Abstract
After severe infection in osteomyelitis patients in the Intensive Care Unit (ICU), there’s a higher risk of mortality. However, limited research exists on predicting prognosis. Develop a predictive model for 1-year mortality risk in ICU-admitted osteomyelitis patients to inform clinical diagnosis and treatment. MIMIC IV database was used to retrieve ICU data for osteomyelitis patients. The data were randomly split into training and validation sets (7:3 ratio). Univariate and multiple logistic regression identified independent predictors of one-year mortality and constructed a risk prediction nomogram in the training set. Predictive value of the nomogram was assessed using C-indexes, ROC curves, DCA, CIC and calibration curves. This study included a total of 1153 osteomyelitis patients, with 137 deaths within one year. These patients were randomly divided into training (N = 807) and validation (N = 346) sets. In the training set, multiple features were identified as key predictors of one-year mortality in osteomyelitis patients in the ICU. These factors were incorporated into the nomogram model, demonstrating good identification performance, with AUCs of 0.872 and 0.826 for the training and validation sets, respectively. The calibration curve and ROC curve indicate excellent predictive accuracy. DCA suggests strong clinical utility and robust predictive efficiency. Further analysis through CIC illustrates the clinical effectiveness of this predictive model. We have developed a nomogram model to predict the 1-year mortality rate for osteomyelitis patients admitted to the ICU, providing valuable predictive information for clinical decision-making.
Similar content being viewed by others
Introduction
Osteomyelitis presents a significant challenge in the fields of orthopedics and trauma surgery1. It is an inflammatory response involving bone tissue destruction, initiated by infectious factors2. The classification of osteomyelitis includes implant-related osteomyelitis (encompassing prosthetic joint infections and infections around internal fixation in the spine), infection related to fractures, acute hematogenous osteomyelitis, infections in diabetic foot ulcers, septic arthritis, and primary osteomyelitis3.
Literature reports indicate that before the introduction of antibiotic therapy, the mortality rate for severe infections of osteomyelitis reached as high as 20%, with an incidence rate of 45–50%4. It wasn’t until the advent of systemic antibiotic treatment, coupled with surgical interventions, after 1945 that these figures rapidly declined. Systemic antibiotic therapy has reduced the occurrence of severe sepsis caused by osteomyelitis. In recent years, the incidence of osteomyelitis has continued to rise, attributed to the increasing prevalence of Methicillin-Resistant Staphylococcus aureus (MRSA) infections, surgical implants, and a surge in traumatic injuries5,6. Research indicates that over the past 20 years, the incidence of pediatric osteomyelitis has increased by 2.8 times7. In the United States, there are 22 cases per 100,000 people annually, and the incidence has been steadily rising over time. This trend is particularly notable among the elderly and diabetic patients, with the number of admissions to the Intensive Care Unit (ICU) also increasing year by year8. When severe infections in patients cannot be controlled, continuous necrosis of bones and tissues occurs, leading to further disruption of the internal environment with inflammation factors, electrolyte imbalances, and an exacerbation of systemic inflammatory response. This progression can result in sepsis accompanied by multiple organ failure, ultimately posing a life-threatening situation9. Therefore, early identification of high-risk factors for adverse outcomes in severely ill osteomyelitis patients in the Intensive Care Unit (ICU) is essential. Clinical prediction models can provide clinicians with effective information for identifying high-risk patients, making clinical decisions, and implementing strategies in a timely manner.
However, there is currently limited research on predicting the one-year mortality rate of severely ill osteomyelitis patients in the Intensive Care Unit (ICU). Therefore, this study has established a model to predict the occurrence of one-year mortality in severely ill osteomyelitis patients in the ICU. The model exhibits good predictive performance and can provide effective predictive information.
Materials and methods
Sources of data
This study is a retrospective observational investigation that extracted medical records of osteomyelitis patients from the MIMIC-IV database. Clinical information of patients hospitalized in the ICU from 2008 to 2019 was retrieved from the MIMIC-IV database, which is a publicly available database of patients admitted to the Beth Israel Deaconess Medical Center (BIDMC) ICU and is freely accessible to researchers. After completing the online training course, the Collaborative Institutional Training Initiative, the authors received a certificate (No. 38821147) and access to the clinical database. The MIMIC database is a publicly available anonymised database, and ethical committee approval was not required.
