapeutic option in clinical practice for the treatment of fibrotic scars, for which definitive treatment protocols have yet to be established 8,9,10,11,12,50. In clinical settings, performing scar resection may present a risk of invasiveness, especially in cases involving extensive scar removal. To secure ethical approval, it is crucial to evaluate the extent of scarring using ultrasound or pharmacological agents and to limit the resection area to a minimum.
In chronic SCI, the survival of transplanted cells is believed to be influenced by scaffold presence and soluble factors from the blood vessel endothelial cells 43. Our study utilized dECM hydrogel as a scaffold in the gap created by scar resection, indicating enhanced blood vessel endothelial cell migration. However, due to the potential inclusion of vascular leakage caused by scar resection, the increase in CD31-positive regions observed in histological analysis alone cannot confirm the reestablishment of the neurovascular unit (NVU). To confirm effective angiogenesis, we examined the Gene Ontology analysis of RNA sequencing. As a result, angiogenesis-related terms that did not increase in the scar resection group alone were found to be upregulated in the scar resection + scaffold group. This supports the effect of the scaffold in promoting angiogenesis. These findings contributed to the significant improvements in the cell engraftment rates at the injury site. Although the transplanted cells successfully engrafted, limitations in cell migration within the scaffold at the epicenter were observed. Additionally, restricted axonal extension originating from the transplanted cells made it challenging to calculate the differentiation ratios. On the other hand, our histological study revealed that the surviving transplanted cells supported the host’s axonal extension. Since previous research has shown that transplanted cells can promote host axon elongation by secreting trophic factors 44,45, a similar stimulation occurred in the present study, contributing to axonal regeneration. In cases of chronic complete SCI where no residual axons remain, encouraging clinical outcomes have been observed through the promotion of host axonal regeneration.
This study has certain limitations. Although the present study observed improvements in the cell engraftment rates and the host’s axonal extension, the recovery of motor function was not achieved. This outcome may be attributed to insufficient graft-derived axonal growth and cell migration at the lesion epicenter where the scaffold was implanted, which likely limited the formation of synapses between the host and graft. Considering that the lesion epicenter is deficient in trophic factors in the present cases of chronic complete SCI, future research should focus on the long-term release of neurotrophic factors or drugs from the scaffold.
In conclusion, combining hNS/PC transplantation with scar resection and a decellularized scaffold may be a prospective treatment approach for chronic SCI. However, further research is needed to improve the functional outcomes.
Methods
Preparation of a kidney-derived dECM hydrogel and collagen I hydrogel
The kidney-derived dECM hydrogel was prepared with minor modifications based on the methods of a previous study 41. Briefly, kidneys derived from minipigs (Oriental Yeast Co., Ltd, Tokyo, Japan) were cut into small pieces, which were further washed in PBS and decellularized with 0.5% sodium dodecyl sulfate (SDS; Fujifilm Wako Pure Chemical Corp.) for 10 h. The decellularized kidney pieces were rinsed with PBS for 4 days at 4 ℃. The rinsing solution was changed four times a day to remove the residual detergent. The tissues were lyophilized and milled into a powder. Moreover, the resulting powder was enzymatically digested in pepsin/HCl solution to prepare a decellularized kidney-derived collagen solution. This solution was further lyophilized and dissolved in water to prepare the hydrogel solutions at concentrations of 8 and 16 mg/ml, respectively. The solution was neutralized to pH 7.4 and subsequently used. Furthermore, collagen I hydrogels (3 mg/ml; Corning, Inc.) were prepared according to the manufacturer’s protocol.
