Abstract
Transition from the manual processes that are performed during the initial research and development (R&D) stage to automated processes for later and commercial stage cell therapy manufacturing can be challenging. It often requires significant effort, time, and costs – which hinders the therapy’s access to the clinic. To ease this transition, we have developed a novel and flexible manufacturing platform, Bioreactor with Expandable Culture Area (BECA), that aims to support both R&D and manufacturing to accelerate cell therapies from bench to bedside. This report introduces two models in this manufacturing platform: BECA-S for manual small-scale operation at R&D phase and BECA-Auto for functionally closed and automated scaled-out operation at manufacturing phase. We employed these two models to streamline transition of the T cell culture process from manual to automated and reported insignificant differences in the culture outcome between the two. Our work represents the first detailed development and demonstration of a standalone cell manufacturing platform that facilitates a seamless transition between manual and automated processing for autologous T cell therapy manufacturing.
Similar content being viewed by others
Introduction
While the use of adoptive T cell therapy for cancer treatment has been in research for decades, its 1st commercial product was only approved by the US Food and Drug Administration (FDA) in 2017. As a result, manufacturing of these therapies only became a focal point of the industry in the last few years1,2,3,4,5. Currently, many of the cell therapy manufacturing processes are performed manually instead of being automated. However, manual processes are not ideal for manufacturing as they pose high risks of contamination and production inconsistencies due to their difficulty in implementing stringent process controls. These issues can be mitigated by transitioning to automated processes which will allow for scalable and standardised T cell therapy manufacturing. In spite of that, cell therapy developer may choose to retain manual processes for manufacturing as the transition to automated processes require significant effort in adaptation or even the development of entirely new processes6, resulting in heightened development costs and impacting the time to market.
The consideration of transitioning to automated process includes choosing the automated culture platform. Currently, automated platforms designed for T cell culture available on the market include Miltenyi’s Prodigy7,8,9, Terumo’s Quantum10,11, Lonza’s Cocoon12, Sartorius’s AMBR13,14, and Cytiva’s Sefia6 and Xuri15,16. These platforms vary widely in their features, including vessel types (e.g., hollow fibre, culture bag, culture chamber), capacity (250 mL to over 5 L), and workflow (e.g., cell selection and expansion). They feature distinct sets of critical process parameters compared to manual counterparts, such as flow shear, gas supply, cell density and media supply12,17,18. The differences between the manual culture vessels and automated platform necessitate substantial effort and considerations to optimise the matched processes, hampering the transition, as mentioned above. The development of a flexible manufacturing platform that can execute both manual and automated workflows would greatly ease process transfer between these two modes of operation.
To address this need, our team has developed the Bioreactor with Expandable Culture Area (BECA)19 platform which is a versatile manufacturing platform consisting of BECA-S – a single-chamber culture vessel, and BECA-Auto – a standalone automated system for T cell culture. BECA-S is a single-use open culture vessel that can be used for manual mode of operation within a Biosafety Cabinet (BSC) while BECA-Auto is an automated and functionally closed benchtop system that utilises BECA-S as the culture vessel and provides optimal culture conditions and sustains sterile culture outside of the BSC and CO2 incubator (Fig. 1A and B). As BECA-Auto and BECA-S utilise the same culture vessel design, manual processes developed with BECA-S can be easily transferred into BECA-Auto to be automated. In this report, we detail the development of the BECA platform and describe its validation, demonstrating the direct translation of manual culture processes performed with BECA-S to automated culture processes performed with BECA-Auto for T cell manufacturing.
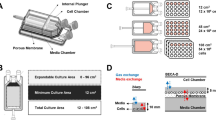
(A) i. BECA-S and ii. BECA-S (Closed) share the same vessel design with an internal movable wall separating the vessel space into culture region and expansion region. Movement of the plunger shifts the internal movable wall into the expansion region resulting in an in-situ expansion of culture region from 19 - 102.4 cm2. The input/output port of BECA-S is replaced by input/output tubing network in BECA-S (Closed). (B) Steps in setting up BECA-Auto for a T cell culture process starting with installation of the single-use kits onto the control units, followed by closing the enclosure and allowing the climate control system to achieve optimum parameters prior to starting the culture process. Graphic user interface controlling BECA-Auto is not depicted. Details of climate control – heating element for temperature control, humidifier for humidity control, and gas solenoid valves for CO2, N2, and pressure control are not depicted. CIFC: Capsule Internal Fluid Controller, DAAS: Device for Automated Aseptic Sampling.
