Abstract
Natural organic sulfides are predominantly found in cruciferous and liliaceous plants. Among these compounds, alliin—an organic sulfide derived from garlic—has garnered significant attention from researchers due to its potential anti-atherosclerotic properties. However, studies specifically investigating the anti-atherosclerotic effects of alliin remain limited. This study aims to elucidate the protective effects of alliin on ox-LDL-injured human umbilical vein endothelial cells (HUVECs) and their underlying mechanisms. Initially, HUVECs were exposed to 80 mg/L oxidized low-density lipoprotein (ox-LDL) for 24 h to establish an ox-LDL injury model. Subsequently, the Cell Counting Kit-8 (CCK8) assay was utilized to assess the effect of alliin on the proliferation of ox-LDL-injured cells at 12, 24 and 48 h, and the levels of total cholesterol (TC) and free cholesterol (Fch) in the HUVECs were measured according to the instructions of TC and Fch kits. Next, quantitative proteomics was then adopted to analyze the differential protein expression in cell samples from the control group (Con), the ox-LDL injury model group (Mod), and the alliin treatment group (Alliin). Among the quantified proteins, a statistical t-test with P < 0.05 was used as a threshold for significance regarding a 1.5-fold change in differential expression. Finally, functional enrichment analysis of the differential proteins in the Alliin/Mod group was performed using Gene Ontology (GO) enrichment and KEGG pathway analysis. Additionally, Western blotting was used to validate the findings. The proteomic analysis identified 6173 identified proteins, of which 5162 were quantifiable. Differential protein analysis revealed that in the Alliin/Con comparison, there were a total of 108 up-regulated proteins and 116 down-regulated proteins; in the Alliin/Mod comparison there were a total of 33 up-regulated proteins and 17 down-regulated proteins; while in the Mod/Con comparison, there were a total of 106 up-regulated proteins and 147 down-regulated proteins. GO enrichment, KEGG pathway analyses and Western blotting verification demonstrated that alliin up-regulates the expression of Low-density lipoprotein receptor (LDLR) (P < 0.05) and apolipoprotein C (ApoC) (P < 0.05) while down-regulating the expression of apolipoprotein B (ApoB) (P < 0.05) to regulate the cholesterol metabolism pathway of the ox-LDL-injured HUVECs. Our findings highlight the importance of cholesterol metabolism in alliin treatment for atherosclerosis. Alliin exerts a protective effect in the ox-LDL-induced HUVEC injury model by modulating the expression of LDLR, ApoC, and ApoB within the cholesterol metabolism pathway. These findings indicate that alliin could potentially serve as a therapeutic agent for the prevention and treatment of atherosclerosis.
Similar content being viewed by others
Introduction
Cardiovascular disease (CVD) is associated with a high mortality rate, and has become one of the major diseases threatening human health in China, with atherosclerosis (AS) serving as the primary pathological basis of this type of disease1. The risk factors of AS include hypertension, diabetes mellitus, hyperlipidemia, obesity, smoking, and pathogen infections2. The pathogenesis of AS involves oxidative stress, inflammatory response, and endothelial dysfunction3. The liver is the central organ for lipid metabolism in the human body, accountable for the synthesis of fatty acids and cholesterol, as well as the intake and secretion of serum lipoprotein4. Serum lipoprotein mainly consists of low-density lipoprotein (LDL), intermediate-density lipoprotein (IDL), and high-density lipoprotein (HDL), while an elevated level of serum LDL is associated with AS5. LDL can be oxidized to form oxidized low-density lipoprotein (ox-LDL) when the human body is in a hyperoxic state6. Ox-LDL cannot be recognized by LDL receptors (LDLR) but is instead recognized by macrophages, resulting in macrophages engulfing an enormous amount of lipids and forming foam cells. Aggregation of foam cells could contribute to lipid streaks and even atherosclerotic plaques.
In ancient China, garlic was traditionally used for prevention and the treatment of AS. In the"Compendium of Materia Medica”, a historical document written by Li Shizhen from the Ming Dynasty, it was recorded that garlic has the effect of promoting blood circulation, eliminating phlegm, and relieving blood stasis. This was closely related to what we now know about the pathogenesis of AS. In addition to historical literature, garlic’s role in the prevention and treatment of AS has also been extensively studied by modern scientists7,8,9. Garlic has been found to contain more than 30 types of natural organic sulfur compounds (OSCs), and most of them are converted from L-cysteine sulfoxides andγ-glutamyl-l-cysteine peptides under different conditions. Alliin (S-allyl-L-cysteine sulfoxide) was found in fresh and dried garlic and could rapidly converted to allicin by alliinase when garlic was chopped, crushed, or chewed10.
Currently, statins are the primary pharmacological agents applied in the treatment of AS on the market. However, some patients are still intolerant, and even long-term use of statins has led to severe hepatic and renal toxicity. To investigate the role of alliin in AS prevention and therapy, a literature search was performed on databases such as PubMed, Google Scholar, and Web of Science using the keywords “alliin anti-AS” and “alliin sulfur compounds anti-AS”. Lu et al. reported that alliin can effectively improve the lipid metabolism disorder induced by 1,3-dichloro propanol in HepG2 by reducing TG and TC levels11. Bombicz et al. discovered that wild garlic leaf lyophilisate (with an alliin content of 0.261%) alleviated cardiovascular damage in hypercholesterolemic rabbits12. Additionally, Li et al. confirmed that alliin exhibits anti-adipogenic activity by downregulating major adipogenic differentiation-related genes Akt expression13. Nevertheless, there is limited research on the protective effect of alliin on ox-LDL-induced HUVECs as well as its mechanism for preventing atherosclerotic lesions.
