Abstract
We conducted a disproportionality analysis of the FDA Adverse Event Reporting System (FAERS) database (2005 Q2–2024 Q3) to evaluate neurological adverse events (NAEs) associated with six glucagon-like peptide-1 receptor agonists (GLP-1 RAs): exenatide, liraglutide, lixisenatide, dulaglutide, semaglutide, and tirzepatide. Among 28,953 NAE reports associated with GLP-1 RAs, 19 distinct NAE signals were identified using reporting odds ratios (RORs), including dizziness, tremor, dysgeusia, lethargy, taste disorder, presyncope, parosmia, allodynia, and hypoglycemic unconsciousness, etc. Time-to-onset analysis revealed a median latency of 32 days (IQR 7–122) for GLP-1 RA-related NAEs, with 45.28% occurring within 30 days of treatment initiation. Sensitivity analyses using proportional reporting ratios (PRRs), information components (ICs), and empirical Bayes geometric means (EBGMs) confirmed robustness of these signals. While these pharmacovigilance findings underscore the need for heightened clinical vigilance, they represent associations rather than causal relationships, constrained by inherent limitations of FAERS such as reporting bias and confounding. Future prospective studies are needed to confirm these associations and clarify underlying mechanisms.
Similar content being viewed by others
Introduction
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are structurally modified analogs of endogenous GLP-1, engineered to resist enzymatic degradation while retaining potent agonism at the GLP-1 receptor (GLP-1R)1. GLP-1Rs exhibit broad tissue distribution, spanning the pancreas, gastrointestinal tract, cardiovascular system, and central nervous system, allowing GLP-1 RAs to impact multiple organ systems2,3. Beyond their well-established benefits in managing type 2 diabetes mellitus (T2DM) and promoting weight reduction, emerging evidence suggests that GLP-1 RAs may also offer neuroprotective, anti-inflammatory, and cardiovascular protective effects4,5,6,7. Nevertheless, the broad spectrum of targets for GLP-1 RAs also introduces certain risks and side effects.
While gastrointestinal AEs such as nausea and vomiting dominate safety reports8 and recent pharmacovigilance studies highlight increasingly recognized risks including acute pancreatitis, nephritis, hypotension, and syncope9,10,11, emerging data suggest that GLP-1 RAs may be associated with neurological adverse events (NAEs)12,13. Despite the potential clinical significance of these findings, research into NAEs associated with GLP-1 RAs remains limited. Besides, patients with diabetes may already have a predisposition to diabetic neuropathy, potentially masking or confounding drug-related NAEs. Given the global increase in the use of GLP-1 RAs and the growing number of patients at risk, further investigation into their neurological safety profile is warranted.
The Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) is a spontaneous reporting database monitoring the safety of drugs and biologics after their market approval14. FAERS collects reports from manufacturers, healthcare professionals, and patients, providing a valuable resource for post-marketing pharmacovigilance14. Using the FAERS database from the second quater (Q2) of 2005 to the third quater (Q3) of 2024, we conducted a comprehensive analysis to investigate the association between GLP-1 RAs and NAEs. NAE reports associated with GLP-1 RAs were extracted, and disproportionality analysis was performed to identify NAEs significantly disproportionately related to these agents, which were defined as GLP-1 RA-related NAEs in this work. This study aims to enhance understanding of the neurological safety profile of GLP-1 RAs and inform clinical practice and patient care.
Methods
Ethical approval and informed consent were waived as this study utilized publicly available, de-identified data from the FAERS database. Statistical analyses were performed in R (v4.4.2)15. This study follows the recommendations of the reporting of a disproportionality analysis for drug safety signal detection using individual case safety reports in pharmacovigilance (READUS-PV)16.
Data source
FAERS database aggregates spontaneous adverse event (AE) reports from healthcare providers, manufacturers, and consumers17. Its structure comprises seven standardized files: demographic data (DEMO), medication records (DRUG), AE descriptions (REAC), patient outcomes (OUTC), reporter information (RPSR), drug therapy timelines (THER), and treatment indications (INDI)14,18,19. Medical concepts are systematically categorized using the Medical Dictionary for Regulatory Activities (MedDRA) terminology, with Preferred Terms (PTs) representing granular AE descriptions, hierarchically grouped into High-Level Terms (HLTs), High-Level Group Terms (HLGTs), and System Organ Classes (SOCs)18,19. Detailed descriptions of the files and relevant terminology are provided in Tables S1 and S2.
Data extraction and processing
We analyzed FAERS reports from 2005 Q2 to 2024 Q3. The starting point coincided with the approval date of the first GLP-1 RA, exenatide (BYETTA), April 28, 2005. Duplicate entries were resolved through a two-step deduplication protocol20: 1) retaining the most recent FDA receipt date (FDA_DT) for identical CASEID entries; 2) selecting the higher PRIMARYID when CASEID and FDA_DT matched.
GLP-1 RAs were identified in the DRUG files using generic or brand names, including exenatide (BYETTA, BYDUREON BCISE), liraglutide (VICTOZA, SAXENDA, XULTOPHY), lixisenatide (LYXUMIA, ADLYXIN, SOLIQUA), dulaglutide (TRULICITY), semaglutide (OZEMPIC, RYBELSUS, WEGOVY), and tirzepatide (MOUNJARO, ZEPBOUND). Withdrawn agents were excluded from the analysis. FDA approval dates for these GLP-1 RAs are detailed in Table S3. Target drugs were identified in the DRUG file, focusing on those marked as primary suspect (PS) drugs in the reported role code (ROLE_COD). NAEs were identified using SOC = “nervous system disorders” (SOC ID: 10,029,205) and primary SOC = “Yes”, encompassing 1075 PTs from MedDRA version 27.0. And then reports of NAEs associated with GLP-1 RAs were filtered. A descriptive analysis evaluated report characteristics including demographics, outcomes, and indications, and identified the top 20 concomitant medications.
