Abstract
Transfer RNA (tRNA) contains modified nucleosides essential for modulating protein translation. One of these modifications is queuosine (Q), which affects NAU codons translation rate. For decades, multiple studies have reported a wide variety of species-specific Q-related phenotypes in different eukaryotes, hindering the identification of a general underlying mechanism behind that phenotypic diversity. Here, through bioinformatics analysis of representative eukaryotic genomes we have predicted: i) the genes enriched in NAU codons, whose translation would be affected by tRNA Q-modification (Q-genes); and ii) the specific biological processes of each organism enriched in Q-genes, which generally in eukaryotes would be related to ubiquitination, phosphatidylinositol metabolism, splicing, DNA repair or cell cycle. These bioinformatics results provide evidence to support for the first time in eukaryotes that the wide diversity of phenotypes associated with tRNA Q-modification previously described in various species would directly depend on the control of Q-genes translation, and would allow prediction of unknown Q-dependent processes, such as Akt activation and p53 expression, which we have tested in human cancer cells. Considering the relevance of the Q-related processes, our findings may support further exploration of the role of Q in cancer and other pathologies. Moreover, since eukaryotes must salvage Q from bacteria, we suggest that changes in Q supply by the microbiome would affect the expression of host Q-genes, altering its physiology.
Similar content being viewed by others
Introduction
An efficient and accurate translation of the genetic information into proteins is required for a correct cellular physiology. This process consists of the decoding of messenger RNA (mRNA) by ribosomes and transfer RNAs (tRNAs). A wide variety of post-transcriptional modifications are present in tRNAs, which are crucial for fine-tuning the translation process1. One of these modifications is queuosine (Q), a hypermodified nucleoside derived from guanine that is located at the wobble anticodon position 34 of tRNAs containing the 5’-GUN-3’ anticodon sequence, those involved in decoding Asn, Asp, His and Tyr codons (AAC/U, GAC/U, CAC/U, UAC/U; NAC/U)2.
Q is found in both bacteria and eukaryotes, although only certain bacterial species can synthesize Q de novo. Eukaryotes must salvage the Q nucleobase queuine (q) from the microbiome and/or nutrient sources3. q can be directly imported into the cell or released from Q-5'-phosphate by the queuosine 5’-phosphate N-glycosylase/hydrolase (QNG1)4. Then, the replacement of the guanine at position 34 by salvaged q to obtain Q-tRNA is catalysed by a tRNA guanine transglycosylase (TGT), a heterodimeric enzyme formed by a catalytic subunit (QTRT1) and a regulatory one (QTRTD1)5.
Numerous studies conducted in eukaryotes over decades have reported a wide variety of species-specific processes in which Q is somehow involved: i) pupae maturation in Drosophila melanogaster6; ii) cell aggregation and development in Dictyostelium discoideum7; iii) virulence in Entamoeba histolytica8; iv) protein folding, ER stress and energy metabolism, neurodegeneration, or autoimmunity in mice9,10,11,12; and v) protein folding, ER stress, cell adhesion, proliferation, cancer, antioxidant defence system or hypoxia in humans9,13,14,15,16,17,18. Due to this variety of phenomena, the underlying role of Q has been difficult to address. Previous studies supported that G34-tRNAs harbouring the GUN anticodon show a strong preference for NAC codons over NAU codons, whereas Q34-tRNAs exhibit no bias for either cognate codon19,20. In addition, reported ribosomal profiling experiments performed in human cells showed that Q especially affects translational speed at NAU codons, whereas translation of NAC or near-cognate codons do not seem to be particularly affected by Q-tRNAs9,12,21. Furthermore, it has been experimentally verified in Escherichia coli and Trypanosoma brucei that the translational efficiency of synthetic genes enriched in NAU codons depends on Q availability22,23. This evidence suggests that Q-tRNAs may prevent the translational codon bias shown by unmodified tRNAs, and indicates that the expression level of proteins encoded by genes enriched in NAU codons (Q-genes) would depend on the degree of tRNA Q-modification23.
In a previous study from our laboratory, the general physiological role of Q in bacteria was addressed23. For that purpose, we started from the assumption that Q-tRNAs mainly modulates the translation of NAU codons, based on previously reported research. In this sense, we analysed the translation of a gene enriched in NAC or NAU codons, and we observed that only the NAU version was dependent on tRNA Q-modification, which affects its translation by a maximum of 20–30%, similar to the results reported in T. brucei in another study22. Since this small change in the translation of a specific gene should not be sufficient to cause a clear phenotype, we hypothesised that cumulative changes in the translation of several functionally related Q-genes could result in higher alterations of certain cellular processes, and therefore, that Q-related processes would depend on the main roles of the Q-genes in each organism. To address this hypothesis, a bioinformatics approach was developed for the identification of the specific Q-genes of different bacterial species, revealing an enrichment in biological processes related to biofilm formation and virulence generally in bacteria. Experimental data verified that those processes were controlled by tRNA Q-modification, demonstrating that the physiological role of Q in bacteria depends on the biological processes in which the Q-genes of each bacterial species are mainly involved, and suggesting that tRNA Q-modification could represent a mechanism for coordinating the expression of functionally related genes23.
Therefore, in this work, we aimed to address whether Q played a similar role in eukaryotes. For that, genomes from different model eukaryotic organisms, including humans, have been submitted to a similar bioinformatics analysis and we report that most of the wide variety of previously reported Q-related phenotypes could also be predicted by this approach. Thus, tRNA Q-modification would also modulate in eukaryotes those biological processes in which the fine-tuning of Q-genes expression is critical, similar to what has been reported in bacteria23. In addition, a second bioinformatics approach was developed to identify the processes predicted to be coordinated by Q generally in eukaryotes. Furthermore, those bioinformatics strategies followed by experimental verification were used to discover new physiological functions of Q, such as the modulation of Akt activation and p53 levels in human cancer cells reported here. Since most of the processes that may be controlled by Q-tRNAs generally in eukaryotes are crucial in carcinogenesis, we discuss the unexplored anti-tumoral potential of Q. Moreover, we addressed the question of whether an impairment of q supply from the microbiome can alter the physiology of a eukaryotic host through the control of host Q-genes expression.
Methods
Bioinformatics analysis of Q-genes
Bioinformatics analysis for identification of Q-genes and prediction of Q-dependent processes was performed as previously described23. Codon data were retrieved from coding sequence (CDS) genomic Reference sequence (RefSeq) database at NCBI24. CDS were associated to their corresponding UniProt IDs using the conversion tool available at the UniProt website. Only CDS with an associated UniProt ID were considered for calculations. When a UniProt ID was associated to several CDS, the consensus CDS (CCDS) was employed in the analysis. In the case that a CCDS was absent, verified RefSeq CDSs were considered as synonymous for calculations. The NAU codons frequencies of all CDS were calculated dividing the number of NAU codons by the total number of codons of each CDS. It is worth noting that NAC or other near-cognate codons were not considered in the analysis as previous reported research indicates that translation of those codons is not particularly modulated by Q9,12,21,22,23. In addition, it must be considered that other factors may modulate the strength of the Q-genes translational regulation exerted by Q-tRNAs, such as the presence of consecutive NAU codons, specific codons next to NAU codons, or the formation of certain mRNA secondary structures. Each NAU codons frequency was compared to the genome-wide NAU codons frequency using right-tail χ2 test followed by false-discovery rate (FDR) correction (α = 0.05) to adjust P-values25. Those genes associated to at least one CDS statistically enriched in NAU codons (P-adj value < 0.05) were considered as Q-genes (Supplementary Data S1). The list of Q-genes of each organism was submitted to functional enrichment analysis using DAVID and STRING tools26,27. Gene Ontology (GO) and KEGG Pathways terms with a P-adj value lower than 0.1 were considered statistically significant and predicted as possible Q-dependent biological processes (Supplementary Data S2)28.
To investigate the general role of Q in eukaryotes, codon data were retrieved from the 64 eukaryotic species harbouring TGT gene homologues and belonging to GO database as described above, and the frequencies of NAU codons of each gene from each organism were calculated. List of GO biological process terms was retrieved from GO database, and only terms with more than 100 annotations among 64 analysed species were selected for the analysis (~2.700 terms). The means of NAU codons frequencies of the genes from each organism belonging to each GO term were calculated. Differences between average frequencies of NAU codons of the genes from each organism and GO term, and the genome-wide frequencies of NAU codons of each organism were determined. The means of those differences were calculated for each GO term (Δ NAU codons), only if the GO term was annotated in more than 2 genes of, at least, the 75% of the analysed species (Supplementary Data S3). This filter was necessary to select the more general GO terms, as the objective of this bioinformatics approach was to address the general role of Q in eukaryotes. Genes of a GO term were considered to have a high frequency of NAU codons when its Δ NAU codons value was higher that 0% and showed an associated P-adj value < 0.05, calculated by one-sample t-test followed by FDR (α = 0.05) to avoid identification of false positives (Supplementary Data S3). Only GO terms with a Δ NAU codons > 1% were considered as terms with genes generally enriched in NAU codons.
