Abstract
Bacterial cell division and plant chloroplast division require selfassembling Filamentous temperature-sensitive Z (FtsZ) proteins. FtsZ proteins are GTPases sharing structural and biochemical similarities with eukaryotic tubulin. In the moss Physcomitrella, the morphology of the FtsZ polymer networks varies between the different FtsZ isoforms. The underlying mechanism and foundation of the distinct networks is unknown. Here, we investigated the interaction of Physcomitrella FtsZ2-1 with FtsZ1 isoforms via co-immunoprecipitation and mass spectrometry, and found protein-protein interaction in vivo. We tagged FtsZ1-2 and FtsZ2-1 with different fluorophores and expressed both in E. coli, which led to the formation of defined structures within the cells and to an influence on bacterial cell division and morphology. Furthermore, we have optimized the purification protocols for FtsZ1-2 and FtsZ2-1 expressed in E. coli and characterized their GTPase activity and polymerization in vitro. Both FtsZ isoforms showed GTPase activity. Stoichiometric mixing of both proteins led to a significantly increased GTPase activity, indicating a synergistic interaction between them. In light scattering assays, we observed GTP-dependent assembly of FtsZ1-2 and of FtsZ2-1 in a protein concentration dependent manner. Stoichiometric mixing of both proteins resulted in significantly faster polymerization, again indicating a synergistic interaction between them. Under the same conditions used for GTPase and light scattering assays both FtsZ isoforms formed filaments in a GTP-dependent manner as visualized by transmission electron microscopy (TEM). Taken together, our results reveal that Physcomitrella FtsZ1-2 and FtsZ2-1 are functionally different, can synergistically interact in vivo and in vitro, and differ in their properties from FtsZ proteins from bacteria, archaea and vascular plants.
Similar content being viewed by others
Introduction
Plants harvest energy from oxygenic photosynthesis, during which light is the energy source for the fixation of atmospheric CO2 in organic compounds, while O2 is produced as a side product. In eukaryotic cells, photosynthesis depends on chloroplasts1. These cell organelles derived from endosymbiosis, where a eukaryote engulfed a free-living, photoautotrophic cyanobacterial-like prokaryote2,3,4. While some genes of prokaryotic origin disappeared over time, or were retained in the organellar genome, respectively, most were transferred to the host nucleus after the endosymbiotic event5. The relation of these organelles to prokaryotes explains why chloroplasts do not develop de novo but divide by binary fission6, while at the same time suggesting that prokaryotic cytokinesis and cell organelle division are conserved mechanisms7,8.
Most bacteria and chloroplasts of bryophytes, comprising mosses, liverworts, and hornworts, are surrounded by a peptidoglycan wall9,10, which needs to be remodelled during bacterial cell division11,12. Proteins involved in bacterial cell division are also responsible for plastid division in plants6,8. Both mechanisms require Filamentous temperature-sensitive Z (FtsZ) proteins as a key component of the division machinery13,14. FtsZ proteins were named after one of the filamentous temperature-sensitive Escherichia coli (E. coli) mutants, which grows filamentous due to their inability to divide at elevated temperatures15. FtsZ proteins assemble into a ring structure, the Z-ring, thus determining the future division site of bacteria and chloroplasts16,17,18. In E. coli and Bacillus subtilis, FtsZ-function is coupled to peptidoglycan synthesis11,19. Remarkably, bacterial cell division as well as division of moss chloroplasts, but not plastid division of vascular plants, is sensitive to beta-lactam antibiotics20.
FtsZ proteins are soluble guanosine triphosphatases (GTPases)21,22,23, that share structural and biochemical similarities with the eukaryotic cytoskeletal protein tubulin24,25. FtsZ proteins have similar functions in bacteria and plastids78,. The nuclear-encoded plant FtsZ proteins are imported into the chloroplast7, where they are required for plastid division as well as plastid shaping26. In most bacteria, FtsZ is encoded by a single gene27,28, while plants and archaea, except the TACK and ASGARD superphyla, encode predominantly two FtsZ isoforms25,29. For instance, Arabidopsis thaliana(Arabidopsis) encodes FtsZ1 and FtsZ27,14. These arose from a gene duplication29, in which plant FtsZ1 arose from FtsZ230,31,32.
In the vascular plant Arabidopsis, both FtsZ isoforms have non-redundant functions since their independent expression inhibition led in both cases to a reduced number of chloroplasts14. The rings formed by Arabidopsis FtsZ1 and FtsZ2 colocalize in vivo at the chloroplast centre17 and form heteropolymers at the future division site upon recombinant expression in the yeast Pichia pastoris (new species name Komagataella phaffii)18. Also, the polymerization behaviour of Arabidopsis FtsZ isoforms has been characterized in vitro33,34. Here, FtsZ1 and FtsZ2 show differences in their GTPase activity and polymerization mechanism and only FtsZ2 polymerized on its own34.
Furthermore, FtsZ1 and FtsZ2 show differences regarding their protein structure. In general, FtsZ proteins contain two domains, a widely conserved N-terminal GTPase domain and a C-terminal region32,35. The GTPase domain comprises a GTP-binding domain and a GTPase-activating domain. Together, they represent the core structure of FtsZ18, since the C-terminal region is more variable36. The N-terminal domain is responsible for GTP binding and hydrolysis35. FtsZ2 proteins contain an additional short motif at the C-terminus that is missing in FtsZ131,32. In Arabidopsis, this motif is responsible for the interaction of FtsZ2 with transmembrane proteins32. Recently, a conserved sequence motif of the C-terminal domain of Arabidopsis FtsZ1 was identified that is involved in membrane binding and interactions with other proteins of the plastid division machinery37. Moreover, the N- as well as the C-terminus of both isoforms are involved in polymer formation of FtsZ1 and FtsZ238.
The moss Physcomitrella (new species name Physcomitrium patens) encodes the high number of five different FtsZ proteins (FtsZ1-1, FtsZ1-2, FtsZ2-1, FtsZ2-2, FtsZ3), which fall in three clades (FtsZ1, FtsZ2, FtsZ3), indicating neofunctionalisation of the different isoforms during evolution30,39,40. Arabidopsis FtsZ1 and FtsZ2 are orthologues to Physcomitrella FtsZ1 and FtsZ239,40. Among those FtsZ isoforms, FtsZ2-1 was proven to be involved in chloroplast division8,41,42.
The Physcomitrella FtsZ proteins assemble in network-like structures inside the chloroplast26,43. Using reverse genetics, the diverse and non-redundant functions of the five FtsZ isoforms in Physcomitrella were investigated42. Here, the analysis of distinct single and double knockout mutants of Physcomitrella ftsZ genes revealed their roles in chloroplast division, chloroplast shaping, as well as their network assembling properties within the chloroplast26,42. Due to these characteristics, the Physcomitrella FtsZ proteins ensure plastid stability and structural integrity26,44. Since this FtsZ network in moss plastids resembles the structure of the eukaryotic cytoskeleton, the term ‘plastoskeleton’ was coined to describe its organization and function in plastids26,45. Subsequently, it was discovered that not only eukaryotes but also bacteria have a highly dynamic cytoskeleton in which FtsZ is a part46,47,48,49.
Unlike described for other plants, proteins of the nuclear ftsZ genes are not only imported into plastids but are dually targeted to chloroplasts and the cytosol in Physcomitrella, suggesting that they have functions beyond plastid division50. Interestingly, this coincides with a low diversification of the cytoskeletal protein family tubulin in Physcomitrella51. Subsequently, Physcomitrella FtsZ proteins were found to be involved in cell patterning, plant development, and gravity sensing42. Additionally, distinct localization-dependent in vivo interactions among four of the five Physcomitrella FtsZ isoforms were revealed by using fluorescence resonance energy transfer (FRET)52.
The morphology of the Physcomitrella FtsZ polymer networks varies between the different isoforms. In vivo network analysis using GFP-tagged FtsZ1-2 and FtsZ2-1 indicated functional differences between them53. While the FtsZ2-1 network is exclusively formed within the chloroplast, FtsZ1-2 networks form long, extraplastidic extensions and may play a role in the formation of stromules53, stroma-filled, tubular connections between chloroplasts54. The FtsZ1-2 network also contains significantly more nodes than the FtsZ2-1 network, while so-called meganodes (extraordinarily large nodes) occur only with FtsZ2-1. Moreover, FtsZ2-1 filaments are more curved in vivo, thicker in segment size and resemble the microtubules of the cytoskeleton more than FtsZ1-2. This resemblance hints towards mechanical properties that might be interesting when using Physcomitrella FtsZ filaments to develop sustainable material systems55. Based on the identified morphological differences of Physcomitrella FtsZ1-2 and FtsZ2-153, the mechanical behaviour and load-bearing responses of the two Physcomitrella FtsZ isoforms were investigated in in silico experiments, relating the structure to the function of the FtsZ protein networks55,56,57. The capability of the plastoskeleton to maintain its load-bearing structure even under distortion is based on its material properties as polymers and more importantly, on the structural features of the network55.