Data collection and definitions
This study extracted the following information: demographic details, vital signs, complications, laboratory parameters, treatment, and severity scores. Demographic information included demographic characteristics such as gender, age, BMI, height, weight, marital status, race, admission and discharge times, time of death, and duration of ICU stay. Vital signs encompassed systolic blood pressure, diastolic blood pressure, mean arterial pressure, oxygen saturation, heart rate, respiratory rate (RR), temperature, and blood glucose. Complications included hypertension, diabetes, chronic obstructive pulmonary disease (COPD), kidney injury, and sepsis. Laboratory parameters encompassed white blood cell count (WBC), neutrophil-to-lymphocyte ratio, hemoglobin, albumin, platelet count, lactate, creatinine, international normalized ratio (INR), PaO2, partial pressure of carbon dioxide (PCO2), C-reactive protein (CRP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), calcium, chloride, potassium, sodium, magnesium, red cell distribution width, wound microbiota detection, and others. Severity scores included the Sequential Organ Failure Assessment (SOFA) score. Treatments included mechanical ventilation and antibiotic use. All data were collected within the first 24 h of the patient’s admission to the ICU.
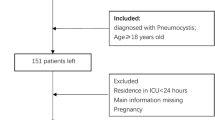
Inclusion criteria: (1) Diagnosis of osteomyelitis; (2) First admission to the ICU. Exclusion criteria: (1) ICU stay less than 24 h. The primary outcome was the all-cause mortality rate within one year, determined by the admission time and recorded death time in the database. For patients with multiple admissions, we used data from the first hospitalization; for repeated examinations, data from the first examination within the first 24 h of admission were used.
Missing data handling
Due to the lack of consensus on a universally accepted percentage for missing values, previous studies have used thresholds of 60% and 70% to define missing data. In our investigation, however, we have chosen a threshold of 50%. Before fitting each model, we assumed that the missing data adhered to a Missing At Random (MAR) pattern. In this research, patients or variables with more than 50% missing values were excluded from the analysis. Subsequently, the missing values in the database were addressed using the "norm. predict" method in Multivariate Imputation by Chained Equation (MICE), wherein each variable is imputed conditionally on all other variables. This approach helped to mitigate the impact of missing data and ensured a more comprehensive and reliable analysis of the dataset.
Statistical analysis
Data extraction and processing were performed using the PostgreSQL database management system, and statistical analysis was conducted using R software (version 4.2.3). The extracted patients were randomly divided into two groups: a training set and a validation set, with a ratio of 7:3. All data extracted from the database were compared between the survival and death groups, as well as between the training and validation sets. Univariate logistic regression analysis was employed to identify predictors of one-year mortality. A multiple logistic regression model with a stepwise regression approach was established using these variables. The goodness of fit for the multiple logistic regression model was assessed using the Hosmer–Lemeshow (HL) test. Finally, a nomogram model with statistically significant parameters was constructed based on the results of the multiple logistic regression.
ROC curves were plotted for the training set to assess the discriminative ability of the nomogram model, and the area under the ROC curve (AUC) was used for evaluation. Calibration curves were generated to assess the consistency between the actual and predicted occurrence rates. Additionally, the calibration was further evaluated using 1000 bootstrap resamples to calculate the corrected concordance index (c-index). Furthermore, Decision Curve Analysis (DCA) was employed to assess the clinical utility of the nomogram model, and Clinical Impact Curve (CIC) analysis was performed to illustrate the clinical effectiveness of the predictive model. The effectiveness of the nomogram was further validated using the validation set.
The normality of continuous variables was assessed using the Shapiro–Wilk test. As the continuous variables did not follow a normal distribution, they were described using the median (interquartile range) [M(Q1, Q₃)]. Between-group comparisons for continuous variables were conducted using non-parametric tests (Mann–Whitney U test or Kruskal–Wallis test). Categorical variables were presented as counts (percentages), and between-group comparisons were performed using the Pearson chi-square test.
