Abstract
Hypertension is a clinical syndrome characterized by elevated systemic arterial blood pressure, which can be accompanied by functional impairment of or organic damage to the heart, brain, kidney and other organs. Hypertensive emergencies occur when significant increases in blood pressure result in persistent target organ damage. Hypertensive encephalopathy is a rare hypertensive emergency. Glymphatic system dysfunction is significantly correlated with the occurrence and development of hypertensive encephalopathy. We found that SHRs had a longer duration of peak contrast intensity and a slower clearance of contrast in the brain compared with WKY rats. Of note, in SHRs, there was a clear difference in contrast signal intensity between the hippocampus and the aqueduct. The results of cluster analysis showed that shr and WKY rats exhibited different patterns of voxel clustering. Progressively expanding brain clusters were observed from the posterior part of the brain near the cisterna magna (CM) to the anterior part of the brain, olfactory bulb, and dorsal cortical areas. In addition, shr showed a significant peak delay within some clusters and a slower rate of signal decay with time. Ex vivo imaging of ISF efflux showed that ISF efflux was also significantly reduced in shr. In vitro studies have shown decreased AQP4 expression in the brainstem and olfactory bulb of shr, accompanied by extensive astrogliosis, which may affect CSF circulation.Our study highlights the changes in glymphatic system function in a hypertension model and provides an important theoretical basis for further elucidating the mechanisms underlying the development and progression of hypertensive encephalopathy.
Similar content being viewed by others
Introduction
Hypertension is a clinical syndrome characterized by an increase in systemic arterial blood pressure, which may be accompanied by functional impairment of or organic damage to the heart, brain, kidneys and other organs. Studies have shown structural and functional changes in the cerebral vasculature system, such as arterial remodelling, loss of integrity of the blood‒brain barrier (BBB), and endothelial dysfunction, in hypertension. Elevated blood pressure is significantly, independently, and linearly associated with the risk of cardiovascular disease and all-cause mortality1. A hypertensive emergency occurs when sustained target organ damage occurs due to a marked increase in blood pressure. Hypertensive encephalopathy is a less common type of hypertensive emergency that manifests as altered mental status and signs of cerebral oedema following the elevation of blood pressure and can be life-threatening in severe cases.
Notably, hypertension has been identified as an important risk factor for neurodegenerative diseases and is associated with several forms of cognitive dysfunction. Hypertension in midlife is significantly associated with cognitive impairment later in life. For example, many features of Alzheimer’s disease (AD), including brain atrophy, cognitive decline, β-amyloid accumulation, and blood‒brain barrier dysfunction, have been observed in animal models of hypertension. Hypertension increases the susceptibility to AD and aggravates the development of AD by increasing oxidative stress and the inflammatory response2,3,4, while antihypertensive drugs have been found to reduce the risk of developing AD5. Women with a history of preeclampsia have more severe cognitive impairment later in life than women with a history of normotensive pregnancy. Furthermore, the pattern of cognitive changes is consistent with that observed in vascular disease/white matter pathology6. Hypertension is associated with a reduction in net cerebrospinal fluid (CSF) flow in the paravascular space (PVS), which may be related to the hardening of the arterial wall7.
The glymphatic system is a whole-brain fluid exchange system that rapidly transports CSF through the PVS as well as interstitial fluid (ISF) and waste solutes into the perivenous space of deep central veins and venous sinuses before exiting the brain parenchyma via authentic lymphatic vessels located at the level of the dura covering the brain and cranial nerves8,9, which ultimately connect to the cervical lymphatic vessels10. Its function relies on aquaporin-4 (AQP4) on astrocytes. AQP4 mainly promotes the exchange of CSF and ISF, as well as the clearance of ISF, and plays a key role in regulating CSF-ISF exchange11,12,13.
The driving force of glymphatic system activity mainly includes pressure generated by the continuous production of CSF, arterial pulsation, and slow vasomotion. The glymphatic system regulates fluid exchange and removes harmful substances such as Aβ, ɑ-synuclein, and tau protein14. Cerebral arterial pulsation accelerates the rapid movement of solutes in the PVS by mixing and diffusion, thereby promoting the exchange of material between the CSF and ISF15. The stronger cerebral arterial pulsation is, the more efficient the material exchange between the CSF and ISF, and vice versa. The elasticity of the vascular wall and heart rate can affect cerebral artery pulsation and change the clearance rate through the glymphatic system16. Notably, an animal model of acute hypertension induced by angiotensin-ii (Ang-ii) exhibits reduced glymphatic transport7. Structural MRI analysis revealed that the cerebroventricular volume is greater and that brain volume is significantly smaller in spontaneously hypertensive rats (SHRs) than in WKY rats, regardless of age. Mortensen et al. observed ventricular reflux of Gd-DOTA in SHRs only, indicating abnormal CSF flow dynamics secondary to innate hydrocephalus. One-tissue compartment analysis revealed impeded glymphatic transport of Gd-DOTA in SHRs compared with WKY rats in both age groups, implying that glymphatic transport, including solute clearance from the brain parenchyma, is impaired during evolving hypertension in young SHRs, a change that worsens under conditions of chronic hypertension17. However, it is still unclear whether the dynamics of the glymphatic system in different brain regions are different under hypertensive conditions.