Results
BECA-S: for manual T cell culture
BECA-S is a single-chamber culture vessel handled similarly to a T-flask (Fig. 1A). It has an internal movable wall that separates the vessel into the culture region and the expansion region. Movement of the internal wall expands the surface area of the culture region, thereby expanding the culture area (19 - 102.4 cm2). Liquid transfer in and out of BECA-S can be done through a port accessing the culture region. Handling of cultures in BECA-S should only be done in the BSC to maintain culture sterility. BECA-S used in this study was made from polycarbonate material, machined by CNC, and sterilised by autoclave at 121ºC, for 20 min. It is designed such that it can be injection moulded and gamma sterilised for mass production.
BECA-Auto: for automated T cell culture
BECA-Auto (Fig. 1B) is a standalone system with a compact physical footprint of 56 (length) x 47 (width) x 42 (height) cm—suitable for use on a laboratory benchtop. It is designed to automate the aseptic handling of BECA-S and maintain a closed sterile environment for cell culture. The system comprises control units and single-use kits which are made up of multiple functional modules, each supporting a specific cell culture process (Table 1). The single-use kits establish a functionally closed sterile environment and the control units enable automation of processes such as sampling, fluid transfer, actuation, and environment control. The following section describes the individual single-use kits and control units in BECA-Auto.
Single-use kit: BECA-S (closed)
BECA-S (Closed) is the system’s culture vessel and is a modified version of BECA-S (Fig. 1A). It retains the same dimension and materials as BECA-S but replaces the open port with a tubing network connecting to other functional modules to establish a closed environment within BECA-Auto.
Single-use kit: Manifold Assembly and Input Manifold
Manifold Assembly is the fluid transfer pathway between BECA-S (Closed), the control units, and the external environment (Figs. 1B and 2A). It consists of a network of tubing and connectors connecting BECA-S (Closed) to (1) Input/Output interface, where the tubing terminate at AseptiQuik® connectors which connect to bags for external input (2 connectors) and output (1 connector), and (2) DAAS (Device for Automated Aseptic Sampling), which extracts small samples from BECA-S (Closed) to a collection tube. In place of directly connecting to the Input/Output interface, bags can be connected to Input Manifolds, which are standalone networks of tubing and connectors that can be used to amplify the connections to the Manifold Assembly (Fig. 2A).
(A) Layout and flow circuit of BECA-Auto. Black outline represents control units, orange outline represents single-use kit, and green outline represents user supplied containers. Direction of fluid flow in the tubing is represented as arrows. Connection of sterile culture bags for seeding to, media feed into, and harvesting from BECA-Auto (Closed) to Manifold Assembly and collection of sample from BECA-S (Closed) to a collection tube through DAAS are shown. (B) Illustration of the direct process mapping between manual handling in BECA-S and automated operation in BECA-Auto. All processes for BECA-Auto were conducted within the Enclosure. Input refers to seeding and media feed. Culture with BECA-S requires multiple transfers between the BSC and incubator, while culture with BECA-Auto takes place entirely within the enclosure.
Control unit: CIFC (Capsule Internal Fluid Controller)
CIFC, consisting of peristaltic pumps and pinch valves, manages fluid movement in and out of BECA-S (Closed). It controls the flow within the Manifold Assembly’s fluid transfer pathway. The sequence of actions within CIFC has been optimised to ensure smooth flow and accuracy of volume drawn or injected into BECA-S (Closed).
Control unit: DAAS (Device for Automated Aseptic Sampling)
DAAS enables automated and aseptic sampling from BECA-S (Closed). It was designed with a series of peristaltic pumps and pinch valves to create an aseptic barrier that allows for small volume samples (0.02 ml to 1 ml) to be drawn repeatedly during cell culture to an open external collection tube without compromising on sterility. The process robustness of DAAS was validated in two bacteria ingression tests20. DAAS is a standalone unit that can also be adapted to systems not limited to BECA-Auto.
Control unit: Actuation Platform
The Actuation Platform has motors, encoders and sensors to actuate the BECA-S (Closed) with three degrees of freedom. It shifts the position of BECA-S (Closed)’s plunger to expand the culture surface area, rocks the vessel along its length from side to side to resuspend the culture, and tilts the vessel along its width and length to consolidate the culture to one corner of the vessel. The platform is equipped with sensors to precisely regulate the extent and speed of these movements.
Control unit: Enclosure and Climate Control
The Enclosure houses all of the above-mentioned functional modules except for DAAS (Fig. 2A). It has a lid which can be opened for the installation of single-use kits and closed to hermetically seal its internal environment. Gas exchanges between its internal and external environment are achieved through solenoid gas valves with HEPA gas filters connected to CO2 or N2 gas supply, and pressure release gas solenoid valve connected to ambient pressure. Fluid exchanges between BECA-S (Closed) and external bags/collection tubes are achieved through Manifold Assembly. The Enclosure’s ability to sustain its internal environment for extended periods of time was validated through a stress test of high CO2 levels (11.3 - 11.8% CO2 for 37 h, data not shown).