Results
Characteristics of the study cohort
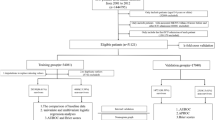
The flow chart of the study is shown in Fig. 1. From the database, 4865 cases of osteomyelitis patients were selected. After excluding non-first-time admissions (N = 2238) and patients with ICU stays less than 24 h (N = 1474), the remaining 1153 patients were included in the study, with 137 deaths and 1016 survivors within one year. There were 712 male and 441 female patients, with a median age of 64.2 [54.9; 72.8] years in the survival group and 73.8 [65.1; 81.7] years in the death group. The characteristics of the death and survival groups are compared in Table 1. Following a 7:3 ratio, the study subjects were randomly divided into a training set (N = 807) and a validation set (N = 346), with both groups having similar characteristics (Supplementary Table 1), supporting their use for development and validation. The median ages in the training and validation sets were 66.1 [56.5; 74.7] years and 63.5 [55.2; 72.9] years, respectively, with one-year mortality rates of 12.6% (102 cases) and 10.1% (35 cases), respectively.
Results of feature selection
We performed univariate logistic regression analysis on the training set to identify factors associated with one-year mortality. Subsequently, using a stepwise backward logistic regression method, we determined independent predictive variables. After excluding variables with poor predictive performance or significant multicollinearity, the following variables with prognostic significance were retained: age, heart rate, systolic blood pressure (SBP), mean blood pressure (MBP), albumin, red cell distribution width (RDW), platelet count (PLT), ICU duration (Los_icu), sepsis, and International Normalized Ratio (INR) (Table 2).
Construction and validation of nomogram
We incorporated the significant predictive factors from the multiple logistic regression analysis of the training set into the nomogram for the prognosis of severe osteomyelitis patients (Fig. 2). The nomogram includes 11 significant predictive factors, and the model’s C-index is 0.867. The model’s effectiveness was confirmed by the Hosmer–Lemeshow test (P = 0.167 > 0.05). By summing the scores for each variable in the nomogram, a total score was obtained, corresponding to the probability of one-year mortality in the predictive model. Validation of the model was conducted through the drawing of ROC curves, calibration curves, Decision Curve Analysis (DCA), and Clinical Impact Curve (CIC). The ROC curve analysis results (Fig. 3) showed that the AUC for the training set and validation set were 0.872 (95% CI 0.836–0.908) and 0.826 (95% CI 0.755–0.898), respectively, indicating that the model has good discriminative ability.
In the calibration curve, the y-axis represents the actual occurrence probability in the study set, and the x-axis represents the estimated probability by the model. As shown in Fig. 4, the estimated probabilities align well with the actual values, demonstrating good consistency. In the Decision Curve Analysis (DCA) (Fig. 5a), the gray diagonal line represents the scenario where all patients receive intervention, and the gray parallel line represents the scenario where no patients receive intervention. In the DCA of the training set, we compared three models: DCA-train1 model included sepsis, RDW2, age, RDW; DCA-train2 model included sepsis, RDW2, age, RDW, Heart Rate, SDP, MDP; DCA-train3 model included sepsis, RDW2, age, RDW, Heart Rate, SDP, MDP, Albumin, Platelets, INR, Los_icu.
In the validation set’s Decision Curve Analysis (DCA), the same three models were employed, as shown in Fig. 5b. Our models demonstrated a substantial net benefit in both datasets. Clinical Impact Curve (CIC) analysis for the training set and validation set further illustrated the clinical effectiveness of the predictive model. When the threshold probability exceeded 70%, the predictive model identified individuals as a high-risk group, closely matching the actual occurrence population, confirming the extremely high clinical effectiveness of the predictive model (Fig. 6).
Discussion
Osteomyelitis, named in 1844, is one of the oldest known diseases in the scientific community. It is an inflammatory response process characterized by the destruction of bone tissue caused by infectious agents1,3. This infection may arise from the bloodstream, neighboring infectious foci, or even as a result of direct bacterial inoculation through traumatic mechanisms. Osteomyelitis can manifest in a single bone tissue or simultaneously involve the bone marrow, bone, periosteum, and surrounding soft tissues. In severe cases, the infection intensifies and spreads, leading to a worsening inflammatory response. As the infection progresses, the inflammatory lesions in osteomyelitis and the inflammatory response further intensify. The continuous necrosis of bones and tissues, along with the disturbance of inflammatory mediators and electrolyte balance, results in sepsis accompanied by multiple organ failure, ultimately posing a life-threatening risk10,11.