Time-to-onset analysis
Time-to-onset was calculated as the duration between GLP-1 RA initiation and the occurrence of a NAE21. Cumulative incidence curves stratified by GLP-1 RA type were generated using time-to-onset data, excluding reports with implausible (onset ≤ 0 days) or missing event dates (Table S4). Differences between GLP-1 RAs were assessed using the log-rank test. Weibull distribution modeling characterized failure patterns of time-to-onset data, where the shape parameter (β) defined three scenarios: the upper limit of 95% confidence interval (CI) of β < 1 (early failure: NAEs clustered in treatment initiation phases), 95% CI of β including 1 (random failure: no temporal association), and the lower limit of 95% CI of β > 1 (wear-out failure: risk escalation with prolonged use)22 (Table S4).
Signal mining and statistical analysis
Disproportionality analysis serves as a crucial tool for pharmacovigilance to identify potential drug-event safety signals23. The reporting odds ratio (ROR) quantifies the relative likelihood of a specific AE being reported for a target drug compared to its reporting frequency with all other drugs in the FAERS database24,25. In this study, we utilized the ROR to identify NAE signals significantly associated with GLP-1 RAs, using contemporaneous FAERS reports of other medications as the reference cohort26,27.
The ROR with 95% CI was calculated as:
In these equations, ‘a’ represents the number of reports with both target drug exposure and target AEs; ‘b’ represents reports with target drug exposure but without target AEs; ‘c’ represents the number of reports without target drug exposure but with target AEs; and ‘d’ represents the number of reports with neither target drug exposure nor target AEs.
NAE signals were considered significant if ≥ 3 reports existed and the lower limit of 95% CI of the ROR exceeded 128. PTs classified under nervous system disorders meeting these thresholds were identified as GLP-1 RA-related NAEs for subsequent analysis. We further examined the top 20 concomitant medications and performed time-to-onset analysis for these cases.
Sensitivity analyses incorporated three validated pharmacovigilance methods: proportional reporting ratio (PRR), information component (IC), and empirical Bayes geometric mean (EBGM). Detailed equations for these methods are provided in Table S5. The PRR identifies potential adverse drug reactions (ADRs) through disproportionate reporting rates, with thresholds of PRR ≥ 2, ≥ 3 cases, and χ2 ≥ 4 indicating significant associations25. Bayesian approaches were featured for their enhanced detection of ADRs. The IC metric quantifies observed-to-expected reporting discrepancies through the Bayesian Confidence Propagation Neural Network framework (BCPNN)29, while EBGM estimates posterior reporting rates by integrating prior reporting patterns and adjusting for cross-drug variability27,30. These methods improve signal detection reliability by mitigating random reporting fluctuations and strengthening true safety signals31.
Results
NAE reports associated with GLP-1 RAs
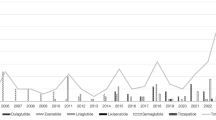
From 2005 Q2 to 2024 Q3, the FAERS database recorded a total of 250,014 AE reports associated with GLP-1 RAs, of which 28,953 (11.58%) were NAEs (Fig. 1). Among these NAEs, exenatide accounted for 10,175 cases (35.14%), liraglutide for 3,686 cases (12.73%), lixisenatide for 256 cases (0.88%), dulaglutide for 6,034 cases (20.84%), semaglutide for 5,935 cases (20.50%), and tirzepatide for 2,867 cases (9.90%). Surveillance data from 2014 to 2024 demonstrated a sustained upward trajectory in NAE reports associated with GLP-1 RAs, peaking in 2023–2024 (Fig. 2A). This growth contrasted with an overall decrease in NAE proportional representation among total AEs, declining from 22.92% (2005) to 9.27% (2024) (Fig. 2B). Despite this relative decline, NAEs persisted as a substantial AE subset, maintaining notable absolute reporting frequencies during the final three observation years (2021–2024) (Fig. 2).
Temporal and pharmacological patterns of NAEs associated with GLP-1 RAs. (A) Annual case counts of NAEs. (B) Annual proportion of NAEs relative to total AE reports. (C) Drug-specific NAE case counts across GLP-1 RAs. (D) Drug-specific NAE proportions relative to total AEs per agent. Abbreviations: NAEs, neurological adverse events; non-NAEs, non-neurological adverse events; AEs, adverse events; GLP-1 RAs, glucagon-like peptide-1 receptor agonists.
Descriptive analysis
Table 1 summarizes the clinical characteristics of 28,953 NAE reports associated with GLP-1 RAs. Females predominated (n = 18,907, 65.3%) over males (n = 8,838, 30.5%). Among 18,464 reports with documented age, most patients (39.9%) were aged 18–65 years. Most reports were submitted by consumers (n = 23,663, 81.7%), with physicians accounting for 8.5% (n = 2,451). Since FAERS is maintained by FDA, 89.6% of reports (n = 25,934) originated from the United States. Diabetes constituted the majority of indications (n = 16,863, 58.2%), followed by weight control (n = 1,352, 4.7%) and non-diabetic blood glucose abnormalities (n = 556, 1.9%). Notably, 10,182 cases (35.2%) were recorded as other indications or lacked documented indications. Regarding clinical outcomes, hospitalization was the most common outcome (n = 3,336, 11.5%), while death (n = 234, 0.8%) and life-threatening events (n = 354, 1.2%) occurred less frequently. Concomitant antidiabetic therapies were frequently reported, with metformin being the most frequently reported concomitant medication (n = 1,879), followed by pioglitazone (n = 478), insulin glargine (n = 474), glipizide (n = 467), and glimepiride (n = 345) (Figure S1).
Time-to-onset analysis of NAEs associated with GLP-1 RAs
The median time-to-onset of NAEs for all GLP-1 RAs was 31 days (interquartile range [IQR] 7–123), with 46.25% occurring within the first 30 days of treatment initiation (Table S6, Figure S2). Substantial variability emerged among individual agents: exenatide demonstrated the longest median latency (54 days, IQR 16–184), while dulaglutide showed the shortest (7 days, IQR 2–34). Intermediate values were observed for liraglutide (11 days, IQR 2–57), tirzepatide (19 days, IQR 3–69), lixisenatide (20 days, IQR 5–116), and semaglutide (27 days, IQR 5–77) (Table S6). A log-rank test confirmed significant inter-agent differences in onset timing (P < 0.001; Figure S3), with distinct onset time distribution curves. The Weibull distribution model demonstrated that upper limits of 95% CIs of β were all below 1 (Table S6), consistent with an early failure profile, indicating NAEs predominantly manifest during initial treatment phases.