Cell culture
HeLa cells were purchased from ATCC (catalog number CCL-2) and maintained in DMEM (Thermo Fisher Scientific; ref: 12100061) supplemented with 10% foetal bovine serum (FBS), 2 mM glutamine and 1 mM sodium pyruvate at 37 °C/5% CO2. To study the effect of q depletion, FBS was replaced with 10% horse serum (HS) (Sigma-Aldrich; ref: H1138). Most animal sera contain q except for HS, which is essentially q-free29,30,31. In fact, growth of HeLa cells with HS was shown to induce Q-tRNAs deficiency, and HS has been used for culturing human cells under q-free conditions in different studies13,14,29,30,31. Cells were incubated with or without q (500 nM) (Toronto Research Chemicals; ref: Q525000) for 14 days.
Western blot
After incubation in the absence or presence of q, cells were scrapped, pelleted, and washed with ice-cold PBS. Cell pellets were lysed with RIPA buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1% Triton, 0.5% sodium deoxycholate, 0.1% SDS) supplemented with proteases and phosphatases inhibitors (1 mM PMSF, 1 mM iodoacetamide and 10 mM NaF, and 1 mM sodium orthovanadate, respectively). Proteins were separated by SDS-PAGE, transferred onto nitrocellulose membranes, and blocked in 5% BSA (in TBS-0.1% Tween20). Membranes were then probed with antibodies against p-AKT (Santa Cruz Biotechnology; ref: p-Akt1 Antibody (5.Ser 473) sc-293125), Akt (Santa Cruz Biotechnology; ref: Akt1 Antibody (B-1) sc-5298), p53 (Santa Cruz Biotechnology; ref: p53 antibody (DO-1) sc-126) and vinculin (Proteintech; ref: 66305-1-Ig). Vinculin was used as a loading control. After three washes in TBS with 0.1% Tween-20, membranes were incubated with specific secondary antibodies conjugated with horseradish peroxidase (Bio-Rad). Chemiluminescence was detected with home-made specific solution (75 mM Tris-HCl, pH 8.8, 2.5 mM luminol, 0.4 mM p-coumaric acid, 0.01% H2O2). Quantification of the bands was performed with ImageJ software32. pAKT signal was normalised with total AKT signal, whereas vinculin was used as a loading control for p53.
Analysis of proteomic studies comparing conventional and germ-free mice
Data were retrieved from the previous studies to date in which proteomic analysis comparing conventional and germ-free mice were performed33,34,35. The mean frequency of NAU codons was calculated as described above for those genes that encoded for down- or upregulated proteins in germ-free mice compared to conventional mice. Proportions of genes considered as Q-genes that encoded for differentially expressed proteins (DEPs) were calculated according to the Q-genes lists (Supplementary Data S1). Genes encoding for downregulated proteins were submitted to functional enrichment analysis as described above.
Quantification and statistical analysis
Right-tail χ2 test followed by FDR correction was performed to identify CDS enriched in NAU codons of different organisms23. Statistical χ2 was calculated using Eq. (1):
where NAUO is the observed number of NAU codons, NAUE is the expected number of NAU codons, calculated by multiplying the number of total codons by the genome-wide frequency of NAU codons, Non-NAUO is the observed number of total codons except NAU codons, and Non-NAUE is the expected number of total codons except NAU codons, calculated by multiplying the number of total codons multiplied by the genome-wide frequency of total codons except NAU codons. Statistical χ2 was submitted to right-tail χ2 distribution for obtaining p-values. FDR correction (α = 0.05) was performed to adjust P-values and avoid the identification of false positives25. GraphPad Prism version 9.00 (GraphPad Software, La Jolla, CA, United States) was used to calculate statistical significance and other parameters, including the values of mean and standard deviation (S.D.) based on the datasets from independent experiments. Statistical significance was calculated using one or two-sided unpaired Student’s t-test.
Results and discussion
Reported species-specific Q-related phenotypes would depend on Q-genes translational modulation exerted by Q-tRNAs
Since the discovery of Q, different eukaryotic model organisms have been used to study the physiological role of this modified nucleoside. However, the underlying function of Q was hard to elucidate due to the wide variety of Q-related phenotypes observed in different organisms. As Q-tRNAs control Q-genes translational modulation, we proposed that those phenotypes could be explained by considering that the physiological role of Q in each organism depends on the specific biological processes in which their Q-genes are involved23. We cannot discard that Q-tRNAs affect the expression of other types of genes, but in this work, we will focus on genes enriched in NAU codons, which are the ones reported to be especially Q-dependent9,12,21. To address that hypothesis, a bioinformatics approach previously developed for bacteria was used for identification of the Q-genes of seven different eukaryotic organisms by statistical comparison of the frequency of NAU codons of each gene with the average frequency of the corresponding genome23 (see ‘Methods’) (Supplementary Data S1). The percentage of predicted Q-genes in the genome of each species ranged from 3 to 21% (average 9%) (Supplementary Data S1). Q-genes of each organism were submitted to functional enrichment analysis, so that ontology terms significantly enriched in Q-genes were predicted to represent Q-dependent processes (Supplementary Data S2). We observed that a wide diversity of the species-specific Q-related phenotypes reported in previous studies over decades were in line with the predicted Q-dependent processes revealed by the bioinformatics analysis.
Dictyostelium discoideum
This species of amoeba undergoes a multicellular developmental cycle, beginning with the aggregation of thousands of cells into a multicellular aggregate that develops in a fruiting body in which sporulation takes place36. Schachner et al. reported that D. discoideum showed enlarged aggregates and fruiting bodies and faster development to spores in the presence of q, whereas q-lacking amoebae lost their ability to differentiate into stalk cells and spores7. The bioinformatics analysis revealed an enrichment of predicted Q-genes in the ontology terms “Multicellular organism development” and “Phosphatidylinositol (PI) signalling system” in this organism (Table 1 and Supplementary Data S2). The absence of q may impair translational efficiency of Q-genes related to multicellular development, which would originate the observed Q-related phenotypes. Furthermore, PI signalling is known to be crucial in D. discoideum development for cell-cell communication36. Therefore, D. discoideum Q-related phenotypes may be caused by Q-modulation of genes related to development and PI signalling.
Entamoeba histolytica
E. histolytica causes intestinal dysentery, a hallmark of amoebiasis, in which destruction of human tissues occurs by death and phagocytosis of human cells. Nagaraja et al. proposed the involvement of Q-tRNA modification in amoebiasis since QTRT1 silencing in E. histolytica reduces its virulence8. In fact, our bioinformatics analysis revealed that E. histolytica Q-genes were especially involved in “Amoebiasis”, “Actin cortical patch assembly”, and “Endocytosis” (Table 1 and Supplementary Data S2). Cortical actin dynamics and endocytosis are crucial processes for host cell phagocytosis37. Therefore, Q would control E. histolytica pathogenesis by modulating the gene expression of Q-genes especially involved in virulence.
Trypanosoma brucei
Another organism in which Q is thought to be related to the transition between different developmental stages is T. brucei. This organism resides in the Tsetse fly vector in a procyclic form. Once T. brucei is transmitted to the mammalian host through bloodsucking, it transforms into the bloodstream form. It has been recently reported that Q-tRNA levels are increased at this developmental stage38. In the bloodstream form, T. brucei can show two morphologies: the high-replicating slender form, and the non-proliferative stumpy form. Controlling the transition between slender and stumpy forms is essential to limit the parasite population size, maintaining the reservoir in the host, but preventing premature death of the host. This transition is known to be regulated by extracellular stumpy induction factors (SIF) together with cAMP signalling39. We have observed that “cAMP biosynthetic process”, “Adenylate cyclase activity”, and “Cellular response to stimulus” ontology terms are enriched in the predicted Q-genes of T. brucei (Table 1 and Supplementary Data S2). Therefore, we propose that tRNA Q-modification is induced in the bloodstream form to properly control of the slender to stumpy transition, by an efficient translation of proteins related to cAMP signalling and SIF response.