Besides the mechanical properties of the FtsZ isoforms, knowledge about the polymer-forming properties of Physcomitrella FtsZ proteins as a GTPdriven structure is of particular interest. Combining the data about the load-bearing structure of the plastoskeleton with knowledge about the biochemical foundations could pave the way for adaptive biomimetic materials58 or 3D-printed biopolymer materials for tissue engineering59. In various tissues, Physcomitrella FtsZ1-2 and FtsZ2-1 have relatively high expression levels, suggesting division-independent, stable networks within the chloroplast53. We examined GTPase activity, polymer assembly, and formation of the Physcomitrella FtsZ network in vitro. We expressed Physcomitrella FtsZ1-2 and FtsZ2-1 in E. coli, purified the proteins, and analysed their GTP-dependent polymerization individually and together. Furthermore, we investigated the interaction of these isoforms in vivo, and visualised both FtsZ isoforms in E. coli cells individually and in combination without potential interaction partners.
Different bacterial FtsZ (e.g., B. subtilis FtsZ and E. coli FtsZ60, Agrobacterium tumefaciens FtsZ61, Synechococcus elongatus23, archaeal FtsZ (Haloferax volcanii29,62), and plant FtsZ (Arabidopsis23,34) were already investigated regarding their assembly and biochemical properties. Here, we provide insights into the GTP-dependent polymerization and the assembly properties of FtsZ1-2 and FtsZ2-1 of the moss Physcomitrella.
Results
In-vivo interaction of Physcomitrella FtsZ2-1 with FtsZ1
Distinct localization-dependent interactions among four of the five Physcomitrella FtsZ isoforms have been revealed by overexpression as GFP fusions52. Here, we independently reassessed these results on a physiological level with special focus on the interactions between FtsZ1 isoforms and FtsZ2-1.
To address this, an in-frame fusion of Physcomitrella FtsZ2-1 with a single linker-GFP (Fig. 1A, Supplemental Figure S1A) at the endogenous locus was generated via targeted knockin (Supplemental Table S1), employing the highly efficient homologous recombination in this moss63,64. Twenty-one selected lines were screened by PCR for the presence of the GFP coding sequence (CDS). From these, sixteen positive candidate lines were selected and the correct integration of the knock-in construct was analysed by PCR (Supplemental Figure S1A, B). From this analysis three of the fifteen positive lines were chosen for a test co-immunoprecipitation (Co-IP, Supplemental Figure S1C). Finally, line #513 was chosen for quantitative co-immunoprecipitations (Co-IPs), performed using GFP-Trap Magnetic Particles, with Physcomitrella wild type (WT) as a negative control. Label-free quantitation values (LFQ) obtained from a MaxQuant search65,66 were used and significant interacting partners were identified at a false discovery rate (FDR) of 0.01% (Fig. 1B). The significant interacting proteins can be found in Supplemental Table S2.
From this, Physcomitrella FtsZ1 isoforms were identified as significant interactors of FtsZ2-1 (Fig. 1). Thus, we confirmed that FtsZ2-1 is interacting with FtsZ1 proteins in vivo. However, due to their high sequence similarity (Fig. 1C) and the limited number of matching peptides, the isoforms FtsZ1-1 and FtsZ1-2 could not be distinguished in these experiments. Hence, we were able to confirm that Physcomitrella FtsZ2-1 is interacting with Physcomitrella FtsZ1 proteins in vivo, but we could not explicitly narrow this down to an interaction with FtsZ1-2. As Co-IPs do not allow to assess spatial information of interactions, they can be direct or indirect.
FtsZ domain structures, sequence identity and analysis of the co-immunoprecipitations (Co-IPs) against GFP-tagged FtsZ2-1 protein. (A) Domain structures of the five Physcomitrella FtsZ proteins and the FtsZ2-1_GFP fusion protein. Depicted are PFAM67 domains (FtsZ family, C-terminal domain: PF12327; Tubulin/FtsZ family, GTPase domain: PF00091; GFP: PF01353) and the poly-G linker in the FtsZ2-1_GFP fusion protein. Arrows indicate predicted chloroplast transit peptide (cTP) cleavage sites (blue: ChloroP1.1; green: TargetP2.0). Predicted cTP cleavage sites of other FtsZ isoforms than FtsZ1-2 and FtsZ2-1 are not shown. The image was created with the R package drawProteins68. (B) The volcano plot shows the log2 ratios of normalized LFQ (label-free quantitation) intensities plotted against log10 of adjusted p-values. Proteins significantly enriched in the GFP-tagged pulldown are shown as red crosses with a false discovery rate (FDR) of 0.01%. Physcomitrella wild type (WT) served as a negative control. Proteins not significant for neither WT nor FtsZ2-1_GFP are visualized as grey crosses. The Co-IP was performed with GFP-Trap Magnetic Particles M-270 (ChromoTek GmbH, Planegg, Germany) and Physcomitrella protonema tissue homogenized from suspension culture. The isoforms of FtsZ1 (FtsZ1-1, FtsZ1-2) could not be distinguished on the basis of the peptides identified in this approach and are thus grouped (FtsZ1). (C) Matrix showing the sequence identity ([%]) between the five different FtsZ isoforms. Identity values were obtained from a multiple sequence alignment using protein sequences done with the UniProt Align tool (https://www.uniprot.org/align).
Expression of Physcomitrella FtsZ1-2 and FtsZ2-1 in E. coli
The respective plastoskeletal morphologies of Physcomitrella FtsZ1-2 and FtsZ2-1, which were elucidated before53, hint towards functional differences of these two isoforms. Therefore, GTPase activity, polymer assembly, and the formation of Physcomitrella FtsZ1-2 and FtsZ2-1 filaments in vitro were studied.
In order to produce Physcomitrella FtsZ1-2 and FtsZ2-1 in E. coli, the coding sequences (CDS) of both FtsZ isoforms were optimized for codon usage in bacteria (Supplemental Figures S2 and S3). In total, 304 bases were changed for FtsZ1-2 and 357 bases were changed for FtsZ2-1. The GC content was concurrently increased from 49 to 51% for FtsZ1-2, and from 49 to 52% for FtsZ2-1. The in silico predicted N-terminal transit peptides for Physcomitrella FtsZ1-2 (Pp3c19_2490) and Physcomitrella FtsZ2-1 (Pp3c11_17860) were 34 aa and 31 aa (ChloroP 1.1, http://www.cbs.dtu.dk/services/ChloroP/), respectively. The predictions of TargetP-2.0 (http://www.cbs.dtu.dk/services/TargetP/) and ChloroP 1.1 differ (Table 1; Fig. 1A) and the exact estimation of cTPs remains challenging. The shorter predictions by ChloroP 1.1 were chosen so as not to affect the functional domains of both proteins (Fig. 1A).
The respective codon-optimized FtsZ1-2 and FtsZ2-1 sequence, fused to a C-terminal 8 x His-tag encoding sequence, were subcloned into the pLATE11 expression vector (Supplemental Tables S3 and S4) and with the correct plasmids subsequently transformed into BL21 Star™ (DE3) cells. Immunoblots of IPTG-induced strains confirmed the expression of both isoforms (Supplemental Figure S4).
Physcomitrella FtsZ proteins localize in foci in elongated E. coli cells
To investigate the behaviour of each isoform individually and in combination within a heterologous system, fluorescent FtsZ fusions were employed. E. coli FtsZ, which is non-functional in Physcomitrella and does not interact with Physcomitrella FtsZ isoforms in FRET experiments51, is not anticipated to interact with Physcomitrella proteins. FtsZ1-2 was fused at the N-terminus with enhanced Green Fluorescent Protein (eGFP), while FtsZ2-1 was fused at the N-terminus with mKusabira-Orange2 (mKOrange2, mKO2). To serve as controls, eGFP-His8 and mKO2-His were used respectively. All CDS were cloned into the inducible pLATE11 expression vector. For the co-expression of eGFP-FtsZ1-2 and mKO2-FtsZ2-1, the latter expression cassette was introduced into the initially generated pLATE11_eGFP-FtsZ1-2 vector. BL21 Star™ (DE3) cells were transformed with the final plasmids. Confocal microscopy of IPTG-induced bacteria revealed that the eGFP-His control was localized in a diffuse pattern within the cell (Fig. 2A). The cells expressing eGFP-His grew normally and were approximately 1–2 μm long. The eGFP-FtsZ1-2 protein was localized in numerous foci within the cell (Fig. 2B), and, in some instances, formed single filaments or filamentous networks (Fig. 3, Supplemental Figures S5A, B, E, and S7C). Cells expressing eGFP-FtsZ1-2 were longer than those expressing eGFP-His, showing a length distribution between 5 and 10 μm (Fig. 2B, and Supplemental Figure S5). The distribution of the eGFP-FtsZ1-2 signal suggests the assembly of functional structures, which is further supported by the filamentous phenotype of the E. coli cells expressing eGFP-FtsZ1-2, which resembles the phenotype of E. coli strains overexpressing FtsZ or ftsZ depletion strains.