Scanning for GLP-1 RA-related NAEs
A heatmap (Fig. 3A) illustrates the RORs for 60 important NAEs associated with GLP-1 RAs. The most frequently reported NAEs included dizziness (n = 7,078), tremor (n = 2,519), dysgeusia (n = 1,469), lethargy (n = 783), taste disorder (n = 482), presyncope (n = 370), allodynia (n = 192), and parosmia (n = 192) (Fig. 3B).
Signal detection of GLP-1 RA-related NAEs. (A) Heatmap visualization of RORs for 60 NEAs meeting case threshold (≥ 3 reports). NAEs labelled in dark red are GLP-1 RA-related NAEs. (B) Case frequency distribution of 19 GLP-1 RA-related NAEs. (C) Methodological concordance analysis using Venn diagram. Abbreviations: ROR, reporting odds ratio; PRR, proportional reporting ratio; EBGM, empirical Bayesian geometric mean; BCPNN, Bayesian confidence propagation neural network; NAEs, neurological adverse events; GLP-1 RAs, glucagon-like peptide-1 receptor agonists.
Disproportionality analysis identified 19 GLP-1 RA-related NAEs using the ROR method. These signals included dizziness (ROR, 1.30, 95% CI 1.27–1.33), tremor (1.37, 1.32–1.42), dysgeusia (1.72, 1.64–1.81), lethargy (1.24, 1.15–1.33), taste disorder (2.55, 2.33–2.80), presyncope (1.39, 1.25–1.54), parosmia (2.39, 2.07–2.75), allodynia (25.55, 21.71–30.08), hypoglycemic unconsciousness (8.11, 6.94–9.46), brain fog (1.78, 1.51–2.10), hyperesthesia (1.40, 1.18–1.66), diabetic neuropathy (1.95, 1.61–2.36), hypoglycemic seizure (3.29, 2.32–4.66), ophthalmic migraine (3.14, 1.88–5.27), Wernicke’s encephalopathy (2.37, 1.39–4.04), diabetic hyperglycemic coma (3.07, 1.68–5.62), hyperglycemic unconsciousness (7.94, 4.27–14.77), vagus nerve disorder (3.75, 1.92–7.32), and hyperglycemic seizure (5.85, 1.81–18.90) (Fig. 4A). Among which, allodynia had the highest ROR value.
Forest plots of signal detection for GLP-1 RA-related NAEs. (A) Results for 19 GLP-1 RA-related NAEs. (B) Recalculation of the RORs after grouping 19 GLP-1 RA-related NAEs in the FAERS database. Abbreviations: PT, Preferred Term; GLP-1 RAs, glucagon-like peptide-1 receptor agonists; NAEs, neurological adverse events; ROR, reporting odds ratio; CI, confidence interval; PRR, proportional reporting ratio; χ2, chi-squared; EBGM, empirical Bayesian geometric mean; EBGM05, the lower limit of 95% CI of EBGM; IC, information component; IC025, the lower limit of 95% CI of the IC. Note: Bold text indicates statistically significant signals per algorithm criteria.
Sensitivity analyses using PRR, IC, and EBGM methodologies produced divergent results. Venn diagram analysis revealed eight consistent signals across all four algorithms: taste disorders, parosmia, allodynia, hypoglycemic unconsciousness, hypoglycemic seizure, hyperglycemic unconsciousness, vagus nerve disorder, and hyperglycemic seizure (Figs. 3C and 4A).
For 19 identified GLP-1 RA-related NAE signals, we recalculated the RORs after grouping these NAEs in the FAERS database. For different GLP-1 RAs, the data limits during data retraction were coinciding with their respective first approval date (Table S3). This analysis confirmed a significant association between GLP-1 RAs and these NAEs (ROR 1.44, 95% CI 1.41–1.46), with variation observed across different GLP-1 RAs (Fig. 4B). Exenatide, liraglutide, dulaglutide, and semaglutide demonstrated significant associations with these 19 NAE signals, whereas lixisenatide and tirzepatide showed no significant associations.
Concomitant medications in cases of GLP-1 RA-related NAEs mirrored patterns observed in all NAE cases, with frequent co-administration of oral antidiabetics and insulin (Fig. 5A). Temporal analysis revealed early onset characteristics, with median time-to-event of 32 days (IQR 7–122). Weibull distribution modeling (β = 0.59, 95% CI 0.58–0.61) indicated these NAEs are likely to occur during initial treatment phases (Table 2, Fig. 5B). Significant inter-agent differences in onset timing were observed (P < 0.001, Fig. 5C).
Discussion
The global diabetes epidemic, affecting approximately 828 million adults in 202232, has driven widespread adoption of GLP-1 RAs due to their dual glycemic and weight management benefits33,34,35. While AEs associated with these agents have been investigated19,36,37, our analysis reveals critical gaps in understanding their NAEs given the central nervous system penetration and expanding indications of GLP-1 RAs. Data from 2005 Q2 to 2024 Q3 suggested NAEs persisted as a substantial AE subset associated with GLP-1 RAs based on FAERS. The analysis identified 19 significant NAE signals associated with GLP-1 RAs, representing 11.58% of all reported AEs associated with these agents between 2005 Q2 and 2024 Q3. Notably, semaglutide and exenatide showed higher NAE proportions (16.97% and 14.44%, respectively) compared to tirzepatide (6.06%). In addition, our analysis demonstrated significant variability in RORs for GLP-1 RA-related NAEs across agents, with semaglutide exhibiting stronger disproportionality signals compared to lixisenatide and tirzepatide. These inter-agent differences may be due to pharmacokinetic and pharmacodynamic distinctions including receptor internalization rates and secondary messenger activation38.
The analyses revealed sex-specific reporting patterns, consumer-driven pharmacovigilance, and early-onset NAE manifestations. Female predominance in AE reports (65.3% vs 30.5% male) persists despite global diabetes prevalence favoring males39, suggesting either biological susceptibility or heightened health-seeking behavior in women. Patient-submitted reports constituted 81.7% of NAEs, reflecting both expanded GLP-1 RA accessibility and growing public awareness of NAEs. Furthermore, GLP-1 RA-related NAEs are likely to occur in the early stages of treatment, mirroring the temporal trajectory of psychiatric19, gastrointestinal36, and ophthalmic37 AEs associated with these agents, and emphasizing vigilance during treatment initiation phase. While early onset may indicate physiological adaptation to receptor activation, detection bias from intensified early monitoring cannot be excluded. Notably, exenatide showed delayed NAE emergence, necessitating relatively prolonged monitoring.