Mark Wilkins introduced the notion of proteomics in the 1990s. The term"proteome” refers to the complete set of proteins in a given organism, while proteomics is a scientific discipline focused on studying the proteome’s composition, structure, function, and connections14,15. This approach challenges conventional experimental thinking and methodology by allowing for the rapid identification of proteins with up- or down-regulated in response to drug interactions with biological systems, as well as the enrichment of these proteins into connected signaling pathways. In this research, the protective effect of alliin on ox-LDL-induced HUVECs and its mechanism were investigated using quantitative proteomics.
Materials and methods
Materials
Alliin (lot number 556-27-4) was purchased from Beijing Solarbio Technology Co., Ltd. DMEM (lot number: C11995500BT) and FBS (lot number:10099-141) were acquired from Gibco, while ox-LDL (lot number:202044) was purchased from Guangzhou Yiyuan Biotech Co., Ltd. PBS (lot number: SH30256.01), penicillin ( lot number: SH30002.01), and 0.5% trypsin solution (lot number: SH30236.01) were purchased from Hyclone. Biosharp Biotechnology LTD supplied the 6-well,96-well plates and dithiothreitol (lot number: BL552A). The CCK8 kit (lot number: AR1160) was obtained from Boster Biological Co., Ltd., and the Oil-Red-O staining kit (lot number: MM202-01) was purchased from Beijing Solarbio Technology Co., Ltd. Additionally, the total cholesterol kit (lot number: A111-1-1) and free cholesterol kit (lot number: A110-1-1) were acquired from Nanjing Jiancheng Bioengineering Institute. The TMT kit (lot number: LM8IHC2733Rb), BCA kit (lot number: P0012S-1), SDS-PAGE Gel Rapid Preparation Kit (lot number: P0012AC), and ultra-sensitive ECL chemiluminescence kit (lot number: P0018AFT) were purchased from Beijing Biyuntian Technology Co., LTD. RIPA Cracking and Extraction buffer (lot number:89900) and PVDF membrane were purchased from Thermo Field, USA. American Abcam company provided the anti-LDLR antibody (lot number: ab30532), anti-ApoB antibody (lot number: ab20737), anti-ApoC antibody (lot number: ab21032), β-actin loading control (lot number: ab8226), and secondary anti-rabbit goat antibody (lot number: ab6702).
HUVECs culture
HUVECs were acquired from Shanghai Zhong Qiao Xin Zhou Biotechnology Co., Ltd. HUVECs were cultured in DMEM supplemented with 15% FBS and 1% penicillin at 37 °C in an atmosphere containing 5% CO2. Experiments were initiated when HUVECs have reached 70% of 6-well plates.
Establishment of ox-LDL-injured HUVECs model
HUVECs were seeded in 6-well plates at a density of approximately 5 × 105 per well. Ox-LDL was dissolved in DMEM and then diluted to a concentration of 80 mg/L. The ox-LDL-injured HUVECs were treated with 2 ml of 80 mg/L ox-LDL for 24 h at 37 °C in a 5% CO2 environment. While the control group obtained DMEM alone. Following the treatment, the ox-LDL-injured HUVECs were then rinsed three times with cold PBS before being fixed in 4% paraformaldehyde for 40 min. Similarly, the cells were rinsed again with PBS before being incubated with an oil-red-O solution for 1 h at 37 °C in the dark, followed by 3 min of hematoxylin staining. Finally, before examining the results under a microscope, the cells were thoroughly rinsed with double-distilled water to remove any loose cells. The levels of total cholesterol (TC) and free cholesterol (Fch) in the ox-LDL-injured HUVECs were measured simultaneously using TC and Fch kits.
HUVECs viability assays
Initially, alliin was dissolved in DMEM and diluted at concentrations of 6.25, 12.5, 25, 50, 100, 200, and 400 mg/L. HUVECs were seeded in 96-well plates at 8 × 103 cells/well. The ox-LDL-injured HUVECs were treated with 80 mg/L of ox-LDL (2 ml) for 24 h at 37 °C in a 5% CO2 environment. The experiment groups were pre-treated with 80 mg/L ox-LDL (2 ml) for 24 h before giving treatments with an alliin solution (150µL) and incubated at 37 °C in a 5% CO2 atmosphere for 12, 24, and 48 h. The control group received only 150µL of DMEM. After treatment, the 96-well plate was emptied with 10µL of CCK8 solution and incubated for 45 min at 37 °C. After the incubation period, the absorbance value of each well was measured with a microplate reader set to 450 nm, and the optical density (OD) of each well was then recorded. Cell viability= (OD value of drug group-OD value of blank group)/(OD value of control group-OD value of blank group)×100%.
Determination of TC and Fch content in HUVECs
HCVECs were inoculated on a 6-well plate with an inoculation density of about 5 × 105 cells per well. The control group had previously received DMEM, while the mod group (ox-LDL-injured HUVECs) was provided with 80 mg/L ox-LDL for 24 h at 37 °C in a 5% CO2 environment. The experiment’s three groups were then pre-treated with 80 mg/L ox-LDL for 24 h before receiving 12.5, 25, and 50 mg/L alliin solutions. Finally, the positive drug group was given 80 mg/L ox-LDL for 24 h, followed by receiving 0.5 mg/L lovastatin solution. After 24 h of incubation in a CO2 incubator, HUVECs were washed three times with cold PBS before being scraped and centrifuged for 8 min at 1300 rpm. Then the supernatant was removed, leaving the cell pellet for further investigation. The levels of TC and Fch in the HUVECs were then measured according to the TC and Fch kit instructions.
