Abstract
The addition of zinc oxide (ZnO) to mineral trioxide aggregate (MTA) has been shown to prevent tooth discoloration; however, its biological effects remain unclear. This study aimed to evaluate the pulpal responses to MTA containing 5% ZnO in full pulpotomy of dogs’ teeth. Forty caries-free premolars from mixed-breed dogs were subjected to full pulpotomy. Exposed pulpal tissues were randomly capped with either Angelus MTA (MTA) or Angelus MTA mixed with 5% ZnO (MTA + ZnO) (n = 20 each). After 4 weeks, the teeth were extracted, processed for histological evaluation, and stained with hematoxylin and eosin. Tissue response data were analyzed using the Mann-Whitney U test at a 95% significance level. The incidence, thickness, and continuity of hard-tissue bridge formation were significantly lower in the MTA + ZnO group (p = 0.007, p = 0.001, and p = 0.002, respectively). Most samples in both groups exhibited no inflammatory cells, and none showed signs of necrosis. Incorporating ZnO into Angelus MTA compromised the quantity and quality of hard-tissue bridge formation following full pulpotomy in dogs’ premolars.
Similar content being viewed by others
Introduction
Vital pulp treatment (VPT) in immature permanent teeth preserves pulp structural integrity and function, fostering continued radicular development and maturation. This healing process involves reorganization of damaged pulpal cells and the formation of a hard-tissue bridge1. In a full pulpotomy (FP), as a VPT modality, the coronal portion of the vital (and inflamed) pulp is amputated to preserve the intact radicular portion according to the definition stated by the American Association of Endodontists (AAE) and European Society of Endodontology (ESE)2,3. Recently, pulpotomy has been advised to treat carious pulpal exposures with a high success rate based on the presence of healthy pulpal structure away from the local damage at the exposure site, removal of possibly infected coronal tissue, and use of biologically active hydraulic calcium silicate-based cements (HCSCs)3. The sustained vitality of the radicular pulp in FP can prevent load-related root fracture through its stress-reducing damping effect via mechanoreceptor and proprioceptor function4.
The introduction of HCSCs has significantly improved the success rate of VPT in recent decades. Among these, mineral trioxide aggregate (MTA) has emerged as the gold standard due to its ability to induce rapid and high-quality calcified bridge formation5. However, MTA has significant clinical limitations, including discoloration, which restricts its use in esthetically sensitive regions. The discoloration is linked to the presence of bismuth oxide as a radiopacifier, which destabilizes upon contact with oxidizing agents such as sodium hypochlorite or amino acids in dentin collagen, leading to esthetic compromise6,7.
To address this, various strategies have been proposed, including substituting bismuth oxide with zirconium oxide or calcium tungstate, or incorporating zinc oxide (ZnO) into MTA’s formulation. ZnO addition has shown promise in preventing bismuth oxide destabilization, thereby reducing discoloration while maintaining critical properties such as radiopacity, setting time, volume stability, and biocompatibility8,9,10. Moreover, the inclusion of 5% ZnO has been reported to enhance calcium ion release, which is crucial for pulp healing9. Despite these advancements, there is a critical lack of data on how ZnO-modified MTA influences the biological response of the pulp, a key factor in the success of VPT.
Given the ethical restrictions of testing novel unlicensed materials in humans, dog models have been widely advocated for histological evaluations of VPT due to similarities in human and canine dento-alveolar histology11. To date, no study has investigated the pulpal response to ZnO-modified MTA in FP procedures. Addressing this gap, the present study aims to evaluate the histopathological pulpal responses following the application of MTA with or without ZnO incorporation in FP after four weeks, using healthy male mongrel dogs’ premolars.
Methods
Ethical consideration
This study was approved by Research Ethics Committee of Tehran University of Medical Science, Tehran, Iran (Ethics code: IR.TUMS.DENTISTRY.REC.1397.169), and all methods were performed in accordance with the relevant guidelines and regulations. This study was also reported in accordance with the Animal Research: Reporting In Vivo Experiments (ARRIVE) guidelines (https://arriveguidelines.org). Regarding the 3 R’s (replacement, reduction, and refinement) every effort was made to minimize animal suffering and reduce the number of animals used12.
Subject selection and sample size estimation
Immunocompetent, caries-free male mongrel dogs sourced from the Research Laboratory of the Faculty of Veterinary Medicine at Tehran University were registered. The dogs had an average age of 2 years and a weight range of 20 to 30 kg. The sample size for the study was calculated using PASS 2021 software, specifically utilizing the paired means power analysis option to identify significant differences between groups. Other included parameters were: α = 0.05, β = 0.20, mean difference = 1.25 and standard deviation = 0.84. Therefore, the result indicated a minimum of 6 samples per group. To account for potential procedural mishaps during the execution of the procedures, ten samples were included in each group.