Drosophila melanogaster
In this organism, it was reported that q induced more pupae maturation into adults when incubated in cadmium-containing medium, and that Q-tRNAs availability dramatically changed during metamorphosis6,40. Bioinformatics analysis of D. melanogaster Q-genes showed that Q would regulate processes such as “Ecdysteroid metabolic process”, “Tissue development”, and “Animal organ morphogenesis” (Table 1 and Supplementary Data S2). Ecdysteroid hormones initiate post-embryonic development, including the metamorphosis of immature forms into adults, and their regulation is mainly controlled by the levels of biosynthetic enzymes at the appropriate developmental times41. Thus, Q may be necessary for temporal fine-tuning of the levels of ecdysteroid metabolic enzymes and other developmental proteins through the translational control of Q-genes, which may explain the observed Q-related phenotypes.
Mus musculus and Rattus norvegicus
The role of Q has also been addressed in rodents in multiple studies. In a recent work, Cirzi et al. showed that NAU codons were translated more slowly than NAC codons in a QTRT1 knockout Q-lacking mice12. In addition, our bioinformatics analyses revealed that 49.4% of genes with high ribosome occupancy (slow translation) were Q-genes, whereas this percentage was only 7.6% in genes with low ribosome occupancy (Supplementary Data S4A). These data suggest that Q-tRNAs especially affect translational efficiency of Q-genes in mice.
In terms of the physiological role of Q in mice, an increase in endoplasmic reticulum (ER) stress, unfolded protein response (UPR) and oxidative stress was reported in the liver of germ‐free mice fed with a q‐free synthetic diet (Q-lacking mice)9. Our bioinformatics analysis of mouse Q-genes showed that Q may regulate processes such as “Protein processing in ER”, “Ubiquitin mediated proteolysis”, or “Regulation of protein stability” (Table 1 and Supplementary Data S2). Thus, absence of Q would impair the expression of several Q-genes involved in these processes, leading to protein misfolding, ER stress and UPR response. In fact, Tuorto et al. showed that downregulated proteins in the liver of Q-lacking mice are especially involved in ER stress and UPR9. Protein misfolding and aggregation increase ROS production, which would in turn cause the reported Q-dependent oxidative stress and worsen protein misfolding42. Protein aggregation is especially associated with neural dysfunction and neurodegeneration11. In this regard, it was reported that q shows protection in in vitro mouse models of neurodegeneration, reducing alpha-synuclein aggregation in a Parkinson’s disease model of synucleinopathy, as well as tau hyperphosphorylation in acute and chronic models of Alzheimer’s disease11. In addition, a recent study proposed that protein mistranslation stress may be responsible for impaired learning and memory formation in QTRT1 knockout mice12. Similar conclusions were obtained in R. norvegicus11, in which we also observed that Q-genes were involved in terms related to protein processing (Table 1 and Supplementary Data S2). Therefore, Q may play a neuroprotective role preventing protein misfolding and aggregation, at least in mice and rats, by modulating the gene expression of their Q-genes involved in protein processing in ER and UPR.
In addition, Zhang et al. reported the downregulation of β-catenin and claudins in the colon of QTRT1 knockout mice43. These proteins play a crucial role in cell adhesion and tight junctions, and we observed that “Cell-cell adhesion” and “Focal adhesion” terms were enriched in mouse Q-genes (Table 1 and Supplementary Data S2). This result suggests that absence of Q-tRNAs may alter the expression of proteins involved in cellular adhesion.
Furthermore, it was shown that q causes a reduction in proliferation of murine cancer cells in vivo44,45, and “Cell cycle”, “Cell division”, and other related terms were enriched in mouse Q-genes, suggesting a dysregulation of the cell cycle depending on Q availability (Table 1 and Supplementary Data S2). Moreover, it was revealed that Q-lacking mice show a reduced number of active ribosomes and a substantially impaired body weight, with a dysregulation in lipid and carbohydrate metabolism9. We observed that Q-genes were also involved in “Ribosome biogenesis”, which may explain the reduction in active ribosomes, “Energy reserve metabolic process”, and other processes related to lipid metabolism and glucose homeostasis, which would support the reduced body weight and lipid and carbohydrate metabolism dysregulation of Q-lacking mice (Table 1 and Supplementary Data S2). Thus, absence of Q may affect the translational efficiency of Q-genes causing the dysregulation of protein processing, cell adhesion, cell cycle, ribosome biogenesis and energy metabolism in mice. This would lead to ER stress, and alteration of tight junctions, proliferation, number of active ribosomes and body weight.
Homo sapiens
Tuorto et al., performed ribosome profiling and proteomic experiments to examine the genome‐wide codon occupancy in HeLa cells, a cell line derived from cervical cancer cells9. In the absence of q, it was shown that NAU codons have a higher ribosomal occupancy, suggesting that their translation is slower than that of NAC codons, and furthermore, genes encoding downregulated proteins are globally enriched in NAU codons, but the relative abundance of these codons in individual genes was not analysed9. According to our bioinformatics analysis, 47.4% of these downregulated proteins were encoded by predicted Q-genes, and this percentage was only 8.4% in the upregulated proteins (Supplementary Data S4B). It is worth noting that these data were very similar to the values above reported in mice (Supplementary Data S4A)12. Altogether, it seems that Q-tRNAs control Q-genes translational efficiency also in humans.
Several studies have attempted to reveal the physiological role of this modified nucleoside in humans. As reported in mice and rats, the absence of q increases ER stress and UPR in HeLa cells9. This phenotype could be explained considering that several human predicted Q-genes involved in “Protein processing in ER”, “Protein folding”, “Ubiquitin-mediate proteolysis”, or “Proteolysis” would be downregulated in the absence of Q-tRNAs (Table 2 and Supplementary Data S2). As protein misfolding and aggregation are crucial in the development of neurodegenerative pathologies and it is known that q would play a protective role in mouse and rat models of Alzheimer´s and Parkinson´s diseases11, we suggest that these findings open the door to the evaluation of the possible neuroprotective function of q, not only in mice, but also in humans.
Other Q-related phenotypes reported in humans could be also explained by our bioinformatics analysis. Reduced Q-tRNAs availability in several human cancer cell lines is known to induce changes in cellular adhesion and tight junctions, altering the expression of β-catenin, cadherins and claudins18,43. In this sense, bioinformatics analysis revealed that human predicted Q-genes, such as the CTNNB1 gene encoding for β-catenin, would be involved in “Adherens junction” and “Adherens junction organization” (Table 2 and Supplementary Data S1 and S2). In addition, several studies have revealed that cells cultured in absence of Q show alterations in proliferation, with different results depending on the cell line or culture condition13,14,18. We observed that human Q-genes were related to ontology terms such as “Cell division”, “Cell cycle”, “Regulation of cell cycle”, “G2/M transition of mitotic cell cycle”, “Negative regulation of G0 to G1 transition”, “Regulation of signal transduction by p53 class mediator”, and others (Table 2 and Supplementary Data S2). Moreover, enrichment analysis predicted that Q may modulate the response to viral infections, showing that Q-genes were especially involved in “Herpes simplex virus 1 infection”, “Viral process”, or “Positive regulation of interferon-alpha production” (Table 2 and Supplementary Data S2). This result may help to understand why interferon seems to control the q uptake in cultured human fibroblasts46.
In summary, we would like to highlight the relevance of showing that our bioinformatics analysis was able to predict most of that wide variety of previously reported Q-related phenotypes observed in different eukaryotic model organisms. The bioinformatics predictions provide evidence to strongly suggest for the first time in eukaryotes that the physiological function of Q is likely to directly depend on the translational control of Q-genes specific to each eukaryotic species. Although we cannot completely discard that any of those Q-related processes may depend on indirect effects of Q, all these matches between bioinformatics predictions and Q-related phenotypes lead to consider highly unlikely that all these matches are random, and to suggest that most of those Q-related phenotypes are caused by the regulation exerted by Q-tRNAs on Q-genes translation. While our bioinformatics data provides evidence supporting this idea, previous studies reporting species-specific Q-related phenotypes were not able to address this question. Therefore, these predictions not only shed light on the mechanism by which Q causes such a wide variety of previously reported phenotypes, but also create a basis for prediction of unknown Q-related processes.