Cell sample Preparation and protein extraction
HUVECs were cultured in 6-well plates at a density of about 5 × 105 cells/well. Ox-LDL-injured HUVECs were treated with 2 mL of 80 mg/L ox-LDL for 24 h at 37 °C in a 5% CO2 atmosphere. At the same time, the alliin group was provided with 80 mg/L ox-LDL (2 ml) for 24 h followed by treatment with 25 mg/L alliin solution (2 ml) for 24 h. Furthermore, the control group received only DMEM. The cells were digested with 300µL of 0.5% trypsin solution before centrifugation at 1200 rpm for 4 min at 4 °C. The supernatant was subsequently removed, and the residual cells were lysed on ice for 30 min in lysis buffer as shown in the product’s instructions, followed by centrifugation at 12,000 rpm at 4 °C for 8 min. The protein content of the supernatants was measured using the BCA Protein Assay Kit.
Trypsin digestion and TMT labeling
Dithiothreitol was initially added to the protein solution and incubated at 56 °C for 30 min. Iodoacetamide was then added and kept to react for 15 min in a dark environment. The sample’s urea concentration was then diluted to 2 mol/L, and trypsin was added in a 1:50 ratio for overnight digestion at 37 °C. Following that, the peptides were labeled using the TMT kit according to the manufacturer’s instructions.
LC-MS/MS analysis and database search
Peptides were dissolved in 0.1% formic acid and separated using an EASY-nLC1200 ultra-high-performance liquid chromatography (UHPLC) system. Following their isolation, the peptides were processed with an NSI source and analyzed using QExactiveTM HF-X mass spectrometry. The LC-MS/MS data were then processed using the MaxQuant search engine (v.1.5.2.8) for protein identification and quantification.
Bioinformatics
We utilized Wolfpsort v.0.2 (http://www.genscript.com/psort/wolf_psort.html) to predicted the subcellular localization of differentially expressed proteins in the Alliin/Mod group. The GO annotation of the proteins was performed using InterProScan (v.5.14-53.0-http://www.ebi.ac.uk/interpro/) and the proteins were classified into three categories: cellular component, molecular function, and biological process. A two-tailed Fisher’s exact test was used to examine the enrichment of differentially expressed proteins for each category. The GO with a corrected p-value < 0.05 was considered significant. The KEGG mapper (V2.5http://www.kegg.jp/kegg/mapper.html) matches the annotated proteins into the associated pathways, and then the significance of pathway enrichment was identified by a two-tailed Fisher’s exact test16,17,18. The pathway with a corrected p-value < 0.05 was considered significant.
Western blotting verification
HUVECs were cultured in 6-well plates at a density of approximately 5 × 105 cells per well. The mod group (ox-LDL-injured HUVECs) was given 80 mg/L (2 ml) for 24 h at 37 °C in a 5% CO2 environment. At a similar period, the alliin group was pre-treated with 80 mg/L ox-LDL (2 ml) for 24 h before receiving 25 mg/L alliin solution (2 ml) for an additional 24 h. The total protein in HUVECs was extracted using a protein extraction reagent. The protein concentration was then determined using a BCA protein concentration measurement kit. The samples were denatured at 95℃ for 10 min, electrophoresed on an SDS-polyacrylamide gel for 1 h, and then transferred to a PVDF membrane. After blocking with 5% nonfat dry milk in TBST for 1 h, the membranes were incubated with the corresponding primary antibodies, LDLR, ApoB, ApoC, and β-actin at dilutions of 1:600, 1:1000, 1:1000, and 1:1000, at 4℃ overnight. The membranes were washed by TBST three times before being incubated with the matching secondary antibodies (1:10000) for 1 h at room temperature. The membranes were then treated with ECL chemiluminescent substrate chemicals and visualized by the 2750 Chemiluminescence Imaging System.
Statistical analysis
GraphPad Prism 5.0 software was used to process the data, all of which were expressed as x ± S. One-way ANOVA analysis was used for multi-group comparison, and T-test was used for pairwise comparison. P < 0.05 or P < 0.01 was considered a significant difference.
Results
Establishment of ox-LDL-injured HUVECs model
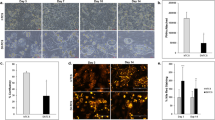
Oil-red-O staining results demonstrated that compared with the cell control group, there were a large amount of red-stained particles in 80 mg/L ox-LDL-injured HUVECs (Fig. 1A, B). On the other hand, the Fch content in the ox-LDL-injured HUVECs group was 503 ± 12.54µmol/L, whereas the TC content was 742 ± 20.78µmol/L, resulting in an Fch/TC proportion that exceeded over 50% (Table 1). These findings indicated a disorder of lipid metabolism. Combined with the results of oil-Red-O staining, it was determined that the ox-LDL-injured HUVECs model has been successfully established.
Cell viability
The proliferative activity of 80 mg/L ox-LDL-injured HUVECs receiving alliin (6.25–400 mg/L) for 12 h, 24 h, and 48 h was measured using the CCK-8 assay. The results (Fig. 2) demonstrated that at concentrations of 12.5–50 mg/L for 24 h, alliin significantly enhanced HUVEC proliferation compared to the ox-LDL-injured HUVECs (P < 0.05). In particular, the most pronounced proliferative effect was shown in Fig. 2 at a concentration of 25 mg/L alliin after 24 h of incubation.
