Abstract
Body mass index (BMI) is associated with chronic obstructive pulmonary disease (COPD) risk. We investigated the association between BMI and the risk of COPD among young individuals. Using the Korean National Health Information Database, we screened individuals aged 20–39 years who participated in the national health examination between 2009 and 2012. We identified 6,304,769 eligible individuals, and 13,784 had newly developed COPD. BMI was categorized according to the Asian BMI criteria. We performed multivariate Cox proportional hazards models to estimate the adjusted hazard ratio (aHR) of risk factors for COPD development. Their mean age was 30.8 ± 5.0 years, and 3,732,656 (59.2%) were men. The incidence rate for developing COPD was 0.22/1,000 person-years. Compared to individuals with normal BMI (18.5–22.9 kg/m2), those who were underweight (< 18.5 kg/m2) had higher risks of COPD development (aHR: 1.37, 95% confidence interval [CI]: 1.29–1.46). Meanwhile, overweight or obese individuals (23–24.9 or 25–29.9 kg/m2) had lower risks for COPD development (aHR 0.90, 95% CI 0.86–0.95, and aHR 0.90, 95% CI 0.85–0.94, respectively). Although males showed tendencies similar to those of the total population, the risk was increased with increasing BMI among females. In the subgroup analysis, the risk reduction was not observed among non-smokers as BMI increased. In young individuals, being underweight was associated with an increased risk for COPD development, whereas being overweight and obese were associated with a decreased risk for COPD.
Similar content being viewed by others
Introduction
Chronic obstructive pulmonary disease (COPD) is defined as a heterogeneous lung condition presenting chronic respiratory symptoms caused by the airways and/or alveoli abnormalities, which cause persistent and progressive airflow limitations1. Traditionally, prolonged exposure to noxious gases, particularly cigarette smoke, is a well-established cause of COPD2,3. However, the recently updated COPD classification in Global Initiative for Chronic Obstructive Lung Disease 2023 guidelines require more attention to risk factors other than cigarette smoking, including biomass fuel, occupational exposures, air pollution, low birth weight, genetic variants, childhood respiratory illness, male sex, low socioeconomic status, respiratory infections, and asthma1,2,3.
The prevalence of COPD increases with aging, and COPD has been regarded as a disease of older adults3. However, some individuals aged < 50 years were also diagnosed with COPD1, and it was estimated that 49.3 million aged 30–39 years had COPD worldwide in 2019, with a prevalence of approximately 4%3. In this context, early recognition of young individuals with modifiable risks for COPD is essential, particularly other than cigarette smoking.
Being underweight is a well-established risk factor for COPD4,5 and is also associated with sarcopenia and malnutrition as risk factors for COPD6,7. On the contrary, the relationship between being overweight/obese and COPD has been inconsistent in previous studies5,8. Obesity causes substantial changes to the mechanics of the respiratory system and is also associated with increased inflammatory cytokines and immune cells that might lead to COPD9. Although obesity is associated with restrictive lung mechanics and diseases9, the obese population had a lower risk for airflow obstruction10. A meta-analysis concluded that being overweight and obese might reduce the risk of COPD; meanwhile, most included studies had limitations in that participants were aged > 40 years, sample sizes were small, and a small number of studies were included in the subgroup analysis stratified by sex4. Therefore, we evaluated the association between body mass index (BMI) and COPD development in young individuals (20–39 years) using the nationwide retrospective cohort, with further comparison by sex and stratified analysis by smoking status.
Materials and methods
Data source
The Korean National Health Insurance Service (NHIS) has provided the Korean National Health Information Database (NHID), which contains data on sociodemographic information, medical treatment, insurance claims information, health care use, national health examination, and mortality of the entire South Korean population since 200111,12. The NHIS is a single insurer, a mandatory universal public health insurance system covering approximately 97% of the Korean population. The other 3% of the population in the lowest income bracket receives public assistance, medical aid; however, the NHIS also oversees all the administrative processes for medical aid beneficiaries.
Since 1995, the NHIS has provided national health examinations for preventing and early detecting diseases13. Until 2018, all adult employees or adults aged ≥ 40 years with national health insurance received a national health examination every two years (every year for manual workers), including anthropometric measurements, laboratory tests, simple chest radiographs, and self-report questionnaires about lifestyle behaviors and medical history12,14.