Animal husbandry
The animals underwent necessary vaccinations and a 3-week preoperative quarantine in standardized cages with normal light, temperature, and humidity conditions. They had access to water and food ad libitum and were closely supervised by a veterinarian. All nutritional recommendations and pre-, intra-, and postoperative animal care were conducted by certified animal technicians under the guidance of a senior veterinarian, adhering to the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals, as well as current international legislation on animal use in experimental research. All aspects of animal care and husbandry were carried out by two experienced veterinarian experts (SFM and MMD).
Procedure
Teeth underwent periodontal pocket depth measurement for assessing periodontal conditions, while pulpal and periapical health was evaluated through intra-oral examinations and periapical radiographs, preoperatively. Dogs having caries and/or periodontal or periapical problems were excluded and 40 healthy permanent premolars with complete root formation were selected. Anesthesia was induced using 5 mg/kg 10% ketamine hydrochloride (Alfasan, Woerden, Netherlands) and 0.27 mg/kg diazepam (Caspian-Tamin Pharmaceuticals, Iran). Dogs were intubated, and inhalation anesthesia was maintained throughout the procedure using 1.5% isoflurane. Animal vital signs were monitored continuously throughout the process. Prophylactic cefazolin (25 mg/kg; Loghman-Pharmaceuticals, Iran) followed by preemptive analgesia (2 mg/kg Tramadol; Caspian-Tamin Pharmaceutical Company; and 0.2 mg/kg Meloxicam; Razak-Pharmaceuticals, Iran) was also provided. Teeth were polished and rinsed with 0.2% chlorhexidine gluconate (Shahre-Daru Pharmaceuticals, Iran). Then, all teeth were locally anesthetized by infiltration for maxillary and mental block in conjunction with local infiltration for mandibular regions using 2% lidocaine with 1:100,000 epinephrine (Daru-Pakhsh Company, Iran). Subsequently, teeth were isolated with a dental dam (Sanctuary-Systems©, Perak, Malays i.a.). Access cavities were prepared coronally using a water-cooled high-speed round bur (DiaDent International, South Korea) and the entire roof of the pulp chamber was removed. Afterwards, the coronal pulps were removed with endodontic excavators at cemento-enamel junction level. Bleeding was controlled by copious saline irrigation and cotton pellet placement using light pressure. All dental procedures were done by a postgraduate endodontics resident (SN) aided by a senior undergraduate dental student(BD) under the supervision of an experienced endodontist (BB).
Materials, experiment groups and randomization
The teeth were subjected to one of the following groups based on the HCSCs used:
-
1.
White Angelus MTA (Angelus, Londrina, Brazil) (MTA).
-
2.
White Angelus MTA mixed with 5% ZnO (Sigma Aldrich, Dorset, UK) (MTA + ZnO).
To incorporate ZnO into Angelus MTA, 0.05 g ZnO was added to 1 g MTA powder and mixed in a Dry Powder Rotator (Glas-Col, Terre Haute, Indiana, USA) to achieve a homogeneous mixture. Cement preparation for each group involved mixing 1 g of each cement powder with 0.33 ml of their respective liquid, following manufacturers’ instructions9. Teeth on one side of the animal’s mouth were randomly assigned to a treatment using an online random generator (www.randomization.com), while the contralateral teeth were then allocated to the alternate treatment group, establishing a split-mouth model. The HCSCs were packed in opaque paper bags to which the operator, assistant, and analyzer were blinded, preoperatively. A 2-mm-thick layer of the cement was gently applied to each cavity. Upon application, each cement mixture in the samples effectively hardened and adhered to the application site. This ensured stability and integrity throughout the procedure, owing to their optimal viscosity and consistency. For the ZnO-MTA mixture, the chemically stable compound formed by this combination significantly contributed to its stabilization, ensuring durability and strength. Additionally, no reflux issues were observed during the application process. The composition of the mixture and the application technique minimized any potential for reflux, ensuring a smooth and effective procedure. Then, a moist cotton pellet was positioned on the cement for 30 min, and the cavity was filled with a restorative resin-modified glass ionomer (GC Corporation, Tokyo, Japan).