The 19 identified GLP-1 RA-related NAEs are not documented in approved labeling, expanding on prior evidence. A 2019 network meta-analysis of 233 trials (n = 147,710) reported elevated risks of dizziness (odds ratios [ORs] 1.92, 1.57, 1.40) and headache (ORs 1.34, 1.41, 1.18) with GLP-1 RAs versus insulin, thiazolidinediones, and placebo40. The effect of GLP-1 RAs on blood flow, blood pressure, and the risk of hypoglycemia may increase risks of dizziness40. Headache has been reported in 4%–25% of patients using GLP-1 RAs13. Although our disproportionality analysis did not identify headache as a statistically significant NAE signal, it represented the most frequently reported adverse event with 7,132 cases.
While our analysis identified significant pharmacovigilance signals, we emphasize these findings represent associations rather than established causation. As per Bradford Hill’s criteria41, the biological plausibility through GLP-1R expression in the nervous system and temporal consistency in time-to-onset analysis support potential causality, but confounding factors inherent to spontaneous reporting systems preclude definitive conclusions. Future longitudinal studies are needed to strengthen causal inference.
Consistent with FDA labeling, GLP-1 RAs amplify hypoglycemia risk when combined with insulin or sulfonylureas42, a complication linked to seizures, syncope, and tremors43. Mitigation strategies include frequent glucose monitoring when changes are made to insulin dosage, oral hypoglycemic medications, meal patterns, physical activity, and in cases of renal or hepatic impairment or hypoglycemia unawareness, with consideration for dose reduction.
Emerging evidence highlights taste and olfactory disturbances as underrecognized NAEs of GLP-1 RA therapy44,45,46. Clinical reports document taste alterations and xerostomia in patients using semaglutide47, with studies implicating GLP-1R expression in mammalian taste buds and their role in modulating gustatory sensitivity48. Notably, Khan et al. demonstrated GLP-1 RAs broadly suppress perception of all five basic taste modalities, potentially mediated through brainstem GLP-1Rs and vagally mediated pathways44. In addition, Brindisi et al. observed enhanced sweet taste sensitivity and reduced hedonic responses to fatty foods in liraglutide-treated T2DM patients49, while Sever et al. reported semaglutide improved taste perception alongside altered tongue gene expression and modified central integration of sweet stimuli50.
Olfactory dysfunction, including parosmia, has been associated with liraglutide use46, likely reflecting GLP-1R-mediated modulation of odor-evoked insulin secretion and foraging behaviors in the olfactory bulb51,52. Central nervous system involvement is further supported by GLP-1 production in caudal brainstem neurons, key integrators of vagus nerve-mediated gut-brain signaling53,54,55,56. Vagal afferent neurons (VANs) expressing GLP-1Rs innervate intestinal mucosa and hepatic portal vasculature, suggesting these pathways may transduce peripheral GLP-1 RA effects to central satiety and glucoregulatory centers57,58,59. This neuroanatomical overlap raises the possibility of vagus nerve disorders, though causal mechanisms require further elucidation.
Wernicke encephalopathy, a life-threatening complication of thiamine deficiency characterized by ophthalmoparesis, ataxia, confusion, and nystagmus60,61, has been reported in patients using semaglutide62. GLP-1 RAs may indirectly precipitate this condition via appetite suppression, which risks inadequate nutritional intake and subsequent thiamine depletion. Proactive patient education on maintaining balanced micronutrient intake during GLP-1 RA therapy is critical to mitigate preventable complications. Wernicke encephalopathy exemplifies the importance of vigilance for rare NAEs. Clinicians must balance therapeutic efficacy with proactive risk management, particularly in vulnerable populations.
Hyperglycemic crises and related neurological sequelae including seizures and altered consciousness may link to suboptimal glycemic control during GLP-1 RA therapy. Central GLP-1R activation in hypothalamic and mesolimbic pathways may lead to fatigue, lethargy, or brain fog63, though such effects might be often transient and dose-dependent. Rare neuro-ophthalmic events such as ophthalmic migraine warrant monitoring despite low incidence64.
Signals including allodynia, hyperesthesia, and diabetic neuropathy have been detected. However, diabetes itself predisposes individuals to neuropathy, potentially confounding the association between GLP-1 RAs and NAEs. As a secondary analysis of the FAERS database, subgroup analyses were unfeasible due to incomplete clinical data. Future prospective studies could better adjust for such confounders. Intriguingly, studies suggest a potential role of GLP-1 RAs in pain relief, including acute and chronic pain65, chronic migraine66, and diabetic neuropathic pain67. These discrepancies highlight the need for further investigation.
GLP-1 and its analogs exhibit blood–brain barrier penetrance, enabling direct modulation of central nervous system function68. GLP-1R expression spans multiple neuroanatomical domains including the basal ganglia (caudate, putamen, globus pallidus), limbic system (amygdala, hippocampus), hypothalamic nuclei, cerebellum, and spinal cord69. GLP-1 influences fundamental neurobiological processes such as mitochondrial function, protein aggregation, and synaptic plasticity70,71,72. Emerging evidence delineates central roles of GLP-1 in appetite regulation, neuroprotection, neuroinflammatory mitigation, enhanced intracellular signaling, and cognitive enhancement72,73,74. These multifaceted roles of central GLP-1R activation may provide a plausible biological framework for understanding the spectrum of reported NAEs associated with GLP-1 RAs.
Our findings support several key clinical and regulatory imperatives. First, clinicians should implement baseline neurological evaluations before initiating therapy, particularly in patients with pre-existing neurological comorbidities. Second, structured surveillance protocols should prioritize the initial treatment month when approximately 46% of NAEs occur, with scheduled follow-ups during this period. Third, regulatory agencies should evaluate updates to prescribing information to reflect emerging safety signals meeting pharmacovigilance thresholds, while maintaining evidentiary standards for causal inference. Other operational recommendations include implementing patient-specific education programs about medication adherence and potential NAEs; establishing longitudinal monitoring frameworks to elucidate dose–response relationships and temporal patterns; and prioritizing prospective studies to validate signal strength and refine risk mitigation strategies. These measures will enable timely dissemination of safety updates while preserving the risk–benefit balance essential for optimal therapeutic deployment.