Study population
Individuals aged 20–39 years who received the national health examination between 2009 and 2012 were eligible for inclusion. Among initially screened 6,891,400 individuals, we excluded 5,265 individuals with any medical insurance claim of International Classification of Diseases 10th Revision (ICD-10) codes for COPD (J43–J44) prior to their health examination (COPD wash-out), 577,714 individuals with insufficient medical records, and 3,652 individuals who were identified with insurance claims of COPD within one year after their health examination (one-year lag period). The one-year lag period was applied to exclude the over-detection of COPD after the health examination.
Subsequently, the remaining 6,304,769 eligible individuals were included and started follow-up one year after the index date. Follow-ups occurred until December 2019, and the follow-ups ceased at (1) COPD development (study outcome), (2) death, or (3) censors (e.g., out-migration). Finally, 13,784 individuals were identified with newly developed COPD (Fig. 1).
COPD and BMI definition
The study outcome was newly diagnosed COPD, which was defined as medical insurance claims of the ICD-10 codes for COPD (J44.x) or emphysema (J43.x), except for J43.0 (unilateral pulmonary emphysema, Macleod’s syndrome) ≥ three times per year for at least two years15,16,17.
Body weight and height were measured during the national health examination within two years prior to the index date. BMI was calculated by dividing body weight by the square of height (kg/m2) and categorized according to the BMI criteria for Asians: underweight (< 18.5 kg/m2), normal (18.5–22.9 kg/m2), overweight (23–24.9 kg/m2), obesity (25–29.9 kg/m2), and severe obesity (≥ 30 kg/m2)18.
Covariates
Information on anthropometric measurements (body weight, height, waist circumference, and blood pressure) and participant’s lifestyle behaviors from self-reported questionnaires were collected during the national health examination within two years prior to the index date. Smoking status was categorized into non-, former, and current smokers. A former smoker was defined as a person who had smoked at least 100 cigarettes or cigars during their lifetime but did not smoke at the time of the health examination. Alcohol consumption was categorized into none, mild (< 30 g/day), and heavy (≥ 30 g/day). Regular exercise was defined as > 30 min of moderate exercise at least five times per week or > 20 min of strenuous exercise at least three times per week19. The household income level was categorized into a quartile (Q1 = the lowest, Q4 = the highest) based on the payer’s annual national health insurance premium. Medical aid beneficiaries were also included in the Q1 category.
Previous respiratory diseases were defined as follows15: (1) asthma, insurance claims of ICD-10 codes for asthma (J45) ≥ three times per year and within five years prior to COPD diagnosis; (2) pneumonia, an insurance claim of hospitalization with ICD-10 codes for pneumonia (J10.0, J11.0, J12–J18, or A481) within five years prior to COPD diagnosis.
Using the NHIS and national health examination data within one year prior to the index date, metabolic syndrome was identified as ≥ three of the following five criteria20: (1) abdominal obesity: waist circumference ≥ 90 cm for males and ≥ 85 cm for females (Korean specific cutoffs)21; (2) elevated triglycerides: fasting blood triglyceride concentration ≥ 150 mg/dL or prescription of medications for elevated triglyceride; (3) reduced high-density lipoprotein cholesterol: blood high-density lipoprotein cholesterol concentration of < 40 mg/dL for males and < 50 mg/dL for females; (4) elevated blood pressure: blood pressure ≥ 130/85 mmHg or prescription of antihypertensive medications; (5) elevated fasting plasma glucose: fasting blood glucose concentration ≥ 100 mg/dL or prescription of anti-diabetes medications.
Statistical analysis
Continuous variables are presented as mean ± standard deviation, and categorical variables are presented as numbers (percentage). Student’s t-test and χ2 test were used to compare continuous and categorical variables, respectively. The incidence rate of COPD was calculated as the ratio between the number of newly developed COPD cases and the number of person-years (PY) at the risk of COPD (per 1,000). A multivariate Cox proportional hazards model was used to evaluate the effect of risk factors on the time-to-event of COPD development. The proportional hazards assumption was checked using the Schoenfeld residuals test. Model 1 was non-adjusted. In Model 2, the covariates included age (as a continuous variable) and sex. Model 3 included the covariates in Model 2 and cigarette smoking, alcohol consumption, regular exercise, asthma, and pneumonia. Model 4 (the main analysis model) contained the covariates in Model 3 and metabolic syndrome. For Model 4, subgroup analysis stratified by smoking status was performed to evaluate any interactive effect of smoking on the association between BMI and COPD. All p-values were two-tailed, with statistical significance set at P < 0.05. All statistical analyses were performed using SAS version 9.4 (SAS institute, Cary, NC, United States), and the PHREG procedure was used for the Cox proportional hazards model.