Follow-up
The animals underwent daily monitoring to ensure lack of intra- or postoperative discomfort. In case of any observed distress, 0.1 mg/kg meloxicam was provided intravenously. Animals were anesthetized after 30 days as previously described. Following comprehensive examinations, the treated teeth were removed atraumatically and immediately placed in a 10% buffered formalin solution (Sigma-Aldrich, Missouri, USA) for further evaluation.
Tissue processing and histological evaluation
The extracted affected teeth were fixed in 10% neutral buffered formalin for 48 h and then decalcified in 20% formic acid until suitable for microtomy. They were thoroughly rinsed in running tap water for several hours to remove residual acid, then processed and embedded in paraffin. Each specimen was serially sectioned at a thickness of 4 μm in a bucco-lingual direction and subsequently stained with Hematoxylin and Eosin (H&E). Afterwards, the specimens were coded and the evaluations were conducted on three consecutive mid-sections of the tissue after dissection to maintain consistency. A single, experienced oral and maxillofacial pathologist, blinded to the group assignments (NKK), performed the analysis to ensure unbiased interpretation. The entire interfacial areas were evaluated under 40x, 100x, and 400x magnifications using a light optical microscope(Olympus BX51, Tokyo, Japan). At lower magnifications (40x and 100x), the overall tissue structure, including the extent of new dentin formation and associated pulpal changes, was evaluated. At higher magnifications (400x), finer details such as cellular infiltration, tissue vitality, and the quality of calcified bridge formation were examined.
Pulpal reactions to the experimental HCSCs and formation of calcified barrier were histologically assessed. Evaluation of pulpal reactions encompassed intensity (quantified by the average count of inflammatory cells), severity, and extent of the inflammatory responses. Additionally, the presence or absence of pulpal congestion, hyperemia, necrosis, and calcification were examined. In assessing the hard-tissue barrier, the focus was on identifying the presence or absence of the bridge, along with analyzing its continuity and thickness13 (Table 1). A single, experienced oral and maxillofacial pathologist (NKK), blinded to the group assignments, performed the analysis to ensure unbiased interpretation.
Statistical analysis
According to the non-normal distribution of the quantitative variable regarding Kolmogorov–Smirnov test as well as ordinal and dichotomous nature of the qualitative variables, the statistical analysis was performed using the Mann-Whitney U test and the significance level was set at p = 0.05 using SPSS 25.0 software (SPSS Inc., Chicago, IL, ).
Results
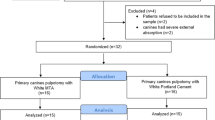
The subjects’ general health condition, including animal weight and food/water consumption, showed no significant differences during the experiment. Due to procedural mishaps (perforations of the pulpal chamber floor while preparing the access cavity), six teeth (three from each group) were excluded from the study. This exclusion left a total of 34 teeth (17 per group) available for histological evaluation using various indices (as detailed in Table 1). The exclusions were necessary to prevent any potential interference with the treatment outcomes. The distribution of samples between groups based on tooth and jaw type is detailed in Table 2, and the procedural steps are illustrated in the PRIASE flowchart (Fig. 1)14.
Evaluation of hard-tissue Bridge formation
Development of hard-tissue Bridge
Significantly more hard-tissue bridges formed in cases treated with MTA at the material interface and radicular pulp entrance compared to MTA + ZnO (p = 0.007) (Table 3).
Hard-tissue Bridge continuity
There was a significant difference between MTA and MTA + ZnO group in terms of calcified bridge continuity (Table 3). The overall continuity of calcified bridge in MTA + ZnO group at the vicinity of the material and at the entrance of radicular pulp was significantly less than that in MTA group (p = 0.002). (Fig. 2a&b)
(a through d) Photomicrographic images of histologic sections in both MTA, and MTA + ZnO groups. (D: dentin, HTB: hard-tissue barrier, P: vital pulp, B: bone, pdl: periodontal ligament, BVC: blood vessel congestion and hyperemia, INF: inflammation, MTA: mineral trioxide aggregate, MTA + ZnO: mineral trioxide aggregate mixed with zinc oxide.) (a) Formation of a continuous hard-tissue barrier in response to MTA (H&E ×40). (b) Incomplete and non-continuous dentin bridge formation in MTA + ZnO group (H&E ×100). (c) Areas of necrosis in the upper root canal area, along with a mild chronic inflammatory process in the remaining viable pulp tissue in the MTA + ZnO group (H&E ×40). (d) Upper regions exhibit chronic lymphocytic inflammatory cell infiltration, while lower areas display pulpal hyperemia, including multiple congested blood vessels (H&E ×400).