The Climate Control controls and maintains the Enclosure’s internal environment at the user-specified parameters for the duration of operation. These parameters are entered through a Graphic User Interface (GUI) which also logs the fluctuations of these parameters during operation. The Climate Control maintains these parameters via sensors and closed-loop feedback controls for temperature, humidity, and CO2 and O2 levels which respectively trigger the heating elements, humidifier, and solenoid gas valves to automatically establish preset conditions. For example, during operation for T cell culture, the Enclosure’s ambient internal environment is maintained at levels similar to environment in conventional CO2 incubators − 37ºC, 90% relative humidity, 5% CO2 level, and 20% O2 level. The Enclosure and Climate Control together can ramp up BECA-Auto internal parameters to these target levels within 45 min of operation (data not shown), with the steady state performance shown in Table 2.
BECA-Auto operation
Figure 1B presents the steps in setting up BECA-Auto and Fig. 2A presents the layout and flow circuit of BECA-Auto.
To set up BECA-Auto, pre-sterilised single-use kits are assembled in the BSC to form a functionally closed flow path which is then installed onto the Actuation Platform, and coupled to DAAS and CIFC. Subsequently, the Enclosure is closed and sealed, and the Climate Control activated to initiate the Environmental Control programme to establish the user-specified environment for temperature, humidity, and O2 and CO2 levels which is maintained for the duration of operation.
To seed a culture into BECA-Auto, a sterile culture bag with the seeding culture is prepared and connected to the Manifold Assembly via AseptiQuik® connectors. BECA-Auto then executes the Seeding programme which initiates (1) CIFC to draw fluid from the bag into BECA-S (Closed), and (2) the Actuation Platform to resuspend the culture by gently rocking BECA-S (Closed) from side to side. Afterwards, the tube connected to the emptied bag is manually sealed and the emptied bag removed using Clipster® (Sartorius), which aseptically seals the tube end.
During the cell culture, media feed is first prepared with a sterile bag in BSC. An Input Manifold is connected to the Manifold Assembly, and the prepared media bag connected to the Input Manifold, both via AseptiQuik® connectors. BECA-Auto executes the Media Feed programme to transfer the fluid from the connected bag into BECA-S (Closed) and resuspend the culture. As the Input Manifolds can be built upon each other as required, there are no limitations on the number of inputs (such as media, cells, culture supplements) that user can add to the culture in BECA-Auto.
To sample the culture, a collection tube is placed at the output tubing of DAAS to collect a user-specified volume of culture that will be removed out of BECA-S (Closed) automatically with DAAS. At the point of sampling, BECA-Auto executes the Automated Sampling programme which initiates (1) the Actuation Platform to gently rock BECA (Closed) to resuspend the culture, and (2) DAAS to extract the specified volume of culture to the placed collection tube.
Similarly to BECA-S, the culture area of BECA-S (Closed) can be expanded to control the culture density (per cm2. As the culture proliferates, the user can determine when and how much the area is to be expanded. To perform the expansion function, BECA-Auto executes the Culture Area Expansion programme which initiates the Actuation Platform to (1) shift the position of the internal movable wall of BECA-S (Closed) to expand the culture region, and (2) gently rock BECA-S (Closed) from side to side to resuspend the culture.
Upon the termination of cell culture, a sterile bag is connected to the Manifold Assembly via AseptiQuik® connector as the harvest bag. BECA-Auto then executes the Harvesting programme which initiates (1) the Actuation Platform to gently rock BECA-S (Closed) to resuspend the culture, and (2) CIFC to draw the contents in BECA-S (Closed) through the Manifold Assembly into the harvest bag. An optional rinsing step can be performed where media from an additionally prepared sterile bag is connected to the Manifold Assembly and used to rinse BECA-S (Closed). BECA-S (Closed) is then gently rocked again and CIFC draws the rinsed media into the harvest bag. The harvest bag is then disconnected from the Manifold Assembly manually using Clipster®.