Dorsal root ganglion isolation and ex vivo culture on the hydrogels
DRG were obtained from Sprague-Dawley (SD) rats at postnatal days 1–5 (P1-5) as previously discussed 51. The DRGs with nerve roots were placed in Hank’s Balanced Salt Solution. After removing the nerve roots under a stereoscopic microscope, the DRGs were seeded onto 8-well plates pre-coated on the previous day with collagen I hydrogel or decellularized kidney hydrogel (8 or 16 mg/ml) (n = 3). To coat the 8-well chamber, 60–80 μl of each type of hydrogel was used: collagen I hydrogel, dECM hydrogel (8 mg/ml), and dECM hydrogel (16 mg/ml). The chambers were then incubated at 37°C with 92% humidity and 5% CO2 for 15–30 min to ensure gelation. The DRGs were cultured for 5 days on each hydrogel in a Neurobasal medium (Gibco) supplemented with 100 ng/ml of nerve growth factor (NGF), 2% B27 (Gibco), 1% L-glutamine (Gibco), and 1% penicillin–streptomycin (Gibco). The medium was changed every day.
Immunocytochemistry
To perform the immunocytochemical analysis, the cells were fixed with 4% paraformaldehyde for 15 min and then rinsed three times with phosphate-buffered saline (PBS) for 5 min each. The cells were then permeabilized and blocked with 5% skim milk and incubated with a primary antibody for βIII-tubulin (mouse IgG2b, 1:500; Sigma, Inc.) at 4 °C for 24 h, followed by incubation with the corresponding secondary antibodies: Fluor 488 (1:400; Abcam, Inc.) and Hoechst 33258 (1:1000, Sigma–Aldrich) at 37 °C for 1 h. The immunofluorescence images of the stained sections were obtained using a fluorescence microscope (Leica Microsystems 173 THUNDER Imager Live Cell, LAS X Version: 3.7.5.24914).
Human neural stem/progenitor cells hNS/PC generation from iPSC
A feeder-free human iPSC line (QHJI01s04) was established at the Center for iPS Cell Research and Application from the peripheral blood cells obtained from HLA homozygous healthy donors under xeno-free and feeder-free conditions via the transduction of reprogramming factors (i.e., OCT3/4, SOX2, KLF4, L-MYC, dominant-negative p53, and EBNA1) using episomal vectors. A clonal working cell bank of MCB003, a master cell bank of QHJI01s04, was used for hNS/PC induction. The iPSCs were supported by applying the feeder-free iPSC culture method. The hNS/PC induction was performed using the methods used in previous research 52. Experiments using hiPSCs were approved by the ethics committee of the Keio University School of Medicine (Approval Numbers: 20030092, 20130146). Informed consent was acquired from the donor from whom the hiPSCs were derived, in accordance with the guidelines outlined in the Declaration of Helsinki. All procedures were conducted following the institutional guidelines.
Animals
Adult female SD rats (pregnant rat, Sankyo Labo Service Corporation, INC., Tokyo, Japan), SD rats at postnatal days 1–5 (P–− 5), and adult (8-week-old) female athymic nude rats (F344/NJcl-rnu/rnu; weight, 110–160 g; CLEA Japan, Inc., Tokyo, Japan) were used for these experiments. The rats were assigned randomly in groups of four per cage (24 × 42 × 24 cm), irrespective of their experimental group. The animals were kept under a controlled 12/12-h light/dark cycle with regulated temperature and humidity, and they had free access to food and water. Antibiotics (orbifloxacin; Sumitomo Dainippon Pharma Animal Health, Inc., Osaka, Japan) were administered for 3 days after the surgeries. Urinary retention occurred after spinal cord transection injury, which required bladder expression for 1–2 weeks. Fluid supplementation was provided as needed for rats with poor food intake. All experimental procedures were authorized by the Experimental Animal Care Committee of Keio University, School of Medicine (assurance no. A2022-214, A2022-127) and conducted in compliance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD). Additionally, the study adhered to the ARRIVE guidelines (Animal Research: Reporting of In Vivo Experiments) to ensure transparency and reproducibility in the reporting of methods and results.
