Abstract
One of the primary reasons for the failure of therapy in nasopharyngeal cancer (NPC) is radio resistance-related localized one, which may lead to tumor residuals or recurrences. Several studies have linked interleukin-10 (IL-10) to crucial functions in cancer development and response to therapy. Its function in NPC’s radio resistance is, however, not well understood. Enzyme-linked immunosorbent assay (ELISA) and quantitative real-time PCR were utilized for confirming IL-10 expression in NPC cell lines. The prognostic significance of IL-10 was also assessed via Kaplan-Meier analysis. CNE2R, a radioresistant NPC cell line, expressed IL-10 at high levels, which were also shown to be considerably elevated in individuals with radioresistant NPC, as measured by ELISA. Moreover, the levels were also linked to poor clinical outcomes and prognosis in cancer cases. We also showed some evidence of a link between hypoxia-inducible factor 1-alpha (HIF-1 A) and serum IL-10 levels in NPC. Meanwhile, we find that IL-10 is up-regulated in CSC. IL-10 enhanced the self-renewal and tumorigenesis of nasopharyngeal CSC. In terms of mechanism, IL-10 enhances nasopharyngeal CSC self-renewal and tumorigenesis by activating STAT3 pathway. In NPC, IL-10/STAT3 Axis Nasopharyngeal Carcinoma Cancer stem cell and radio resistance.
Similar content being viewed by others
Introduction
Nasopharyngeal cancer (NPC) is among the most often diagnosed cancers in China1,2,3,4. Non-metastatic NPC is often treated with radiotherapy because of the cancer’s extreme susceptibility to ionizing radiation and the nasopharyngeal inconvenient anatomical placement, which makes surgical removal of the tumor challenging5,6. However, intrinsic or acquired radio resistance might lead to distant failure or tumor recurrence, reducing the effectiveness of radiation and worsening the prognosis of NPC patients7. Therefore, there is an immediate need to study radio resistance’s underlying mechanism for NPC.
Recent study suggested that cancer stem cells (CSCs) adaptation may contribute to radio resistance8. CSCs characteristic has been recognized as the clinical explanation for the observed tumor metastasis. Interleukin-10(IL-10), other cytokines in this family include IL19, IL20, IL22, IL24, IL26, IL28A, IL28B, and IL299,10,11,12. Animal models and people with IL-10/IL-10R axis mutations have shown that IL-10 has a role in a wide variety of diseases13,14,15. Although IL-10 has been linked to NPC radio resistance, its precise function in this process is not still completely understood16. In this work, we evaluated IL-10 for its application as a biomarker of radiosensitivity and recurrence in NPC CSCs.
Materials and methods
Cell lines
We cultured NPC cell lines (CNE-1 and − 2 with its radioresistant cell type CNE2R SUNE1, HNOE1, and HNE1) in DMEM medium (Invitrogen, California, USA) supplemented with 1% penicillin-streptomycin (HyClone, Utah, USA) and 10% fetal bovine serum (Gibco, New York, USA) from the Sun Yat-Sen University Cancer Center in Guangzhou, China. The cells were grown in a humidified 37 °C environment containing 5% CO2.
Vectors and cell transfection
Vectors expressing IL-10(LV-NC) and Negative control (LV-NC) were provided by GeneCopoeia, Inc. All cell transfections were performed using Lipofectamine® 3000 (Invitrogen; Thermo Fisher Scientific, Inc.) with all operations performed in strict accordance with the manufacturer’s protocol.
Virus knock IL-10 (IL-10-KD) and Negative control (Control-KD) were provided by GeneCopoeia, Inc. All cell transfections were performed using Lipofectamine® 3000 (Invitrogen; Thermo Fisher Scientific, Inc.) with all operations performed in strict accordance with the manufacturer’s protocol. Expression of IL-10 was detected using RT-qPCR 24 h after transfection as aforementioned. The primers were listed in supplement Table 1.
Patients
A total of 25 radioresistant NPC patients and 35 radiosensitive primary-diagnosed NPC serum specimens were obtained from Shijiazhuang People’s Hospital (Hebei, China) between February 2016 and November 2019 to evaluate serum IL-10 level with NPC radiosensitivity via enzyme-linked immunosorbent assay analysis (ELISA). Eventually, from February 2011 through November 2015, 105 blood samples from cases with locally advanced or metastatic NPC were collected from the Shijiazhuang People’s Hospital (Hebei, China) for determining the correlation between serum IL-10 level and NPC prognosis via ELISA. NPC cases’ radiosensitivity was determined using the criteria mentioned in the preceding Sect17. Patients were considered to have radioresistant NPC if their lesions did not completely disappear after radical irradiation, if they still had detectable tumors more than 6 weeks after treatment ended, or if they had a local or regional recurrence. were considered radiosensitive NPC if their tumors completely shrank following radiation treatment or did not return when radiotherapy was stopped18. Informed consent was obtained from all subjects and/or their legal guardian(s). The Shijiazhuang People’s Hospital Ethics Committee approved the utilization of NPC serums.