Direct manual to automated process transition using BECA-S and BECA-Auto
To assess BECA platform’s performance in direct manual to automated process transition, manual and automated workflows were matched and executed respectively in BECA-S and BECA-Auto in the same environment (Table 3). The matched workflows were identical except that manual workflow was conducted within a BSC and a CO2 incubator while the automated workflow was conducted within BECA-Auto on a laboratory workbench (Fig. 2B). The workflow consisted of a 10-day T cell culture with cell count and viability assessed on Day 3, 5, 7, and 10, as well as cell surface phenotype assessed on Day 0, 7, and 10. Cell density was maintained at 1 × 106 cells/cm2, and expansion of culture was performed when cell density exceeded 1 × 106 cells/cm2 (Fig. 3A). To maintain the proliferating culture, IL-2 supplementation was performed on Day 3, 5, and 7, and media top-up was performed on Day 3, 5, and 7 to achieve media height of 5 mm (Day 3) or 10 mm (Day 5 and 7). The experiment was performed with three different donor PBMCs.
(A) Overview of the experimental plan comparing T cell culture with BECA-S and BECA-Auto. (B) Cell population (solid line) and cell viability (dashed line) in BECA-S and BECA-Auto. Inverted triangle indicates expansion of culture for BECA-S (black) and BECA-Auto (green). (C) Cell population in BECA-S and BECA-Auto. (D) Left: Percentage population of CD3 + cells in culture as analysed by flow cytometry on Day 0, Day 7, and Day 10 for each donor. Level of 80% live lymphocytes is indicated by red dotted line. Right: Mean and standard deviation of values shown in Left. (E) Percentage population of CD4 and CD8 T cells in culture as analysed by flow cytometry on Day 0, Day 7, and Day 10. (F) Ratio of CD4/CD8 T cells as calculated from values in 3E. (G) Heatmap of percentage population of memory T cells in culture as analysed by flow cytometry on Day 0, Day 7, and Day 10 for each donor. TN: CCR7 + CD45RA+, TCM: CCR7 + CD45RA-, TEM: CCR7-CD45RA-, TEMRA: CCR7-CD45RA+. (H) Percentage population of TIM3 + PD1 + exhausted T cells. P-values indicated were calculated using paired t-test. n.s. not significant, * P < 0.05.
BECA-Auto achieved increasing cell growth and high cell viability similar to BECA-S
The two culture platforms achieved similar cell numbers on Day 10 across all three donors (50.7 ± 6 × 106 cells in BECA-S, 45.7 ± 9.8 × 106 cells in BECA-Auto). In Donor 2 and 3, cell numbers in both BECA-S and BECA-Auto had a similar trend – decreased from Day 0 to Day 3 and increased exponentially from Day 3 to Day 10 (Fig. 3B). However, the cultures of Donor 1 in BECA-Auto did not follow the similar exponential growth curve, instead had a linear growth curve until Day 5 when the cell number plateaued and achieved a similar yield as BECA-S on Day 10. Despite this discrepancy, there was overall no statistically significant difference in cell numbers between the cultures on the two platforms throughout the cell culture period (Fig. 3C).
Cultures in both platforms achieved > 85% viability on Day 10 (95.79 ± 1.41% in BECA-S, 91.05 ± 3.93% in BECA-Auto) (Fig. 3B). Both platforms resulted in similar viability for Donor 1 and 2. However, for Donor 3, viability in BECA-Auto dropped from 93.0% on Day 0 to 74.7% on Day 3 while it maintained at 90.4% in BECA-S. This drop in viability was echoed in lower cell numbers in BECA-Auto on Day 3 (2.7 × 106 cells compared to 4.6 × 106 cells in BECA-S), which could have led to the overall lower cell yield on Day 10 for BECA-Auto (39.2 × 106 cells) compared to BECA-S (57.4 × 106 cells).
BECA-Auto supported similar CD3 cell proliferation with no significant difference in CD4 and CD8 T cell populations compared to BECA-S
Cultures in both BECA-S and BECA-Auto across all three donors were enriched in CD3 T cells on both Day 7 (> 80%) and Day 10 (> 75%) with no significant difference between them (Fig. 3D). The cultures in BECA-S and BECA-Auto were observed to have a decrease in CD4 and an increase in CD8 population as the cell culture progressed (Fig. 3E). There was no significant difference between the population of CD4 and CD8 T cells in both platforms across all three donors. The CD4/CD8 ratios in BECA-Auto were not consistently higher or lower than that in BECA-S during the 10-day cell culture period across all three donors, suggesting that the platforms did not selectively promote the proliferation of CD4 or CD8 cells within the cultures (Fig. 3F).
BECA-Auto supported similar memory T cell differentiation and cell exhaustion levels compared to BECA-S
Memory T cell subpopulations were assessed to evaluate the culture differences in BECA-Auto and BECA-S. Trends in memory subpopulations for CD4 population matched that of CD8 population within the same donor (Fig. 3G). There were also no distinct differences in distribution of memory T cell subpopulations between cultures in BECA-Auto and BECA-S.