It is noteworthy that, in this work, we propose to study the frequency of NAU codons of each gene for the identification of the genes controlled by Q-tRNAs since tRNA Q-modification is known to particularly affects the translation of NAU codons. However, some authors proposed to consider NAU/NAC ratio or some amino acid bias towards His, Asp, Asn and Tyr instead of just NAU codon frequency. The NAU/NAC ratio would not necessarily reveal information about whether a gene can be regulated by Q or not. First, because translation of the NAC codons does not seem to be affected by Q9,12,21,22,23, and second, considering that there are genes that could have a high NAU/NAC ratio but only a few NAU codons, which makes unlikely that their expression is Q-dependent. Attending to amino acids bias, certain proteins may be enriched in Asn, Asp, His and/or Tyr amino acids, but it does not necessarily imply that they are encoded by genes with a high NAU codons content. Indeed, since mainly NAU codons depend on Q, it would be more reasonable to think that these amino acids were encoded particularly by NAC codons to ensure the correct expression of those proteins even if there are low levels of Q, and in this case the translation of those genes would not be Q-dependent, as previously shown in T. brucei and bacteria22,23. The fact that the presence of genes with NAU codon enrichment has been conserved during evolution in species using Q-tRNAs, may support a beneficial Q-regulation of certain genes.
Genes involved in protein ubiquitination, PI metabolism, splicing, regulation of gene expression, DNA repair and cell cycle are enriched in NAU codons generally in eukaryotes
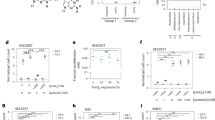
tRNA Q-modification is thought to enhance the translational speed at NAU codons and therefore the translational efficiency of Q-genes in bacteria and eukaryotes9,22,23. Previous studies from our group suggested that Q-tRNAs control biofilm formation and virulence generally in bacteria due to Q-genes are mainly involved in those processes23. In eukaryotes, as described above, diverse species-specific Q-related phenotypes have been reported since the discovery of Q, and most of them could be explained assuming that Q controls the expression of Q-genes. However, the identification of a general role of Q in eukaryotes remains elusive. We have developed another bioinformatics strategy to investigate which cellular processes can be affected by Q-tRNAs generally in eukaryotes. For that, we evaluated the average frequency of NAU codons of the genes belonging to each GO term in 64 species from the four eukaryotic kingdoms Protista, Fungi, Plantae and Animalia (see ‘Methods’) (Supplementary Data S3). We hypothesise that the biological processes generally controlled by Q would be those associated to the GO terms that include genes with an average frequency of NAU codons statistically higher and conserved along most of analysed organisms. This bioinformatics analysis revealed 41 GO biological process terms containing genes with a statistically high average frequency of NAU codons conserved in at least the 75% of eukaryotic species analysed (Fig. 1, Supplementary Data S3). Those GO terms could be grouped in ubiquitination and other protein modifications, PI metabolism, splicing and RNA processing, regulation of gene expression, DNA repair, and cell cycle and division (Fig. 1). As a high NAU codons frequency of the genes involved in these processes would be conserved in eukaryotes, and Q controls translational efficiency of NAU codons, we propose that Q may be involved in those processes generally in eukaryotes. It should be noted that apart from these general processes, Q-tRNAs may affect other species-specific processes, as described above. Focusing on the kingdom Animalia, we observed that GO terms with an associated high frequency of NAU codons were related to the same general processes, except for 7 terms including ‘Homophilic cell adhesion via plasma membrane adhesion molecules’ (Figure S1). In the kingdom Plantae, apart from the general processes, we observed GO terms related to transport and cell organization, signalling, sugar metabolism, and others (Figure S2). In summary, these results may support that reported ER stress and UPR enhancement by q depletion may be caused by an altered expression of proteins involved in ubiquitination, and that inefficient expression of proteins involved in cell cycle, division and cell adhesion may produce the reported Q-dependent alterations in proliferation and adhesion/migration. It is noteworthy that the conservation of a high frequency of NAU codons in genes related to these processes in phylogenetically different eukaryotes may underline the relevance of this additional layer of translational regulation performed by Q-tRNAs, and may discard that the enrichment in NAU codons conserved in specific groups of genes is random. On the other hand, it has been suggested that proteins encoded by genes with low or high content of guanine and cytosine in the third codon position (GC3) present a different subcellular spatial distribution, precisely GC3-rich gene products tended to be associated with the plasma membrane and cell periphery, while GC3-poor genes products were localized in the nucleus and organelles47,48. Therefore, we cannot rule out that a low GC3 content might partially contribute to the conservation of an enrichment of NAU codons in some Q-genes encoding proteins with a certain subcellular localization. Further research will be required to verify this general predicted role of Q in eukaryotes.
Genes involved in ubiquitination and other protein modifications, PI metabolism, splicing and RNA processing, regulation of gene expression, DNA repair, and cell cycle and division are enriched in NAU codons generally in eukaryotes. The frequencies of NAU codons of each gene from the 64 eukaryotic species harbouring TGT gene homologues and belonging to the GO database were calculated. The means of NAU codons frequencies of the genes from each organism belonging to each GO term were determined. Differences between the average frequencies of NAU codons of the genes from each organism and GO term, and the genome-wide frequencies of NAU codons of each organism were calculated and graphed in box-and-whiskers plots. Boxes represent the median ± interquartile range (IQR), whiskers denote observations within ± 1.5 times the IQR, and open circles represent outliers. The means of those differences were calculated for each GO term (Δ NAU codons), only if the GO term was annotated in more than 2 genes of, at least, the 75% of the species analysed (Supplementary Data S3). Black lines and their error bars represent the Δ NAU codons values ± 95% confidence interval, which were used to reveal the GO terms with genes with a high NAU codons frequency (Δ NAU codons > 1%; P-adj < 0.05).
tRNA Q-modification affects Akt activation and p53 levels in cancer cells
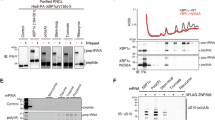
We have shown that a wide diversity of Q-related phenotypes reported in different eukaryotes may be explained attending to the roles of the Q-genes of each species, and we have indicated some biological processes that may be Q-regulated in general in eukaryotes. Now, we wondered whether the bioinformatics approach could be used to predict unknown processes regulated by Q-tRNA. In this sense, PI metabolism and PI dephosphorylation were processes predicted to be generally controlled by tRNA Q-modification (Fig. 1), and particularly in humans (Table 2 and Supplementary Data S2). It is widely known that PI metabolism is crucial for a correct regulation of the PI 3-kinase (PI3K)/Akt pathway, an intracellular signalling pathway involved in the regulation of the cell cycle49. PI is phosphorylated by different PI3K to form PI-3,4,5-trisphosphate [PI(3,4,5)P3], which works as an anchorage for Akt and its upstream activating kinase, PI-dependent kinase-1 (PDK-1). PI(3,4,5)P3 can be dephosphorylated by phosphatases such as PTEN. Recruitment of both Akt and PDK1 facilitates PDK1-mediated phosphorylation and consequent activation of Akt. This straightforward mechanism allows to use Akt phosphorylation to analyse changes in PI metabolism (Fig. 2A)49,50. Therefore, we hypothesized that Q could control the expression of Q-genes involved in PI metabolism, resulting in altered Akt phosphorylation. This bioinformatics prediction was validated in HeLa cancer cells, in which we observed an increase in the phosphorylation of Akt when cells were incubated in q-free medium (Fig. 2B,C). In addition, a recent study has reported a dysregulation of PI metabolism in human embryonic kidney cells, HEK293T, when genes encoding the subunits QTRT1 or QTRTD1 of the queuine tRNA-ribosyltransferase are mutated51. Akt contributes to a wide variety of molecular mechanisms that are critical in modulating many aspects of cellular function, especially in cancer cells, such as proliferation, survival, metabolism, apoptosis, or differentiation (Fig. 2A)52. First, Akt promotes the Warburg effect, a metabolic switch from oxidative phosphorylation to aerobic glycolysis with lactate production, necessary for the maintaining of a high proliferative cellular state53. In this sense, it was reported in HeLa cells that q depletion increases Warburg effect and lactate production, while q addition impairs lactate dehydrogenase (LDH) expression14,17. Second, previous evidence supports that q downregulates the expression of the oncogenes c-myc and bcl2 in a model of T-cell lymphoma45,54. Both c-Myc and Bcl-2 are activated by PI3K/Akt pathway and are involved in cell survival and apoptosis inhibition49. Third, PI3K/Akt pathway activation is also critical for stemness regulation and maintaining an undifferentiated state55. Reduced Q-tRNA levels have been reported in fast growing undifferentiated cells, embryonic tissues and regenerating hepatocytes, and increased tRNA Q-modification is related to organism development, aging and cellular differentiation15,56,57,58,59,60. Thus, all these reported effects of Q together with our findings suggest that Q would reduce Akt activation in cancer cells, possibly by controlling the translational efficiency of Q-genes involved in PI metabolism and dephosphorylation, as predicted by bioinformatics. We propose that, in the absence of q, small decreases in the expression of PI phosphatases encoded by Q-genes, such as PTEN, would result in increased activation of PI(3,4,5)P3 and Akt. In a previous study, Cirzi et al. reported that the gene encoding for the PI phosphatase PTEN showed a high ribosome occupancy in the absence of Q-tRNAs, meaning that this gene was translated slower under these conditions (Supplementary Data S4C)12. This dysregulation of Akt activation by Q depletion would promote proliferation, survival and the Warburg effect, key processes in carcinogenesis (Fig. 2A). A detailed analysis of genes encoding for PI phosphatases and other PI regulators may be required to verify the hypothesis.