As with eGFP-His, the mKO2-His control exhibited a diffuse localization throughout the cell (Fig. 2C). Cells transformed with mKO2-His also grew normally and were approximately 1–2 μm long. The mKO2-FtsZ2-1 protein was distributed in foci throughout the whole cell. The cells were elongated, ranging between 5 and 20 μm in length (Fig. 2D, Supplemental Figure S6). In analogy to eGFP-FtsZ1-2, we infer that mKO2-FtsZ2-1 also assembles in functional structures when expressed in E. coli cells.
In general, the signal of mKO2-FtsZ2-1 expressed in E. coli was weaker than the signal of eGFP-FtsZ1-2. Furthermore, it was observed that the foci of the mKO2-FtsZ2-1 were less frequent than in eGFP-FtsZ1-2 and were situated at regular intervals inside the cell. In contrast to eGFP-FtsZ1-2, no filaments between the foci of mKO2-FtsZ2-1 were observed.
Cellular positioning of Physcomitrella FtsZ1-2 and FtsZ2-1 fluorescent fusion proteins in transformed E. coli cells. Confocal fluorescence microscopy of (A) eGFP-His, (B) eGFP-tagged Physcomitrella FtsZ1-2, (C) mKOrange2-His and (D) mKOrange2-tagged Physcomitrella FtsZ2-1 in E. coli. Scale bars 5 μm.
Confocal microscopy of E. coli cells containing the double construct eGFP-FtsZ1-2_mKO2-FtsZ2-1 confirmed the heterologous expression of eGFP-FtsZ1-2 and mKO2-FtsZ2-1 in E. coli (Fig. 3). Cells expressing eGFP-FtsZ1-2_mKO2-FtsZ2-1 were elongated and ranged between 5 and 20 μm in length (Fig. 3, Supplemental Figure S7). In these cells, eGFP-FtsZ1-2 was once again localized in foci and filamentous networks. Correspondingly, the distribution pattern of mKO2-FtsZ2-1 was similar as observed in the cells transformed with the single constructs: mKO2-FtsZ2-1 was distributed in foci throughout the whole cell. Remarkably, it was observed that eGFP-FtsZ1-2 and mKO2-FtsZ2-1 co-localize in these foci within the cell (Fig. 3) suggesting a direct or indirect interaction between the two proteins. Furthermore, it was observed that bacterial cells expressing eGFP-FtsZ1-2, mKO2-FtsZ2-1 and the construct with both FtsZ fusion proteins together exhibited highly aberrant phenotypes (Figs. 2 and 3, Supplemental Figures S5, S6 and S7), suggesting that expression of the two moss FtsZ proteins affected bacterial cell division and shaping.
Cellular positioning of combined expression of Physcomitrella FtsZ1-2 and FtsZ2-1 fluorescent fusion proteins in E. coli. Confocal fluorescence microscopy of E. coli cells co-expressing eGFP-tagged Physcomitrella FtsZ1-2 and mKOrange2-tagged Physcomitrella FtsZ2-1. Filamentous eGFP-FtsZ1-2-derived network formation is highlighted with an arrow. Scale bars 5 µm.
Optimized purification of recombinant Physcomitrella FtsZ proteins
To investigate whether Physcomitrella FtsZ1-2 and FtsZ2-1 can form plastoskeleton-like structures in the absence of other proteins, we aimed to purify both proteins recombinantly produced in E. coli. Overexpression and initial attempts to purify the C-terminally His-tagged Physcomitrella FtsZ1-2 and FtsZ2-1 from E. coli revealed that both proteins were partially localized in inclusion bodies. Purification of the soluble fractions using standard affinity purification protocols resulted in relatively low yields and highly contaminated protein samples. Consequently, we sought to optimize the expression strategies for both FtsZ isoforms. To enhance the stability of the FtsZ proteins and reduce inclusion body formation, Physcomitrella FtsZ1-2 and FtsZ2-1, lacking the N-terminal transit peptides of 86 and 42 amino acids, respectively (Table 1), were fused to an N-terminal His6-SUMO-tag, which was cleaved off after the first purification step. Various expression conditions were tested, with the highest amounts of soluble protein obtained after growth in a simplified version of autoinduction medium at 25 °C, as described by69. The buffer composition used during the purification process was subsequently optimized. Both proteins exhibited significant aggregation at lower and neutral pH values, as well as at higher salt concentrations. Stabilization was achieved in the presence of GDP and glycerol, whereas the presence of sodium inhibited activity after purification. The optimized purification buffer compositions were as follows: for FtsZ1-2, 50 mM glycine (pH 9.5), 150 mM KCl, 1 mM MgCl₂, and 5% glycerol; for FtsZ2-1, 25 mM glycine (pH 10.5), 50 mM KCl, 1 mM MgCl₂, and 5% glycerol. This resulted in highly enriched FtsZ1-2 or FtsZ2-1, respectively (Supplemental Figure S8).
In vitro polymerization of Physcomitrella FtsZ1-2 and FtsZ2-1
To evaluate the activity of both FtsZ proteins, polymer formation was assessed using light-scattering assays. This method is highly effective for monitoring FtsZ filament formation in real-time, as the intensity of light scattering is directly proportional to the mass of polymers formed70. The light-scattering experiments were performed using the optimized purification buffers. For both proteins, a protein concentration-dependent increase in light scattering was observed upon the addition of GTP, indicating the formation of FtsZ filaments (Fig. 4A and B). Interestingly, both proteins exhibited an initial lag phase following GTP addition, during which no increase in light scattering was detected. Attempts to enhance the polymerization rate of the two FtsZ proteins by altering the buffer conditions to those typically favouring polymerizations in other FtsZ proteins, such as lowering the pH or increasing the potassium concentration, resulted in protein precipitation. However, when equal concentrations of the two FtsZ proteins were combined, a significantly faster increase in light scattering was observed after GTP addition, indicating a synergistic interaction between the two proteins (Fig. 4C).
Light scattering experiments with FtsZ1-2 and FtsZ2-1, individually and in combination of 5 mM. Individual light scattering experiments were performed using 1.875 µM, 3.5 µM, 7.5 µM or 15 µM of either of FtsZ1-2 in buffer C (A) or FtsZ2-1 in buffer B (B). The combined light scattering experiments were performed in a buffer containing 37.5 mM glycine (pH 10), 100 mM KCl, 5 mM MgCl₂, and 5% glycerol. For these experiments, 7.5 µM of each FtsZ1-2 and FtsZ2-1 were mixed and analysed in technical triplicates. Additionally, 7.5 µM of each FtsZ variant was measured separately in triplicates under the same buffer conditions (C). Light scattering in counts per seconds (cps).
Physcomitrella FtsZ proteins show GTPase activity in vitro
To further investigate the synergistic interaction between FtsZ1-2 and FtsZ2-1, the GTPase hydrolysis rates of the proteins were measured. Both FtsZ proteins exhibited low but significant GTPase activities. FtsZ1-2, which demonstrated a lower and slower increase in light scattering, displayed higher GTPase activity compared to FtsZ2-1. Stoichiometric mixing of both FtsZ isoforms led to a significant increase in GTPase activity, exceeding the activity observed for the individual proteins (Fig. 5). This observation further supports the existence of a synergistic interaction between the two FtsZ isoforms.
GTPase activity of Physcomitrella FtsZ1-2 and FtsZ2-1, individually and in combination. GTPase assays were performed at 25 °C using either 7.5 µM of FtsZ1-2 or 7.5 µM of FtsZ2-1 individually, or a mixture of 7.5 µM FtsZ1-2 with 7.5 µM FtsZ2-1. The assays were performed in a buffer containing 37.5 mM glycine (pH 10), 100 mM KCl, 5 mM MgCl₂, and 5% glycerol in the presence of 1 mM GTP and conducted in technical triplicates. To account for background GTP auto-hydrolysis, parallel reactions without FtsZ variants were included for each replicate. Background values were subtracted from the corresponding experimental measurements. Absorbance was measured at 620 nm after a 10-minute incubation. The amount of free phosphate (µM) released per minute was determined using a phosphate standard calibration curve. Data are presented as mean ± standard deviation from three technical replicates.