There was also no distinct difference observed between BECA-Auto and BECA-S cultures in terms of exhausted T cell populations (Fig. 3H).
Discussion
Conventional culture vessels such as well plates and T-flasks are widely used during cell therapy R&D due to their low cost, ease of use, and adaptability to process changes. BECA-S, the culture vessel used in this study, is designed to have the same ease of use and adaptability, allowing cell therapy developers to easily incorporate it into their R&D workflow. In addition, BECA-S is devised with an expandable culture region that caters to T cell sensitivity and keeps T cell-to-cell contact optimal for culture and expansion21. The culture region of BECA-S starts at a small surface area of 19 cm2 up to 102.4 cm2, making it suitable for expansion of cultures with low initial cell numbers, such as those for therapies where the target cell population are rare or low due to prior lines of treatments. This range of culture area is flexible in accommodating protocols with varying seeding and maintenance culture densities within the same vessel. The in-situ culture expansion eliminates the need to transfer the culture across multiple or increasingly larger vessels as the culture proliferates, allowing for controlled expansion process with minimum culture disturbance and handling steps. The omission of the vessel transfer step allows for the entire culture to be housed within a single vessel, enabling the automation of BECA-S in BECA-Auto. Automating T cell culture is crucial for the manufacturing of cell therapies as it reduces manpower, process deviation, contamination risks, and costs22. To support the automated manufacturing and the scale-out manufacturing model typically employed for autologous T cell therapies, BECA-Auto is designed as a compact standalone benchtop system adopting a “GMP-in-a-box” concept. It utilises single-use kits and various control units to provide a functionally closed and controlled cell culture environment that can be employed in C-grade clean rooms. BECA-Auto has a relatively small footprint that fits on a laboratory workbench, providing flexibility in space utilization and allowing for the installation of multiple units even in space-constrained manufacturing suites.
BECA-S and BECA-Auto are part of the BECA platform, designed to streamline the transition from manual to automated processes for cell therapy manufacturing. They help minimise the process development and/or validation effort typically required for such major workflow changes. We have successfully matched manual processes performed with BECA-S and transited them to automated processes performed with BECA-Auto for T cell expansion. The design of BECA-S prevents the execution of media change processes without centrifugation. To avoid the complexity of additional steps with centrifugation while fulfilling the requirement of supplying nutrients to and diluting waste from the growing culture, the experiment included a media top up from 5 mm to 10 mm on Day 5. The increase in media height has minimal impact on the culture as 10 mm is still well within the commonly used media height in culture flasks and well-plate. This limitation can be overcome by using a culture vessel capable of filtering cells from the media (such as BECA-D23, enabling automated media change when used with BECA-Auto. The direct manual to automated process transfer using BECA-S and BECA-Auto achieved non-significant difference in cell yield across three donor cells. The cultures were also observed to have similar phenotypes.
While BECA-Auto and BECA-S share similar workflow and utilise an identical culture vessel design, they do exhibit differences within certain processes such as culture resuspension (rocking of vessel versus pipetting of culture), temperature control (consistent temperature in the Enclosure versus intermittent temperature change between BSC and CO2 incubator), and fluid flow (peristaltic pumping versus pipetting). These could have led to the discrepancies observed in BECA-Auto individual donor runs, including the higher cell population on Day 2 and 4 for Donor 1 cells, and the dip in cell viability on Day 2 for Donor 3 cells, compared to BECA-S. Additionally, possible inadequate culture resuspension with BECA-Auto could be another factor that have caused the discrepancies in our observation of the cultures, leading to collection of a sample that is not representative of the culture at that point in time. Refining the movements of the Actuation Platform can help improve the culture resuspension and ensure the collection of representative samples of the culture. To demonstrate the robustness of BECA platform in supporting manual to automated process transition, additional studies with more donors and a variety of different cell culture protocols can be conducted in the future.
The diversity of cell therapy automation solutions has grown significantly in recent years to suit the needs of cell therapy developers. In terms of costs and scalability, the fully developed BECA platform is expected to be similar to currently available automated T cell culture platforms such as Lonza’s Cocoon, Cytiva’s Sefia, and Miltenyi Biotec’s CliniMACS Prodigy. These fully closed platforms have relatively lower setup costs due to their lower requirements for facilities but do require recurring costs for single-use consumable sets with each manufacturing run. Their limited scale in culture volume imposes an upper limit on culture capacities, making these platforms unsuitable for the scale-up required for allogenic cell therapy products. Despite these similarities, BECA distinguishes itself from the other automated platforms by its ability to accommodate a wide range of culture capacities (due to its expandable culture area), thus automating workflows of varying starting cell numbers including rare populations with low starting numbers.