Thickness of hard-tissue Bridge
The thickness of the hard-tissue bridge formed among the samples ranged from 0 to 157 μm. A significantly greater mean thickness of the hard-tissue bridge was noted after MTA application compared to MTA + ZnO (p = 0.001) (Table 3).
Evaluation of pulpal reactions
In the MTA group, 41.2% (n = 7) and in the MTA + ZnO group, 23.5% (n = 4) exhibited varying degrees of subjacent pulpal inflammatory reactions to the formed barrier (Fig. 2c). Details of the histopathological evaluation of pulpal reactions are provided below.
Inflammation intensity
Less intense inflammatory reactions were observed in teeth treated with MTA + ZnO compared to MTA alone (Table 3) (p = 0.370). In the MTA group, scores 1 and 2 were more frequent than in MTA + ZnO in terms of inflammatory reaction intensity, while score 3 was more frequent in MTA + ZnO (p > 0.05).
Extent of inflammatory cell infiltration
Deeper inflammatory cell penetration was noted in teeth treated with MTA + ZnO. Although, there were no statistically significant differences in the extent of inflammatory cell infiltration between the two groups. (Table 3) (p = 0.466).
Diffuse pulpal calcification
More than half of the samples in the MTA group showed intrapulpal calcification, but the difference was not statistically significant (p = 0.307) (Table 3).
Pulpal necrosis
No pulp tissue necrosis, indicated by the absence of denatured proteins within the intrapulpal space, was observed in the MTA group (Table 3). However, these features were encountered in 2 cases treated with MTA + ZnO throughout the entire radicular pulp (11.8%). (p = 0.151)
Pulpal hyperemia
Extravasated erythrocytes from pulpal blood vessels were observed in over half of the samples in both experimental groups, but the difference was not statistically significant. (Fig. 2d) (p = 0.734) (Table 3).
Discussion
In VPT, applying a biocompatible material to promote healing is mandatory. FP, involves removing the coronal pulp following carious or traumatic pulpal exposure, while preserving the radicular pulp. Key factors in selecting this treatment modality include pulp health, exposure size, symptoms, restorability, and patient factors like age. HCSCs such as MTA are preferred to be used as pulpotomy agents for their bioactive properties15. Treatment strategy in FP focuses on promoting pulpal wound repair and preventing contamination, similar to other VPT modalities. This involves disinfection and proper sealing of the wound environment using HCSCs. This well-documented procedure, stimulates the recruitment and proliferation of pulpal stem cells that differentiate into odontoblasts and odontoblast-like cells capable of hard-tissue barrier formation2. MTA is widely favored as a VPT material due to its bioactive properties. However, its drawbacks including tooth discoloration, poor handling, and extended setting time have made researchers to enhance its physical and biological properties. To reduce tooth discoloration, Marciano et al. (13) recommended incorporating ZnO into Angelus MTA. They found that adding ZnO prevented the destabilization of bismuth oxide, the main cause of MTA-induced discoloration upon exposure to oxidizing agents9. Testing various ZnO concentrations with MTA, authors identified that a 5% concentration effectively prevented cement-induced discoloration without compromising its physical, chemical, or biological properties. However, as per the authors’ current knowledge, pulpal responses to this mixture have not been elucidated ex vivo, representing a crucial area of inquiry.
It is imperative to consider several pivotal factors during histological assessments of responses to biomaterial application in animal models. Although use of small animals e.g. mice and rats can provide researchers with some advantages such as ease of handling and comparably lower costs of experimentation, utilizing large animal models such as dogs or monkeys can be beneficial in their closer approximation with human body conditions16. Correspondingly, dogs are widely regarded as a popular animal model for studying the biological reactions of HCSCs in VPT and regenerative endodontics. Their tooth sizes, comparable to humans, stand as one of the most crucial factors in choosing them as a suitable research model11. However, basic structural differences between human and any kind of animal model used in pre-clinical studies cannot be overemphasized in interpretation of the translational data. In the current investigation canine premolars were used because of their close similarities with their human counterparts in terms of dental anatomy, pulp-dentin complex structure and development. Additionally, resemblances between human and canine immune reactions and genetic factors were of utmost importance in this regard17,18. In addition, the decision to observe the effects of HCSCs on VPT for four weeks was made because this timeframe was deemed sufficient for the pulpal tissue to adequately show its primary responses1,19.