GTP-dependent filament formation of FtsZ1-2 and FtsZ2-1
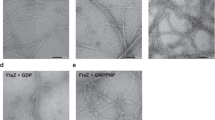
The formation of FtsZ filaments was further investigated using negative-stain transmission electron microscopy. Under the same conditions used for the GTPase and light-scattering experiments, filaments were observed for both FtsZ1-2 and FtsZ2-1 when incubated in the presence of 2 mM GTP (Fig. 6), while no filaments were detected in the absence of GTP (Supplemental Figure S9). FtsZ1-2 formed small protofilaments, which further assembled into wider filaments, adopting a straight, bundle-like conformation. In contrast, FtsZ2-1 mainly polymerized into curved filaments with straight filaments being observed only occasionally. While filament bundles formed from FtsZ1-2 were wider, those formed from FtsZ2-1 were notably thinner. When FtsZ1-2 and FtsZ2-1 were co-incubated in the presence of GTP, both curved and straight filaments were observed with occasionally single protofilaments also present. Overall the appearance of the filaments more closely resembled that of FtsZ2-1. However, it was not possible to distinguish between FtsZ1-2, FtsZ2-1, or potential hybrid FtsZ1-2/FtsZ2-1 filaments (Fig. 6). In conclusion, purified Physcomitrella FtsZ1-2 and FtsZ2-1 are active and capable of forming FtsZ filaments in the presence of GTP.
Negative stain transmission electron microscopies of Physcomitrella FtsZ filaments. 7.5 µM of FtsZ1-2 (left), 7.5 µM FtsZ2-1 (middle) and 7.5 µM FtsZ1-2 mixed with 7.5 µM FtsZ2-1 (right) were incubated at room temperature for 5 min with 2 mM GTP and then imaged by transmission electron microscopy. Representative images are shown. Scale bars as indicated. Experiments were repeated with two independent purifications with similar results.
Discussion
FtsZ proteins are GTPases and GTP binding is a prerequisite for their polymerization, and GTP hydrolysis supports filament dynamics and turnover21,22,34. The moss Physcomitrella encodes five different FtsZ proteins, suggesting neofunctionalisation of the different isoforms during evolution30,39,40. The interplay among the five FtsZ isoforms results in the formation of a filament network inside the chloroplasts, where each isoform exhibits distinct filament characteristics52. Two of the five isoforms (FtsZ1-2, FtsZ2-1) have relatively high expression levels in various moss tissues, indicating division-independent, stable networks within the chloroplast53. Knowledge about the polymer-forming properties of FtsZ1-2 and FtsZ2-1 as a GTP-driven non-equilibrium structure is of particular interest to develop sustainable material systems71. Here, we expressed FtsZ1-2 and FtsZ2-1 in E. coli. We studied the localization of fluorescence-tagged Physcomitrella FtsZ in E. coli cells, established a purification protocol for the recombinant FtsZ isoforms, and characterized their GTPase activity and polymerization in vitro. Expression of fluorescence-tagged Physcomitrella FtsZ1-2 and FtsZ2-1 in E. coli resulted in fluorescent foci and elongated cells. The distribution pattern of fluorescence tagged Physcomitrella FtsZ is similar to E. coli FtsZ tagged with GFP72. Elevated levels of E. coli FtsZ tagged with GFP also localize in foci, and overexpression of E. coli FtsZ at least 10-fold above wild-type levels inhibits cell division, leading to filamentous cell shapes. In contrast, mild overexpression induces the formation of minicells72,73. The foci at higher FtsZ concentrations were suggested to represent FtsZ nucleation sites72. In contrast, Synechococcus elongatus FtsZ tagged with mCerulean and Arabidopsis FtsZ1 tagged with mVenus localize to long curved filaments when expressed separately in yeast23. The localization of Physcomitrella FtsZ1-2 and FtsZ2-1 in E. coli indicates that the FtsZ function is evolutionary conserved and that Physcomitrella FtsZ interferes with cell division and morphology when overexpressed in E. coli.
To analyse the in vitro-polymerization of both Physcomitrella FtsZ isoforms individually and in combination, GTPase assays and light scattering assays were performed. GTPase and light scattering assays are FtsZ-concentration dependent and are generally performed at concentrations between 1 and 15 µM. For example, concentrations ranging up to 8 µM for Agrobacterium tumefaciens FtsZ29 and 12 µM for Bacillus subtilis FtsZ and E. coli FtsZ60 have been used previously. Notably, E. coli FtsZ polymerizes rapidly and reaches a steady-state within only 30 s74. Indeed, both Physcomitrella FtsZ proteins exhibited concentration-dependent polymerization at concentrations higher than ~ 2 µM. While the individual proteins exhibited a short lag phase prior to polymerization, a mixture of both proteins initiated polymerization immediately. Although strong polymerization was observed, the GTPase activity (~ 0.2–0.4 GTP/min) was relatively low compared to that reported for FtsZ proteins from other organisms60. This may, however, be attributed to the instability of both FtsZ proteins at low and neutral pH as well as under high-salt conditions, limiting the assays to high-pH and low-salt conditions. These conditions deviate from both the natural environment and the conditions under which most FtsZ proteins exhibit optimal polymerization activity. GTP hydrolysis is essential for depolymerization, and consistent with this, no depolymerization of the FtsZ proteins was observed during the 10-minute duration of the experiments. Alternatively, our results support the idea23 that a low GTPase activity of plant FtsZ proteins compared to bacterial FtsZ is a conserved feature. One reason might be that bacteria divide faster than chloroplasts, and thus require a higher GTPase activity and faster assembly of the Z-ring. In contrast to bacteria, chloroplasts are not able to divide on their own as chloroplast division relies on additional nuclear-encoded proteins75. This implies that only the initiation of chloroplast division itself might take as much time as the whole division process in bacteria. Furthermore, plant-specific components of the division machinery are involved in the rate of chloroplast division, e.g. PLASTID DIVISION (PDV) proteins. The overexpression of PDV proteins in both Arabidopsis and Physcomitrella led to an increase in chloroplast number and a decrease in chloroplast size75. Additionally, the rates of chloroplast division might vary depending on the developmental stage: during leaf development the level of PDV proteins decreased, leading to a decrease in the chloroplast division rate75. This indicates another mechanism determining the rate of chloroplast division, which might explain why chloroplasts take more time to divide, therefore not depending on a high FtsZ GTPase activity. Thus, the relatively slow GTP-dependent polymerization of the Physcomitrella FtsZ proteins at relatively high concentrations observed here is in accordance with their participation in a plastoskeleton, which persists independent of the division process.
By performing Co-IPs, we confirmed that Physcomitrella FtsZ2-1 interacts with Physcomitrella FtsZ1 proteins in vivo. In FRET experiments, the in vivo interaction between Physcomitrella FtsZ2-1 and FtsZ1-1 was observed before52. Our Co-IPs were performed with an in-frame fusion of Physcomitrella FtsZ2-1 with GFP at the endogenous locus and not with overexpression lines. Due to the relatively low abundance and high similarity of these isoforms, the specific interacting FtsZ1 isoform could not be unequivocally identified based on the detected peptides. Consequently, it was decided not to follow up on the approach to estimate the ratio of FtsZ1-2 and FtsZ2-1 forming (hetero)polymers but to perform GTPase assays and polymerization assays with a mixture of Physcomitrella FtsZ1-2 and FtsZ2-1 in a 1:1 ratio. This mixing resulted in a significantly enhanced overall GTPase activity, suggesting that there is a synergistic interaction of the two FtsZ isoforms. This result is different from findings regarding Arabidopsis FtsZ proteins34, where no synergistic effects were observed.