In addition, BECA offers a matched manual vessel that facilitates process transfer from manual handling to automation — a key consideration for cell therapy developers when choosing an automation solution. The use of an unsuitable platform could result in significant redevelopment and revalidation of the manufacturing process, leading to increased development cost and time. Currently, there is a lack of technologies that focus on this process transfer. The BECA platform is well-positioned to address this gap and has the potential in lowering total cost and expediting the development timelines, ultimately improving business efficiency. Aside from the BECA platform, this gap of manual to automated transition in cell therapy manufacturing may also be addressed using robotic automation, which consists of robotic arms and modular stations, such as those from Multiply Labs24. However, these solutions are distinctively different from BECA in these aspects: (1) their station-based mode of operations with robotic arms take up significant large footprint compared to BECA-Auto, (2) they are highly customisable with regards to types of culture vessels or processes that can be adapted to the system compared to BECA which is specialised to the BECA-S vessel, and (3) users require advanced training and engineering training to program the robotic arms and integrate different platforms. Due to these differences, robotic automation and BECA address the needs of different cell therapy developers and manufacturers - robotic automation being more suitable for use in cell therapy manufacturing facilities that cater to a wide variety of culture processes for multiple cell products and BECA being more suitable for an independent cell therapy developer looking to control their end-to-end therapy development from R&D to manufacturing.
The BECA platform, currently consisting of BECA-S, BECA-D23, and BECA-Auto, can accommodate different process needs and automation process controls. Although this report focuses on transition of manual process from BECA-S, those developed on BECA-D, such as previously published virus-specific T cells workflow23, can also be automated by BECA-Auto. Additionally, while the reported data in this study has focused on T cell expansion, the BECA platform can potentially be used for the culture of other non-adherent cell types (e.g. natural killer (NK) cells) and support a wider manufacturing workflow beyond expansion. Doing so, we aim to achieve a fully automated end-to-end cell therapy manufacturing system with direct manual to automated process transfer. To streamline and further automate tangential workflows such as monitoring of culture parameters including population, density, viability, and metabolites, BECA-Auto can be integrated with analysis equipment (e.g., automated cell counter, flow cytometers, metabolite analyser) through DAAS. The analysis data can be fed into feedback control for BECA-Auto to optimise the reagent input and harvesting plan, further improving the efficiency of the cell culture. We are currently working on beta-testing BECA-Auto and DAAS on a GMP workflow with further plans on exploring the above applications.
Methods
Development of BECA-S and BECA-Auto
BECA-S was developed as a single-use cell culture vessel for manual handling in the BSC. Its key design feature is the in-situ expansion of cell culture area from 19 to 102.4 cm2.
BECA-Auto was developed as a standalone, climate-controlled automated system for automated cell manufacturing.
Workflow Analysis and Design Thinking was used to define the essential functions and design requirements for BECA-S and BECA-Auto (Table 4).
Cell culture
For all studies (3 donors, BECA-S and BECA-Auto), cryopreserved human PBMCs (STEM Cell Technologies) were thawed at 37 °C and seeded at 0.5 × 106 cells/cm2 (0.5 mL/cm2) with a starting culture area of 19 cm2. Cells were activated on Day 0 with 25 µL/mL ImmunoCult™ Human CD3/CD28 T Cell Activator (STEMCELL Technologies) and maintained for 10 days in complete media consisting of RPMI-1640 (Thermo Fisher Scientific), 10% heat-inactivated Fetal Bovine Serum (FBS) (HyClone), and 1× GlutaMAX (Thermo Fisher Scientific), with supplementation of 50 IU/mL Interlukin-2 (IL-2) (STEMCELL Technologies). Gentle rocking of vessels was performed to resuspend culture after seeding and prior to sample extractions for cell count and cell surface phenotype assessments. Cell counts and viability were assessed through trypan blue exclusion on Days 3, 5, 7, and 10 and cell surface phenotypes were assessed using flow cytometry on Days 0, 7, and 10.
Use of BECA-S and BECA-Auto for cell culture
Fluid handling steps for BECA-S were manually performed in the BSC and culture was incubated in standard CO2 incubators at 37ºC under 5% CO2. BECA-Auto was operated as a benchtop equipment placed on a workbench. Fluid handling steps and culture incubation for BECA-Auto were performed by the equipment at 37ºC under 5% CO2. All manual steps in the workflow (seeding, sampling, media feed, expansion, and harvesting) for BECA-S were replicated and performed through automation in BECA-Auto.