Detection results of TC and Fch contents in HUVECs
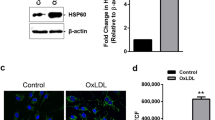
The experimental results revealed that Alliin at 25 mg/L significantly reduced TC and Fch levels in HUVECs compared to the Mod group (P < 0.01) (Fig. 3). On the other hand, in comparison with the control group, the levels of TC and Fch in the model group were significantly elevated (P < 0.01), suggesting that exposure to 80 mg/L ox-LDL for 24 h effectively disrupted the cholesterol metabolic process in HUVECs. Based on these findings, as well as the proliferation assay results, which demonstrate the dose-dependent effects of alliin (6.25–400 mg/L) on HUVEC viability, a concentration of 25 mg/L was chosen to treat HUVECs and proceed with the experiment. This optimal concentration was applied in a 24-hour incubation of ox-LDL-injured HUVECs for further investigation.
Results of TC and Fch content in HUVECs. (A) TC content detection results in the control, mod, alliin, and lovastatin groups; (B) Fch content detection results in the control, mod, alliin, and lovastatin groups. Data are presented as means ± S.D. (n = 3). Compared to the mod group: *P < 0.05, **P < 0.01; compared to the control group: #P < 0.05, ##P < 0.01.
Differentially expressed proteins statistics
The differential protein expression was then analyzed using quantitative proteomics in three groups, the control group treated with DMEM (Con), the 80 mg/L ox-LDL-injured HUVECs (Mod), and the 25 mg/L alliin experimental group pretreated with 80 mg/L ox-LDL over a 24-hour period (Alliin). Among the quantified proteins, a statistical t-test with P < 0.05 was used as a threshold for significance regarding a 1.5-fold change in differential expression. Figure 4A reveals the differentially expressed proteins identified in the Alliin/Con, Alliin/Mod, and Mod/Con comparison groups. In the Alliin/Con group, 108 proteins were up-regulated and 116 proteins were down-regulated, while in the Alliin/Mod group, 33 proteins were up-regulated and 17 proteins were down-regulated. Similar to that, in the Mod/Con group, 106 proteins were up-regulated and 147 were down-regulated. As demonstrated, 25 mg/L of alliin significantly up-regulated the expression of 33 proteins while down-regulated the expression of 17 proteins in ox-LDL-injured HUVECs over a 24-hour period. In the subsequent examinations, we will investigate the bioinformatics analysis of the differentially expressed proteins in the Alliin/Mod group.
(A) The number and distribution of differentially expressed proteins between comparison groups are shown in the histogram, with red representing up-regulated proteins while green indicating down-regulated proteins. (B) Subcellular structure localization map of differentially expressed proteins, the numbers in the figure indicate the number of differential proteins.
Subcellular localization
Figure 4B exhibited the subcellular distribution of differentially expressed proteins in the Alliin/Mod group. 15 proteins were identified in the extracellular space,14 proteins in the nucleus,10 proteins in the plasma membrane,7 proteins in the cytoplasm, and 2 proteins in the peroxisome and endoplasmic reticulum, indicating that the function of the differential proteins may be primarily involved in cell-cell interactions.
GO enrichment
As depicted in Fig. 5A, GO enrichment analysis of up-regulated proteins suggested that alliin may predominantly target apolipoprotein particles and intercellular spaces, altering molecular functions such as molecular binding, lipid transport, and cellular regulation. These effects are most likely mediated by regulating biological processes such as cholesterol metabolism, secondary alcohol metabolism, and steroid metabolism, which causes protective effects on ox-LDL-injured HUVECs. Among these processes, cholesterol metabolism was found to be the most strongly related. It was observed that alliin’s protection of ox-LDL-injured HUVECs could be linked to some essential proteins involved in cholesterol metabolism. As illustrated in Fig. 5B, the down-regulated proteins largely target cellular components associated with cytoplasmic vesicle membranes, that affect cardiovascular system function via signal transduction. This demonstrates that the biological functions of these down-regulated proteins could be mediated by endocytosis.
Enrichment distribution diagram of differentially expressed proteins in GO functional classification, the vertical axis was the functional classification or pathway, and the horizontal axis value was the value of P-value-Log10 conversion obtained by Fisher’s accurate test. (A) The up-regulated proteins in GO enrichment (Alliin/Mod group); (B) the down-regulated protein in GO enrichment (Alliin/Mod group).
KEGG pathway enrichment
As illustrated in Fig. 6, each bubble in the figure represents a specific pathway, and its size is proportional to the number of proteins identified inside that pathway. The color gradient of the bubbles, which ranges from light to deep red, represents the statistical significance of protein differential expression, with deeper red hues suggesting more intense and statistically significant variations in protein expression levels. This visualization efficiently highlights both the quantitative and qualitative features of pathway enrichment, providing an understandable overview of the most significantly impacted biological processes in response to Alliin/Mod medical treatment.
As previously stated, the KEGG pathway enrichment analysis revealed that differentially expressed proteins in the Alliin/Mod group were significantly enriched in eight signaling pathways, three of which were associated with cardiovascular disease treatment, including the PPAR signaling pathway, steroid biosynthesis, and cholesterol metabolism. Among them, it is worth noting that cholesterol metabolism is the most essential signaling pathway for atherosclerosis prevention. This finding highlights the potential therapeutic value of Alliin/Mod intervention in cardiovascular disease management by modulating these critical metabolic pathways.