Osteomyelitis poses a significant public health challenge, primarily associated with adverse outcomes1. Early identification of osteomyelitis patients in the critical stage is crucial for their prognosis. Currently, there is no unified standard for the early identification of critically ill patients, leading to delayed management and potentially negative impacts on clinical outcomes. Therefore, this study establishes and validates, for the first time, a predictive model for the one-year mortality risk of critically ill osteomyelitis patients admitted to the ICU. As a visual model, the nomogram quantifies the impact of each predictive variable on the outcome. The nomogram model is simple, applicable, and facilitates better and more effective clinical decision-making12.
In this study, we retrospectively analyzed data from 1153 patients with osteomyelitis admitted to the ICU. Based on demographic information, laboratory test indicators, and complications of these patients, we employed univariate and multiple logistic regression to identify independent risk factors. Subsequently, a nomogram predicting the one-year mortality risk of critically ill osteomyelitis patients was constructed. The nomogram model included 11 significant predictive factors: age, heart rate, systolic blood pressure (SBP), mean blood pressure (MBP), albumin, red cell distribution width (RDW), platelet count (PLT), ICU duration (LOS), sepsis, International Normalized Ratio (INR), and RDW < 14.5%. The nomogram model based on these variables demonstrated an AUC greater than 0.8 in both the training set and the validation set, indicating good predictive accuracy.
Our results indicate that age, heart rate, systolic blood pressure (SBP), mean blood pressure (MBP), albumin, red cell distribution width (RDW), platelet count (PLT), ICU duration (LOS_ICU), and International Normalized Ratio (INR) are important predictors of one-year mortality in osteomyelitis patients in the ICU. Additionally, patients with osteomyelitis who also have sepsis are at a higher risk of mortality.
Age is a significant prognostic factor for severe osteomyelitis patients8,13. According to the literature, patient age is closely associated with higher mortality, more adverse events, and prolonged hospitalization14,15. The risk of mortality increases with age, with patients aged 80 and above having an odds ratio (OR) of 1.81. In this study, we found that age is an independent risk factor for the prognosis of severe osteomyelitis patients (OR = 1.073, 95% CI 1.05–1.097, P < 0.001). As age increases, patients experience a decline in physiological function and immune capacity, an increase in the number of comorbidities, and reduced resilience to stress from trauma, anesthesia, and surgery. This results in a higher incidence of complications and an increased mortality rate following osteomyelitis.
When osteomyelitis infection becomes severe and leads to systemic inflammatory response syndrome (SIRS), changes in heart rate and blood pressure may become more pronounced. Research suggests that abnormalities in blood pressure indices and heart rate are independent risk factors for severe infections, significantly associated with patient mortality and ICU hospitalization duration. They are also important indicators for determining the prognosis of patients with severe infections19,20. Our study confirmed that heart rate (OR = 1.021, 95%CI 1.003–1.039, P = 0.021), systolic blood pressure (OR = 0.968, 95%CI 0.942–0.994, P = 0.017), and mean arterial pressure (OR = 1.075, 95% CI 1.004–1.156, P = 0.042) are independent risk factors for the prognosis of severe osteomyelitis patients.
Reduced serum albumin levels are commonly observed in critically ill patients21. As a multifunctional blood component, albumin plays a crucial role in maintaining plasma osmotic pressure and capillary permeability. It serves as a carrier and ligand for both endogenous and exogenous compounds, and also exhibits anti-inflammatory, antioxidant, and antiplatelet aggregation effects22. Numerous international studies indicate that hypoalbuminemia decreases disease tolerance in patients and is an independent risk factor for increased in-hospital and post-discharge mortality, as well as poor prognosis in critically ill patients23,24,25,26. In our study, a statistically significant difference in albumin levels was observed between the deceased and surviving groups (p < 0.05). Single-factor logistic regression analysis indicated that albumin is a risk factor for the one-year mortality rate in severe osteomyelitis patients. After adjusting for confounding factors in the multiple logistic regression analysis, albumin (OR = 0.359, 95% CI 0.241–0.525, P < 0.001) remained an independent risk factor. As the condition worsens, the synthesis of albumin by the liver significantly decreases. The longer the duration of low albumin levels, the more severe the condition, leading to a poorer prognosis.