Surgical procedures
For surgical treatment, the rats were anesthetized via the subcutaneous injection of 0.4 mg/kg medetomidine hydrochloride, 2 mg/kg midazolam, and 2.5 mg/kg butorphanol. Laminectomy was performed at T10, and the dura mater was opened longitudinally. Complete spinal cord transection at this level was then performed using microscissors. A total of 90 rats were used for the in vivo experiments. The exclusion criteria were as follows: rats displaying a BBB score of ≥ 7 within 6 weeks after SCI, rats that developed soft tissue infections, rats that died. The composition of each group was as described below: TP (+ hydrogel injection) without scar resection series (BBB score of ≥ 7 rats: n = 6, infected rats: n = 1, dead rats: n = 2), control (BBB score of ≥ 7 rats: n = 4), scar resection (BBB score of ≥ 7 rats: n = 3, infected rats: n = 1, dead rats: n = 2), TP (BBB score of ≥ 7 rats: n = 2), TP + scaffold (BBB score of ≥ 7 rats: n = 3, dead rats: n = 1).
Forty-two days after SCI, the hNS/PCs (1 × 106 cells) were transplanted into the lesion epicenter (cell transplantation: TP group) with or without an 8 μl injection of dECM hydrogel (8 mg/ml). The hNS/PCs were washed thrice with PBS and suspended in 2 μl of PBS. Subsequently, 3.3 × 105 cells/2 μl or 2 μl of PBS were injected into three locations (the epicenter and areas 1 mm rostral and caudal to it) using a 27G metal needle and a microstereotaxic injection system (KDS310; Muromachi-Kikai Co., Ltd.). Moreover, the injection depth was 0.6–0.8 mm, whereas the injection speed was 1 μl/min.
For the scar resection model, the scar tissue (1 mm wide at the lesion epicenter) was resected 42 days after SCI using microscissors. Either 8 μl of PBS (scar resection group) or dECM hydrogel (8 mg/ml) was injected into the gap created by the scar resection (scar resection + scaffold group). After the hydrogel administration, the rat was left undisturbed on a 37 °C warming incubator for about 20 min to confirm gelation. In these groups, hNS/PCs (1 × 106 cells) were injected into three locations at the lesion epicenter one week after scar resection. Conversely, the control group used a model without scar resection.
Immunohistochemistry and picrosirius staining
The rats were anesthetized, exsanguinated transcardially with heparinized saline, and euthanized using a 4% PFA solution. The spinal cord tissues were dissected and postfixed in 4% PFA followed by sequential soaking in 10% and 30% sucrose solutions. Subsequently, the tissues were then embedded in a frozen section compound and sectioned at a thickness of 16 μm for the sagittal plane. The sections were then processed for histological analysis using the following primary antibodies: anti-HNA (MAB4383, mouse IgG1, 1:100, Millipore, Inc.), anti-GFAP (16825-1-AP, rabbit IgG, 1:500, Proteintech, Inc.; mouse IgG2a, Thermo Fisher, Inc.), anti-CS56 (C8035, rabbit IgG, 1:200, Sigma–Aldrich), anti-Arg1 (ab60176, goat IgG, 1:400, Abcam, Inc.), anti-Iba1 (019-19741, rabbit IgG, 1:1000, Wako), anti-CD31 (AF3628, goat IgG, 1:100, R&D Systems, Inc.), anti-ELAVL3/4 (Hu C/D) (A21271, mouse IgG2b, 1:500, Molecular Probes, Inc.), anti-APC (ab16794, mouse IgG2b, 1:300, Abcam, Inc.), STEM121 (Y40420, mouse IgG1, 1:200, Takara Bio, Inc.), anti-NF-H (rodent-specific) (ab8135, rabbit IgG, 1:500, Abcam, Inc.), and anti-βIII-tubulin (mouse IgG2b, 1:500, Sigma, Inc.). The sections were then incubated with Alexa Fluor-conjugated secondary antibodies (1:400; Abcam, Inc.) and Hoechst 33258 (1:1000, Sigma–Aldrich). Picrosirius staining was performed using a staining kit (ScyTek Laboratories, Inc.). The images were captured using a fluorescence microscope (Leica Microsystems 173 THUNDER Imager Live Cell (LAS X Version: 3.7.5.24914) and BZ-X710 (Keyence, Osaka, Japan)) or confocal laser-scanning microscope (LSM 780; Carl Zeiss, Jena, Germany). All the image analyses were conducted using the ImageJ software (version 2.1.0/1.53c).