KEGG pathway analysis of cholesterol metabolism
The cholesterol metabolic pathway is visually shown in Fig. 7. The red bars reflect up-regulated proteins, while the green bars show down-regulated proteins. ApoC is the primary component of the lipoproteins in HDL, Lp(a), and chylomicron. As showed in Fig. 8, ApoC expression in the cholesterol metabolic pathway has been significantly up-regulated. In the contrary, apolipoprotein B-100 (ApoB-100), a primary component of LDL, Lp(a), IDL, and very low-density lipoprotein (VLDL), followed by apolipoprotein B-48 (ApoB-48), a major component of chylomicrons and chylomicron remnants, are both significantly down-regulated in the cholesterol metabolism pathway. Additionally, the expression of the low-density lipoprotein receptor (LDLR) is significantly up-regulated.
Increased LDLR expression promotes LDLR binding to circulating LDL cholesterol (LDL-C), lowering peripheral LDL-C levels and effectively reducing the risk of AS. Furthermore, increased LDLR expression stimulates the degradation of proprotein convertase subtilisin/kexin type 9 (PCSK9). ApoB-100 is the major apolipoprotein component of LDL, and cellular uptake of LDL is mostly accomplished by its recognition. The decreased level in ApoB-100 expression demonstrates a reduction in cellular LDL uptake, lowering the risk of AS development. In one word, the results of the KEGG pathway analysis of cholesterol metabolism revealed that alliin’s protective impact on HUVECs with ox-LDL-induced damage was mostly achieved by up-regulating the expression of LDLR and ApoC while down-regulating the expression of ApoB (ApoB-100, ApoB-48).
Western blotting verification
Western blotting verification (Fig. 8) revealed that Alliin increased the expression of LDLR and ApoC compared to the Mod group (P < 0.05) while decreasing the expression of ApoB (Fig. 8) (P < 0.05).
Discussion
OSCs were produced from China’s cash crops, including garlic and onions. Their studies and innovations could stimulate economic growth, create more job possibilities, and raise living standards. Stoll and Seebeck discovered alliin, which has the chemical formula S-allyl-L-cysteine sulfoxide, in 1947. It is now recognized as the principal active component of garlic due to its lipid-lowering, anti-inflammatory, and antioxidant activities19. Previous investigations found that alliin dramatically reduced plasma and liver cholesterol levels in SD rats fed a diet containing 10% hydrogenated coconut oil, 1% cholesterol, and 0.2% cholic acid20. Furthermore, Sanchez-Sanchez et al. demonstrated that alliin substantially improves lipid metabolism in diet-induced obese C57BL/6J mice21.
In our present research, we used proteomics and bioinformatics to investigate the proteins and signaling pathways associated with alliin’s protective mechanism against ox-LDL-induced HUVEC injury. For the proteins quantified in the Alliin/mod group, 33 were up-regulated, while 17 were up-regulated. Subcellular localization analysis revealed that the differential proteins of the Alliin/Mod group were mainly distributed in the extracellular space, implying that their function is predominantly involved in intercellular interactions. ApoB, the main structural protein of LDL, is an important indicator of the amount of LDL in the blood22. ApoB is synthesized in the liver and transports cholesterol to peripheral tissues by crossing endothelial barriers into the extracellular space23. Studies have shown that the accumulation and retention of ApoB beneath the endothelium play a crucial part in the pathogenesis of inflammatory response and AS24.
GO enrichment analysis indicated that the functions of the differentially expressed proteins in the Alliin/Mod group were primarily enriched in the biological process of cholesterol metabolism (Fig. 6A). Alliin has been shown to reduce blood cholesterol and triglyceride levels effectively25,26. In addition, Ali et al. discovered that garlic powder (13% alliin) effectively lowered blood cholesterol and triglyceride levels in rats fed a high-cholesterol diet27. Lu et al. reported that alliin at 80µM and 160µM effectively reduced 1,3-DCP-induced TC and TG levels in HepG2 cells11. Consistent with these findings, our results show that Alliin at 25 mg/L significantly reduced TC and Fch levels in the ox-LDL-injured HUVECs.These findings confirm our experimental results of GO enrichment, which suggest that alliin performs a protective role in ox-LDL-induced injured cells by modulating the cholesterol metabolism pathway.
The KEGG pathway enrichment analysis illustrated that differential proteins in the Alliin/Mod group were strongly enriched in the signaling pathways for PPAR, sterol biosynthesis, and cholesterol metabolism. The activation of PPAR has a close connection to glucose and lipid metabolism28. Yang et al. found that increases in PPARγexpression improved glucose and lipid metabolism in obese mice on a high-fat diet29. Activating PPARγand PPARαhas been shown to inhibit the development of AS30,31. However, bioinformatics research demonstrated that alliin up-regulated the expression of PPARβ/δin ox-LDL-injured HUVECs. In the future, more research is needed to find the relationship between PPARβ/δup-regulation and anti-atherosclerosis activities.
Early epidemiological investigations revealed that elevated levels of LDLR are significantly associated with AS and vascular risk prevention32. LDL is abundant in free cholesterol, constituting 50% of its total weight and serving as the primary agent responsible for cholesterol transport33,34. The LDLR, located on hepatocyte surfaces, specifically binds to LDL and subsequently internalizes LDL-cholesterol via endocytosis, thereby reducing plasma LDL-cholesterol levels and preventing excessive accumulation within the vascular wall that leads to AS lesions35,36. Garlic-derived organosulfur compounds, including S-Ethyl-L-cysteine, alliin, and diallyl trisulphide, have been shown to decrease PCSK-9 activity, increase LDLR function, and lower LDL cholesterol levels37. In this current research, we discovered that alliin up-regulated LDLR expression of ox-LDL injury HUVECs in the cholesterol metabolism pathway, which leads to an increase in the combination of LDLR and LDL, enhancing cholesterol endocytosis for cell metabolism and LDL-cholesterol removal.