In this study, we also found that red cell distribution width (RDW) (OR = 1.183, 95% CI 1.008–1.388, P = 0.039) is associated with the one-year mortality rate in severe osteomyelitis patients, with a lower one-year mortality rate observed in patients with RDW < 14.5%. Studies have indicated that a higher RDW is predictive of an increased risk of mortality in patients with severe infections27,28. The elevation of RDW has been linked to elevated inflammatory markers such as C-reactive protein, erythrocyte sedimentation rate, and interleukins29. Inflammatory cytokines can influence the survival of red blood cells in circulation, promoting deformability of red blood cell membranes, inhibiting red blood cell maturation, leading to the entry of younger and larger reticulocytes into the peripheral circulation, thereby increasing RDW30,31. This may be the reason for the elevated red cell distribution width (RDW) in severe osteomyelitis patients with systemic infections. RDW has also emerged as an important predictive factor for mortality in patients with severe infections.
Coagulation dysfunction is commonly present in patients with severe infections. In the early stages of infection, the body’s inflammatory response activates the coagulation system disparately. The inflammatory response and coagulation reactions are closely interconnected, mutually promoting each other, thereby initiating a cascade reaction in the coagulation system, leading to coagulation dysfunction32,33. The coagulation dysfunction in patients with severe infections is a dynamic and continuous pathological process. The pathogenesis involves an imbalance driven by the upregulation of procoagulant mechanisms, downregulation of natural anticoagulant mechanisms, and inhibition of the fibrinolysis system. This imbalance results in microvascular fibrin deposition, thrombus formation, microcirculatory dysfunction, and may ultimately lead to multiple organ dysfunction syndrome (MODS)34,35. Multifactor logistic regression analysis in this study found statistically significant differences in platelet count (OR = 0.996, 95%CI 0.994–0.998, P < 0.001) and the international normalized ratio (INR) (OR = 1.345, 95%CI 0.999–1.796, P = 0.046) with respect to the mortality rate in patients with severe osteomyelitis infections. The clinical condition of patients with severe osteomyelitis infections is often complex, and their ICU stay is generally prolonged.
Yagdiran et al. reported a retrospective analysis of 155 patients with pyogenic vertebral osteomyelitis, finding that 21.9% of patients exhibited symptoms of sepsis, with a high in-hospital mortality rate of 12.9%36,37. The 1-year and 2-year mortality rates were 20% and 23%, respectively. They identified organ dysfunction as a risk factor for increased mortality. This is consistent with our study, where there was a statistically significant difference in the occurrence of sepsis between the survival and non-survival groups (P < 0.05). The probability of sepsis in severely ill osteomyelitis patients is considerable, emphasizing the critical importance of early recognition of sepsis in patients with severe osteomyelitis infections.
Our predictive model’s AUC is greater than 0.7, indicating good predictive capability. It fills the gap in predicting the one-year mortality rate of severe bone marrow infection patients admitted to the ICU. Our predictive model enables clinicians to identify the risk of death in severe bone marrow infection patients early on and develop targeted treatment plans based on risk factors, thereby reducing the occurrence of mortality and improving patient prognosis.
However, this study has some limitations. Firstly, our model has only been validated internally and has not yet been externally validated in a concentrated dataset. We plan to conduct further research in the future. Secondly, due to the limited types of variables in public databases, some variables of interest, such as data on procalcitonin and erythrocyte sedimentation rate (ESR), were not included in the study.
Conclusion
According to our study results, age, heart rate, systolic blood pressure (SBP), mean blood pressure (MBP), albumin, red cell distribution width (RDW), platelet count (PLT), ICU duration (Los_icu), International Normalized Ratio (INR), and sepsis are all significant predictors of one-year mortality in severe osteomyelitis patients. Based on these risk factors, we have established and validated a nomogram model. Clinicians can use this model to identify high-risk patients and make optimal medical decisions for treatment and recovery.
Data availability
The dataset used in this study can be found on the online website of the MIMIC-IV database. Anyone who meets the requirements for database usage can access the database.
References
Alt, V. & Giannoudis, P. V. Musculoskeletal infections—A global burden and a new subsection in Injury. Injury 50, 2152–2153 (2019).
Rupp, M. et al. Terminology of bone and joint infection. Bone Joint Res. 10, 742–743 (2021).
Masters, E. A. et al. Skeletal infections: Microbial pathogenesis, immunity and clinical management. Nat. Rev. Microbiol. 20(7), 385–400. https://doi.org/10.1038/s41579-022-00686-0 (2022).