Behavior analysis of the hindlimb locomotor functions
The motor function of the rats’ lower extremities was assessed weekly until 42 days after transplantation using the Basso–Beattie–Bresnahan (BBB) scale 53. Behavioral analysis was conducted by two investigators who were blinded to the experimental groups.
RNA-seq analysis
Three mm-long dissected spinal cord samples that were collected one week after scar resection (49 days after SCI) were used for the analysis, and the samples were pooled in the control (PBS, n = 3), scar resection (scar resection + PBS, n = 4), and scar resection + scaffold (scar resection + dECM hydrogel, n = 4) groups (total: n = 11). The total RNA was extracted using the RNeasy Mini Kit (Qiagen) according to the manufacturer’s instructions, with slight modifications. The RNA from each sample was purified, and the mRNA libraries were prepared following the TruSeq Stranded mRNA LT Sample Prep Kit (Illumina, San Diego, CA, United States) protocol and sequenced on the HiSeq 2500 System (Illumina) to obtain single-end reads. Raw reads were trimmed for quality and read length using Trimmomatic 0.38. Read mapping to the reference genome (rn6) was performed, and transcript counts were obtained using StringTie version 2.1.3b. The read count of each sample was normalized to fragments per kilobase of the transcript per million mapped reads (FPKM) and transcripts per kilobase million (TPM). A differentially expressed gene (DEG) analysis was conducted on two comparison pairs as requested using DESeq2. The raw count data were normalized with DESeq2’s geometric mean-based size factors, and statistical analysis was carried out using the log2 fold change (FC) and Wald test for each pair. Significant results were identified based on a FC of ≥ 2 or ≤ 0.5 and a raw p value of < 0.05 from the Wald test.
GO enrichment analysis
In this study, GO enrichment analysis was performed using the gProfiler tool (g:GOSt: e111_eg58_p18_f463989d). The genes with an adjusted p value below 0.05 were considered significant using the Bonferroni test.
Quantification of staining
Immunohistochemical staining across all sections was quantified using ImageJ software, with threshold values kept constant throughout all analyses by observers blinded to the experimental groups and conditions. Moreover, in each DRG on the hydrogel, the five longest neurites were measured and averaged. CSPG was evaluated by measuring the CS56-positive area in the sagittal sections (2000 μm-wide regions). The average numbers of the microglia/macrophages were calculated in the sagittal sections (2000 μm-wide regions). Neovascularization was evaluated by measuring the CD31-positive area in the sagittal sections (1000 μm-wide regions). The graft volume was estimated by assessing the HNA + area. Based on the previously reported methods 54, both graft and lesion volumes were measured in entire sagittal sections. The number of regenerating host neurons positive for rodent-specific NF-H at the injury epicenter was quantified in the sagittal sections (1000 μm-wide regions). For the calculation of graft volume, we analyzed all sagittal sections to ensure accurate volume measurements. Quantification of other tissues was performed using the midsagittal section. In cases where the total number of sections was even, or when quantification on the midsagittal section was not feasible, the adjacent section was used for analysis.
Experimental designs and statistical analysis
All statistical analyses were conducted using SPSS Statistics (version 26.0.0.0; Japan IBM, Tokyo, Japan). Data were expressed as mean ± SEM. The in vitro and in vivo immunohistochemistry (IHC) staining results of three and five groups were compared using the one-way ANOVA and Tukey–Kramer test, respectively. The in vivo immunohistochemistry (IHC) staining results of two groups were compared using the Mann–Whitney U tests. Statistical significance was set at a P value of < 0.05 (as *P < 0.05).
Data availability
The datasets generated and/or analysed during the current study are available in the GEO repository, under accession number GSE278491.
References
Nagoshi, N. et al. Phase I/II study of intrathecal administration of recombinant human hepatocyte growth factor in patients with acute spinal cord injury: A double-blind, randomized clinical trial of safety and efficacy. J. Neurotrauma 37, 1752–1758 (2020).
Honmou, O. et al. Intravenous infusion of auto serum-expanded autologous mesenchymal stem cells in spinal cord injury patients: 13 case series. Clin. Neurol. Neurosurg. 203, 106565 (2021).