Immunohistochemical
A two-step immunoperoxidase method was employed for the immunohistochemistry. Primary antibody was a 1:60 dilution of a polyclonal antibody against HIF1A (Proteintech Group, Inc., Chicago, USA). In brief, antigen retrieval was achieved by heating the sections in 10 mmol/L citrate solution, following which the sections were incubated at room temperature for an hour with the primary antibody and subsequently the secondary antibody. The sections were then seen under a microscope after being prepared in diaminobenzidine solution and counterstained with hematoxylin. Three examiners blinded to patient information evaluated the immunohistochemistry stains. Both the cytoplasm and the nucleus showed strong staining for HIF1A. The staining intensity of positive cells was rated as negative, intermediate, or positive, and the results were recorded with the corresponding expression. Disagreements in scoring were debated until a resolution was achieved.
Spheroid assay
The NPC cells were seeded in 96-well ultra-low attachment culture plates (Corning Incorporated Life Sciences) (300 cells per well) and following performing assays were as previous reported26.
In vitro limiting dilution assay
The NPC cells were seeded in 96-well ultra-low attachment culture plates (Corning Incorporated Life Sciences) and following performing assays were as previous reported26.
Flow-cytometric analysis (identified and distinguished CSCs)
For CD133 and CD90 positive cells sorting (identified and distinguished CSCs), primary NPC patients’ cells and NPC cells were incubated with the primary anti-CD133 (Biolegend, Inc., San Diego, CA) or anti-CD90 (Biolegend, Inc., San Diego, CA) for 30 min at room temperature and following performing assays were as previous reported26.
Statistical analyses
The analyses findings will be presented as means ± SDs. When comparing continuous parameters, we employed either the student’s t-test. We employed the chi-square test for assessing the ways in which clinicopathological characteristics could be distributed across groups with high and low IL-10 expression. The Kaplan-Meier technique could be utilized for generating survival curves, and the log-rank test could be applied for evaluating the differences between them. Recurrence-free survival (RFS) was defined as the absence of any local or regional recurrence. From the initial day of therapy to the date of death from any cause, overall survival (OS) could be calculated. After controlling for potential confounders, the data was subjected to multivariate analysis via the Cox proportional hazards model. The chi-square test could be applied for analyzing the correlation significance among HIF-1A and IL-10 expressions. When the P-value was less than 0.05, the correlation was determined significant. SPSS 26.0 (SPSS IBM, Chicago, USA) had been approached for all statistical tests.
Results
IL-10 gene is linked to NPC radio resistance and can potentially predict NPC radio resistance
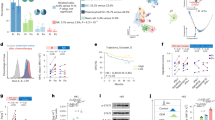
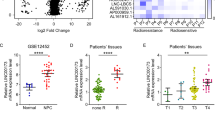
The findings demonstrated that CNE2R cells (Radiation resistant strain) had the greatest IL-10 expression compared to other NPC cells (Fig. 1A and B). While CNE1 is an EBV-negative cell line generated from a well-differentiated NPC, HNE1 is an EBV-positive cell line produced from a poorly-differentiated squamous cell carcinoma. Highly differentiated tumor cells often show moderate to low susceptibility to radiation19,20, as stated by Bergonié and Tribondeau law. Infection with EBV is a major contributor to radio resistance in non-small-cell lung cancer21,22. Figure 1A and B shows that IL-10 expression was comparatively high in the CNE1 and HNE1 cell lines. Following, our results indicated that radioresistant NPC cells have elevated levels of IL-10 expression when comparing radioresistant and radiosensitive NPC patients (n = 25 and 35, respectively, Fig. 1C). We employed receiver operating characteristic (ROC) curves for evaluating the efficacy of serum IL-10 level in differentiating between cases with radioresistant and radiosensitive NPC and the area under the curve (AUC) values were 0.821 (Fig. 1D). Based on these findings, IL-10 expression level might accurate predictor of NPC radio resistance.