Horch, R. E. et al. Osteomyelitis : Behandlungskonzepte aus Sicht der plastischen Chirurgie [Osteomyelitis: treatment concepts from the plastic surgeon’s point of view]. Chirurg. 84(11), 962–9. https://doi.org/10.1007/s00104-013-2623-8 (2013) (German).
Arnold, S. R. et al. Changing patterns of acute hematogenous osteomyelitis and Staphylococcus aureus. J. Pediatr. Orthop. 26, 703–708 (2006).
Honkanen, M. et al. Periprosthetic joint infections as a consequence of bacteremia. Open Forum Infect. Dis. 6, ofz218 (2019).
Gafur, O. A. et al. The impact of the current epidemiology of pediatric musculoskeletal infection on evaluation and treatment guidelines. J. Pediatr. Orthop. 28, 777–785 (2008).
Kremers, H. M. et al. Trends in the epidemiology of osteomyelitis: A population-based study, 1969 to 2009. J. Bone Joint Surg. Am. 97(10), 837–845. https://doi.org/10.2106/JBJS.N.01350 (2015).
Marques, P. et al. Chronic hypocalcemia dueto anti-calcium sensing receptor antibodies. Acta Med. Port. 27(3), 399–402 (2014).
Zimmerli, W. Clinical practice. Vertebral osteomyelitis. N. Engl. J. Med. 362, 1022–1029 (2010).
McHenry, M. C., Easley, K. A. & Locker, G. A. Vertebral osteomyelitis: Long-term outcome for 253 patients from 7 Cleveland-area hospitals. Clin. Infect. Dis. 34, 1342–1350 (2002).
Park, S. Y. Nomogram: An analogue tool to deliver digital knowledge. J. Thorac. Cardiovasc. Surg. 155(4), 1793 (2018).
Courjon, J. et al. Pyogenic vertebral osteomyelitis of the elderly: Characteristics and outcomes. PLoS ONE 12, e0188470 (2017).
Akiyama, T. et al. Incidence and risk factors for mortality of vertebral osteomyelitis: A retrospective analysis using the Japanese diagnosis procedure combination database. BMJ Open 3, e002412 (2013).
Cheung, W. Y. & Luk, K. D. Pyogenic spondylitis. Int. Orthop. 36, 397–404 (2012).
Chae, H. J., Kim, J. & Kim, C. Clinical characteristics of spinal epidural abscess accompanied by bacteremia. J. Korean Neurosurg. Soc. 64, 88–99 (2021).
Vettivel, J. et al. Pyogenic vertebral column osteomyelitis in adults: Analysis of risk factors for 30-day and 1-year mortality in a Single Center Cohort Study. Asian Spine J. 13, 608–614 (2019).
Zarrouk, V. et al. Increased mortality in patients aged 75 years or over with pyogenic vertebral osteomyelitis. Infect. Dis. 50, 783–787 (2018).
Deep, A. et al. Evolution of acute Kidney injury and its association with systemic hemodynamics in children with fluid-refractory septic shock. Crit. Care Med. 46(7), 7e677-7e683. https://doi.org/10.1097/CCM.0000000000003156 (2018).
Holder, A. L. et al. Predictors of early progression to severe sepsis or shock among emergency department patients with nonsevere sepsis. Int. J. Emerg. Med. 9(1), 10. https://doi.org/10.1186/s12245-016-0106-7 (2016).
Artero, A. et al. Prognostic factors of mortality in patients with community-acquired bloodstream infection with severe sepsis and septic shock. J. Crit. Care 25(2), 276–281. https://doi.org/10.1016/j.jcrc.2009.12.004 (2010).
Paar, M. et al. Anticoagulant action of low, physiologic, and high albumin levels in whole blood. PLoS ONE 12(8), e0182997. https://doi.org/10.1371/journal.pone.0182997 (2017).
Ison, J. J. et al. Serum albumin level as a predictor of incident coronary heart disease: The Atherosclerosis Risk in Communities (ARIC) study. Am. J. Epidemiol. 151(5), 468–477. https://doi.org/10.1093/oxfordjoumals.aje.a010232 (2000).