Fehlings, M. G. et al. Rho inhibitor VX-210 in acute traumatic subaxial cervical spinal cord injury: Design of the SPinal Cord Injury Rho INhibition InvestiGation (SPRING) Clinical Trial. J. Neurotrauma 35, 1049–1056 (2018).
Grossman, R. G. et al. A prospective, multicenter, phase I matched-comparison group trial of safety, pharmacokinetics, and preliminary efficacy of riluzole in patients with traumatic spinal cord injury. J. Neurotrauma 31, 239–255 (2014).
Sugai, K. et al. First-in-human clinical trial of transplantation of iPSC-derived NS/PCs in subacute complete spinal cord injury: Study protocol. Regen. Ther. 18, 321–333 (2021).
Hashimoto, S., Nagoshi, N., Nakamura, M. & Okano, H. Regenerative medicine strategies for chronic complete spinal cord injury. Neural Regeneration Res. 19, 818–824 (2024).
Li, X. et al. Comparison of subacute and chronic scar tissues after complete spinal cord transection. Exp. Neurol. 306, 132–137 (2018).
Dias, D. O. et al. Pericyte-derived fibrotic scarring is conserved across diverse central nervous system lesions. Nat. Commun. 12, 5501 (2021).
Dias, D. O. et al. Reducing pericyte-derived scarring promotes recovery after spinal cord injury. Cell 173, 153-165.e22 (2018).
Göritz, C. et al. A pericyte origin of spinal cord scar tissue. Science 333, 238–242 (2011).
Dorrier, C. E. et al. CNS fibroblasts form a fibrotic scar in response to immune cell infiltration. Nat. Neurosci. 24, 234–244 (2021).
Sofroniew, M. V. Inflammation drives fibrotic scars in the CNS. Nat. Neurosci. 24, 157–159 (2021).
Barnett, S. C. & Linington, C. Myelination: Do astrocytes play a role?. Neuroscientist 19, 442–450 (2013).
Bradbury, E. J. & Burnside, E. R. Moving beyond the glial scar for spinal cord repair. Nat. Commun. 10, 3879 (2019).
Pearson, C. S., Mencio, C. P., Barber, A. C., Martin, K. R. & Geller, H. M. Identification of a critical sulfation in chondroitin that inhibits axonal regeneration. Elife 7, (2018).
Nishimura, S. et al. Time-dependent changes in the microenvironment of injured spinal cord affects the therapeutic potential of neural stem cell transplantation for spinal cord injury. Mol. Brain 6, 3 (2013).
Tran, A. P., Warren, P. M. & Silver, J. The biology of regeneration failure and success after spinal cord injury. Physiol. Rev. 98, 881–917 (2018).
Keirstead, H. S. et al. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J. Neurosci. 25, 4694–4705 (2005).
Ikegami, T. et al. Chondroitinase ABC combined with neural stem/progenitor cell transplantation enhances graft cell migration and outgrowth of growth-associated protein-43-positive fibers after rat spinal cord injury. Eur. J. Neurosci. 22, 3036–3046 (2005).
Nori, S. et al. Human oligodendrogenic neural progenitor cells delivered with chondroitinase ABC facilitate functional repair of chronic spinal cord injury. Stem Cell Rep. 11, 1433–1448 (2018).
Yoshida, T. et al. Chronic spinal cord injury regeneration with combined therapy comprising neural stem/progenitor cell transplantation, rehabilitation, and semaphorin 3A inhibitor. eNeuro 11, (2024).
Shibata, T. et al. Rehabilitative training enhances therapeutic effect of human-iPSC-derived neural stem/progenitor cells transplantation in chronic spinal cord injury. Stem Cells Transl. Med. 12, 83–96 (2023).
Shinozaki, M. et al. Combined treatment with chondroitinase ABC and treadmill rehabilitation for chronic severe spinal cord injury in adult rats. Neurosci. Res. 113, 37–47 (2016).
Li, X. et al. Transplantation of hUC-MSCs seeded collagen scaffolds reduces scar formation and promotes functional recovery in canines with chronic spinal cord injury. Sci. Rep. 7, 43559 (2017).