IL-10 gene is linked to NPC radio resistance and can potentially predict NPC radio resistance. (A, B) The amounts of IL-10 mRNA (A) and IL-10 secretion into the supernatant were analyzed via RT-qPCR and ELISA in NPC cells, respectively. The enzyme GAPDH could be applied as a reference standard. (C) Individuals with radioresistant NPC (n = 25) had an elevated serum IL-10 level compared to those with radiosensitive NPC (n = 30). (D) Predictive value of serum IL-10 in differentiating radioresistant NPC from radiosensitive NPC shown by ROC curves. (E) Kaplan–Meier analysis of the RFS and OS between high and low serum IL-10 cohorts. (F) Uni-and multivariate analyses in RFS and OS. (G) Up panel: Percentage of HIF1α-negative, HIF1α-moderate, and HIF1α-high cases in High serum IL-10 or Low serum IL-10 group. Down panel: The representative images of HIF1α-negative, HIF1α-moderate, and HIF1α-high cases from NPC tissues. scale bars: 2000µM. For(A-C) Student’s t-test, (D)ROC test, (E) the log-rank test, (F) Single factor and multiple factor logistic regression. Error bars show mean ± SD derived from n = 3 independent experiments, *p < 0.05.
We next analyzed a group of 105 NPC patients divided into two groups(high/low) based on the mean level of blood IL-10 level to see whether there was a connection between the blood IL-10 level and the clinical indicators, and founded that Lymphatic metastasis (P = 0.006), big tumor size (P = 0.033), and advanced tumor stage (P = 0.008) were all strongly correlated with elevated IL-10 levels (Table 1). Low RFS and OS were seen in cases with elevated IL-10 expression (Fig. 1E). As shown in Fig. 1F, Tables 2 and 3, IL-10 was shown to be an unfavorable and independent prognostic predictor for RFS and OS in NPC patients when subjected to further multivariate analysis. Subsequently, serum IL-10 level may be a useful biomarker for predicting outcomes in NPC.
Serum IL-10 level and HIF-1α correlation
Since tumor hypoxic microenvironment is linked to radio resistance23, we hypothesized that IL-10 expression would be correlated with hypoxia-related gene expression. HIF-1 A expression levels in 105 NPC tissues were compared to the levels of blood IL-10. The findings showed a remarkable correlation between the expression of serum IL-10 and that of HIF-1α (Fig. 1G).
IL-10 is upregulated in Nasopharyngeal CSCs.
The Cancer Stem Cells (CSCs), a small sub-population with the capacity of self-renewal and differentiation into diverse types of cancer cells, are considered as strong drivers of radioresistance24. As shown in Fig. 2A and B, IL-10 mRNA levels were positively correlated with the expression of CD133 and CD90 as well-known nasopharyngeal CSCs markers19 in tumor cells isolated from 30 primary NPC tissues. Next, IL-10 mRNA levels were significantly upregulated in isolated CD133 + or CD90 + NPC cells compared with CD133- or CD90-NPC cells (Fig. 2C and D). Moreover, IL-10 expression was increased in NPC spheres compared with adherent NPC cells (Fig. 2E). Notably, IL-10 expression was gradually increased in serial passages of NPC spheroids (Fig. 2F). Besides, we found that the proportion of CD133 + and CD90 + population in CNE2R was higher than CNE1 (Figure S1A).
IL-10 is upregulated in Nasopharyngeal CSCs.A and B. The correlation between the level of IL-10, CD133 and CD90 in primary NPC (n = 30). C. IL-10 expression in CD133+/- NPC cells were analyzed. D. IL-10 expression in CD90+/- NPC cells were analyzed. E. IL-10 expression in NPC spheroids and adherent cells was determined. F. IL-10 expression in serial passages of NPC spheroids was determined. For(A-B) Pearson correlation, (C-F) Student’s t-test. Error bars show mean ± SD derived from n = 3 independent experiments, *p < 0.05.
IL-10 enhanced Nasopharyngeal CSCs self-renewal and tumorigenesis.
To explore the potential biological role of IL-10 in Nasopharyngeal CSCs, NPC cells were infected with IL-10 knockdown virus (Fig. 3A). As shown in Fig. 3B and C, IL-10 knockdown cells showed decreased CSCs markers. Moreover, IL-10 knockdown cells exhibited inhibited self-renewal capacity and decreased the proportion of CSCs (Fig. 3D and E).