The FAERS remains pivotal for post-marketing pharmacovigilance, yet its dependence on spontaneous reports introduces critical methodological constraints. Underreporting may exist due to voluntary submissions. Reporting bias arises from disproportionate U.S. submissions and consumer-driven reports, skewing signal detection. Lack of denominator data precludes incidence calculations. Systematic differential reporting patterns influenced by patient awareness, outcome severity, and regulatory activities create substantial ascertainment bias, and underreporting of mild events and chronic risks may persist18. These limitations may distort signal-to-noise ratios in risk quantification. Furthermore, the predominant representation of U.S. healthcare ecosystems of this database limits generalizability to global populations, and missing data elements complicate accurate benefit-risk assessments. Future surveillance frameworks should prioritize multinational data harmonization and integration with real-world evidence streams to enhance validity.
Strengths and limitations
While causal relationships between GLP-1 RAs and NAEs remain unproven and underlying mechanisms remain uncertain, the temporal association observed in our analysis necessitates enhanced clinical vigilance for early detection and management. Our analysis faces several limitations. First, FAERS spontaneous reporting architecture introduces selection biases in ethnicity, geographic representation, and temporal reporting patterns tied to drug approval timelines and public awareness cycles18. These inherent constraints preclude causal inference between GLP-1 RAs and NAEs while preventing incidence rate calculations75. Second, despite employing sensitivity analyses, disproportionality metrics carry inherent false-positive risks that require confirmation through controlled studies76. Third, insufficient information on medication histories and confounding by indication limits differentiation between drug effects and underlying disease progression and complications. Concomitant antidiabetic medication use may also confound the observed neurological event associations. Fourth, as diabetes itself predisposes individuals to neurological complications, confounding from disease progression cannot be excluded in this disproportionality analysis. The absence of longitudinal clinical data in FAERS precludes adjustment for these factors. Fifth, disproportionality analyses do not account for temporal trends in GLP-1 RA prescription rates or cumulative drug exposure. Sixth, while the FAERS database provides valuable insights, exclusion of international pharmacovigilance databases such as the EudraVigilance or WHO VigiBase limits global generalizability, though accessibility constraints justify this approach. Finally, several identified safety signals lack mechanistic validation or replication in clinical studies, emphasizing the exploratory nature of this complete-case analysis. Prospective trials incorporating longitudinal monitoring of neurological parameters and biomarker profiling are critically needed to validate these pharmacovigilance signals while elucidating dose–response relationships and underlying mechanisms.
Conclusion
This pharmacovigilance study leveraging FAERS real-world data identified 19 GLP-1 RA-related NAEs through disproportionality analysis. These NAEs constituted over 5% of all reported AEs for this drug class, encompassing more than 12,000 cases. While the observed associations do not establish causality, they highlight the importance of vigilance for potential NAEs during treatment, particularly given the heterogeneous temporal patterns of onset across events. Clinicians should prioritize early recognition and targeted monitoring of high-risk neurological manifestations. The exploratory nature of this analysis underscores the necessity for validation in prospective longitudinal study. Further population-based studies may quantify incidence rates, characterize dose-dependent relationships, and investigate mechanistic pathways to optimize risk stratification and therapeutic decision-making.
Data availability
The datasets generated and analyzed during the current study are available in the U.S. FAERS database (https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html). These datasets will also be available from the corresponding author upon reasonable request.
Abbreviations
- AEs:
-
Adverse events
- NAEs:
-
Neurological adverse events
- FDA:
-
The Food and Drug Administration
- FAERS:
-
The FDA Adverse Event Reporting System
- GLP-1 RAs:
-
Glucagon-like peptide-1 receptor agonists
- ADRs:
-
Adverse drug reactions
- PTs:
-
Preferred Terms
- SOCs:
-
System Organ Classes
- IQR:
-
Interquartile range
- ROR:
-
Reporting odds ratio
- CI:
-
Confidence interval
- PRR:
-
Proportional reporting ratio
- BCPNN:
-
Bayesian Confidence Propagation Neural Network
- EBGM:
-
Empirical Bayesian geometric mean
- EBGM05:
-
The lower limit of 95% CI of EBGM
- IC:
-
Information component
- IC025:
-
The lower limit of 95% CI of the IC
- VANs:
-
Vagal afferent neurons
- T2DM:
-
Type 2 diabetes mellitus
- OR:
-
Odds ratios
References
Targher, G., Mantovani, A. & Byrne, C. D. Mechanisms and possible hepatoprotective effects of glucagon-like peptide-1 receptor agonists and other incretin receptor agonists in non-alcoholic fatty liver disease. lancet. Gastroenterol. Hepatol. 8, 179–191. https://doi.org/10.1016/s2468-1253(22)00338-7 (2023).
Drucker, D. J. & Holst, J. J. The expanding incretin universe: From basic biology to clinical translation. Diabetologia 66, 1765–1779. https://doi.org/10.1007/s00125-023-05906-7 (2023).
López-Ojeda, W. & Hurley, R. A. Glucagon-Like Peptide 1: An Introduction and Possible Implications for Neuropsychiatry. J. Neuropsychiatry Clin. Neurosci. 36, A4-86. https://doi.org/10.1176/appi.neuropsych.20230226 (2024).
Zheng, Z. et al. Glucagon-like peptide-1 receptor: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 9, 234. https://doi.org/10.1038/s41392-024-01931-z (2024).
Barritt, A. S. T., Marshman, E. & Noureddin, M. Review article: Role of glucagon-like peptide-1 receptor agonists in non-alcoholic steatohepatitis, obesity and diabetes-what hepatologists need to know. Alimentary Pharmacol. Ther. 55, 944–959. https://doi.org/10.1111/apt.16794 (2022).
Colhoun, H. M. et al. Long-term kidney outcomes of semaglutide in obesity and cardiovascular disease in the SELECT trial. Nat. Med. 30, 2058–2066. https://doi.org/10.1038/s41591-024-03015-5 (2024).