On the other side, the results from quantitative proteomics and Western blotting verification identified that alliin could increase ApoC expression meanwhile decreasing ApoB expression. ApoC and ApoB are members of the lipid carrier family, which transports lipids and cholesterol throughout the body38. Up-regulation of ApoC expression has been shown to increase lipoprotein lipase activity and promote the clearance of triglyceride-rich lipoproteins39. Alliin increases the expression of ApoC in the mod group, which confirms that it might contribute to preventing atherosclerosis by enhancing the clearance of triglyceride-rich lipoproteins. ApoB could be classified into two subtypes: ApoB-100 and ApoB-48, which differ in their amino acid composition40. ApoB-100 exists in LDL, lipoprotein(a) [Lp(a)], IDL, and VLDL, whereas ApoB-48 is mainly discovered in chylomicrons (CM) and CM remnants. ApoB-100, which has the greatest relationship with LDL, transfers cholesterol from the liver to peripheral organs41. Elevated ApoB-100 levels have been associated with the accumulation of cholesterol on arterial walls, which promotes the formation of atherosclerotic plaques42,43. Alliin’s downregulation of ApoB expression suggests that it may reduce the synthesis of atherogenic lipoproteins, possibly slowing down the progression of AS.
Currently, lovastatin is the primary pharmacological agent for the clinical treatment of AS. Statins are well known for their ability to regulate cholesterol metabolism by inhibiting the synthesis of HMG-CoA reductase44. While HMG-CoA reductase inhibitors are known to decrease cholesterol levels, they also disrupt the enzyme’s critical physiological activities, which may pose long-term health risks45,46. In this present investigation, GO enrichment and KEGG pathway enrichment analysis demonstrated that the cholesterol metabolism pathway was substantially related to alliin’s protective effect against ox-LDL damage in HUVECs. Furthermore, our findings indicated that alliin could regulate the cholesterol metabolic pathway of ox-LDL-injured HUVECs through increasing LDLR and ApoC protein expression all while decreasing ApoB protein expression.
This investigation holds significant social implications by exploring the pharmacodynamic basis and mechanisms underlying alliin’s anti-AS effects. We employ Western blotting to validate the differentially expressed proteins identified through proteomics. The validation results were consistent with the proteomics data, which indicates that proteomics provides a novel approach to exploring the pharmacological properties of drugs. However, this finding is based solely on in vitro experiments; further in vivo studies and clinical validation are necessary to substantiate these results. Additionally, this research lacks direct Oil Red O staining to confirm lipid accumulation changes after alliin therapy due to retroactive experimental restrictions. Future research should prioritize this visualization. We believe that further investigations are expected to yield more comprehensive insights into alliin’s therapeutic targets and efficacy in atherosclerosis management.
Conclusion
In conclusion, the research we performed found that alliin regulates the cholesterol metabolic pathway by up-regulating the expression of LDLR and ApoC while down-regulating the expression of ApoB, which protects HUVECs from ox-LDL-induced injury, indicating the potential mechanism of alliin alleviating endothelial dysfunction and anti-atherosclerosis.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Fan, J. & Watanabe, T. A. Known Unkn. Pathol. Int. ;72(3):151–160. https://doi.org/10.1111/pin.13202 (2022).
LibbyP The changing landscape of atherosclerosis. Nature 592 (7855), 524–533. https://doi.org/10.1038/s41586-021-03392-8 (2021).
Pedro-Botet, J. Climent E,Benaiges d.atherosclerosis and inflammation.new therapeutic approaches.arteriosclerosis e Inflamación.Nuevos enfoques terapéuticos. Med. Clin(Barc). 155 (6), 256–262. https://doi.org/10.1016/j.medcli.2020.04.024 (2020).
Jeon, S. & Carr, R. Alcohol effects on hepatic lipid metabolism. J. Lipid Res. 61 (4), 470–479. https://doi.org/10.1194/jlr.R119000547 (2020).
Gylling, H., Hallikainen, M., Simonen, P., Miettinen & HE,Nissinen MJ,Miettinen TA.Serum and lipoprotein sitostanol and non-cholesterol sterols after an acute dose of plant Stanol ester on its long-term consumption. Eur. J. Nutr. 51 (5), 615–622. https://doi.org/10.1007/s00394-011-0249-5 (2012).
Parthasarathy, S., Raghavamenon, A. & Garelnabi, M. O. Santanam N.Oxidized low-density lipoprotein. Methods Mol. Biol. 610, 403–417. https://doi.org/10.1007/978-1-60327-029-8_24 (2010).
Li, M. et al. Roles and mechanisms of Garlic and its extracts on atherosclerosis: A review. Front. Pharmacol. 13, 954938. https://doi.org/10.3389/fphar.2022.954938 (2022).
Sobenin, I. A. Myasoedova VA,Iltchuk MI,Zhang DW,Orekhov an.therapeutic effects of Garlic in cardiovascular atherosclerotic disease. Chin. J. Nat. Med. 17 (10), 721–728. https://doi.org/10.1016/S1875-5364(19)30088-3 (2019).