Hashimoto, S. et al. Microenvironmental modulation in tandem with human stem cell transplantation enhances functional recovery after chronic complete spinal cord injury. Biomaterials 295, 122002 (2023).
Huang, L. et al. Anisotropic alginate hydrogels promote axonal growth across chronic spinal cord transections after scar removal. ACS Biomater. Sci. Eng. 6, 2274–2286 (2020).
Zhao, C. et al. Chronic spinal cord injury repair by NT3-chitosan only occurs after clearance of the lesion scar. Signal Transduct. Target Ther. 7, 184 (2022).
Nakamura, M., Houghtling, R. A., MacArthur, L., Bayer, B. M. & Bregman, B. S. Differences in cytokine gene expression profile between acute and secondary injury in adult rat spinal cord. Exp. Neurol. 184, 313–325 (2003).
Liu, B., Liu, G., Li, C., Liu, S. & Sun, D. Resection of scar tissue in rats with spinal cord injury can promote the expression of βIII-tubulin in the injured area. World Neurosurg. 170, e115–e126 (2023).
Yagi, H. & Kitagawa, Y. Decellularized scaffold as a platform for novel regenerative therapy. Nihon Geka Gakkai Zasshi 113, 419–423 (2012).
Tajima, K. et al. An organ-derived extracellular matrix triggers in situ kidney regeneration in a preclinical model. NPJ Regen. Med. 7, 18 (2022).
Ikegami, Y. & Ijima, H. Decellularization of nervous tissues and clinical application. Adv. Exp. Med. Biol. 1345, 241–252 (2021).
Jiang, W. et al. Decellularized extracellular matrix in the treatment of spinal cord injury. Exp. Neurol. 368, 114506 (2023).
Tukmachev, D. et al. Injectable extracellular matrix hydrogels as scaffolds for spinal cord injury repair. Tissue Eng. Part A 22, 306–317 (2016).
Hong, J. Y. et al. Decellularized brain matrix enhances macrophage polarization and functional improvements in rat spinal cord injury. Acta Biomater. 101, 357–371 (2020).
Sun, J.-H. et al. Decellularization optimizes the inhibitory microenvironment of the optic nerve to support neurite growth. Biomaterials 258, 120289 (2020).
Cerqueira, S. R. et al. Decellularized peripheral nerve supports Schwann cell transplants and axon growth following spinal cord injury. Biomaterials 177, 176–185 (2018).
Xu, Y. et al. Understanding the role of tissue-specific decellularized spinal cord matrix hydrogel for neural stem/progenitor cell microenvironment reconstruction and spinal cord injury. Biomaterials 268, 120596 (2021).
Hammarström, P. & Nyström, S. Porcine prion protein amyloid. Prion 9, 266–277 (2015).
Ye, K. et al. In vitro study of decellularized rat tissues for nerve regeneration. Front. Neurol. 13, 986377 (2022).
Kushige, H. et al. Injectable extracellular matrix hydrogels contribute to native cell infiltration in a rat partial nephrectomy model. J. Biomed. Mater. Res. B Appl. Biomater. 111, 184–193 (2023).
Zhang, H. et al. Nerve growth factor improves functional recovery by inhibiting endoplasmic reticulum stress-induced neuronal apoptosis in rats with spinal cord injury. J. Transl. Med. 12, 130 (2014).
Shen, Q. et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science 304, 1338–1340 (2004).
Nori, S. et al. Long-term safety issues of iPSC-based cell therapy in a spinal cord injury model: Oncogenic transformation with epithelial-mesenchymal transition. Stem Cell Rep. 4, 360–373 (2015).
Kawabata, S. et al. Grafted human iPS cell-derived oligodendrocyte precursor cells contribute to robust remyelination of demyelinated axons after spinal cord injury. Stem Cell Rep. 6, 1–8 (2016).
Sakamoto, K., Ozaki, T., Suzuki, Y. & Kadomatsu, K. Type IIa RPTPs and glycans: Roles in axon regeneration and synaptogenesis. Int. J. Mol. Sci. 22, (2021).