q depletion affects Akt activation. (A) Graphical scheme of the proposed effects that tRNA Q-modification may exert on the PI3K/Akt pathway. PI derivatives are phosphorylated by different PI3K to form PI-3,4,5-trisphosphate [PI(3,4,5)P3], which works as an anchorage for Akt and its upstream activating kinase, PI-dependent kinase-1 (PDK1). PI(3,4,5)P3 can be dephosphorylated by phosphatases such as PTEN. Recruitment of both Akt and PDK1 facilitates PDK1-mediated phosphorylation and subsequent activation of Akt. An increase in the activation of Akt was observed when cells were incubated in q-free medium (B,C). This result suggests that PI phosphatases encoded by Q-genes like PTEN may be downregulated in the absence of q. (B) Representative Western blots showing levels of p-Akt and total Akt in HeLa cells cultured in q-free medium in the presence (+ q) or absence (−q) of 500 nM q for 14 days. (C) Quantification of the ratio between p-Akt and total Akt. For gel source data, see Figure S3. Data represents the mean ± S.D. of five independent samples. Statistical differences were analysed by two-sample t-test (**P < 0.01).
Furthermore, we have described above that bioinformatics analyses showed that genes involved in the regulation of cell cycle seem to be enriched in NAU codons generally in eukaryotes (Fig. 1), and revealed an enrichment in human Q-genes in the ontology term “Regulation of signal transduction by p53 class mediator” (Table 2), which includes genes that are involved in the regulation of the levels of the tumour suppressor p53 protein (Supplementary Data S2). Therefore, we wondered whether p53 expression was affected by Q availability. For evaluation of p53 levels, the anti-p53 antibody DO-1 was used, which not only recognizes p53 wildtype (p53α), but also the alternative splicing variants p53β and p53γ (Fig. 3A)61. We observed that particularly the protein levels of 48 kDa p53β/γ isoforms seemed to be impaired when Q was depleted (Figs. 3B–D). p53 is mainly controlled by ubiquitination and proteasomal degradation, and certain p53 regulators are involved in p53 stabilization, preventing its degradation. The proteins most frequently associated to p53 stabilization are ATM, ATR, CHK1, CHK2, DNA-PK or USP762. We observed that ATM, ATR, CHK1 and USP7 are encoded by Q-genes and belong to the ontology term “Regulation of signal transduction by p53 class mediator” (Supplementary Data S1 and S2). Considering that p53 isoforms are differentially ubiquitinated and degraded63, we hypothesise that the downregulation of those Q-genes in the absence of q may increase p53 degradation. Other explanation for the observed changes in p53 expression in the absence of q would be that Q-tRNAs modulate the expression of certain proteins involved in the regulation of alternative splicing of specific genes, such as TP53. In this regard, we observed that Q-tRNAs were predicted to control the expression of genes involved in splicing generally in eukaryotes (Fig. 1, Table 2). In addition, it was reported that certain splicing regulators such as serine/arginine-rich splicing family (SRSF) proteins can alter the expression of p53β/γ isoforms, while p53α levels are not affected61,64. Furthermore, in a previous study, Tuorto et al. reported that the gene encoding for the splicing regulator kinase CLK1 showed a high ribosome occupancy in the absence of Q-tRNAs, indicating a slower translation of this gene (Supplementary Data S4D)9. CLK1 kinase is a major regulator of several splicing factors, particularly those SRSF proteins that are known to alter the expression of p53β/γ isoforms65,66. Moreover, Chen et al. reported that p53α and p53β showed different interactomes, with 180 proteins demonstrating greater binding to p53β than to p53α, and most of these proteins were involved in RNA processing, especially spliceosome components67. Altogether, we hypothesize that tRNA Q-modification may control in cancer cells the translation of certain Q-genes encoding for proteins involved in the stabilization of p53 levels or for splicing regulators involved in the expression of p53β and/or p53γ isoforms (Fig. 3A). Further characterization of ATM, ATR, CHK1 and USP7, together with certain splicing regulators and the analysis of the mRNA levels of the p53 splicing variants will be required to clarify whether the observed changes in the protein levels of p53 isoforms were due to the alteration of the protein stability or the mRNA splicing. In any case, it is worth noting that p53β isoform is known to enhance the transcriptional activity of p53, promoting apoptosis and senescence68,69. In addition, upregulation of p53β and p53γ was reported to restore p53 activity in p53-deficient tumours70. Clinically, p53β and p53γ are downregulated in most of breast cancer tumours68,71, and their expression is related to a better clinical outcome in patients suffering from breast cancer, acute myeloid leukaemia, and renal cell carcinoma67,69,71. Thus, the proposed downregulation of p53β/γ isoforms by Q depletion would promote cell cycle alteration and carcinogenesis.
q depletion affects p53 expression. (A) TP53 encodes for the full-length p53 form (p53α), but also the alternative splicing variants p53β and p53γ61. 48 kDa p53β/γ isoforms appeared to be impaired when Q was depleted (B–D). Anti-p53 antibody DO-1 was used in these experiments, because it allows to identify p53α and p53β/γ isoforms61. One explanation to this phenomenon may be that Q modulates the expression of certain proteins encoding for p53 stabilizers such as ATM, ATR, CHK1 and USP7, encoded by Q-genes. These proteins would prevent p53 from ubiquitination by E3-ubiquitin ligases and proteasome degradation. Other possibility is that Q affects expression of proteins involved in the regulation of alternative splicing of specific genes, such as, TP53, as Q-tRNAs were predicted to control the expression of genes involved in splicing generally in eukaryotes (Fig. 1, Table 2). (B) Representative Western blots showing levels of p53α, p53β/γ isoforms and vinculin in HeLa cells cultured in q-free medium in the presence (+ q) or absence (-q) of 500 nM q for 14 days. Vinculin was used as a loading control. (C,D) Quantification of the levels of p53α (C) and p53β/γ isoforms (D). For gel source data, see Figure S3. p53α and p53β/γ isoforms levels in the absence of q were normalized against the levels observed in the presence of q (fold change). Data represent the mean ± S.D. from four independent samples. Statistical differences were analysed by two-sample t-test (*P < 0.05, ns: not significant).
The role of Q in cancer has been addressed multiple times but it is still poorly understood and controversial. It is thought that neoplastic state is not directly caused by the absence of Q16. However, diminished tRNA Q-modification has been observed in several neoplastic tissues and cancer cell lines of colon, ovarian, brain, liver and lung cancer, and in leukaemia and lymphoma16. In addition, a low degree of Q-modified tRNAs positively correlates to the grade of tumour malignancy72,73,74,75. Moreover, gut microbiome Q biosynthesis has been positive correlated to a better clinical response to anti-PD-1 based immunotherapy in hepatobiliary cancers76. Therefore, it is thought that Q may play an anti-tumoral role somehow, although the presence of other alterations further from Q depletion would be required for cancer development. Bioinformatics data and previously reported evidences summarised in this work indicate that: i) tRNA Q-modification may control diverse processes critical in carcinogenesis, such as, ubiquitination, ER stress and UPR, cell adhesion, proliferation, splicing, PI metabolism or DNA repair; ii) diminished tRNA Q-modification was observed in multiple types of tumours; iii) Akt activation and p53 expression were dysregulated by Q depletion in human cells (Fig. 4). Thus, all these data support a potential anti-tumoral role of Q, and we propose that Q may exert this role through orchestrated translational modulation of functionally related genes enriched in NAU codons and involved in those biological processes. It is worth noting that, without more data, we cannot discard that some of these phenomena and other processes affected by Q may be due to indirect effects. One possibility is the alteration of the expression of some Q-genes encoding for transcriptional factors, leading to additional indirect effects on the transcription of the genes controlled by them. Another possibility is the activation of cellular responses due to the physiological alterations caused by the lack of Q that may alter a wide variety of process in a cell, such as the ER stress observed in the absence of Q. Further studies, such as recoding of multiple Q-genes related to Akt activation, p53 expression and other processes affected by Q, will be needed to address this question. However, while it is possible that some processes affected by Q are due to indirect effects, we believe that most of them are likely to be directly regulated by Q. Here, we would like to underline that our bioinformatics approach was able to predict a wide variety of previously reported Q-related phenotypes observed in diverse eukaryotic organisms, as previously done in bacteria23. Bearing in mind that our bioinformatics approach is based on the assumption that Q-tRNAs mainly regulates Q-genes translation, this fact leads to consider highly unlikely that all these matches between predictions and phenotypes are random.