IL-10 knockdown suppresses Nasopharyngeal CSCs self-renewal and tumorigenesis. A. IL-10 knockdown effect was checked. B and C. The CD133 and CD90 in indicated NPC cells. D. Spheroids formation assay of indicated NPC cells. Representative images of spheres are shown. scale bars: 200µM. E. The proportion of Nasopharyngeal CSCs in indicated NPC cells in vitro limiting dilution assay. For(A-E) Student’s t-test. Error bars show mean ± SD derived from n = 3 independent experiments, *p < 0.05.
Moreover, we overexpression IL-10 in NPC cells with LV-IL-10 virus (Fig. 4A). qPCR results indicated that overexpression IL-10 increased CSCs markers-CD133 and CD90 mRNA expression (Fig. 4B and C), enhanced self-renewal capacity (Fig. 4D), and increased the proportion of CSCs (Fig. 4E).
Finally, we found that knock down IL-10 decrease CD133 and CD90 protein level and overexpression IL-10 increase CD133 and CD90 protein level in NPC cells (Figure S1B). Besides, we found that knock down IL-10 decrease the proportion of CD133 + and CD90 + population and overexpression increase the proportion of CD133 + and CD90 + population in NPC (Figure S1C).
IL-10 overexpression promotes Nasopharyngeal CSCs self-renewal and tumorigenesis. A. IL-10 overexpression was checked. B and C. The CD133 and CD90 in indicated NPC cells. D. Spheroids formation assay of indicated NPC cells. Representative images of spheres are shown. scale bars: 200µM. E. The proportion of Nasopharyngeal CSCs in was indicated NPC cells compared by in vitro limiting dilution assay. For(A-E) Student’s t-test. Error bars show mean ± SD derived from n = 3 independent experiments, *p < 0.05.
IL-10 enhanced Nasopharyngeal CSCs self-renewal and tumorigenesis via activating STAT3 Pathway.
STAT3 pathway has been identified as one of the most frequent events occurring in CSCs and radio resistance23,24, and we wonder IL-10 enhanced Nasopharyngeal CSCs via activating STAT3 pathway. We found that knockdown IL-10 or the STAT3 inhibitor (Stattic) could significant inhibited STAT3 pathway in NPC cells (Figure.S2). As shown Fig. 5A and B, the STAT3 inhibitor (Stattic) diminished the increased self-renewal ability, and abolished the increasing proportion of CSCs between IL-10 overexpression and control NPC cells. Following, we found that IL-10, CD133, and CD90 mRNA expression was increased under hypoxia in PNC cells, while the STAT3 inhibitor (Stattic) offset the increased CD133 and CD90 protein expression effect under hypoxia(Fig. 5C). Besides, STAT3 pathway activity was enhanced under hypoxia in NPC cells, while the STAT3 inhibitor (Stattic) offset the enhanced effect under hypoxia (Fig. 5C).
IL-10 enhanced Nasopharyngeal CSCs self-renewal and tumorigenesis via activating STAT3 Pathway. (A) Spheroids formation assay of indicated NPC cells. Representative images of spheres are shown. scale bars: 200µM. (B) The proportion of nasopharyngeal CSCs in was indicated NPC cells compared by in vitro limiting dilution assay. (C) WBs in indicated NPC cells. For(A-E) Student’s t-test. Error bars show mean ± SD derived from n = 3 independent experiments, *p < 0.05.
Discussion
Tumor recurrence often stems from a lack of response to radiation therapy25. In our study, we found that high IL-10 level was positive associated with radio resistance in NPC. Meanwhile, we also found that high IL-10 level was predicted poor prognosis in NPC. Following, we found that IL-10 enhanced Nasopharyngeal CSCs self-renewal and tumorigenesis. In this work, we indicated that IL-10 for its application as a biomarker of radiosensitivity and recurrence in NPC.
Because of their high energy needs and poor vasculature, solid tumors often have low oxygen levels (hypoxia) in their microenvironment. Hypoxia may make cancer cells resistant to radiation23,26. Previous research indicated that whereas HPV-infected head and neck tumor cells were much more radiosensitive than HPV-negative cells, under hypoxic settings, the radio resistance capacity of both cell types was similar27. HIF-1, which governs adaptive responses to hypoxia, has been linked to oxygen deprivation-induced natural radio resistance in malignant cells. It is the master regulator of hypoxia adaptations in tumors28,29. Serum IL-10 levels were shown to positively correlate with HIF-1 A expression in our investigation of 105 NPC tissues. Since HIF-1 A has been linked to cancer cells’ resistance to radiation, we hypothesize that IL-10 interacts with HIF-1 A to promote radio resistance in NPC. More research is needed to understand the mechanisms by which HIF-1 A and IL-10 control radio resistance in NPCs.