We were able to visualize GTP-dependent filament formation for both Physcomitrella FtsZ isoforms using negative stain TEM, revealing distinct structural characteristics. For FtsZ1-2 small protofilaments and larger filaments with a bundle-like architecture were observed, indicating that FtsZ1-2 primarily polymerizes into small protofilaments, which subsequently assemble into straight filament bundles. In contrast, FtsZ2-1 predominantly formed curved filaments, with straight filaments being less frequent. No protofilaments were observed for this isoform. Additionally, a higher abundance of FtsZ2-1 filaments was observed in TEM images compared to FtsZ1-2. This is consistent with the light-scattering data, which demonstrated that FtsZ2-1 achieved a higher scattering intensity (measured in counts per second) than FtsZ1-2. The structural differences extended to filament thickness, where FtsZ1-2 bundles were notably broader than those of FtsZ2-1. When both isoforms were co-incubated in the presence of GTP, the resulting filaments displayed characteristics of both straight and curved morphologies, along with occasional single protofilaments. Interestingly, the overall filament appearance was more similar to FtsZ2-1. However, it was not possible to definitively attribute the observed filaments to FtsZ1-2, FtsZ2-1, or hybrid assemblies. Differences in the polymer-forming abilities of plant FtsZ proteins have been previously reported. Arabidopsis FtsZ2 forms filaments similar to those of Physcomitrella FtsZ1-2 as observed by TEM, whereas Arabidopsis FtsZ1 does not independently assemble into filaments under the tested conditions34. In Physcomitrella, FtsZ2-1 is directly involved in plastid division8,41 leading to enlarged plastids in basal chloronema cells of the ΔftsZ2-1 mutant41. Furthermore, chloroplasts of ΔftsZ2-1 cells show complete loss of chloroplast integrity53. In contrast, chloroplasts in the chloronema cells of the ΔftsZ1-2 mutant did not show morphological differences compared to WT cells53. This indicates that FtsZ2-1 is essential for maintaining chloroplast shape and integrity, while FtsZ1-2 might be less important. While filaments of FtsZ2-1 are exclusively formed within the chloroplast, FtsZ1-2 filaments apparently need to be longer, as they form long, extraplastidic extensions to connect multiple chloroplasts via stromules43,53. In general, it is reasonable to assume that differences in filament morphology are due to the underlying differences in GTPase activity and polymerization dynamics.
Conclusions
We observed GTPase activity and in vitro polymerization of Physcomitrella FtsZ1-2 and FsZ2-1. We confirmed the polymer formation of both FtsZ isoforms using TEM and show that Physcomitrella FtsZ1 isoforms were identified as significant interactors of Physcomitrella FtsZ2-1 in vivo. We present the heterologous expression of eGFP-tagged Physcomitrella FtsZ1-2 and mKO2-tagged FtsZ2-1 in E. coli. The observation that the overexpression of both Physcomitrella FtsZ isoforms in E. coli leads to elongated bacterial cells confirms that the FtsZ function for bacterial and organelle division is evolutionary conserved. Furthermore, our results provide insights into the polymer-forming properties of Physcomitrella FtsZ1-2 and FtsZ2-1. Based on the mutant phenotypes42,53 the localization of Physcomitrella FtsZ in E. coli(this study), the in vivo (53 and this study) and our in vitro studies, we postulate different roles for FtsZ1-2 and FtsZ2-1 regarding the assembly of the plastoskeleton. These diverse functions of FtsZ1-2 and FtsZ2-1, as well as their interaction, impact the formation of the Physcomitrella plastoskeleton, abidingly shaping chloroplast morphology.
Methods
Design of the expression vectors FtsZ1-2 and FtsZ2-1
The coding sequences (CDS) for PpFtsZ1-2 (Pp3c19_2490) and PpFtsZ2-1 (Pp3c11_17860) according to the Physcomitrella genome76 were optimized for bacterial expression. Changes in the CDS were made accordingly to a codon usage table derived from the analysis of 16,000 E. coli genes using a custom Perl script and the GC content was concurrently increased (script written by Dr. Oguz Top). The putative N-terminal transit peptides for PpFtsZ1-2 (Pp3c19_2490) and PpFtsZ2-1 (Pp3c11_17860) were determined using the ChloroP 1.1 Prediction Server (http://www.cbs.dtu.dk/services/ChloroP/). The codon-optimized sequences lacking the predicted N-terminal transit peptides (34 and 32 amino acids, respectively, Table 1) were synthesized by Eurofins Genomics Germany GmbH, Ebersberg, Germany. For cloning of the final construct of FtsZ2-1, the primers were designed starting at the codon for amino acid 33. The fragments for FtsZ1-2 and FtsZ2-1 were amplified via PCR using the respective primers (Supplemental Tables S3 and S4). The final expression vectors were subcloned into the pLATE11 bacterial expression vector via Ligation Independent Cloning (LIC, aLICator LIC Cloning and Expression Kit 1, untagged; Thermo Fisher Scientific, Waltham, USA) following the manufacturer’s instructions. The pLATE11 expression vector allows high levels of target protein expression. The 8 x His-tag was integrated into the primer and added to the C-terminus of the CDS.
Design of the expression vectors pLATE_eGFP_His, pLATE_eGFP_FtsZ1-2_His, pLATE_mKO2_His, pLATE_mKO2_FtsZ2-1_His
To generate the expression vectors pLATE_eGFP_His, pLATE_eGFP_FtsZ1-2_His, pLATE_mKO2_His, pLATE_mKO2_FtsZ2-1_His, the CDS of FtsZ1-2, FtsZ2-1, eGFP and mKOrange2 (mKO2) were amplified via PCR using the respective primers (Supplemental Table S3 and S4). The mKO2 template was kindly provided by Dr. Roland Nitschke. The inserts were then assembled via Gibson cloning77 and subcloned into the pJET1.2 vector (CloneJET PCR cloning kit, Thermo Fisher Scientific) following the manufacturer’s instructions. After identification of the recombinant clones, the correct construct was used as a template to be amplified using the respective primers for LIC cloning (Supplemental Table S3). The PCR fragments were subcloned into the pLATE11 expression vector via Ligation Independent Cloning following the manufacturer’s instructions. To create the construct containing both fluorescence-tagged FtsZ isoforms, the pLATE_eGFP_FtsZ1-2_His vector was linearized using Nde I. Subsequently, the mKO2-FtsZ2-1 expression cassette, amplified from the pLATE_mKO2_FtsZ2-1_His vector, was inserted into the corresponding site by Gibson cloning.
Design of the expression vectors His6_SUMO_FtsZ1-2 and His6_SUMO_FtsZ2-1
The CDS of FtsZ1-2 and FtsZ2-1 lacking the N-terminal transit peptides (86 and 42 amino acids, respectively) were amplified via PCR (Supplemental Table S4) and subcloned into the linearized pSVA13429 vector75 via Gibson cloning77. The final vectors were sequenced with SUMO_fwd and T7_rev (Supplemental Table S4).
Transformation of E. c oli cells
BL21 Star™ (DE3) One Shot® (Invitrogen, Thermo Fisher Scientific) or NiCo (Invitrogen, Thermo Fisher Scientific) cells were transformed with vectors listed in Table 2 according to the manufacturer’s instructions.
Generation of a FtsZ2-1-eGFP fusion line
The coding sequence of eGFP combined with a flexible 18 bp linker78 fused FtsZ2-1 at the endogenous locus via homologous recombination as described79. Homologous flanks for the integration were chosen to remove the endogenous stop codon. Restriction sites for Esp3I to release the linearized transfection construct from the final vector backbone were included at the 5’ and the 3’ end of the knock-in construct. All necessary parts were amplified with Phusion polymerase (Thermo Fisher Scientific) and assembled into the pJet1.2 vector backbone (Thermo Fisher Scientific) via triple template PCR as described78. PCRs were performed with the primers listed in Supplemental Table S1. The plasmid linearized viaAlw26I digestion as well as a plasmid for co-transfection containing a neomycin resistance cassette80 were purified via ethanol precipitation and transfection and selection was performed as described63,81. Plants surviving the selection procedure were screened by PCR (Phire Polymerase, Thermo Fisher Scientific) for targeted integration of the knock-in construct at the desired locus as described79. Sequences of primers used for cloning and screening are listed in Supplemental Table S3. Physcomitrella WT as well as the employed eGFP fusion line #513 is available from the International Moss Stock Center (IMSC, www.moss-stock-center.org) with accessions 40,095 and 40,960, respectively. The plasmid containing the knock-in construct is also available from the IMSC under accession P1779.
Co-immunoprecipitation
Co-immunoprecipitations of the GFP-tagged FtsZ2-1 line was performed in biological triplicates using GFP-Trap Magnetic Particles M-270 (ChromoTek, Planegg-Martinsried, Germany). In brief, 300 mg homogenized protonema of the bait line (GFP-tagged FtsZ2-1, line #513) and WT were dissolved in 2 mL ice-cold extraction buffer (25 mM HEPES-KOH, pH 7.5, 2 mM EDTA, 100 mM NaCl, 200 nM DTT, 0.5% Triton X-100, 1% plant protease inhibitor cocktail (PPI, P9599, Sigma Aldrich, St. Louis, USA)). All further steps were performed as described79. MS analyses were performed on a Q-Exactive Plus instrument (Thermo Fisher Scientific) coupled to an UltiMate 3000 RSLCnano system (Dionex, Thermo Fisher Scientific) as described82. A database search of the test Co-IPs was performed with Mascot Server V2.7.0 (Matrix Science). Results were subsequently loaded into the Scaffold™ 5 software (V5.0.1, Proteome Software) and proteins were accepted at an FDR = 1 and peptides at an FDR = 0.5. Database search and label free quantitation of the quantitative Co-IP were performed as described79 using MaxQuant (2.1.4.065). The database contained all Physcomitrella V3.3 protein models76 as well as a fusion sequence of FtsZ2.1 (Pp3c11_17860) and eGFP Data analysis was performed in Perseus (V2.0.10.080). Proteins with at least two LFQ values in at least one group (FtsZ or WT) were used and missing values were imputed from a normal distribution with a down-shift of 1.8 and distribution width of 0.5. True interaction partners were accepted at an FDR of 0.01% and a p-value < 0.01. The resulting table containing significantly interacting proteins is available from Supplemental Table S2.