Results
Baseline characteristics
Table 1 shows the baseline characteristics of the study population according to the BMI category. The mean age of all participants was 30.84 ± 5.00 years, with men accounting for 59.2%. Underweight individuals were predominantly female (79.2%) and non-smokers (77.9%), whereas overweight, obese, and severely obese individuals were predominantly male (75.2%, 82.5%, and 77.3%, respectively) and current smokers (40.5%, 47.0%, and 48.6%, respectively). Compared by sex, 30.8% of males were non-smokers, whereas 90.8% of females were non-smokers. These proportions of smoking status were consistent regardless of BMI category (Supplementary Tables 1, 2, 3).
Risk of COPD development according to BMI category
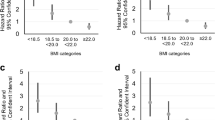
Compared with individuals with normal BMI, underweight individuals had an increased risk of developing COPD (adjusted hazard ratio [aHR] 1.37, 95% confidence interval [CI] 1.29–1.46). Whereas overweight and obese individuals had decreased risks of developing COPD (aHR 0.90, 95% CI 0.86–0.95, and aHR 0.90, 95% CI 0.85–0.94, respectively), severely obese individuals had an increased risk of developing COPD (aHR 1.11, 95% CI 1.02–1.21). Compared by sex, males showed similar tendencies to the total population, whereas females showed increased risks even in overweight and obese individuals (Table 2; Fig. 2, Supplementary Tables 4, 5, 6).
Incidence rate and adjusted hazard ratio of COPD. Incidence rates are presented as a bar graph. Adjusted hazard ratios were presented with 95% confidence intervals as a line graph. Body mass index 21.0–21.9 kg/m2 was the reference value for the hazard ratio. The value of the X-axis represents the body mass index value as a 1 kg/m2 interval (e.g., 20 represents 20–20.9 kg/m2). (A) total, (B) male, (C) female. IR: incidence rate, aHR: adjusted hazard ratio, BMI: body mass index.
Risk of COPD development according to BMI category stratified by smoking status
Underweight individuals showed consistently increased risks of developing COPD regardless of smoking status. Among overweight and obese individuals, former and current smokers showed decreased risks of developing COPD, similar to the total population, while non-smokers showed a tendency to increase the risk of developing COPD as BMI increased (Table 3). Regardless of smoking status, underweight males showed increased risks of developing COPD, which decreased among overweight and obese males. Meanwhile, the risks were not significantly different among females (Supplementary Tables 7, 8).
Discussion
In this study, we investigated the association between BMI and COPD development among young individuals in South Korea. Compared to individuals with a normal BMI, individuals who were underweight and severely obese had increased risks of developing COPD. Males showed a trend of risks for COPD similar to those of the total population, whereas females showed increasing risks for COPD as BMI increased over the normal range. In the subgroup analysis, underweight individuals had consistently increased risks of COPD regardless of smoking status. Being overweight and obese were associated with decreased risks of COPD in the total population and males; meanwhile, the risk reduction was not observed in females. These results demonstrate that being underweight is a significant risk factor for developing COPD among young individuals, and the risk might vary in overweight and obese individuals according to sex and smoking status.
Being underweight is a well-established risk factor for COPD4,5, and the risk might be associated with reduced skeletal muscle mass, including respiratory and non-respiratory muscles22. Furthermore, being underweight might be related to malnutrition and sarcopenia. Malnutrition was common among patient with COPD and associated with poor lung function7. Malnutrition also contributes to muscle dysfunction and modifies the local muscular microenvironment, resulting in protein imbalance, local inflammation and injury, and oxidative stress23. Sarcopenia was associated with weight loss, physical inactivity, inflammatory cytokines, inadequate energy intake, oxidative stress, and reduced circulation to muscles, resulting in limb muscle dysfunction and loss of muscle strength6.