Pulpal protection against microleakage plays a pivotal role in achieving success in VPTs. While this protection is offered by the HCSCs, the sustained success relies on the durability and consistency of the bridge formed, which offers supplementary safeguarding for the pulp. With time, the quality of this bridge might enhance, potentially reducing its permeability20,21,22. Regarding the current study, not only was the hard-tissue bridge formed in MTA group more frequently observed (p = 0.007), but also the resultant bridge showed a thicker and more continuous structure. In addition, the barrier formed in MTA group was significantly thicker than that of the MTA + ZnO group. Furthermore, 41.2% of the samples in the MTA group showed complete hard-tissue barrier formation. Therefore, it can be concluded that the addition of 5% ZnO to Angelus MTA had an adverse effect on the quantity and quality of the hard-tissue barrier formation.
Calcium hydroxide formation is considered as a consequence of hydration reaction of MTA23,24. Calcite crystals are subsequently formed due to the reaction of this calcium hydroxide with the carbon dioxide present in pulp tissue25, serving as the initiating step in the formation of hard-tissue barrier26. Moreover, Ca++ ion release during MTA hydration promotes odontoblastic differentiation27. Ca++ directly reacts with phosphate ions in tissue fluid to form hydroxyapatite crystals28, which are subsequently incorporated into the formed hard-tissue barrier. Gawlicki et al. (33) demonstrated that adding ZnO to Portland cement forms amorphous zinc hydroxide, creating an impermeable layer around tricalcium silicate and potentially hindering cement hydration. Given the similarities between Portland cement and MTA in composition and hydration, this adverse effect might occur in MTA, leading to reduced calcium hydroxide formation and, consequently, impaired calcite crystal creation, adversely affecting hard-tissue barrier formation29.
The study revealed that most MTA group samples exhibited minimal inflammatory reactions beneath the cements, primarily limited to the coronal thirds of the pulp. Moreover, no signs of necrosis were observed in MTA-treated samples. These findings align with previous studies on biological responses to MTA1,30,31,32,33. It can be suggested that MTA provides a suitable environment that allows healing, owing to its biocompatibility. In case of MTA + ZnO, similar results were seen regarding inflammation; however, in 11.8% of the samples, signs of tissue necrosis throughout the pulpal tissue was observed. Cytotoxic effects of zinc remain to be controversial, nonetheless, ZnO-mediated apoptosis and chromosome aberrations have been reported in human pulpal cells34,35.
In more than half of the MTA group samples, diffused intrapulpal calcification was observed. Studies have reported canal obliteration after MTA pulpotomy36, and this was seen in less than half of the MTA + ZnO group samples, potentially linked to a higher rate of necrosis. Since intrapulpal calcification may hinder re-entry in cases of VPT failure, additional research is needed to understand effective management strategies for this issue.
The observed reduction in the thickness and continuity of hard-tissue bridge formation with ZnO-MTA compared to MTA alone can be attributed to several factors related to the physicochemical properties and biological responses elicited by these materials. MTA is known for its excellent biocompatibility and ability to promote reparative dentinogenesis. It interacts favorably with the surrounding biological environment, facilitating the formation of a mineralized barrier through the release of calcium ions, which are crucial for odontoblast differentiation and matrix mineralization37. In contrast, the addition of ZnO may alter these properties, potentially affecting the material’s overall reactivity and its ability to stimulate hard tissue formation. Studies have shown that while ZnO can provide some beneficial effects, it may also inhibit certain biological processes that are critical for effective dentin bridge formation38,39. The cellular response to MTA is characterized by a robust inflammatory reaction that is generally mild, promoting healing and dentin bridge formation. The introduction of ZnO may modulate this response, leading to a less favorable environment for odontoblast-like cell activity. Research indicates that MTA alone often results in a higher percentage of complete dentin bridge formation compared to combinations with ZnO, which may lead to reduced cellular activity in the reparative process40,41. Histological evaluations have demonstrated that specimens treated with MTA alone typically exhibit thicker and more continuous hard-tissue bridges compared to those treated with ZnO-MTA. In one study, the incidence and quality of hard-tissue bridge formation were significantly lower in the MTA + ZnO group, indicating that the addition of ZnO compromises the material’s effectiveness in promoting reparative processes38,39. This reduction in quality may be due to changes in the microenvironment around the pulp tissue, which can affect cell signaling and matrix deposition. While both MTA and ZnO-MTA are designed to minimize inflammation, studies suggest that ZnO may provoke a different inflammatory response that could hinder optimal healing. The inflammatory response plays a crucial role in tissue repair; therefore, any alteration in this response can impact the overall effectiveness of dentin bridge formation40.