Lingvay, I. et al. Semaglutide for cardiovascular event reduction in people with overweight or obesity: SELECT study baseline characteristics. Obesity (Silver Spring, Md.) 31, 111–122. https://doi.org/10.1002/oby.23621 (2023).
Newsome, P. N. et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N. Engl. J. Med. 384, 1113–1124. https://doi.org/10.1056/NEJMoa2028395 (2021).
He, L. et al. Association of glucagon-like peptide-1 receptor agonist use with risk of gallbladder and biliary diseases: A systematic review and meta-analysis of randomized clinical trials. JAMA Intern. Med. 182, 513–519. https://doi.org/10.1001/jamainternmed.2022.0338 (2022).
Bezin, J. et al. GLP-1 receptor agonists and the risk of thyroid cancer. Diabete. Care 46, 384–390. https://doi.org/10.2337/dc22-1148 (2023).
Xie, Y., Choi, T. & Al-Aly, Z. Mapping the effectiveness and risks of GLP-1 receptor agonists. Nat. Med. https://doi.org/10.1038/s41591-024-03412-w (2025).
Zhou, J. et al. Exploration of the potential association between GLP-1 receptor agonists and suicidal or self-injurious behaviors: A pharmacovigilance study based on the FDA adverse event reporting system database. BMC Med. 22, 65. https://doi.org/10.1186/s12916-024-03274-6 (2024).
Filippatos, T. D., Panagiotopoulou, T. V. & Elisaf, M. S. Adverse effects of GLP-1 receptor agonists. Rev. Diabet. Stud.: RDS 11, 202–230. https://doi.org/10.1900/rds.2014.11.202 (2014).
The Food and drug administration (FDA), FDA adverse event reporting system (FAERS) database, https://www.fda.gov/drugs/drug-approvals-and-databases/fda-adverse-event-reporting-system-faers-database Accessed 2 December 2024.
R Core Team R: A Language and environment for statistical computing. r foundation for statistical computing, Vienna, Austria. https://www.R-project.org/(2024).
Fusaroli, M. et al. The reporting of a disproportionality analysis for drug safety signal detection using individual case safety reports in pharmacovigilance (READUS-PV): Development and statement. Drug Saf. 47, 575–584. https://doi.org/10.1007/s40264-024-01421-9 (2024).
U.S. Food and Drug Administration, FDA Adverse Event Reporting System (FAERS) Database, https://www.fda.gov/drugs/drug-approvals-and-databases/fda-adverse-event-reporting-system-faers-database, Accessed 2 November 2024.
Zhou, C. et al. Psychiatric disorders associated with immune checkpoint inhibitors: A pharmacovigilance analysis of the FDA Adverse Event Reporting System (FAERS) database. EClinicalMedicine 59, 101967. https://doi.org/10.1016/j.eclinm.2023.101967 (2023).
Chen, W., Cai, P., Zou, W. & Fu, Z. Psychiatric adverse events associated with GLP-1 receptor agonists: A real-world pharmacovigilance study based on the FDA adverse event reporting system database. Front. Endocrinol. 15, 1330936. https://doi.org/10.3389/fendo.2024.1330936 (2024).
Zhai, Y. et al. Endocrine toxicity of immune checkpoint inhibitors: A real-world study leveraging US food and drug administration adverse events reporting system. J. Immunother. Cancer 7, 286. https://doi.org/10.1186/s40425-019-0754-2 (2019).
Kinoshita, S., Hosomi, K., Yokoyama, S. & Takada, M. Time-to-onset analysis of amiodarone-associated thyroid dysfunction. J. Clin. Pharm. Ther. 45, 65–71. https://doi.org/10.1111/jcpt.13024 (2020).
Sauzet, O., Carvajal, A., Escudero, A., Molokhia, M. & Cornelius, V. R. Illustration of the weibull shape parameter signal detection tool using electronic healthcare record data. Drug Saf. 36, 995–1006. https://doi.org/10.1007/s40264-013-0061-7 (2013).
Caster, O., Aoki, Y., Gattepaille, L. M. & Grundmark, B. Disproportionality analysis for pharmacovigilance signal detection in small databases or subsets: Recommendations for limiting false-positive associations. Drug Saf. 43, 479–487. https://doi.org/10.1007/s40264-020-00911-w (2020).
Rothman, K. J., Lanes, S. & Sacks, S. T. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol. Drug Saf. 13, 519–523. https://doi.org/10.1002/pds.1001 (2004).
Evans, S. J., Waller, P. C. & Davis, S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol. Drug Saf. 10, 483–486. https://doi.org/10.1002/pds.677 (2001).
Sakaeda, T., Kadoyama, K. & Okuno, Y. Statin-associated muscular and renal adverse events: Data mining of the public version of the FDA adverse event reporting system. PLoS ONE 6, e28124. https://doi.org/10.1371/journal.pone.0028124 (2011).
Bate, A. & Evans, S. J. Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol. Drug Saf. 18, 427–436. https://doi.org/10.1002/pds.1742 (2009).
Yan, Y. D. et al. Toxicity spectrum of immunotherapy in advanced lung cancer: A safety analysis from clinical trials and a pharmacovigilance system. EClinicalMedicine 50, 101535. https://doi.org/10.1016/j.eclinm.2022.101535 (2022).
DuMouchel, W. Bayesian data mining in large frequency tables, with an application to the FDA spontaneous reporting system. Am. Stat. 53, 177–190 (1999).
Szarfman, A., Tonning, J. M. & Doraiswamy, P. M. Vol. 24 1099–1104 (Wiley Online Library, 2004).
Liu, W. H. et al. Real-world study of adverse events associated with triptan use in migraine treatment based on the U.S. food and drug administration (FDA) adverse event reporting system (FAERS) database. J. Headache pain 25, 206. https://doi.org/10.1186/s10194-024-01913-0 (2024).
Worldwide trends in diabetes prevalence and treatment from 1990 – 2022. A pooled analysis of 1108 population-representative studies with 141 million participants. Lancet (London, England) 404, 2077–2093. https://doi.org/10.1016/s0140-6736(24)02317-1 (2024).