BECA-S was assembled, autoclaved, and air-dried overnight for seeding of culture on the next day. Expansion of culture area was performed on Days 3, 5, and 7 if culture density exceeded 1 × 106 cells/cm2. The in-situ expansion of culture area at 10 cm2/cm was achieved through pulling of the plunger which shifts the internal movable wall of the vessel. Media height within the vessel was kept at 5 mm from Day 0 to 5, and 10 mm from Day 5 to 10. Media top-ups with supplementation of IL-2 (50 IU/mL at total media volume) were performed on Day 3, 5, and 7 to maintain the required media height.
BECA-S (Closed) and Manifold Assembly of BECA-Auto were sterilised by gamma irradiation under 25 kGy and assembled in the BSC. They were installed onto the Actuation Platform, and coupled to DAAS and CIFC. PBMCs were prepared in a sterile culture bag in the BSC and automatically loaded into BECA-S (Closed) within BECA-Auto using the Seeding programme. A sample was extracted using the Automated Sampling programme on Days 3, 5, and 7, which was used for cell count, and cell surface phenotyping (Day 7). Expansion of culture area was performed on Days 3, 5, and 7 if culture density exceeded 1 × 106 cells/cm2 through the Culture Area Expansion programme. Media height within the vessel was kept at 5 mm from Day 0 to 5, and 10 mm from Day 5 to 10. Media top-ups with supplementation of IL-2 (50 IU/mL at total media volume) were performed on Day 3, 5, and 7 with media prepared in sterile culture bags using the Media Feed programme to maintain the required media height. Culture was harvested on Day 10 using the Harvesting programme.
Cell surface phenotyping
Flow cytometry was performed using the following antibodies (Miltenyi Biotec). Viobility 405/520 Fixable Dye (130-109-814), CD3-VioBlue (130-114-519), CD8-VioBlue (130-110-683), CD4-APC-Vio770 (130-113-223), CD197 (CCR7)-PE (130-120-463) CD45RA-PerCP-Vio700 (130-113-368), TIM-3-FITC (130-125-979) and CD279 (PD1)-APC (130-120-383). Populations of T cells were defined as follows: helper T cells (CD3 + CD4+), cytotoxic T cells (CD3 + CD8+), central memory T cells (TCM) (CCR7 + CD45RA-), effector memory T cells (TEM) (CCR7-CD45RA-), naive T cells (TN) (CCR7 + CD45RA+), and terminally differentiated T cells (TEMRA) (CCR7-CD45RA+), exhausted T cells (CD3 + Tim-3 + PD-1+). MACSQuantX Flow Analyser (Miltenyi Biotec) was used to acquire the data and MACSQuantify Software (Miltenyi Biotec) was used for analyses.
Statistical analysis
Statistical analyses were performed using Microsoft Excel (Office 365). Paired Student’s two-tailed t-tests were performed to assess the statistical significance of differences between two groups within each of the donor sets. A p-value of < 0.05 obtained from the test indicates a significant difference.
Data availability
The datasets generated during and/or analysed during the current study are provided within the manuscript. Should any data files be required in another format they can be made available from the corresponding author on reasonable request.
References
Eyles, J. E. et al. Cell therapy products: focus on issues with manufacturing and quality control of chimeric antigen receptor T-cell therapies. J. Chem. Technol. Biotechnol. 94, 1008–1016 (2019).
Silva, D. N. et al. Process development for adoptive cell therapy in academia: A pipeline for Clinical-Scale manufacturing of multiple TCR-T cell products. Front. Immunol. 13, 896242 (2022).
Missan, D. S. et al. Cytotherapy 26, S138 (2024).
Dai, X., Mei, Y., Nie, J. & Bai, Z. Scaling up the manufacturing process of adoptive T cell immunotherapy. Biotechnol. J. 14, 1800239 (2019).
Song, H. W., Somerville, R. P., Stroncek, D. F. & Highfill, S. L. Scaling up and scaling out: advances and challenges in manufacturing engineered T cell therapies. Int. Rev. Immunol. 41, 638–648 (2022).
Reyes, C. F. et al. End-to-end automated manufacturing of autologous car t cell therapies with the new sefiaTM cell therapy manufacturing platform. Cytotherapy 26, S166–S167 (2024).
Mock, U. et al. Automated manufacturing of chimeric antigen receptor T cells for adoptive immunotherapy using clinimacs prodigy. Cytotherapy 18, 1002–1011 (2016).
Zhu, F. et al. Closed-system manufacturing of CD19 and dual-targeted CD20/19 chimeric antigen receptor T cells using the clinimacs prodigy device at an academic medical center. Cytotherapy 20, 394–406 (2018).