Preparation of transformed BL21 Star™ (DE3) cells for confocal microscopy
For confocal microscopy, 3 mL of LB medium containing 50 mg/mL ampicillin (amp) and 1% glucose was inoculated with a colony of respective transformed BL21 Star™ (DE3) cells, picked from a LB/amp agarose plate. The inoculated culture was then incubated overnight at 37° C while shaking. On the subsequent day, 200 µL of this overnight culture was transferred to 2.8 mL of LB/amp and further incubated for 3 h at 37° C. Subsequently, 0.5 mM isopropyl ß-D-1-thiogalactopyranoside (IPTG) was added, and the culture was incubated for an additional 45 min. For confocal microscopy, 1–5 µL of the respective bacterial solution was transferred to a microscopy glass slide. Then the samples were embedded using ProLong™ Glass Antifade Mountant (Thermo Fisher Scientific), covered with a cover slide, and stored in darkness for 24 h prior to confocal microscopy.
Confocal microscopy of transformed BL21 Star™ (DE3) cells
All images were taken with a Zeiss LSM880 NLO microscope (Carl Zeiss Microscopy GmbH, Jena, Germany) using Plan-Apochromat 40x/1.4 Oil DIC(UV)VIS-IR objective with a zoom factor of 2. For the excitation the laser was applied at 488 nm (eGFP) or 561 nm (mKO2) with an intensity of 2% or 5%, respectively. For eGFP images the pinhole was adjusted to 100.5 AU (12.11 μm), and for mKO2 the pinhole was set to 115.4 AU (18.54 µM), while no emission filter was used. The detection range was set to 500–550 nm for the eGFP fluorescence and 560–600 nm for the mKO2 fluorescence.
Overexpression and purification of His6_SUMO_FtsZ1-2 and His6_SUMO_FtsZ2-1
NiCo cells (Invitrogen, Thermo Fisher Scientific) were transformed with the plasmids His₆_SUMO_FtsZ1-2 and His₆_SUMO_FtsZ2-1, then directly transferred to 200 mL of LB medium containing 50 mg/L kanamycin and 0.025% glucose. Cultures were grown overnight at 37 °C and subsequently used to inoculate 1 L of a simplified autoinduction medium (based on69) in a 5 L baffled flask, to an OD₆₀₀ of 0.025. The medium contained 100 mg/L kanamycin, 6 g/L Na₂HPO₄, 3 g/L KH₂PO₄, 10 g/L tryptone, 5 g/L yeast extract, and 5 g/L NaCl. Glucose, lactose, and glycerol were autoclaved separately as a 20× stock and added to achieve final concentrations of 3 g/L glucose, 8 g/L lactose, and 11 g/L glycerol. The culture was grown at 37 °C until reaching an OD₆₀₀ of 1.0, after which the temperature was lowered to 22 °C, and growth continued overnight. The next day, OD₆₀₀ was monitored hourly until it remained constant for 2 h. Cells were harvested by centrifugation at 7,500 rpm (SS-34 rotor) at 4 °C, washed with either buffer A (50 mM glycine, pH 9.5, 50 mM KCl, 1 mM MgCl₂, 5% glycerol) containing 20 mM imidazole for FtsZ1-2, or buffer B (25 mM glycine, pH 9.5, 150 mM KCl, 1 mM MgCl₂, 5% glycerol) containing 20 mM imidazole for FtsZ2-1, and centrifuged again. The supernatant was removed, and the cell pellets were flash-frozen in liquid nitrogen and stored at −80 °C. Frozen pellets were thawed on ice and resuspended in their respective buffers (A or B) containing 20 mM imidazole. All subsequent steps, including purification, were performed on ice or at 4 °C. Cells were lysed by passing them through a French press three times, followed by centrifugation at 7,500 rpm (SS-34 rotor) and then at 45,000 rpm (Ti-60 rotor). Purification was performed using an ÄKTA Purifier system (Cytiva). The supernatant was loaded onto a 5 mL HisTrap FF column (Cytiva) equilibrated with buffer A or B containing 20 mM imidazole at a flow rate of 0.5 mL/min. The column was washed with the same buffer at 2 mL/min until the OD₂₈₀ stabilized. Proteins were eluted with buffer A or B containing 250 mM imidazole at 0.5 mL/min, and fractions containing protein were collected and pooled. The His₆-SUMO-tag was removed by cleavage with His-Ulp1 protease (80 ng/mL) for 6 h at 4 °C in the presence of 2 mM DTT, 0.1% ND-40, and 500 µM GDP. SUMO protease was produced in-house83,84. Proteins were concentrated using Pierce™ Protein Concentrators PES (10 kDa MWCO, Thermo Fisher Scientific). The concentrated proteins were applied to a HiLoad® 16/600 Superdex® 200 pg (Cytiva) size-exclusion column equilibrated with buffer C (50 mM glycine, pH 10.5, 50 mM KCl, 1 mM MgCl₂, 5% glycerol) for FtsZ1-2 or buffer B for FtsZ2-1. Protein concentrations in fractions containing FtsZ1-2 and FtsZ2-1 were determined using the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific) according to the manufacturer’s instructions.
GTP hydrolysis assay
The GTPase activity was assessed using the malachite green assay. Experiments were conducted in a buffer containing 37.5 mM glycine (pH 10), 100 mM KCl, 5 mM MgCl₂, and 5% glycerol, with a total reaction volume of 50 µL. At 25 °C, released phosphate increased linearly for at least 15 min and therefore the assay was performed at 25 °C for 10 min. The reaction was initiated by adding GTP to a final concentration of 1 mM and stopped by the addition of EDTA to a final concentration of 10 mM. A 20 µL aliquot of the reaction mixture was incubated with 80 µL of malachite green working reagent. Control reactions lacking either GTP or protein were performed under identical conditions. Absorbance at 620 nm was measured using a plate reader (CLARIOstar® Plus, BMG LABTECH GmbH, Ortenberg, Germany), and the amount of free phosphate in each sample was calculated using a phosphate standard calibration curve.
Light scattering assays
Experiments were conducted using a temperature-controlled FluoroMax-4 spectrofluorometer (Horiba Scientific) at 25 °C. The excitation and emission wavelengths were set to 350 nm, with an excitation bandwidth of 1 nm and an emission bandwidth of 0.5 nm. Filament formation was initiated by the addition of GTP to a final concentration of 5 mM. FtsZ1-2 and FtsZ2-1 were measured individually in buffers C and B in the presence of 5 mM MgCl₂, and for the experiments were FtsZ1-2 and FtsZ2-1 were mixed in a buffer containing 37.5 mM glycine (pH 10), 100 mM KCl, 5 mM MgCl₂, and 5% glycerol.
Transmission electron microscopy of FtsZ filaments
For polymerization studies, FtsZ proteins (7.5 µM FtsZ1-2, 7.5 µM FtsZ2-1, or 7.5 µM each of FtsZ1-2 and FtsZ2-1) were incubated at 25 °C for 10 min with or without 5 mM GTP in a buffer containing 37.5 mM glycine (pH 10), 100 mM KCl, 5 mM MgCl₂, and 5% glycerol, in a total volume of 60 µL. A 5 µL aliquot of each sample was immediately applied to freshly glow-discharged carbon/Formvar-coated copper grids (300 mesh; Plano GmbH) and incubated for 30 s. Excess liquid was blotted away, and the grids were washed three times with ddH₂O, followed by negative staining with 2% uranyl acetate. Images were acquired using a Hitachi HT7800 transmission electron microscope operated at 100 kV and equipped with an EMSIS Xarosa 20-megapixel CMOS camera.
Data availability
All relevant data is contained in the manuscript and in the supplementary materials. Physcomitrella WT as well as the employed eGFP fusion line #513 is available from the International Moss Stock Center (IMSC, www.moss-stock-center.org) with accessions 40095 and 40960, respectively. The plasmid containing the knock-in construct is also available from the IMSC under accession P1779. The list of proteins significantly interacting with Physcomitrella FtsZ2-1 as determined by Co-IP and subsequent MS analysis (Table S2) can be downloaded from Zenodo (https://zenodo.org/records/10648779).
References
Leister, D. Genetic engineering, synthetic biology and the light reactions of photosynthesis. Plant Phys. 179, 778–793 (2019).