The limitations of this study primarily stem from the short evaluation period and the absence of long-term effects on pulpal response. The four-week observation period, although sufficient to assess the primary responses of the pulpal tissue, may not fully capture the long-term behavior and stability of the materials used. Longer evaluation periods are essential to understand the durability of the hard-tissue barriers formed, their resistance to microleakage, and their potential for continued mineralization or degradation over time. Additionally, the long-term effects of ZnO-MTA on pulpal health and the overall success of vital pulp therapy (VPT) remain unexplored in this study. Future research should include extended observation periods to monitor the progression of pulpal healing, the integrity of the hard-tissue bridges, and any potential late-stage inflammatory responses or tissue necrosis. This would provide a more comprehensive understanding of the long-term efficacy and safety of ZnO-MTA compared to MTA alone.
The long-term stability and integrity of ZnO-MTA in preventing microleakage is crucial for dental applications like pulp capping and root-end filling. Research shows that ZnO-MTA’s compressive strength is comparable to pure MTA over time, retaining adequate mechanical properties for clinical use, although initial strength is lower42. The ability to prevent microleakage, essential for avoiding bacterial infiltration and failure, may be compromised with ZnO incorporation, potentially leading to increased microleakage over time, unlike MTA which exhibits superior sealing properties43.
The impact of ZnO-MTA on adhesion and bond strength should not be overemphasized in dental restorations. Studies show that bond strength of HCSCs varies with different restorative materials and timings, indicating that ZnO-MTA can perform better with specific strategies. Delayed bonding to MTA improves shear bond strength due to increased stability and reduced moisture interference. Adhesive systems also affect bond strength, with some adhesive systems outperforming others with an added hydrophobic bond layer formation improving performance. SEM analysis shows these factors influence adhesion effectiveness and stability. Overall, careful material choice and timing can enhance ZnO-MTA or MTA adhesion44,45,46.
The incorporation of ZnO into MTA is a novel approach in endodontics, aiming to address MTA’s limitations like tooth discoloration without compromising its bioactivity. This innovative modification advances dental materials and techniques in VPT. ZnO-MTA’s potential to prevent tooth discoloration while maintaining MTA’s beneficial properties is highly relevant to clinical practice, offering insights that could enhance patient satisfaction and treatment outcomes. A comprehensive histological evaluation provides detailed insights into the biological interactions between ZnO-MTA and dental tissues, enhancing understanding of its effectiveness in promoting hard-tissue barrier formation and pulpal health. The use of canine premolars as the animal model adds robustness to the study, as their dental anatomy and pulpal characteristics closely resemble human teeth, increasing the translational value of the findings. The controlled experimental design, including a standardized observation period and systematic comparison between MTA and ZnO-MTA, ensures the reliability and validity of the results, enhancing the credibility of the study’s conclusions.
Future studies should evaluate the long-term biological seal of ZnO-MTA and its effectiveness in preventing bacterial infiltration. These studies could include extended observation periods to assess durability, microleakage tests under various conditions, and comparative studies with other materials like pure MTA and Biodentine. Detailed histological analyses and advanced imaging techniques, such as micro-CT and SEM, would provide insights into tissue responses and seal integrity. Clinical trials could monitor long-term outcomes, including patient satisfaction and success rates. In vivo animal models could simulate clinical conditions, offering relevant translational data. This research would contribute significantly to understanding ZnO-MTA’s long-term effectiveness and potential advantages in dental restorations.
Conclusion
Given the constraints of this study, application of Angelus MTA with 5% ZnO might negatively impact pulp reactions following FP in dogs’ premolars. This combination may not be suitable for further translational clinical exploration in human teeth.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Lee, H. et al. Comparative study of pulpal responses to pulpotomy with proroot MTA, RetroMTA, and theracal in dogs’ teeth. J. Endod. 41 (8), 1317–1324 (2015).
Taha, N. A. & Al-Khatib, H. 4-Year Follow-up of full pulpotomy in symptomatic mature permanent teeth with carious pulp exposure using a stainproof calcium Silicate-based material. J. Endod. 48 (1), 87–95 (2022).
Duncan, H. et al. European society of endodontology position statement: management of deep caries and the exposed pulp. Int. Endod. J. 52 (7), 923–934 (2019).
Li, Y. et al. Pulpotomy for carious pulp exposures in permanent teeth: A systematic review and meta-analysis. J. Dent. 84, 1–8 (2019).
Asgary, S. Mineral Trioxide Aggregate and evidence-based Practice, in Mineral Trioxide Aggregate in Dentistry: from Preparation To Application, 173–199 (Springer, 2014).
Camilleri, J. Color stability of white mineral trioxide aggregate in contact with hypochlorite solution. J. Endod. 40 (3), 436–440 (2014).