Graphical scheme of the processes that are predicted to be dysregulated by Q-tRNAs depletion in cancer cells. Several studies reported that tRNA Q-modification is impaired in different types of tumours. Data presented in this work and in previous studies indicate that Q-tRNAs may control diverse processes critical in carcinogenesis, such as, ubiquitination, ER stress and UPR, cell adhesion, proliferation, splicing, PI metabolism or DNA repair, and we suggest that Akt activation and p53 expression could be dysregulated by Q depletion in human cells.
Certain health defects of germ-free organisms could derive from impaired q supply
As shown in this work, Q-tRNAs seem to be involved in certain important physiological processes in eukaryotes. It is known that eukaryotes must obtain q from microbiome or diet77. Therefore, in germ-free organisms, used for decades to study the physiological effects of the microbiome, the q supply could be reduced, which may affect Q-dependent processes and cause health defects. To address this hypothesis, we analysed the data from the three studies available to date in which authors compared the proteomes of different tissues of conventional and germ-free mice routinely fed under standard conditions (Supplementary Data S5A-C)33,34,35. We observed an increased frequency of NAU codons in genes encoding downregulated proteins in germ-free mice, with Q-genes being a high proportion of them, and genes encoding upregulated proteins showed opposite behaviour (Fig. 5). Therefore, these data suggest that the relative contribution of the microbiome to q supply would be high, and that q dietary supply may be not enough to fully satisfy q requirements, at least in mice fed under standard conditions in animal house facilities. In addition, functional enrichment analysis showed that those downregulated proteins in germ-free mice were especially involved in energy reserve, lipid and glucose metabolism, or mitochondrion morphogenesis, similar to the results obtained when all mouse Q-genes were submitted to the same analysis (Table 1, and Supplementary Data S2 and S5D). Moreover, these germ-free organisms are known to exhibit impaired total body fat and body weight, increased insulin sensitivity, and dysregulation of energy homeostasis, lipid metabolism, and mitochondrial metabolism, and Q-lacking mice were reported to show impaired body weight, with dysregulation in lipid and carbohydrate metabolism9,78,79. Altogether, it seems that germ-free and Q-lacking mice share some characteristics in terms of phenotype and NAU codon enrichment in genes encoding for downregulated proteins. In another model organism such as D. melanogaster, Suyama et al. recently reported that germ-free D. melanogaster flies showed impaired egg maturation due to a reduction in ecdysone hormone80 and, as described above, D. melanogaster Q-genes are particularly involved in the “Ecdysteroid metabolic process” (Table 1 and Supplementary Data S2). Thus, on the bases of these results, we hypothesise that absence of microbiome may result in at least a reduction in q supply and therefore in the downregulation of host Q-genes, which could lead to the dysfunction of certain biological processes known to be altered in germ-free organisms. The analysis of q supply by the microbiome or diet in different germ-free and q-free models will help to deepen this concept.
Absence of microbiome may reduce Q-genes expression in germ-free mice. (A) Mean frequency of NAU codons in genes encoding for down- or upregulated proteins in germ-free mice compared to conventional mice. (B) Percentage of those differentially expressed proteins (DEPs) encoded by mouse Q-genes. Data was obtained from previous studies33,34,35. Dashed lines indicate the genome-wide frequency of NAU codons (A) or the genome-wide proportion of Q-genes (B). Statistical differences were analysed by two-sample t-test (*P < 0.05). Data represents the mean ± S.D. from three independent studies.
Conclusion
Evidence supports that translational efficiency of Q-genes depends on Q-tRNAs availability9,20,22,23. In this sense, it was reported that in bacteria the biological processes affected by Q are those in which Q-genes predicted by bioinformatics of each organism are mainly involved23. For addressing whether Q plays a similar role in eukaryotes, a bioinformatics analysis of the genomes of different model organisms was performed to identify their specific Q-genes and biological processes in which they would be involved 23. We observed that bioinformatics analysis could predict a wide diversity of Q-related processes reported in several eukaryotic organisms: multicellular organism development in D. discoideum, pupae maturation in D. melanogaster, T. brucei life cycle, virulence in E. histolytica, ER stress, cell cycle, cell adhesion and energy metabolism in mice, and protein processing in ER, cellular adhesion, or cell cycle control in humans. Therefore, we propose that Q may control in eukaryotes all these species-specific physiological processes by fine-tuning of Q-genes translation, as described in bacteria. Although some Q-related phenotypes might be caused by indirect effects, all those matches between our bioinformatics predictions and previous observations lead to strongly suggest for the first time that most of Q-related phenotypes are likely to directly depend on the translational regulation of the species-specific Q-genes exerted by Q-tRNAs, and allow the prediction of novel processes affected by Q. Moreover, a high frequency of NAU codons in genes involved in protein ubiquitination, PI metabolism and dephosphorylation, RNA splicing, regulation of gene expression, DNA repair, and cell cycle, would be conserved among widely different eukaryotic species, and may suggest a general role of Q in these processes. All these data underline that translational regulation by Q-tRNAs would not only represent an additional layer of gene expression regulation, but also may allow for the coordinated control of functionally related genes in both bacteria and eukaryotes.
Furthermore, it is known that Q-tRNAs hypomodification may not be a primary cause of cancer but could promote tumour progression16,72,73,74,75,76. Bioinformatics analysis predicted that Q could affect Akt activation and p53, and experimental data showed that absence of q increased Akt activation and impaired p53β/γ expression. Considering also the previously reported ER stress and dysregulation of proliferation and cell adhesion by Q depletion, and the predicted general involvement of Q in protein ubiquitination, RNA splicing, DNA repair and cell cycle, we suggest a potential anti-tumoral role of Q and propose that control of Q-genes expression through tRNA Q-modification may be involved in cancer hallmarks such as proliferation, survival, DNA damage, protein misfolding or cell adhesion. This hypothesis is also supported by the fact that low abundancy of Q-tRNAs was observed in multiple types of tumours16,72,73,74,75, and that higher expression of QNG1, involved in the release of free q from Q precursors, positively correlates with a better prognosis of renal cancer, pancreatic ductal adenocarcinoma, acute myeloid leukaemia, and colorectal cancer metastasis to lymph nodes4,81,82,83,84. Moreover, the role of Q in protein processing seems to be crucial in neurodegenerative diseases and cognitive performance in rodents11,12, and bioinformatics analyses suggests that it could be also relevant in humans. Altogether, further studies are required for clarifying the unexplored anti-tumoral, neuroprotective potential of Q and how cells can regulate the degree of tRNA Q-modification. Additionally, bearing in mind that Q-genes expression would depend on Q-tRNAs, and that q supply by gut microbiome seems to be crucial for host Q-genes expression, we suggest that Q would represent a new molecule by which microbiome could affect host physiology through translational control of host Q-genes. Finally, considering that Q may control a wide variety of relevant physiological processes in eukaryotic organisms, we strongly suggest that Q is recognized as an essential nutrient especially for germ-free organisms or in vitro cell cultures.
Declaration
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
References
Agris, P. F., Narendran, A., Sarachan, K., Väre, V. Y. P. & Eruysal, E. The importance of being modified. In RNA Modification (eds Agris, P. F. et al.) (Elsevier, 2017).
Harada, F. & Nishimura, S. Possible anticodon sequences of tRNAHis, tRNAAsn, and tRNAAsp from Escherichia coli. Universal presence of nucleoside O in the first position of the anticodons of these transfer ribonucleic acids. Biochemistry 11, 301–308 (1972).