CSCs exhibiting self-renewal and reproductive abilities can lead to radiotherapy (RT) failure30, resulting in poor prognosis for NPCs receiving clinical radiotherapy31. Hypoxia can stimulate the malignant characteristics of NPC cells, thereby promoting the radiation resistance of CSCs32,33. In this study, we indicated that the IL-10/STAT3 axis NPC cancer stem cell and this may be a potential reason for the failure of IL-10 to promote radiotherapy for NPC.
There were several limitations in the study, such as are. However, our sample size is still limited, and a small number of cases may have unclear definitions of radiotherapy sensitivity or insensitivity in clinical features. Therefore, only a sufficiently large sample size can reduce the impact of such factors. Besides, the limitations inherent in translating findings from in vitro models to clinical outcomes. Meanwhile, our study defined IL-10’s putative role in regulating radio resistance, our study lacked to define whether a STAT3 inhibitor can sensitize resistant tumors to radiotherapy. This is worth exploring in future research.
Conclusion
We showed that IL-10 was correlated with increased radio resistance in NPC cells under hypoxia induced by HIF1a, and IL-10 enhanced Nasopharyngeal CSCs self-renewal and tumorigenesis via activating STAT3 pathway. Collectively, these findings suggest that IL-10 may serve as a predictor of radio resistance in NPC personalized therapy, which warrants further investigation.
Data availability
The data in the current study are available from the corresponding authors upon reasonable request.
References
Li, W. et al. Immunotherapeutic approaches in EBV-associated nasopharyngeal carcinoma. Front. Immunol. 13, 1079515 (2023).
Su, Z. Y., Siak, P. Y., Leong, C. O. & Cheah, S. C. The role of Epstein-Barr virus in nasopharyngeal carcinoma. Front. Microbiol. 14, 1116143 (2023).
Zhao, F., Yang, D. & Li, X. Effect of radiotherapy interruption on nasopharyngeal cancer. Front. Oncol. 13, 1114652 (2023).
Wang, S., Chen, S., Zhong, Q. & Liu, Y. Immunotherapy for the treatment of advanced nasopharyngeal carcinoma: a promising new era. J. Cancer Res. Clin. Oncol. 149 (5), 2071–2079 (2023).
Zhang, J. et al. Application of small extracellular vesicles in the diagnosis and prognosis of nasopharyngeal carcinoma. Front. Cell. Dev. Biol. 11, 1100941 (2023).
Yang, X., Wu, J. & Chen, X. Application of Artificial Intelligence to the Diagnosis and Therapy of Nasopharyngeal Carcinoma. J. Clin. Med. 12 (9), 3077 (2023).
Qian, X., Chen, H. & Tao, Y. Biomarkers predicting clinical outcomes in nasopharyngeal cancer patients receiving immune checkpoint inhibitors: A systematic review and meta-analysis. Front. Immunol. 14, 1146898 (2023).
Mills, K. H. G. IL-17 and IL-17-producing cells in protection versus pathology. Nat. Rev. Immunol. 23 (1), 38–54 (2023).
Saraiva, M. & O’Garra, A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 10 (3), 170–181 (2010).
Wei, H., Li, B., Sun, A. & Guo, F. Interleukin-10 Family Cytokines Immunobiology and Structure. Adv. Exp. Med. Biol. 1172, 79–96 (2019).
Moore, K. W., de Waal Malefyt, R., Coffman, R. L. & O’Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19, 683–765 (2001).
Ouyang, W., Rutz, S., Crellin, N. K., Valdez, P. A. & Hymowitz, S. G. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu. Rev. Immunol. 29, 71–109 (2011).
Ouyang, W. & O’Garra, A. IL-10 Family Cytokines IL-10 and IL-22: from Basic Science to Clinical Translation. Immunity 50 (4), 871–891 (2019).
Mannino, M. H. et al. The paradoxical role of IL-10 in immunity and cancer. Cancer Lett. 367 (2), 103–107 (2015).
Wang, X., Wong, K., Ouyang, W., Rutz, S. & Targeting IL-10 Family Cytokines for the Treatment of Human Diseases. Cold Spring Harb Perspect. Biol. 11 (2), a028548 (2019).
Neumann, C., Scheffold, A. & Rutz, S. Functions and regulation of T cell-derived interleukin-10. Semin Immunol. 44, 101344 (2019).