Marciano, M. A. et al. Assessment of color stability of white mineral trioxide aggregate Angelus and bismuth oxide in contact with tooth structure. J. Endod. 40 (8), 1235–1240 (2014).
Meraji, N. et al. Prevention of tooth discoloration due to Calcium-Silicate cements: A review. Dent. Hypotheses. 10 (1), 4 (2019).
Marciano, M. A. et al. Zinc oxide inhibits dental discoloration caused by white mineral trioxide aggregate Angelus. J. Endod. 43 (6), 1001–1007 (2017).
Bolhari, B. et al. Evaluation of the properties of mineral trioxide aggregate mixed with zinc oxide exposed to different environmental conditions. Bioactive Mater. 5 (3), 516–521 (2020).
Tucker, R. L. & Ha, W. N. A systematic review comparing mineral trioxide aggregate to other commercially available direct pulp capping agents in dogs. J. Vet. Dent. 38 (1), 34–45 (2021).
Percie du Sert, N. et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. J. Cereb. Blood Flow. Metabolism. 40 (9), 1769–1777 (2020).
Farzad-Mohajeri, S. et al. Direct pulp capping with autologous bone marrow derived stem cells in dogs. in Veterinary Research Forum. Faculty of Veterinary Medicine, Urmia University, Urmia, Iran. (2022).
Nagendrababu, V. et al. PRIASE 2021 guidelines for reporting animal studies in endodontology: a consensus-based development. Int. Endod. J. 54 (6), 848–857 (2021).
Hatipoğlu, Ö. et al. Factors affecting the Decision-making of direct pulp capping procedures among dental practitioners: A multinational survey from 16 countries with Meta-analysis. J. Endod. 49 (6), 675–685 (2023).
Nakashima, M. et al. Animal models for stem cell-based pulp regeneration: foundation for human clinical applications. Tissue Eng. Part. B: Reviews. 25 (2), 100–113 (2019).
Felsburg, P. Overview of immune system development in the dog: comparison with humans. Hum. Exp. Toxicol. 21 (9–10), 487–492 (2002).
Parker, H. G., Shearin, A. L. & Ostrander, E. A. Man’s best friend becomes biology’s best in show: genome analyses in the domestic dog. Annu. Rev. Genet. 44, 309 (2010).
Liu, S., Wang, S. & Dong, Y. Evaluation of a bioceramic as a pulp capping agent in vitro and in vivo. J. Endod. 41 (5), 652–657 (2015).
Mohammadi, Z. & Khademi, A. An evaluation of MTA cements as coronal barrier. Iran. Endod J. 1 (3), 106–108 (2006).
Divya, K. T. et al. Comparative evaluation of sealing ability of four different restorative materials used as coronal sealants: an in vitro study. J. Int. Oral Health. 6 (4), 12–17 (2014).
Tselnik, M., Baumgartner, J. C. & Marshall, J. G. Bacterial leakage with mineral trioxide aggregate or a resin-modified glass ionomer used as a coronal barrier. J. Endod. 30 (11), 782–784 (2004).
Ashofteh Yazdi, K. et al. Microstructure and chemical analysis of four calcium silicate-based cements in different environmental conditions. Clin. Oral Investig. 23 (1), 43–52 (2019).
Grazziotin-Soares, R. et al. Crystalline phases involved in the hydration of calcium silicate-based cements: Semi-quantitative Rietveld X-ray diffraction analysis. Aust Endod J. 45 (1), 26–32 (2019).
Koh, E. T. et al. Cellular response to mineral trioxide aggregate. J. Endod. 24 (8), 543–547 (1998).
Seux, D. et al. Odontoblast-like cytodifferentiation of human dental pulp cells in vitro in the presence of a calcium hydroxide-containing cement. Arch. Oral Biol. 36 (2), 117–128 (1991).
Tran, X. V. et al. Effect of a calcium-silicate-based restorative cement on pulp repair. J. Dent. Res. 91 (12), 1166–1171 (2012).
Sarkar, N. K. et al. Physicochemical basis of the biologic properties of mineral trioxide aggregate. J. Endod. 31 (2), 97–100 (2005).
Gawlicki, M. & Czamarska, D. Effect of ZnO on the hydration of Portland cement. J. Therm. Anal. 38 (9), 2157–2161 (1992).
Bakhtiar, H. et al. Human pulp responses to partial pulpotomy treatment with theracal as compared with Biodentine and proroot MTA: A clinical trial. J. Endod. 43 (11), 1786–1791 (2017).