Djoussé, L. et al. Serum albumin and risk of myocardial infarction and all-cause mortality in the Framingham Offspring Study. Circulation 106(23), 2919–2924. https://doi.org/10.1161/01.cir.0000042673.07632.76 (2002).
Plakht, Y., Gilutz, H. & Shiyovich, A. Decreased admission serum albumin level is an independent predictor of long-term mortality in hospital survivors of acute myocardial infarction. Soroka Acute Myocardial InfaretionII(SAMI-II) project. Int. J. Cardiol. 219, 20–24. https://doi.org/10.1016/j.ijcard.2016.05.067 (2016).
Islam, M. S. et al. Serum albumin levdland in-hospital outcome of patients with first attack acute myocardial infarction. Mymensingh Med. J. 28(4), 744–751 (2019).
Sičaja, M. et al. Red blood cell distribution width as a prognostic marker of mortality in patients on chronic dialysis: A single center, prospective longitudinal study. Croat. Med. J. 54(1), 25–32. https://doi.org/10.3325/cmj.2013.54.25 (2013).
Martínez-Velilla, N., Ibáñez, B., Cambra, K. & Alonso-Renedo, J. Red blood cell distribution width, multimorbidity, and the risk of death in hospitalized older patients. Age 34(3), 717–723. https://doi.org/10.1007/s11357-011-9254-0 (2012).
Claessen, B. E. et al. Impact of intravascular ultrasound imaging on early and late clinical outcomes following percutaneous coronary intervention with drug-eluting stents. JACC Cardiovasc. Interv. 4(9), 974–981. https://doi.org/10.1016/j.jcin.2011.07.005 (2011).
Scharte, M. & Fink, M. P. Red blood cell physiology in critical illness. Crit. Care Med. 31(12 Suppl), S651–S657. https://doi.org/10.1097/01.CCM.0000098036.90796 (2003).
Felker, G. M. et al. Red cell distribution width as a novel prognostic marker in heart failure: Data from the CHARM Program and the Duke Databank. J. Am. Coll. Cardiol. 50(1), 40–47. https://doi.org/10.1016/j.jacc.2007.02.067 (2007).
Levi, M., Schultz, M. & van der Poll, T. Sepsis and thrombosis. Semin. Thromb. Hemost. 39(5), 559–566. https://doi.org/10.1055/s-0033-1343894 (2013).
Van Deventer, S. J. et al. Experimental endotoxemia in humans: Analysis of cytokine release and coagulation, fibrinolytic, and complement pathways. Blood 76(12), 2520–2526 (1990).
Wang, J. G. et al. Levels of microparticle tissue factor activity correlate with coagulation activation in endotoxemic mice. J. Thromb. Haemost. 7(7), 1092–1098. https://doi.org/10.1111/j.1538-7836.2009.03448.x (2009).
Semeraro, F. et al. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: Involvement of platelet TLR2 and TLR4. Blood 118(7), 1952–1961. https://doi.org/10.1182/blood-2011-03-343061 (2011).
Yagdiran, A. et al. Quality of life and mortality after surgical treatment for vertebral osteomyelitis (VO): A prospective study. Eur. Spine J. 30(6), 1721–1731. https://doi.org/10.1007/s00586-020-06519-z (2021).
Lang, S. et al. Is there a difference in clinical features, microbiological epidemiology and effective empiric antimicrobial therapy comparing healthcare-associated and community-acquired vertebral osteomyelitis?. Antibiotics 10(11), 1410. https://doi.org/10.3390/antibiotics10111410 (2021).
Funding
Ningbo Public Welfare Fund Project (2023S125), Ningbo Top Medical and Health Research Program (2022020405).
Author information
Authors and Affiliations
Contributions
All authors were members of the research team and made substantial contributions to the conception and design, acquisition, analysis and interpretation of data. All authors were involved in drafting the manuscript and gave approval for the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
The establishment of MIMIC-IV(v2.0) was approved by the institutional review boards of the Beth Israel Deaconess Medical Center (Boston, MA) and Massachusetts Institute of Technology (Cambridge, MA). Thus, this study was granted a waiver of informed consent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, Y., Zheng, Y. & Ding, S. Development and validation of a prognostic nomogram model for severe osteomyelitis patients. Sci Rep 15, 318 (2025). https://doi.org/10.1038/s41598-024-83418-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-83418-z