In this study, the risk of COPD development was decreased in overweight and obese individuals. High BMI may indicate not only adipose tissue but skeletal muscle masses, while BMI was inversely correlated with airflow limitation and lung function decline24. Among patients with COPD, lung hyperinflation induces shortening and flattening of the diaphragm, resulting in the contractile force weakening25. Obese individuals with high respiratory and skeletal muscle masses could cope with increased airway resistance in COPD25. In this context, being overweight or obese might have protective effects against COPD.
Interestingly, this protective effect of obesity was observed in our results in males but not females. Similarly, a previous cross-sectional study in China also reported that the risk of airflow limitation was decreased with BMI in obese males, whereas the risk was correlated with BMI in obese females26. The limitations of BMI as a clinical parameter can explain this. BMI has limited utility in describing the distribution of adipose tissue and muscles, which may affect lung mechanics and metabolic dysfunction9. Abdominal obesity tends to have a greater effect on lung mechanics and metabolic dysfunction than peripheral obesity9. Furthermore, sexually different metabolic profiles among patients with COPD were reported as males representing metabolic syndrome features, enhanced systemic inflammation and upregulated muscular protein breakdown, and females representing severe muscle wasting and weakness, lower fat mass, and suppressed protein metabolism27. In this study, metabolic syndrome was observed in 15.04% of males; meanwhile, only 3.20% of females had metabolic syndrome. Moreover, the proportion of non-smokers and regular exercisers differed between sexes (Supplementary Table 1). Although we adjusted for these factors in the multivariate analysis, the risk of developing COPD was different by sex among overweight and obese individuals. To further elucidate the risk of developing COPD among the obese population, future research should investigate body composition (adipose tissue, muscle, and bone), diet, and metabolic profiles27.
This study has some notable strengths. First, a large sample size and nationwide population-based cohort enhanced the statistical power to describe the associations between BMI and COPD development in young individuals. Second, using the NHID and National Health Examination Database, we analyzed smoking history, previous respiratory illnesses, and metabolic syndrome as covariates, thus, allowing us to draw complex relationships between BMI and COPD according to sex and smoking status.
This study also has some limitations. First, COPD was defined based on the health insurance claims using ICD-10 diagnostic codes, and lung function data could not be collected because a spirometry test was not included in the health examination. Furthermore, medication history could not be collected due to the limitations of the source data. To overcome this limitation, we defined COPD as insurance claims of COPD for ≥ three times per year for at least two years to identify patients with regular hospital visits for COPD15,16,17. Second, potential other risk factors for COPD, which are related to BMI, such as sarcopenia, lean body mass, diet, and nutritional status, could not be evaluated as covariates owing to the limitation in the source data6,7. Additionally, nutritional markers (e.g., pre-albumin, albumin, etc.) were not included in the health examination. Furthermore, BMI may have limited utility in understanding the effect of obesity on the respiratory function and disease9. Although we applied metabolic syndrome as a covariate in the multivariate analysis to adjust for the effect of metabolic dysfunction in obesity, future research should be performed based on more detailed information on body composition. Third, obese individuals might have dyspnea symptoms, even without airflow limitation10. This might cause an overdiagnosis of COPD among obese individuals, particularly under the operational definition based on insurance claims. Thus, further prospective large-scale cohort studies with spirometry-based lung function data are required for the obese population. Finally, applying our results to other ethnicities requires caution because this study used the Korean population, whose BMI category and body fat distribution differ from those of Caucasians28.
In conclusion, underweight young individuals had significantly increased risks for developing COPD regardless of sex and smoking status. Although overweight and obese young individuals showed decreased risks for developing COPD, this risk reduction was observed only in males and not females or non-smokers. Complex interactions between BMI, smoking status, and sexual differences might influence the risks of developing COPD, particularly in overweight and obese populations.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- aHR:
-
adjusted hazard ratio
- BMI:
-
body mass index
- CI:
-
confidence interval
- COPD:
-
Chronic obstructive pulmonary disease
- ICD-10:
-
International Classification of Diseases 10th Revision
- NHID:
-
Korean National Health Information Database
- NHIS:
-
Korean National Health Insurance Service
- PY:
-
person-years
References
Agusti, A. et al. Global initiative for chronic obstructive lung disease 2023 report: GOLD executive summary. Eur. Respir J. 61. https://doi.org/10.1183/13993003.00239-2023 (2023).