Gould, S. B., Waller, R. F. & McFadden, G. I. Plastid evolution. Ann. Rev. Plant. Biol. 59, 491–517 (2008).
Keeling, P. J. The number, speed, and impact of plastid endosymbioses in eukaryotic evolution. Ann. Rev. Plant Biol. 64, 583–607 (2013).
Zimorski, V., Ku, C., Martin, W. F. & Gould, S. B. Endosymbiotic theory for organelle origins. Curr. Op. Microbio. 22, 38–48 (2014).
Martin, W. et al. Gene transfer to the nucleus and the evolution of chloroplasts. Nature 393, 162–165 (1998).
McFadden, G. I. Endosymbiosis and evolution of the plant cell. Curr. Op. Plant. Bio. 2, 513–519 (1999).
Osteryoung, K. W. & Vierling, E. Conserved cell and organelle division. Nature 376, 473–474 (1995).
Strepp, R., Scholz, S., Kruse, S., Speth, V. & Reski, R. Plant nuclear gene knockout reveals a role in plastid division for the homolog of the bacterial cell division protein FtsZ, an ancestral tubulin. PNAS 95, 4368–4373 (1998).
Hirano, T. et al. Moss chloroplasts are surrounded by a peptidoglycan wall containing D-amino acids. Plant Cell 28, 1521–1532 (2016).
MacLeod, A. I., Knopp, M. R. & Gould, S. B. A mysterious cloak: the peptidoglycan layer of algal and plant plastids. Protoplasma 261, 173–178 (2023).
Bisson-Filho, A. W. et al. Treadmilling by FtsZ filaments drives peptidoglycan synthesis and bacterial cell division. Science 355, 739–743 (2017).
Barrows, J. M. & Goley, E. D. FtsZ dynamics in bacterial division: what, how, and why? Curr. Op. Cell Biol. 68, 163–172 (2021).
Dai, K. & Lutkenhaus, J. ftsZ is an essential cell division gene in Escherichia coli. J. Bac. 173, 3500–3506 (1991).
Osteryoung, K. W., Stokes, K. D., Rutherford, S. M., Percival, A. L. & Lee, W. Y. Chloroplast division in higher plants requires members of two functionally divergent gene families with homology to bacterial ftsZ. Plant Cell 10, 1991–2004 (1998).
Lutkenhaus, J. F., Wolf-Watz, H. & Donachie, W. D. Organization of genes in the ftsa-enva region of the Escherichia coli genetic map and identification of a new fts locus (ftsZ). J. Bac. 142, 615–620 (1980).
Bi, E. & Lutkenhaus, J. FtsZ ring structure associated with division in Escherichia coli. Nature 354, 161–164 (1991).
Vitha, S., McAndrew, R. S. & Osteryoung, K. W. FtsZ ring formation at the chloroplast division site in plants. J. Cell Bio. 153, 111–119 (2001).
Yoshida, Y., Mogi, Y., TerBush, A. D. & Osteryoung, K. W. Chloroplast FtsZ assembles into a contractible ring via tubulin-like heteropolymerization. Nat. Plants 2, 16095 (2016).
Yang, X. et al. GTPase activity–coupled treadmilling of the bacterial tubulin FtsZ organizes septal cell wall synthesis. Science 355, 744–747 (2017).
Kasten, B. & Reski, R. beta-lactam antibiotics inhibit chloroplast division in a moss (Physcomitrella patens) but not in tomato (Lycopersicon esculentum). J. Plant Phys. 150, 137–140 (1997).
de Boer, P., Crossley, R. & Rothfield, L. The essential bacterial cell-division protein FtsZ is a GTPase. Nature 359, 254–256 (1992).
Smith, A. G., Johnson, C. B., Vitha, S. & Holzenburg, A. Plant FtsZ1 and FtsZ2 expressed in a eukaryotic host: GTPase activity and self-assembly. FEBS Lett. 584, 166–172 (2010).
Porter, K. J., Cao, L. & Osteryoung, K. W. Dynamics of the Synechococcus elongatus cytoskeletal GTPase FtsZ yields mechanistic and evolutionary insight into cyanobacterial and chloroplast FtsZs. J. Biol. Chem. 299, 102917 (2023).
Erickson, H. P. FtsZ, a prokaryotic homolog of tubulin? Cell 80, 367–370 (1995).
Santana-Molina, C., del Saz-Navarro, D. & Devos, D. P. Early origin and evolution of the FtsZ/tubulin protein family. Front. Microbio. 13, 1100249 (2023).
Kiessling, J. et al. Visualization of a cytoskeleton-like FtsZ network in chloroplasts. J. Cell Bio. 151, 945–950 (2000).
Lutkenhaus, J. & Addinall, S. G. Bacterial cell division and the Z ring. Ann. Rev. Biochem. 66, 93–116 (1997).
Osteryoung, K. W. & Nunnari, J. The division of endosymbiotic organelles. Science 302, 1698–1704 (2003).
Ithurbide, S., Gribaldo, S., Albers, S. V. & Pende, N. Spotlight on FtsZ-based cell division in archaea. Trends Microbio. 30, 665–678 (2022).
Rensing, S. A., Kiessling, J., Reski, R. & Decker, E. L. Diversification of ftsZ during early land plant evolution. J. Mol. Evol. 58, 154–162 (2004).
Miyagishima, S. et al. Two types of FtsZ proteins in mitochondria and red-lineage chloroplasts: the duplication of FtsZ is implicated in endosymbiosis. J. Mol. Evol. 58, 291–303 (2004).
TerBush, A. D., Yoshida, Y. & Osteryoung, K. W. FtsZ in chloroplast division: structure, function and evolution. Curr. Op Cell Bio. 25, 461–470 (2013).
Olson, B. J. S. C., Wang, Q. & Osteryoung, K. W. GTP-dependent heteropolymer formation and bundling of chloroplast FtsZ1 and FtsZ2. J. Biol. Chem. 285, 20634–20643 (2010).
Porter, K. J. et al. The Arabidopsis thaliana Chloroplast division protein FtsZ1 counterbalances FtsZ2 filament stability in vitro. J. Biol. Chem. 296, 100627 (2021).
Löwe, J. & Amos, L. A. Crystal structure of the bacterial cell-division protein FtsZ. Nature 391, 203–206 (1998).
Osteryoung, K. W. & McAndrew, R. S. The plastid division machine. Ann. Rev. Plant Phys. Plant Mol. Bio. 52, 315–333 (2001).
Liu, X. et al. A novel amphiphilic motif at the C-terminus of FtsZ1 facilitates chloroplast division. Plant Cell 34, 419–432 (2022).
TerBush, A. D., Porzondek, C. A. & Osteryoung, K. W. Functional analysis of the chloroplast division complex using Schizosaccharomyces Pombe as a heterologous expression system. Micros. Microanal. 22, 275–289 (2016).
Martin, A. et al. A uniquely high number of ftsZ genes in the moss Physcomitrella patens. Plant Bio. 11, 744–750 (2009).
Grosche, C. & Rensing, S. A. Three rings for the evolution of plastid shape: a tale of land plant FtsZ. Protoplasma 254, 1879–1885 (2017).
Khraiwesh, B., Ossowski, S., Weigel, D., Reski, R. & Frank, W. Specific gene silencing by artificial microRNAs in Physcomitrella patens: an alternative to targeted gene knockouts. Plant Phys. 148, 684–693 (2008).
Martin, A. et al. Targeted gene knockouts reveal overlapping functions of the five Physcomitrella patens FtsZ isoforms in chloroplast division, chloroplast shaping, cell patterning, plant development, and gravity sensing. Mol. Plant 2, 1359–1372 (2009).
Suppanz, I., Sarnighausen, E. & Reski, R. An integrated physiological and genetic approach to the dynamics of FtsZ targeting and organisation in a moss, Physcomitrella patens. Protoplasma 232, 1–9 (2007).
Reski, R. Challenges to our current view on chloroplasts. Biol. Chem. 390, 731–738 (2009).
Reski, R. Rings and networks: the amazing complexity of FtsZ in chloroplasts. Trends Plant Sci. 7, 103–105 (2002).
Shih, Y. L. & Rothfield, L. The bacterial cytoskeleton. Microbio. Mol. Bio Rev. 70, 729–754 (2006).
Erickson, H. P., Anderson, D. E. & Osawa, M. FtsZ in bacterial cytokinesis: Cytoskeleton and force generator all in one. Microbio. Mol. Biol. Rev. 74, 504–528 (2010).
Loose, M. & Mitchison, T. J. The bacterial cell division proteins FtsA and FtsZ self-organize into dynamic cytoskeletal patterns. Nat. Cell Bio. 16, 38–46 (2014).