Hill, J. J., Jin, K., Mao, X. O., Xie, L. & Greenberg, D. A. Intracerebral chondroitinase ABC and heparan sulfate proteoglycan glypican improve outcome from chronic stroke in rats. Proc. Natl. Acad. Sci. USA 109, 9155–9160 (2012).
Anderson, M. A. et al. Astrocyte scar formation aids central nervous system axon regeneration. Nature 532, 195–200 (2016).
Fournier, A. E., GrandPre, T. & Strittmatter, S. M. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature 409, 341–346 (2001).
Holl, D. et al. Distinct origin and region-dependent contribution of stromal fibroblasts to fibrosis following traumatic injury in mice. Nat. Neurosci. https://doi.org/10.1038/s41593-024-01678-4 (2024).
Sabouni, F., Firouzi, M., Taghikhani, M., Ziaee, A. A. & Semnanian, S. Neurite outgrowth of dorsal root ganglia is delayed and arrested by aspirin. Biochem. Biophys. Res. Commun. 248, 165–167 (1998).
Kamata, Y. et al. A robust culture system to generate neural progenitors with gliogenic competence from clinically relevant induced pluripotent stem cells for treatment of spinal cord injury. Stem Cells Transl. Med. 10, 398–413 (2021).
Basso, D. M. et al. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J. Neurotrauma 23, 635–659 (2006).
Renault-Mihara, F. et al. Beneficial compaction of spinal cord lesion by migrating astrocytes through glycogen synthase kinase-3 inhibition. EMBO Mol. Med. 3, 682–696 (2011).
Acknowledgements
We appreciate the assistance and instruction provided by Drs. T. Kitagawa, M. Kawai, K. Ago, T. Shibata, T. Nishijima, T. Yoshida, R. Ogaki, A. Toga, Y. Ichihara, T. Shimizu, J. I, all of whom are members of the Spinal Cord Research Team at the Department of Orthopedic Surgery and Physiology, Keio University School of Medicine, Tokyo, Japan. We thank Prof. S. Yamanaka (Kyoto University, Kyoto, Japan) for the human iPSC clones. We also thank the JSR-Keio University Medical and Chemical Innovation Center (JKiC) for providing the decellularized hydrogel. We also thank K. Yasutake, T. Kobayashi, Y. Sato, S. Kawashima, M. Akizawa, and T. Harada for their assistance with the experiments and animal care.
Funding
This research was supported by the following grants: Research Center Network for Realization of Regenerative Medicine by AMED Japan (grant Nos. JP24ym0126118, 24bm1223008, 23hk0102089h0001), the General Insurance Association of Japan Medical Research Grants 2023 and the Japan Society for the Promotion of Science (JSPS) (KAKENHI grant number 23K24464 to N.N).
Author information
Authors and Affiliations
Contributions
Conceptualization, K.I., M.S., S.H., Y.S., Y.S., T.T., K.N., H.Y., S.S., Y.K., H.O., M.N., J.K., and N.N.; Methodology, M.S., K.N., H.Y., J.K., and N.N.; Investigation, S.H., Y.S., Y.S., and T.T., Resources, K.I., M.S., K.N., H.Y., S.S., Y.K., H.O., M.N., J.K., and N.N.; Data Curation, J.K., M.S., K.N., H.Y., and S.S.; Writing – Original Draft, K.I., J.K., and N.N.; Editing, K.I., M.S., K.N., H.Y., S.S., Y.K., H.O., M.N., J.K., and N.N.; Supervision, K.I., H.O., M.N., J.K., and N.N.; Project Administration, K.I., H.O., M.N., J.K., and N.N.; Funding Acquisition, K.I., H.O., M.N., J.K., and N.N.; All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ito, K., Shinozaki, M., Hashimoto, S. et al. Histological effects of combined therapy involving scar resection, decellularized scaffolds, and human iPSC-NS/PCs transplantation in chronic complete spinal cord injury. Sci Rep 14, 31500 (2024). https://doi.org/10.1038/s41598-024-82959-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-82959-7