Vogelmeier, C. F. et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am. J. Respir Crit. Care Med. 195, 557–582. https://doi.org/10.1164/rccm.201701-0218PP (2017).
Adeloye, D. et al. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: A systematic review and modelling analysis. Lancet Respir Med. 10, 447–458. https://doi.org/10.1016/S2213-2600(21)00511-7 (2022).
Zhang, X., Chen, H., Gu, K., Chen, J. & Jiang, X. Association of body mass index with risk of chronic obstructive pulmonary disease: A systematic review and meta-analysis. COPD 18, 101–113. https://doi.org/10.1080/15412555.2021.1884213 (2021).
Zhong, N. et al. Prevalence of chronic obstructive pulmonary disease in China: A large, population-based survey. Am. J. Respir Crit. Care Med. 176, 753–760. https://doi.org/10.1164/rccm.200612-1749OC (2007).
Benz, E. et al. Sarcopenia in COPD: A systematic review and meta-analysis. Eur. Respir Rev. 28. https://doi.org/10.1183/16000617.0049-2019 (2019).
Deng, M. et al. Global prevalence of malnutrition in patients with chronic obstructive pulmonary disease: Systemic review and meta-analysis. Clin. Nutr. 42, 848–858. https://doi.org/10.1016/j.clnu.2023.04.005 (2023).
Fuller-Thomson, E., Howden, K. E. N., Fuller-Thomson, L. R. & Agbeyaka, S. A strong graded relationship between level of obesity and COPD: Findings from a National Population-based study of lifelong nonsmokers. J. Obes. 2018 (6149263). https://doi.org/10.1155/2018/6149263 (2018).
Dixon, A. E. & Peters, U. The effect of obesity on lung function. Expert Rev. Respir Med. 12, 755–767. https://doi.org/10.1080/17476348.2018.1506331 (2018).
Sin, D. D., Jones, R. L. & Man, S. F. Obesity is a risk factor for dyspnea but not for airflow obstruction. Arch. Intern. Med. 162, 1477–1481. https://doi.org/10.1001/archinte.162.13.1477 (2002).
Cheol Seong, S. et al. Data resource profile: The National Health Information Database of the National Health Insurance Service in South Korea. Int. J. Epidemiol. 46, 799–800. https://doi.org/10.1093/ije/dyw253 (2017).
Seong, S. C. et al. Cohort profile: The National Health Insurance Service-National Health Screening Cohort (NHIS-HEALS) in Korea. BMJ Open. 7, e016640. https://doi.org/10.1136/bmjopen-2017-016640 (2017).
Lee, J., Lee, J. S., Park, S. H., Shin, S. A. & Kim, K. Cohort profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int J Epidemiol 46, e15. (2017). https://doi.org/10.1093/ije/dyv319
Shin, D. W., Cho, J., Park, J. H. & Cho, B. National General Health screening program in Korea: History, current status, and future direction. Precis Future Med. 6, 9–31. https://doi.org/10.23838/pfm.2021.00135 (2022).
Chung, C., Lee, K. N., Han, K., Shin, D. W. & Lee, S. W. Effect of smoking on the development of chronic obstructive pulmonary disease in young individuals: A nationwide cohort study. Front. Med. (Lausanne). 10, 1190885. https://doi.org/10.3389/fmed.2023.1190885 (2023).
Chung, C. et al. Does rheumatoid arthritis increase the risk of COPD? A nationwide retrospective cohort study. Chest https://doi.org/10.1016/j.chest.2024.02.014 (2024).
Chung, C., Lee, K. N., Shin, D. W., Lee, S. W. & Han, K. Low household income increases risks for chronic obstructive pulmonary disease in young population: A nationwide retrospective cohort study in South Korea. BMJ Open. Respir Res. 11. https://doi.org/10.1136/bmjresp-2024-002444 (2024).
Anuurad, E. et al. The new BMI criteria for asians by the regional office for the western pacific region of WHO are suitable for screening of overweight to prevent metabolic syndrome in elder Japanese workers. J. Occup. Health. 45, 335–343. https://doi.org/10.1539/joh.45.335 (2003).