Wagstaff, J. & Löwe, J. Prokaryotic cytoskeletons: protein filaments organizing small cells. Nat. Rev. Microbio. 16, 187–201 (2018).
Kiessling, J. et al. Dual targeting of plastid division protein FtsZ to chloroplasts and the cytoplasm. EMBO Rep. 5, 889–894 (2004).
Jost, W. et al. Isolation and characterization of three moss-derived beta-tubulin promoters suitable for recombinant expression. Curr. Gen. 47, 111–120 (2005).
Gremillon, L. et al. Filamentous temperature-sensitive Z (FtsZ) isoforms specifically interact in the chloroplasts and in the cytosol of Physcomitrella patens. New Phytol. 176, 299–310 (2007).
Özdemir, B. et al. Cytological analysis and structural quantification of FtsZ1-2 and FtsZ2-1 network characteristics in Physcomitrella patens. Sci. Rep. 8, 11165 (2018).
Köhler, R. H., Cao, J., Zipfel, W. R., Webb, W. W. & Hanson, M. R. Exchange of protein molecules through connections between higher plant plastids. Science 276, 2039–2042 (1997).
Asgharzadeh, P. et al. A NanoFE simulation-based surrogate machine learning model to predict mechanical functionality of protein networks from live confocal imaging. Comp. Struct. Biotech. J. 18, 2774–2788 (2020). (2020).
Asgharzadeh, P., Özdemir, B., Reski, R., Röhrle, O. & Birkhold, A. I. Computational 3D imaging to quantify structural components and assembly of protein networks. Acta Biomat. 69, 206–217 (2018).
Özdemir, B. & Reski, R. Automated and semi-automated enhancement, segmentation and tracing of cytoskeletal networks in microscopic images: a review. Comp. Struc. Biotech. J. 19, 2106–2120 (2021).
te Brinke, E. et al. Dissipative adaptation in driven self-assembly leading to self-dividing fibrils. Nat. Nanotech. 13, 849–855 (2018).
Kalyan, B. G. P. & Kumar, L. 3D printing: applications in tissue engineering, medical devices, and drug delivery. AAPS PharmSciTech. 23, 92 (2022).
Król, E. & Scheffers, D. J. FtsZ polymerization assays: simple protocols and considerations. J. Vis. Exp. 81, 50844 (2013).
Howell, M. et al. Agrobacterium tumefaciens divisome proteins regulate the transition from polar growth to cell division. Mol. Microbio. 111, 1074–1092 (2019).
Liao, Y., Ithurbide, S., Evenhuis, C., Löwe, J. & Duggin, I. G. Cell division in the archaeon Haloferax volcanii relies on two FtsZ proteins with distinct functions in division ring assembly and constriction. Nat. Microbio. 6, 594–605 (2021).
Hohe, A. et al. An improved and highly standardised transformation procedure allows efficient production of single and multiple targeted gene-knockouts in a moss, Physcomitrella patens. Curr. Gen. 44, 339–347 (2004).
Wiedemann, G. et al. RecQ helicases function in development, DNA repair and gene targeting in Physcomitrella patens. Plant Cell 30, 717–736 (2018).
Cox, J. & Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotech. 26, 1367–1372 (2008).
Cox, J. et al. Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol. Cell. Prot. 13, 2513–2526 (2014).
Mistry, J. et al. Pfam: the protein families database in 2021. Nucl. Acids Res. 49, D412–D419 (2021).
Brennan, P. drawProteins: a Bioconductor/R package for reproducible and programmatic generation of protein schematics. F1000Res 7, 1105 (2018).
Studier, F. W. Stable expression clones and auto-induction for protein production in E. Coli. Meth. Mol. Bio. 1091, 17–32 (2014).
Mukherjee, A. & Lutkenhaus, J. Analysis of FtsZ assembly by light scattering and determination of the role of divalent metal cations. J. Bac. 181, 823–832 (1999).
Özdemir, B., Asgharzadeh, P., Birkhold, A., Röhrle, O. & Reski, R. The plastid skeleton: a source of ideas in the nano range in Biomimetics for Architecture: Learning from Nature (eds. Knippers, J., Schmid, U., Speck, T.) 163–166 Birkhauser Verlag AG, (2019).
Ma, X., Ehrhardt, D. W. & Margolin, W. Colocalization of cell division proteins FtsZ and FtsA to cytoskeletal structures in living Escherichia coli cells by using green fluorescent protein. PNAS 93, 12998–13003 (1996).
Ward, J. E. Jr. & Lutkenhaus, J. Overproduction of FtsZ induces minicell formation in E. Coli. Cell 42, 941–949 (1985).
Mukherjee, A. & Lutkenhaus, J. Dynamic assembly of FtsZ regulated by GTP hydrolysis. EMBO J. 17, 462–469 (1998).
Okazaki, K. et al. The PLASTID DIVISION1 and 2 components of the chloroplast division machinery determine the rate of chloroplast division in land plant cell differentiation. Plant Cell 21, 1769–1780 (2009).
Lang, D. et al. The Physcomitrella patens chromosome-scale assembly reveals moss genome structure and evolution. Plant J. 93, 515–533 (2018).
Gibson, D. G. et al. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 6, 343–345 (2009).
Zupan, J. R., Cameron, T. A., Anderson-Furgeson, J. & Zambryski, P. C. Dynamic FtsA and FtsZ localization and outer membrane alterations during polar growth and cell division in Agrobacterium tumefaciens. PNAS 110, 9060–9065 (2013).
Hoernstein, S. N. W. et al. A deeply conserved protease, acylamino acid-releasing enzyme (AARE), acts in ageing in Physcomitrella and Arabidopsis. Comm. Biol. 6, 61 (2023).
Mueller, S. J. et al. Quantitative analysis of the mitochondrial and plastid proteomes of the moss Physcomitrella patens reveals protein macrocompartmentation and microcompartmentation. Plant Phys. 164, 2081–2095 (2014).
Hohe, A. & Reski, R. Optimisation of a bioreactor culture of the moss Physcomitrella patens for mass production of protoplasts. Plant Sci. 163, 69–74 (2002).
Top, O. et al. Expression of a human cDNA in moss results in spliced mRNAs and fragmentary protein isoforms. Comm. Biol. 4, 964 (2021).
Nußbaum, P. et al. Proteins containing photosynthetic reaction centre domains modulate FtsZ-based archaeal cell division. Nat. Microbio. 3, 698-711 (2024).
Lau, Y. K. et al. Discovery and engineering of enhanced SUMO protease enzymes. J. Biol. Chem. 293, 13224–13233 (2018).
Acknowledgements
This project was funded by the German Research Foundation DFG under Germany’s Excellence Strategy (livMatS - EXC-2193/1, Project ID 390951807, and CIBSS – EXC-2189, Project ID 390939984) to R.R.. S.W.L.M. was supported by a state-funded completion scholarship of the Landesgraduiertenförderung LGFG. M.J. was supported by a grant to Sonja-Verena Albers by the Collaborative Research Centre SFB1381 funded by the DFG Project ID 403222702 SFB 1381. The TEM (Hitachi HT7800) was funded by the DFG grant (project number 426849454) and is operated by the University of Freiburg, Faculty of Biology, as a partner unit within the Microscopy and Image Analysis Platform (MIAP) and the Life Imaging Center (LIC), Freiburg.We thank Oğuz Top for codon optimizing the CDS of Physcomitrella FtsZ1-2 and FtsZ2-1 for bacterial expression. We also thank the Life Imaging Center (LIC) of the University of Freiburg for help with confocal microscopy. We thank Sonja-Verena Albers for providing the equipment for purification of Physcomitrella FtsZ proteins and her overall support of this project, and Anne Katrin Prowse for language editing.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
S.W.L.M. designed the research, performed the experiments, analysed the data, and wrote the manuscript. M.J. performed transmission electron microscopy. L.L.B. performed confocal microscopy, designed figures and helped writing the manuscript. S.N.W.H. performed quantitative Co-IPs, analysis of MS data and helped writing the manuscript. B.Ö. generated the FtsZ2-1_eGFP fusion line. E.L.D. helped designing research and writing the manuscript. C.v.d.D. designed and performed experiments, analysed data and helped writing the manuscript. R.R. designed and supervised the research, acquired funding, and wrote the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Milferstaedt, S.W.L., Joest, M., Bohlender, L.L. et al. Differential GTP-dependent in-vitro polymerization of recombinant Physcomitrella FtsZ proteins. Sci Rep 15, 3095 (2025). https://doi.org/10.1038/s41598-024-85077-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-85077-6
Keywords
This article is cited by
-
Production of human papillomavirus type 16 virus-like particles in Physcomitrella photobioreactors
Plant Cell Reports (2025)