Castella, M. et al. Point-of-care CAR T-cell production (ARI-0001) using a closed semi-automatic bioreactor: experience from an academic phase i clinical trial. Front. Immunol. 11, 1–13 (2020).
Coeshott, C., Vang, B., Jones, M. & Nankervis, B. Large-scale expansion and characterization of CD3 + T-cells in the Quantum® cell expansion system. J. Transl Med. 17, 1–13 (2019).
Startz, T. et al. Expansion of T-Cells in an Automated, Functionally Closed Hollow-Fiber Bioreactor System (QUANTUM ® CELL EXPANSION SYSTEM, 2016).
Trainor, N. et al. Automated production of gene-modified chimeric antigen receptor T cells using the cocoon platform. Cytotherapy 000, 1–12 (2023).
Costariol, E. et al. Demonstrating the manufacture of human CAR-T cells in an automated Stirred-Tank bioreactor. Biotechnol. J. 15, (2020).
Costariol, E. et al. Establishing the scalable manufacture of primary human T-cells in an automated stirred-tank bioreactor. Biotechnol. Bioeng. 116, 2488–2502 (2019).
Ismail, R., Janas, M., Stone, S., Marenghi, A. & Sauvage, V. Expansion of T-cells using the Xuri TM cell expansion system W25 and WAVE bioreactor TM 2 / 10 system. 44, 20526253 (2013).
Smith, T. A. CAR-T Cell Expansion in a Xuri Cell Expansion System W25 BT - Chimeric Antigen Receptor T Cells: Development and Production. in (eds. Swiech, K., Malmegrim, K. C. R. & Picanço-Castro, V.) 151–163Springer US, New York, NY, (2020). https://doi.org/10.1007/978-1-0716-0146-4_11
Sin, W. X. et al. A high-density microfluidic bioreactor for the automated manufacturing of CAR T cells. Nat. Biomed. Eng. https://doi.org/10.1038/s41551-024-01219-1 (2024).
Song, H. W. et al. CAR-T cell expansion platforms yield distinct T cell differentiation States. Cytotherapy 26, 757–768 (2024).
Wu, Y. Y., Liu, D. & Naing, M. W. Development of a closed and automated bioreactor technology for cell therapy manufacturing - a sharing of our journey. Regen. Med. 15, 2335–2340. https://doi.org/10.2217/rme-2020-0142 (2020).
Dan, L., Ying, Y., Akshaya, W. & Ahmad Amirul, V. P. B. A. R. & Jia Sheng Zach, L. Device for automated aseptic sampling (DAAS): automated sampling solution for future cell and gene manufacturing. Front. Bioeng. Biotechnol. 12, (2024).
Lapteva, N. & Vera, J. F. Optimization manufacture of virus- and tumor-specific T cells. Stem Cells Int. 17-20, 2011 (2011).
Wu, Y. Y., Yong, D. & Naing, M. W. Automated cell expansion: trends and outlook of critical technologies. Cell. Gene Ther. Insights 843–863. https://doi.org/10.18609/cgti.2018.086
Chen, S. et al. In-situ scalable manufacturing of Epstein–Barr virus-specific T-cells using bioreactor with an expandable culture area (BECA). Sci. Rep. 12, (2022).
Melocchi, A. et al. Development of a robotic cluster for automated and scalable cell therapy manufacturing. Cytotherapy 000, 1–10 (2024).
Acknowledgements
This work is funded by core fund from Bioprocessing Technology Institute (BTI), Singapore Agency for Science, Technology and Research (A*STAR), and Integrated Manufacturing Programme for Autologous Cell Therapy (IMPACT), an Industry Alignment Fund—Pre-Positioning Programme (Grant Number: H18/01/a0/022) funded by Agency for Science, Technology and Research (A*STAR).
Author information
Authors and Affiliations
Contributions
J.S.Z.L. and A.V.P. performed the validation study. Y.Y.W., J.S.Z.L., and A.A.A.R. designed and developed the BECA platforms used in this study. M.W.N., D.L., Y.Y.W. and S.C. conceptualised the BECA platforms and this study. S.C., A.A.A.R., D.L. and A.V.P. analysed the results and designed the experiments for the study. S.C., D.L., A.V.P., J.S.Z.L., and A.A.A.R. wrote the manuscript text. S.C., A.A.A.R., Z.L.J.S., and A.V.P. prepared the figures and the table. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lee, J.S.Z., Prabhu, A.V., Wu, Y.Y. et al. Transition from manual to automated processes for autologous T cell therapy manufacturing using bioreactor with expandable culture area. Sci Rep 15, 15819 (2025). https://doi.org/10.1038/s41598-025-00015-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-00015-4