Piercy, K. L. et al. The physical activity guidelines for americans. JAMA 320, 2020–2028. https://doi.org/10.1001/jama.2018.14854 (2018).
Grundy, S. M. et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National heart, lung, and blood institute scientific statement. Circulation 112, 2735–2752. https://doi.org/10.1161/CIRCULATIONAHA.105.169404 (2005).
Lee, S. Y. et al. Appropriate waist circumference cutoff points for central obesity in Korean adults. Diabetes Res. Clin. Pract. 75, 72–80. https://doi.org/10.1016/j.diabres.2006.04.013 (2007).
Li, J. et al. Association between adiposity measures and COPD risk in Chinese adults. Eur. Respir J. 55. https://doi.org/10.1183/13993003.01899-2019 (2020).
Gea, J., Agusti, A. & Roca, J. Pathophysiology of muscle dysfunction in COPD. J. Appl. Physiol. (1985). 114, 1222–1234. https://doi.org/10.1152/japplphysiol.00981.2012 (2013).
Sun, Y. et al. BMI is associated with FEV(1) decline in chronic obstructive pulmonary disease: A meta-analysis of clinical trials. Respir Res. 20, 236. https://doi.org/10.1186/s12931-019-1209-5 (2019).
Gea, J., Pascual, S., Casadevall, C., Orozco-Levi, M. & Barreiro, E. Muscle dysfunction in chronic obstructive pulmonary disease: Update on causes and biological findings. J. Thorac. Dis. 7, E418–438. https://doi.org/10.3978/j.issn.2072-1439.2015.08.04 (2015).
Huang, H. et al. Sex-specific non-linear associations between body mass index and impaired pulmonary ventilation function in a community-based population: Longgang COPD study. Front. Pharmacol. 14, 1103573. https://doi.org/10.3389/fphar.2023.1103573 (2023).
Engelen, M. et al. Sex related differences in muscle health and metabolism in chronic obstructive pulmonary disease. Clin. Nutr. 42, 1737–1746. https://doi.org/10.1016/j.clnu.2023.06.031 (2023).
Oh, S. W., Shin, S. A., Yun, Y. H., Yoo, T. & Huh, B. Y. Cut-off point of BMI and obesity-related comorbidities and mortality in middle-aged koreans. Obes. Res. 12, 2031–2040. https://doi.org/10.1038/oby.2004.254 (2004).
Acknowledgements
Not applicable.
Funding
This study was supported by a National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (2023R1A2C2006688), the Bio&Medical Technology Development Program of the NRF funded by the MSIT (2022M3A9G8017220 and RS-2023-00222687), the National Institute of Health research project (2024ER080600), a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health & Welfare, Republic of Korea (HI23C0679), and Medical Research Promotion Program through the Gangneung Asan Hospital funded by the Asan Foundation (2024II0008).
Author information
Authors and Affiliations
Contributions
Chiwook Chung, Hajeong Kim, Kyu Na Lee, Dong Wook Shin, Sei Won Lee, and Kyungdo Han conceived and designed the study. Kyu Na Lee and Kyungdo Han contributed to the data collection and analysis. The data were interpreted by Chiwook Chung, Hajeong Kim, Kyu Na Lee, Dong Wook Shin, Sei Won Lee, and Kyungdo Han. Chiwook Chung and Hajeong Kim drafted the manuscript. All authors revised and approved the final manuscript. All authors accept responsibility for the accuracy of the content in the final manuscript. Generative artificial intelligence was not used in any portion of the manuscript writing.
Corresponding authors
Ethics declarations
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Ethics statement
The ethical approval in this study was waived by the Institutional Review Board of Asan Medical Center, Seoul, Republic of Korea (IRB No. 2022 − 1593). The requirement for informed consent was waived, as it was a retrospective study, and the data used were anonymized. This study complied with the guidelines stipulated in the Declaration of Helsinki, and all methods were performed in accordance with the relevant guidelines.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chung, C., Kim, H., Lee, K.N. et al. Association between body mass index and chronic obstructive pulmonary disease in young individuals: a nationwide population‑based cohort study. Sci Rep 14, 31976 (2024). https://doi.org/10.1038/s41598-024-83648-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-83648-1