Sandsdal, R. M. et al. Combination of exercise and GLP-1 receptor agonist treatment reduces severity of metabolic syndrome, abdominal obesity, and inflammation: A randomized controlled trial. Cardiovasc. Diabetol. 22, 41. https://doi.org/10.1186/s12933-023-01765-z (2023).
Ma, X. et al. GLP-1 receptor agonists (GLP-1RAs): Cardiovascular actions and therapeutic potential. Int. J. Biol. Sci. 17, 2050–2068. https://doi.org/10.7150/ijbs.59965 (2021).
Neuen, B. L. et al. Estimated lifetime cardiovascular, kidney, and mortality benefits of combination treatment with SGLT2 inhibitors, GLP-1 receptor agonists, and nonsteroidal mra compared with conventional care in patients with type 2 diabetes and albuminuria. Circulation 149, 450–462. https://doi.org/10.1161/circulationaha.123.067584 (2024).
Liu, L., Chen, J., Wang, L., Chen, C. & Chen, L. Association between different GLP-1 receptor agonists and gastrointestinal adverse reactions: A real-world disproportionality study based on FDA adverse event reporting system database. Front. Endocrinol. 13, 1043789. https://doi.org/10.3389/fendo.2022.1043789 (2022).
Zhou, J. et al. Risk of ophthalmic adverse drug reactions in patients prescribed glucagon-like peptide 1 receptor agonists: A pharmacovigilance study based on the FDA adverse event reporting system database. Endocrine https://doi.org/10.1007/s12020-024-04112-8 (2024).
Gentilella, R., Pechtner, V., Corcos, A. & Consoli, A. Glucagon-like peptide-1 receptor agonists in type 2 diabetes treatment: Are they all the same?. Diabete. Metab. Res. Rev. 35, e3070. https://doi.org/10.1002/dmrr.3070 (2019).
Institute for Health Metrics and Evaluation, GBD Results, https://vizhub.healthdata.org/gbd-results/, Accessed 24 November 2024.
Gao, L. et al. Neurological manifestation of incretin-based therapies in patients with type 2 diabetes: A systematic review and network meta-analysis. Aging Dis. 10, 1311–1319. https://doi.org/10.14336/ad.2019.0303 (2019).
Fedak, K. M., Bernal, A., Capshaw, Z. A. & Gross, S. Applying the Bradford Hill criteria in the 21st century: How data integration has changed causal inference in molecular epidemiology. Emerg. Theme. Epidemiol. 12, 1–9 (2015).
Zhao, Z. et al. Hypoglycemia following the use of glucagon-like peptide-1 receptor agonists: A real-world analysis of post-marketing surveillance data. Ann. Transl. Med. 9, 1482. https://doi.org/10.21037/atm-21-4162 (2021).
Aljihmani, L. et al. In 2019 IEEE International Smart Cities Conference (ISC2). 268–271 (IEEE).
Khan, R. & Doty, R. L. GLP-1 receptor agonists significantly impair taste function. Physiol. Behav. 291, 114793. https://doi.org/10.1016/j.physbeh.2024.114793 (2025).
Okada, S. et al. Hot water eliminates the bitter taste of oral semaglutide: A report of four cases. Int. Med. (Tokyo, Japan) 63, 2297–2300. https://doi.org/10.2169/internalmedicine.2608-23 (2024).
Merino, D. et al. Drug-associated parosmia: New perspectives from the WHO safety database. J. clin. Med. https://doi.org/10.3390/jcm11164641 (2022).
Back, H.-M., Choi, S.-A. & Kim, M. G. Adverse events of GLP-1 receptor agonists for weight loss: Twitter and a national pharmacovigilance database. Drug Targ. Ther. 2, 41–48 (2023).
Martin, B. et al. Modulation of taste sensitivity by GLP-1 signaling in taste buds. Ann. N. Y. Acad. Sci. 1170, 98–101. https://doi.org/10.1111/j.1749-6632.2009.03920.x (2009).
Brindisi, M. C. et al. Proof of concept: Effect of GLP-1 agonist on food hedonic responses and taste sensitivity in poor controlled type 2 diabetic patients. Diabet. Metab. Syndr. 13, 2489–2494. https://doi.org/10.1016/j.dsx.2019.06.021 (2019).
Sever, M. J. et al. 6468 Once-Weekly Semaglutide and Taste Perception in Women with Obesity. J. Endocr. Soc. 8(bvae163), 013 (2024).
Montaner, M. et al. The local GLP-1 system in the olfactory bulb is required for odor-evoked cephalic phase of insulin release in mice. Mol. Metab. 73, 101738. https://doi.org/10.1016/j.molmet.2023.101738 (2023).
Montaner, M. et al. A neuronal circuit driven by GLP-1 in the olfactory bulb regulates insulin secretion. Nat. Commun. 15, 6941. https://doi.org/10.1038/s41467-024-51076-4 (2024).
Brierley, D. I. et al. Central and peripheral GLP-1 systems independently suppress eating. Nat. Metab. 3, 258–273. https://doi.org/10.1038/s42255-021-00344-4 (2021).
Holt, M. K. et al. Preproglucagon neurons in the nucleus of the solitary tract are the main source of brain GLP-1, mediate stress-induced hypophagia, and limit unusually large intakes of food. Diabetes 68, 21–33. https://doi.org/10.2337/db18-0729 (2019).
Llewellyn-Smith, I. J., Reimann, F., Gribble, F. M. & Trapp, S. Preproglucagon neurons project widely to autonomic control areas in the mouse brain. Neuroscience 180, 111–121. https://doi.org/10.1016/j.neuroscience.2011.02.023 (2011).
McLean, B. A. et al. Revisiting the complexity of GLP-1 action from sites of synthesis to receptor activation. Endocr. Rev. 42, 101–132. https://doi.org/10.1210/endrev/bnaa032 (2021).
Nakagawa, A. et al. Receptor gene expression of glucagon-like peptide-1, but not glucose-dependent insulinotropic polypeptide, in rat nodose ganglion cells. Auton. Neurosci.: Basic. Clin. 110, 36–43. https://doi.org/10.1016/j.autneu.2003.11.001 (2004).