Zhu, Y., Anand, R., Geng, X. & Ding, Y. A mini review: Garlic extract and vascular diseases. Neurol. Res. 40 (6), 421–425. https://doi.org/10.1080/01616412.2018.1451269 (2018).
El-Saber Batiha, G. et al. Chemical constituents and Pharmacological activities of Garlic(Allium sativum L.): A review. Nutrients 12 (3), 872. https://doi.org/10.3390/nu12030872 (2020). Published 2020 Mar 24.
Lu, J. C. B. et al. Alliin attenuates 1,3-dichloro-2-propanol-induced lipogenesis in HepG2 cells through activation of the AMP-activated protein kinase-dependent pathway. Life Sci. 195, 19–24. https://doi.org/10.1016/j.lfs.2017.12.040 (2018).
Bombicz, M. P. D. et al. Anti-Atherogenic properties of Allium ursinum liophylisate: impact on lipoprotein homeostasis and cardiac biomarkers in hypercholesterolemic rabbits. Int. J. Mol. Sci. 17 (8), 1284. https://doi.org/10.3390/ijms17081284 (2016). Published 2016 Aug 10.
Li, N. et al. Alliin inhibits adipocyte differentiation by downregulating Akt expression: implications for metabolic disease. Exp. Ther. Med. 21 (6), 563. https://doi.org/10.3892/etm.2021.9995 (2021).
Rozanova, S. et al. Quantitative mass Spectrometry-Based proteomics: an overview. Methods Mol. Biol. 2228, 85–116. https://doi.org/10.1007/978-1-0716-1024-4_8 (2021).
Hanash, S. D. Proteom. Nat. ;422(6928):226–232. https://doi.org/10.1038/nature01514 (2003).
Kanehisa, M., Furumichi, M., Sato, Y., Matsuura, Y. & Ishiguro-Watanabe, M. KEGG: biological systems database as a model of the real world. Nucleic Acids Res. 53 (D1), D672–D677. https://doi.org/10.1093/nar/gkae909 (2025).
Kanehisa, M. Toward Understanding the origin and evolution of cellular organisms. Protein Sci. 28 (11), 1947–1951. https://doi.org/10.1002/pro.3715 (2019).
Kanehisa, M. & Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28 (1), 27–30. https://doi.org/10.1093/nar/28.1.27 (2000).
Francioso, A. & Baseggio Conrado, A. Mosca L,Fontana m.chemistry and biochemistry of sulfur natural compounds:key intermediates of metabolism and redox biology. Oxid. Med. Cell. Longev. 2020, 8294158. https://doi.org/10.1155/2020/8294158 (2020).
Uto-Kondo, H. et al. S-Allyl-L-cysteine sulfoxide, a Garlic odor precursor, suppresses elevation in blood ethanol concentration by accelerating ethanol metabolism and preventing ethanol absorption from the gut. Biosci. Biotechnol. Biochem. 82 (4), 724–731. https://doi.org/10.1080/09168451.2018.1447357 (2018).
Sánchez-Sánchez, M. A. et al. Alliin,an Allium sativum nutraceutical,reducesmetaflammation markers in DIO mice.nutrients.;12(3):624. (2020). https://doi.org/10.3390/nu12030624
Contois, J. H. Langlois MR,Cobbaert C,Sniderman ad.standardization of Apolipoprotein B,LDL-Cholesterol,and Non-HDL-Cholesterol. J. Am. Heart Assoc. 12 (15), e030405. https://doi.org/10.1161/JAHA.123.030405 (2023).
Johannesen, C. D. L. Mortensen MB,Langsted A,Nordestgaard BG.ApoB and Non-HDL cholesterol versus LDL cholesterol for ischemic stroke risk. Ann. Neurol. 92 (3), 379–389. https://doi.org/10.1002/ana.26425 (2022).
Marchini, T., Hansen, S. & Wolf, D. CD4 + T Cells Mouse Hum. Atherosclerosis Cells ;10(2):446. https://doi.org/10.3390/cells10020446 (2021).
Ashraf, R., Sarwar, M., Kamil, N., Wahid, S. & Qureshi, A. Analysis of dose and duration dependent effects of Allium sativum Linn and other hypocholesterolemic agents exhibited on dyslipidemia in patients with essential hypertension.pak. J. Pharm. Sci. 35 (3), 777–784 (2022).
Iciek, M. & KwiecieńI Włodek L.Biological properties of Garlic and Garlic-derived organosulfur compounds. Environ. Mol. Mutagen. 50 (3), 247–265. https://doi.org/10.1002/em.20474 (2009).
Ali, M., Al-Qattan, K. K., Al-Enezi, F., Khanafer, R. M. & Mustafa, T. Effect of allicin from Garlic powder on serum lipids and blood pressure in rats fed with a high cholesterol diet.prostaglandins Leukot. Essent. Fat. Acids. 62 (4), 253–259. https://doi.org/10.1054/plef.2000.0152 (2000).
Nakagawa, Y. & Shimano, H. C. R. E. B. H. Regulates systemic glucose and lipid metabolism. Int. J. Mol. Sci. 19 (5), 1396. https://doi.org/10.3390/ijms19051396 (2018).
Yang, M. Y. et al. Anti-adipogenesis and anti-obesity potential of alliin mediated by modulating glycolipid metabolism via activating PPARγsignaling. Naunyn Schmiedebergs Arch. Pharmacol. 397 (11), 8707–8723. https://doi.org/10.1007/s00210-024-03181-w (2024).