De Rossi, A. et al. Comparison of pulpal responses to pulpotomy and pulp capping with Biodentine and mineral trioxide aggregate in dogs. J. Endod. 40 (9), 1362–1369 (2014).
Nowicka, A. et al. Response of human dental pulp capped with Biodentine and mineral trioxide aggregate. J. Endod. 39 (6), 743–747 (2013).
Eghbal, M. J. et al. MTA pulpotomy of human permanent molars with irreversible pulpitis. Aust Endod J. 35 (1), 4–8 (2009).
Kobayashi, M. et al. Sensitivity of human dental pulp cells to eighteen chemical agents used for endodontic treatments in dentistry. Odontology 101 (1), 43–51 (2013).
Someya, H. et al. Clastogenic activity of seven endodontic medications used in dental practice in human dental pulp cells. Mutat. Res. 650 (1), 39–47 (2008).
Godhi, B. & Tyagi, R. Success rate of MTA pulpotomy on vital pulp of primary molars: A 3-Year observational study. Int. J. Clin. Pediatr. Dent. 9 (3), 222–227 (2016).
Okiji, T. & Yoshiba, K. Reparative dentinogenesis induced by mineral trioxide aggregate: a review from the biological and physicochemical points of view. Int. J. Dent. 2009 (1), 464280 (2009).
Basir, L. et al. Comparison of pulpal response following direct pulp capping using MTA and Zinc-doped bioglass. J. Babol Univ. Med. Sci. 25 (1), 58–69 (2023).
Nair, P. et al. Histological, ultrastructural and quantitative investigations on the response of healthy human pulps to experimental capping with mineral trioxide aggregate: a randomized controlled trial. Int. Endod. J. 41 (2), 128–150 (2008).
Yoon, J. H. et al. Hard Tissue Formation after Direct Pulp Capping with Osteostatin and MTA in Vivo 46(2) (Restorative Dentistry & Endodontics, 2021).
Fransson, H., Wolf, E. & Petersson, K. Formation of a hard tissue barrier after experimental pulp capping or partial pulpotomy in humans: an updated systematic review. Int. Endod. J. 49 (6), 533–542 (2016).
Eskandarinezhad, M. et al. Effect of incorporating hydroxyapatite and zinc oxide nanoparticles on the compressive strength of white mineral trioxide aggregate. J. Dent. 21 (4), 300 (2020).
Mokhtari, F., Akhondzadeh-Kashani, L. & Modaresi, J. Vitro assessment of push-out bond strength of cold ceramic and mineral trioxide aggregate to root dentin. Dent. Res. J. 21 (1), 43 (2024).
Palma, P. J. et al. Does delayed restoration improve shear bond strength of different restorative protocols to calcium silicate-based cements? Materials 11 (11), 2216 (2018).
Xavier, M. T. et al. Evaluation of the interfaces between restorative and regenerative biomaterials used in vital pulp therapy. Materials 14 (17), 5055 (2021).
Palma, P. J. et al. Effect of restorative timing on shear bond strength of composite resin/calcium silicate–based cements adhesive interfaces. Clin. Oral Invest. 25, 3131–3139 (2021).
Acknowledgements
The authors wish to express their sincere appreciation to Dr. Mohammad Javad Kharrazifard for his outstanding support and expertise in performing the statistical analyses.
Author information
Authors and Affiliations
Contributions
BB: contributed to the conceptualization of the study, designed the methodology, performed software corrections, validated the data, performed the formal analysis of raw data, evaluated the current literature, validated the included animals and procedures, performed data curation, helped in writing the original and revised draft, visualized histological sections, supervised the project, and performed project administration and registration within the ethical committee. NKK: created, evaluated and interpreted the histological sections and evaluated and edited the final manuscript draft. HA: participated in the conceptualization of the study, critically appraised the methodology, validated the data, critically revised the original and final draft of the manuscript. SFM: critically appraised the methodology, supervised animal care, surgery and sacrifice. MMD: critically appraised study concepts and methodology, Validated animal care and operations. SN: performed animal surgery and sacrifice, and performed imaging. Helped in writing original draft. BD: performed animal surgery and sacrifice, and performed imaging. Helped in writing original draft, evaluated the data.8. VN, HFD, AH, AS, and SB: critically appraised study concepts, methodology, and data analysis. Helped in preparing original draft and finalized manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Bolhari, B., Khouzestani, N.K., Assadian, H. et al. Pulpal responses to mineral trioxide aggregate with and without zinc oxide addition in mature canine teeth after full pulpotomy. Sci Rep 15, 15957 (2025). https://doi.org/10.1038/s41598-025-00061-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-00061-y