Zallot, R. et al. Plant, animal, and fungal micronutrient queuosine is salvaged by members of the DUF2419 protein family. ACS Chem. Biol. 9, 1812–1825 (2014).
Hung, S. H. et al. Structural basis of Qng1-mediated salvage of the micronutrient queuine from queuosine-5′-monophosphate as the biological substrate. Nucleic Acids Res. 51, 935–951 (2023).
Chen, Y. C., Kelly, V. P., Stachura, S. V. & Garcia, G. A. Characterization of the human tRNA-guanine transglycosylase: Confirmation of the heterodimeric subunit structure. RNA 16, 958–968 (2010).
Siard, T. J., Jacobson, K. B. & Farkas, W. R. Queuine metabolism and cadmium toxicity in Drosophila melanogaster. Biofactors 3, 41–47 (1991).
Schachner, E., Aschhoff, H. J. & Kersten, H. Specific changes in lactate levels, lactate dehydrogenase patterns and cytochrome b559 in Dictyostelium discoideum caused by queuine. Eur. J. Biochem. 139, 481–487 (1984).
Nagaraja, S. et al. Queuine is a nutritional regulator of Entamoeba histolytica response to oxidative stress and a virulence attenuator. mBio https://doi.org/10.1128/mBio.03549-20 (2021).
Tuorto, F. et al. Queuosine-modified tRNAs confer nutritional control of protein translation. EMBO J. https://doi.org/10.15252/embj.201899777 (2018).
Varghese, S. et al. In vivo modification of tRNA with an artificial nucleobase leads to full disease remission in an animal model of multiple sclerosis. Nucleic Acids Res. 45, 2029–2039. https://doi.org/10.1093/nar/gkw847 (2016).
Richard, P. et al. Queuine, a bacterial-derived hypermodified nucleobase, shows protection in in vitro models of neurodegeneration. PLoS One 16, e0253216 (2021).
Cirzi, C. et al. Queuosine-tRNA promotes sex-dependent learning and memory formation by maintaining codon-biased translation elongation speed. EMBO J. https://doi.org/10.15252/embj.2022112507 (2023).
Langgut, W., Reisser, T., Nishimura, S. & Kersten, H. Modulation of mammalian cell proliferation by a modified tRNA base of bacterial origin. FEBS Lett. 336, 137–142 (1993).
Reisser, T., Langgut, W. & Kersten, H. The nutrient factor queuine protects HeLa cells from hypoxic stress and improves metabolic adaptation to oxygen availability. Eur. J. Biochem. 221, 979–986 (1994).
Vinayak, M. & Pathak, C. Queuosine modification of tRNA: its divergent role in cellular machinery. Biosci. Rep. 30, 135–148 (2010).
Fergus, C., Barnes, D., Alqasem, M. & Kelly, V. The queuine micronutrient: Charting a course from microbe to man. Nutrients 7, 2897–2929 (2015).
Hayes, P. et al. Queuine micronutrient deficiency promotes warburg metabolism and reversal of the mitochondrial ATP synthase in hela cells. Nutrients 12, 871 (2020).
Zhang, J. et al. tRNA queuosine modification enzyme modulates the growth and microbiome recruitment to breast tumors. Cancers (Basel) 12, 628 (2020).
Meier, F., Suter, B., Grosjean, H., Keith, G. & Kubli, E. Queuosine modification of the wobble base in tRNAHis influences ‘in vivo’ decoding properties. EMBO J. 4, 823–827 (1985).
Morris, R. C., Brown, K. G. & Elliott, M. S. The effect of queuosine on tRNA structure and function. J. Biomol. Struct. Dyn. 16, 757–774 (1999).
Zhao, X. et al. Glycosylated queuosines in tRNAs optimize translational rate and post-embryonic growth. Cell 186, 5517-5535.e24 (2023).
Kulkarni, S. et al. Preferential import of queuosine-modified tRNAs into Trypanosoma brucei mitochondrion is critical for organellar protein synthesis. Nucleic Acids Res. 49, 8247–8260 (2021).
Díaz-Rullo, J. & González-Pastor, J. E. tRNA queuosine modification is involved in biofilm formation and virulence in bacteria. Nucleic Acids Res. 51, 9821–9837. https://doi.org/10.1093/nar/gkad667 (2023).
O’Leary, N. A. et al. Reference sequence (RefSeq) database at NCBI: current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 44, D733–D745 (2016).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.) 57, 289–300 (1995).
Huang, D. W., Sherman, B. T. & Lempicki, R. A. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 37, 1–13 (2009).
Szklarczyk, D. et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 47, D607–D613 (2019).
Kanehisa, M., Furumichi, M., Sato, Y., Kawashima, M. & Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 51, D587–D592 (2023).
Langgut, W. & Kersten, H. The deazaguanine-derivative, queuine, affects cell proliferation, protein phosphorylation and the expression of the proto-oncogenes c- fos and c- myc in HeLa cells. FEBS Lett. 265, 33–36 (1990).
Rakovich, T. et al. Queuosine deficiency in eukaryotes compromises tyrosine production through increased tetrahydrobiopterin oxidation. J. Biol. Chem. 286, 19354–19363 (2011).
Marczinke, B., Hagervall, T. & Brierley, I. The Q-base of asparaginyl-tRNA is dispensable for efficient−1 ribosomal frameshifting in eukaryotes. J. Mol. Biol. 295, 179–191 (2000).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Lichtman, J. S., Marcobal, A., Sonnenburg, J. L. & Elias, J. E. Host-centric proteomics of stool: A novel strategy focused on intestinal responses to the gut microbiota. Mol. Cell Proteom. 12, 3310–3318 (2013).
Manes, N. P. et al. Multi-omics comparative analysis reveals multiple layers of host signaling pathway regulation by the gut microbiota. mSystems https://doi.org/10.1128/mSystems.00107-17 (2017).
Tung, Y. T. et al. Characterization of the serum and liver proteomes in gut-microbiota-lacking mice. Int. J. Med. Sci. 14, 257–267 (2017).
Dormann, D., Weijer, G., Parent, C. A., Devreotes, P. N. & Weijer, C. J. Visualizing PI3 kinase-mediated cell-cell signaling during dictyostelium development. Curr. Biol. 12, 1178–1188 (2002).
Marion, S., Laurent, C. & Guillén, N. Signalization and cytoskeleton activity through myosin IB during the early steps of phagocytosis in Entamoeba histolytica: a proteomic approach. Cell Microbiol. 7, 1504–1518 (2005).
Dixit, S. et al. Dynamic queuosine changes in tRNA couple nutrient levels to codon choice in Trypanosoma brucei. Nucleic Acids Res. 49, 12986–12999 (2021).
Vassella, E., Reuner, B., Yutzy, B. & Boshart, M. Differentiation of African trypanosomes is controlled by a density sensing mechanism which signals cell cycle arrest via the cAMP pathway. J. Cell Sci. 110, 2661–2671 (1997).
White, B. N., Teneb, G. M., Holden, J. & Suzuki, D. T. Activity of a transfer RNA modifying enzyme during the development of Drosophila and its relationship to the su(s) locus. J. Mol. Biol. 74, 635–651 (1973).
Takeuchi, H., Rigden, D. J., Ebrahimi, B., Turner, P. C. & Rees, H. H. Regulation of ecdysteroid signalling during Drosophila development: identification, characterization and modelling of ecdysone oxidase, an enzyme involved in control of ligand concentration. Biochem. J. 389, 637–645 (2005).
Chong, W., Shastri, M. & Eri, R. Endoplasmic reticulum stress and oxidative stress: A vicious nexus implicated in bowel disease pathophysiology. Int. J. Mol. Sci. 18, 771 (2017).
Zhang, J. et al. Disruption to tRNA modification by queuine contributes to inflammatory bowel disease. Cell Mol. Gastroenterol. Hepatol. 15, 1371–1389 (2023).
Katze, J. R. & Beck, W. T. Administration of queuine to mice relieves modified nucleoside queuosine deficiency in Ehrlich ascites tumor tRNA. Biochem. Biophys. Res. Commun. 96, 313–319 (1980).
Pathak, C., Jaiswal, Y. K. & Vinayak, M. Possible involvement of queuine in regulation of cell proliferation. BioFactors 29, 159–173 (2007).
Elliott, M. S. & Crane, D. L. Interferon induced inhibition of queuine uptake in cultured human fibroblasts. Biochem. Biophys. Res. Commun. 171, 384–392 (1990).