Montaigne, D. & Butruille, L. Staels B.PPAR control of metabolism and cardiovascular functions. Nat. Rev. Cardiol. 18 (12), 809–823. https://doi.org/10.1038/s41569-021-00569-6 (2021).
Zhang, M. et al. Oridonin attenuates atherosclerosis by inhibiting foam macrophage formation and inflammation through FABP4/PPARγsignalling. J. Cell. Mol. Med. 27 (24), 4155–4170. https://doi.org/10.1111/jcmm.18000 (2023).
FerenceBA et al. Association of Triglyceride-Lowering LPL variants and LDL-C-Lowering LDLR variants with risk of coronary heart disease. JAMA 321 (4), 364–373. https://doi.org/10.1001/jama.2018.20045 (2019).
Hartley, A., Haskard, D. & Khamis, R. Oxidized LDL and anti-oxidized LDL antibodies in atherosclerosis-Novel insights and future directions in diagnosis and therapy < sup/>. Trends Cardiovasc. Med. 29 (1), 22–26. https://doi.org/10.1016/j.tcm.2018.05.010 (2019).
Zhou, Y. X. et al. Delivery of low-density lipoprotein from endocytic carriers to mitochondria supports steroidogenesis. Nat. Cell. Biol. 2023, 25(7):937–949. https://doi.org/10.1038/s41556-023-01160-6
Barale, C., Melchionda, E., Morotti, A. & Russo, I. P. C. S. K. 9 Biology and Its Role in Atherothrombosis.Int J Mol Sci.;22(11):5880.Published 2021 May 30. (2021). https://doi.org/10.3390/ijms22115880
Defesche, J. C., Gidding, S. S. & Harada-Shiba, M. Hegele RA,Santos RD,Wierzbicki AS.Familial hypercholesterolaemia. Nat. Rev. Dis. Primers. 3, 17093. https://doi.org/10.1038/nrdp.2017.93 (2017).
Ahmad, P. et al. Naturally occurring organosulfur compounds effectively inhibits PCSK-9 activity and restrict PCSK-9-LDL-receptor interaction via in-silico and in-vitro approach. Nat. Prod. Res. 38 (22), 3924–3933. https://doi.org/10.1080/14786419.2023.2269465 (2024).
Mahley, R. W., Innerarity, T. L. & Rall, S. C. Jr Weisgraber KH.Plasma lipoproteins: Apolipoprotein structure and function. J. Lipid Res. 25 (12), 1277–1294 (1984).
Borén, J., Packard, C. J. & Taskinen, M. R. The roles of ApoC-III on the metabolism of Triglyceride-Rich lipoproteins in humans. Front. Endocrinol(Lausanne). 11, 474. https://doi.org/10.3389/fendo.2020.00474 (2020).
Mehta, A. & Shapiro, M. D. Apolipoproteins in vascular biology and atherosclerotic disease. Nat. Rev. Cardiol. 19 (3), 168–179. https://doi.org/10.1038/s41569-021-00613-5 (2022).
Löffler, T. et al. Impact of ApoB-100 expression on cognition and brain pathology in wild-type and hAPPsl mice. Neurobiol. Aging. 34 (10), 2379–2388. https://doi.org/10.1016/j.neurobiolaging.2013.04.008 (2013).
SnidermanAD, TremblayAJ, De Graaf, J. & Couture Calculation of LDL ApoB. Atherosclerosis 234 (2), 373–376. https://doi.org/10.1016/j.atherosclerosis.2014.03.012 (2014).
De SilvaGS et al. Circulating serum fatty acid synthase is elevated in patients with diabetes and carotid artery stenosis and is LDL-associated. Atherosclerosis 287, 38–45. https://doi.org/10.1016/j.atherosclerosis.2019.05.016 (2019).
Henwood, J. M. & Heel, R. C. Lovastatin.A preliminary review of its pharmacodynamic properties and therapeutic use in hyperlipidemia.drugs.1988;36(4):429–454. https://doi.org/10.2165/00003495-198836040-00003
Schumacher, M. M. & DeBose-Boyd, R. A. Posttranslational regulation of HMG coa reductase,the Rate-Limiting enzyme in synthesis of cholesterol. Annu. Rev. Biochem. 90, 659–679. https://doi.org/10.1146/annurev-biochem-081820-101010 (2021).
Mahdavi, A., Bagherniya, M., Fakheran, O., ReinerŽ, Xu, S. & Sahebkar, A. Medicinal plants and bioactive natural compounds as inhibitors of HMG-CoA reductase: A literature review.biofactors.2020;46(6):906–926. https://doi.org/10.1002/biof.1684
Acknowledgements
We are grateful to Hangzhou Jingjie Biological Company in China and Nanchong Chuanbei Medical College in Sichuan Province, China, for their technical assistance with this experiment.
Funding
This work was supported by the Natural Science Foundation of Hainan Province (No.823MS148), the Foundation of Hainan Health Committee (No.22A200257), the Foundation of Hainan Educational Committee (No.Hnky2023-25), and the Foundation of the Affiliated Hospital of Chengdu University of Traditional Chinese Medicine (No.23TS03).
Author information
Authors and Affiliations
Contributions
Yuan-yuan Tang. and De Lv. wrote the main manuscript text and Yuan-yuan Tang prepared Figs. 1, 2, 3, 4, 5, 6, 7 and 8. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tang, Yy., Lv, D. Quantitative proteomic analysis of the protective effect exerted by alliin on ox-LDL-injured HUVECs. Sci Rep 15, 17072 (2025). https://doi.org/10.1038/s41598-025-01677-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-01677-w