Ronveaux, C. C., de Lartigue, G. & Raybould, H. E. Ability of GLP-1 to decrease food intake is dependent on nutritional status. Physiol. Behav. 135, 222–229. https://doi.org/10.1016/j.physbeh.2014.06.015 (2014).
Berthoud, H. R. & Neuhuber, W. L. Functional and chemical anatomy of the afferent vagal system. Auton. Neurosci. : Basic clin. 85, 1–17. https://doi.org/10.1016/s1566-0702(00)00215-0 (2000).
Sinha, S., Kataria, A., Kolla, B. P., Thusius, N. & Loukianova, L. L. Wernicke encephalopathy-clinical pearls. Mayo Clin. Proc. 94, 1065–1072. https://doi.org/10.1016/j.mayocp.2019.02.018 (2019).
Sechi, G. & Serra, A. Wernicke’s encephalopathy: New clinical settings and recent advances in diagnosis and management. Lancet. Neurol. 6, 442–455. https://doi.org/10.1016/s1474-4422(07)70104-7 (2007).
Sheth, K., Garza, E., Saju, A., Nazir, N. & Agarwal, A. Wernicke encephalopathy associated with semaglutide Use. Cureus 16, e61783. https://doi.org/10.7759/cureus.61783 (2024).
Arillotta, D. et al. GLP-1 receptor agonists and related mental health issues; insights from a range of social media platforms using a mixed-methods approach. Brain sci. https://doi.org/10.3390/brainsci13111503 (2023).
Halloum, W., Dughem, Y. A., Beier, D. & Pellesi, L. Glucagon-like peptide-1 (GLP-1) receptor agonists for headache and pain disorders: A systematic review. J. Headache Pain 25, 112. https://doi.org/10.1186/s10194-024-01821-3 (2024).
Go, E. J. et al. GLP-1 and its derived peptides mediate pain relief through direct TRPV1 inhibition without affecting thermoregulation. Exp. Mol. Med. 56, 2449–2464. https://doi.org/10.1038/s12276-024-01342-8 (2024).
Jing, F., Zou, Q. & Pu, Y. GLP-1R agonist liraglutide attenuates pain hypersensitivity by stimulating IL-10 release in a nitroglycerin-induced chronic migraine mouse model. Neurosci. Lett. 812, 137397. https://doi.org/10.1016/j.neulet.2023.137397 (2023).
Zhang, Q. et al. Glucagon-like peptide-1 receptor agonist attenuates diabetic neuropathic pain via inhibition of NOD-like receptor protein 3 inflammasome in brain microglia. Diabetes Res. Clin. Pract. 186, 109806. https://doi.org/10.1016/j.diabres.2022.109806 (2022).
Yaribeygi, H., Rashidy-Pour, A., Atkin, S. L., Jamialahmadi, T. & Sahebkar, A. GLP-1 mimetics and cognition. Life Sci. 264, 118645. https://doi.org/10.1016/j.lfs.2020.118645 (2021).
Muscogiuri, G., DeFronzo, R. A., Gastaldelli, A. & Holst, J. J. Glucagon-like peptide-1 and the central/peripheral nervous system: Crosstalk in diabetes. Trend. Endocrinol. Metab. 28, 88–103. https://doi.org/10.1016/j.tem.2016.10.001 (2017).
Merchenthaler, I., Lane, M. & Shughrue, P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J. Comp. Neurol. 403, 261–280. https://doi.org/10.1002/(sici)1096-9861(19990111)403:2%3c261::aid-cne8%3e3.0.co;2-5 (1999).
Pyke, C. et al. GLP-1 receptor localization in monkey and human tissue: Novel distribution revealed with extensively validated monoclonal antibody. Endocrinology 155, 1280–1290. https://doi.org/10.1210/en.2013-1934 (2014).
Athauda, D. & Foltynie, T. The glucagon-like peptide 1 (GLP) receptor as a therapeutic target in Parkinson’s disease: Mechanisms of action. Drug Discov. Today 21, 802–818. https://doi.org/10.1016/j.drudis.2016.01.013 (2016).
Müller, T. D. et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 30, 72–130. https://doi.org/10.1016/j.molmet.2019.09.010 (2019).
Seufert, J. & Gallwitz, B. The extra-pancreatic effects of GLP-1 receptor agonists: A focus on the cardiovascular, gastrointestinal and central nervous systems. Diabet. Obes. Metab. 16, 673–688. https://doi.org/10.1111/dom.12251 (2014).
Yin, G., Song, G., Xue, S. & Liu, F. Adverse event signal mining and serious adverse event influencing factor analysis of fulvestrant based on FAERS database. Sci. Rep. 14, 11367. https://doi.org/10.1038/s41598-024-62238-1 (2024).
Cutroneo, P. M. et al. Conducting and interpreting disproportionality analyses derived from spontaneous reporting systems. Front. Drug Saf. Regul. https://doi.org/10.3389/fdsfr.2023.1323057 (2024).
Funding
This study was funded by the High Level Chinese Medical Hospital Promotion Project (HLCMHPP2023089), the Special Programme for the Cultivation of Outstanding Young Scientific and Technological Talents (Innovation Category) under the Basic Scientific Research Fund of China Academy of Chinese Medical Sciences (ZZ13-YQ-019), and the Fundamental Research Funds for the Central Public Welfare Research Institutes (ZZ-YQ2023004). The study sponsors were not involved in any stage of research design, execution, data analysis, manuscript preparation, or publication decisions. This includes no influence on methodological frameworks, data interpretation, or final reporting of results.
Author information
Authors and Affiliations
Contributions
He Chen and Sixing Liu: Formal Analysis, Methodology, Software, Writing − Original Draft. Shuai Gao, Hangyu Shi, and Yan Yan: Investigation, Formal Analysis, Software, Writing − Original Draft. Yixing Xu, Jiufei Fang, Weiming Wang, and Huan Chen: Data Curation, Visualization, Writing − Review & Editing. Zhishun Liu: Conceptualization, Supervision. All authors have approved the manuscript for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, H., Liu, S., Gao, S. et al. Pharmacovigilance analysis of neurological adverse events associated with GLP-1 receptor agonists based on the FDA Adverse Event Reporting System. Sci Rep 15, 18063 (2025). https://doi.org/10.1038/s41598-025-01206-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-01206-9