Shen, W. et al. GC3-biased gene domains in mammalian genomes. Bioinformatics 31, 3081–3084 (2015).
Benisty, H. et al. Genes enriched in A/T-ending codons are co-regulated and conserved across mammals. Cell Syst. 14, 312–323 (2023).
Fruman, D. A. et al. The PI3K pathway in human disease. Cell 170, 605–635 (2017).
Manning, B. D. & Toker, A. AKT/PKB signalling: Navigating the network. Cell 169, 381–405 (2017).
Rashad, S. et al. Translational response to mitochondrial stresses is orchestrated by tRNA modifications. bioRxiv https://doi.org/10.1101/2024.02.14.580389 (2024).
Yu, J. S. L. & Cui, W. Proliferation, survival and metabolism: the role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development 143, 3050–3060 (2016).
Hoxhaj, G. & Manning, B. D. The PI3K–AKT network at the interface of oncogenic signalling and cancer metabolism. Nat. Rev. Cancer 20, 74–88 (2020).
Pathak, C., Jaiswal, Y. K. & Vinayak, M. Modulation in the activity of lactate dehydrogenase and level of c-Myc and c-Fos by modified base queuine in cancer. Cancer Biol. Ther. 7, 85–91 (2008).
Madsen, R. R. PI3K in stemness regulation: from development to cancer. Biochem. Soc. Trans. 48, 301–315 (2020).
Frazer, J. M. & Yang, W. K. Isoaccepting transfer ribonucleic acids in liver and brain of young and old BC3F1 mice. Arch. Biochem. Biophys. 153, 610–618 (1972).
Lin, V. K., Farkas, W. R. & Agris, P. F. Specific changes in Q-ribonucleoside containing transfer RNA species during Friend leukemia cell erythroid differentiation. Nucleic Acids Res. 8, 3481–3489 (1980).
Shindo-Okada, N., Terada, M. & Nishimura, S. Changes in amount of hypo-modified tRNA having guanine in place of queuine during erythroid differentiation of murine erythroleukemia cells. Eur. J. Biochem. 115, 423–428 (1981).
Singhal, R. P., Kopper, R. A., Nishimura, S. & Shindo-Okada, N. Modification of guanine to queuine in transfer RNAs during development and aging. Biochem. Biophys. Res. Commun. 99, 120–126 (1981).
Chen, Y. L. & Wu, R. T. Altered queuine modification of transfer RNA involved in the differentiation of human K562 erythroleukemia cells in the presence of distinct differentiation inducers. Cancer Res. 54, 2192–2198 (1994).
Marcel, V., Fernandes, K., Terrier, O., Lane, D. P. & Bourdon, J.C. Modulation of p53β and p53γ expression by regulating the alternative splicing of TP53 gene modifies cellular response. Cell Death Differ. 21, 1377–1387 (2014).
Abuetabh, Y. et al. DNA damage response revisited: the p53 family and its regulators provide endless cancer therapy opportunities. Exp. Mol. Med. 54, 1658–1669 (2022).
Camus, S. et al. The p53 isoforms are differentially modified by Mdm2. Cell Cycle 11, 1646–1655 (2012).
Chen, J., Crutchley, J., Zhang, D., Owzar, K. & Kastan, M. B. Identification of a DNA damage-induced alternative splicing pathway that regulates p53 and cellular senescence markers. Cancer Discov. 7, 766–781 (2017).
Duncan, P., Stojdl, D., Marius, R. & Bell, J. In vivo regulation of alternative pre-mRNA splicing by the Clk1 protein kinase. Mol. Cell. Biol. 17, 5996–6001 (1997).
Colwill, K. P. et al. The Clk/Sty protein kinase phosphorylates SR splicing factors and regulates their intranuclear distribution. EMBO J. 15, 265–275 (1996).
Chen, J. et al. DNA-damage-induced alternative splicing of p53. Cancers (Basel) 13, 251 (2021).
Bourdon, J.-C. et al. p53 isoforms can regulate p53 transcriptional activity. Genes Dev. 19, 2122–2137 (2005).
Zhao, L. & Sanyal, S. p53 isoforms as cancer biomarkers and therapeutic targets. Cancers (Basel) 14, 3145 (2022).
Gudikote, J. P. et al. Inhibition of nonsense-mediated decay rescues p53β/γ isoform expression and activates the p53 pathway in MDM2-overexpressing and select p53-mutant cancers. J. Biol. Chem. 297, 101163 (2021).
Bourdon, J.-C. et al. p53 mutant breast cancer patients expressing p53γ have as good a prognosis as wild-type p53 breast cancer patients. Breast Cancer Res. 13, R7 (2011).
Emmerich, B. et al. Relationship of queuine-lacking transfer RNA to the grade of malignancy in human leukemias and lymphomas. Cancer Res. 45, 4308–4314 (1985).
Huang, B. S., Wu, R. T. & Chien, K. Y. Relationship of the queuine content of transfer ribonucleic acids to histopathological grading and survival in human lung cancer. Cancer Res. 52, 4696–4700 (1992).
Baranowski, W., Dirheimer, G., Jakowicki, J. A. & Keith, G. Deficiency of queuine, a highly modified purine base, in transfer RNAs from primary and metastatic ovarian malignant tumors in women. Cancer Res. 54, 4468–4471 (1994).
Aytaç, U. & Gündüz, U. Q-modification of tRNAs in human brain tumors. Cancer Biochem. Biophys. 14, 93–98 (1994).
Mao, J. et al. Gut microbiome is associated with the clinical response to anti-PD-1 based immunotherapy in hepatobiliary cancers. J. Immunother. Cancer 9, e003334 (2021).
Kersten, H. The nutrient factor queuine: biosynthesis, occurrence in transfer RNA and function. Biofactors 1, 27–29 (1988).
Boulangé, C. L., Neves, A. L., Chilloux, J., Nicholson, J. K. & Dumas, M.-E. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 8, 42 (2016).
Rabot, S. et al. Germ-free C57BL/6J mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism. FASEB J. 24, 4948–4959 (2010).
Suyama, R., Cetraro, N., Yew, J. Y. & Kai, T. Microbes control Drosophila germline stem cell increase and egg maturation through hormonal pathways. Commun. Biol. 6, 1287 (2023).
Uhlen, M. et al. A pathology atlas of the human cancer transcriptome. Science 357, 6352 (2017).
Hu, D. et al. Proteomic analyses identify prognostic biomarkers for pancreatic ductal adenocarcinoma. Oncotarget 9, 9789–9807 (2018).
Sweetser, D. A. et al. Delineation of the minimal commonly deleted segment and identification of candidate tumor-suppressor genes in del(9q) acute myeloid leukemia. Genes Chromosom. Cancer 44, 279–291 (2005).
Zhuang, A. et al. Proteomic characteristics reveal the signatures and the risks of T1 colorectal cancer metastasis to lymph nodes. Elife 12, 82959 (2023).
Acknowledgements
Jorge Díaz-Rullo was supported by a FPI fellowship from the Universidad de Alcalá (Spain) and by a FPU fellowship (FPU18/03583) from the Spanish Ministry of Universities. Luis González-Moreno was supported by a FPU fellowship (FPU18/01109) from the Spanish Ministry of Universities. This research was supported by: i) the Spanish Ministry of Science and Innovation (MICINN) PID2021-126114NB-C43 (METACIRCLE), which also included European Regional Development Fund (FEDER); and ii) the Spanish Ministry of Science and Innovation (MICINN) PID2020-114499RB-I00 (to AdelA). We thank Dr. Carolina González de Figueras (Centro de Astrobiología, Spain) for technical assistance, and Universidad de Alcalá, Universidad Autónoma de Madrid and Universidad de Castilla-La Mancha (Spain) for its support to Jorge Díaz-Rullo and Luis González-Moreno during their PhD studies.
Author information
Authors and Affiliations
Contributions
J.D.R.: experiments design and performance, bioinformatic analyses, manuscript writing and reviewing. L.G.M.: experiments involving HeLa cell cultures and western blots, and manuscript reviewing. A.D.A.: manuscript reviewing. J.E.G.P.: experiments design, and manuscript writing and reviewing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Díaz-Rullo, J., González-Moreno, L., Del Arco, A. et al. Decoding the general role of tRNA queuosine modification in eukaryotes. Sci Rep 15, 345 (2025). https://doi.org/10.1038/s41598-024-83451-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-83451-y