Trbovich, A. M., Sherry, N. K., Henley, J., Emami, K. & Kontos, A. P. The utility of the Convergence Insufficiency Symptom Survey (CISS) post-concussion. Brain Inj. 33 (12), 1545–1551 (2019).
Tsang, R. K. et al. Sensitivity and specificity of Epstein-Barr virus IGA titer in the diagnosis of nasopharyngeal carcinoma: a three-year institutional review. Head Neck. 26 (7), 598–602 (2004).
Vogin, G. & Foray, N. The law of Bergonié and Tribondeau: a nice formula for a first approximation. Int. J. Radiat. Biol. 89 (1), 2–8 (2013).
Ritenour, E. R. Health effects of low level radiation: carcinogenesis, teratogenesis, and mutagenesis. Semin Nucl. Med. 16 (2), 106–117 (1986).
Lei, F. et al. Radio-Susceptibility of Nasopharyngeal Carcinoma: Focus on Epstein- Barr Virus, MicroRNAs, Long Non-Coding RNAs and Circular RNAs. Curr. Mol. Pharmacol. 13 (3), 192–205 (2020).
Tan, W. L. et al. Advances in systemic treatment for nasopharyngeal carcinoma. Chin. Clin. Oncol. 5 (2), 21 (2016).
Guan, X. et al. Nanoparticle-enhanced radiotherapy synergizes with PD-L1 blockade to limit post-surgical cancer recurrence and metastasis. Nat. Commun. 13 (1), 2834 (2022).
Leiser, D. et al. Role of caveolin-1 as a biomarker for radiation resistance and tumor aggression in lung cancer. PLoS One. 16 (11), e0258951 (2021).
Huang, J. et al. Characterization of the mechanism of Scutellaria baicalensis on reversing radio-resistance in colorectal cancer. Transl Oncol. 24, 101488 (2022).
Slominski, R. M. et al. Melanoma, Melanin, and Melanogenesis: The Yin and Yang Relationship. Front. Oncol. 12, 842496 (2022).
Baumeister, P., Zhou, J., Canis, M. & Gires, O. Epithelial-to-Mesenchymal Transition-Derived Heterogeneity in Head and Neck Squamous Cell Carcinomas. Cancers (Basel). 13 (21), 5355 (2021).
Sun, Y., Zhou, Z., Yang, S. & Yang, H. Modulating hypoxia inducible factor-1 by nanomaterials for effective cancer therapy. Wiley Interdiscip Rev. Nanomed. Nanobiotechnol. 14 (1), e1766 (2022).
Sarnella, A. et al. Inhibition of carbonic anhydrases IX/XII by SLC-0111 boosts cisplatin effects in hampering head and neck squamous carcinoma cell growth and invasion. J. Exp. Clin. Cancer Res. 41 (1), 122 (2022).
Olivares-Urbano, M. A., Griñán-Lisón, C., Marchal, J. A. & Núñez, M. I. CSC Radioresistance: A Therapeutic Challenge to Improve Radiotherapy Effectiveness in Cancer. Cells 9 (7), 1651 (2020).
Atashzar, M. R. et al. Cancer stem cells: A review from origin to therapeutic implications. J. Cell. Physiol. 235 (2), 790–803 (2020).
Hoque, S. et al. Cancer stem cells (CSCs): key player of radiotherapy resistance and its clinical significance. Biomarkers 28 (2), 139–151 (2023).
Schulz, A., Meyer, F., Dubrovska, A. & Borgmann, K. Cancer Stem Cells and Radioresistance: DNA Repair and Beyond. Cancers (Basel). 11 (6), 862 (2019).
Funding
N/A.
Author information
Authors and Affiliations
Contributions
H-H Z conceived the study. All authors helped to organize and perform the study. Statistical analysis was undertaken by All authors. All authors read and approved the final version of the manuscript. All authors wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical statement
All methods were performed in accordance with the relevant guidelines. Informed consent was obtained from all subjects and/or their legal guardian(s). The experimental protocols were approved by The Shijiazhuang People’s Hospital Ethics Committee.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, Lj., Zhao, Y., Wang, Ys. et al. The IL-10/STAT3 Axis Nasopharyngeal Carcinoma Cancer stem cell and radio resistance. Sci Rep 14, 31943 (2024). https://doi.org/10.1038/s41598-024-83423-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-83423-2
Keywords
This article is cited by
-
The role of immune checkpoints in modulating cancer stem cells anti-tumor immune responses: implications and perspectives in cancer therapy
Journal of Experimental & Clinical Cancer Research (2025)