In this study, we used in vitro imaging techniques to monitor the dynamic functional state of the glymphatic system in multiple brain regions in SHRs (an established model recapitulating the effects of hypertension on cerebral microvasculature) and normotensive control rats (Wistar Kyoto (WKY) rats).The contrast agent exhibited the peak signal intensity for longer and was cleared at a a slower rate in SHRs than in WKY rats. Moreover, the distribution of the contrast agent in the brain was more uniform and the tendency for contrast agent to accumulate on the nasal side was significantly higher in SHRs than in WKY rats. Notably, in the SHRs, there was a significant difference in the signal intensity of the contrast agent between the hippocampus and the aqueduct. The results of cluster analysis showed that the SHRs and WKY rats exhibited different patterns of voxel clustering. Ex vivo imaging of ISF efflux showed that the efflux of ISF was also significantly reduced in SHRs. In vitro evaluation revealed reduced AQP4 expression in the brainstem and olfactory bulb in the SHRs, accompanied by extensive astrogliosis, which may have affected CSF circulation.We demonstrate that glymphatic system dynamics were impaired in SHRs and provide a theoretical basis for the clinical treatment of cerebral oedema, cognitive dysfunction and other complications of hypertension (Fig. 1).
Timeline of the experiment. Dynamic contrast-enhanced MRI (DCE-MRI) was performed after injection of Gd-DTPA into the cisterna, and then OVA647 was injected into the brain parenchyma of the animals for ex vivo imaging. Finally, the animals were sacrificed under anesthesia and immunofluorescence staining was performed.
Methods
Ethical statement
All the animal studies were conducted according to the protocol approved by the Animal Care Welfare Committee of Guizhou Medical University (ID: 2200973). The study was conducted in compliance with the ARRIVE guidelines. All experiments were performed in accordance with relevant guidelines and regulations.
Animals
Male SHRs and WKY rats aged 18–20 weeks were used, All rats were purchased from Beijing Weitonglihua Experimental Animal Technology Co, LTD. All rats were housed in a temperature and humidity-controlled environment on a 12-h light-dark cycle and given standard food and water.
The SHRs were generated as first described by Okamoto and Aoki (1963); as these rats exhibit a condition very similar to human progressive hypertension, the SHR model is the most commonly used animal model of cardiovascular diseases18,19. At 4–6 weeks of age, SHRs spontaneously develop hypertension without any stress stimulation (physical, drug, surgery, or other factors). At age 6–8 weeks, their systolic blood pressure increases rapidly, finally reaching 200–220 mmHg at age 14 weeks20. In contrast, WKY rats have no genetic factors related to hypertension, and their blood pressure is normal in the absence of special circumstances.
Blood pressure monitoring.
Caudal arterial blood pressure was measured using a small animal noninvasive blood pressure metre. During the measurement process, the rats were placed on a heating pad for at least 20 min to heat their tails. The tail sleeve was placed on the root of the tail of the rats, and measurements were taken for 15 consecutive periods to determine the systolic and diastolic blood pressure. After the measurements, the data were exported for analysis (Fig. 2).
Statistical analysis of systolic and diastolic blood pressurein SHRs and WKYs. Independent-samples T Test was used for comparison between the two groups, and P < 0.05 was considered statistically significant.Levels of significance are defined as *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001; ****p ≤ 0.0001, n=10
Surgery
Using a previously reported protocol, after T2-weighted (T2W) imaging, the rats were subjected to dynamic contrast-enhanced (DCE) T1-weighted (T1W) MRI. Eighty microlitres of Gd-DTPA (469 mg/mL, MW 938 Da, Bayer) was microinjected into the cisterna magna (CM) at a rate of 1.6 µL/min through a PE-10 tube connected to a microlitre syringe (Hamilton) controlled by a microsyringe pump (Pump 11, Harvard). The rats were anaesthetized with 2% isoflurane in oxygen21,22,23. The rats were placed on a stereotaxic apparatus and anesthetized with 2% isoflurane, and their heads were fixed so that they faced downwards. A midline incision was made from the occipital crest to the first cervical vertebra. Then, the subcutaneous muscle and fascia were gently separated with two curved tweezers, exposing the CM24.
The CM appears as a small inverted triangle between the cerebellum and the medulla and is covered by a translucent dura mater. After the CM was exposed, a PE-10 tube was inserted 2–3 mm deep into the CM and fixed in place with cyanoacrylate glue. The temperature was maintained at 37.0 ± 0.5 °C using a thermostatic water bed. Physiological parameters such as respiratory rate, oxygen saturation, heart rate, and body temperature were recorded by a magnetic resonance-compatible physiological detection system.
MRI acquisition
After surgery, the rats were placed on an MRI-compatible bed (with the animal in the prone position) in an MRI-compatible rat stereotactic device, and a nose cone was used to deliver 2% isoflurane. The rats were allowed to breathe spontaneously during MRI acquisition. All imaging protocols were performed on a 9.4T Biospec 94/20 USR (Bruker BioSpin, Germany) instrument connected to a Bruker Advance console, which was controlled by Paravision 5.0 software (Bruker Corporation, Billerica, MA, USA; http://www.bruker.com). Imaging was performed with the use of a 40 mm (internal diameter; ID) volume coil.
First, sagittal two-dimensional T2W Turbo RARE images were acquired to ensure consistency of the slice position (RARE; TR/TE 4705/33 ms; 45 slices of 1 mm; FOV = 31 × 28 mm2; matrix = 256 × 256). Then, 3D T1W FLASH sequences (TR = 74.9 ms, TE = 1.7 ms, flip angle = 15°, NA = 1, FOV = 35 × 35 mm2, matrix size = 128 × 128) were obtained in the sagittal plane. The scanning protocol included three baseline scans. Then, a total of 80 µl of the paramagnetic contrast agent Gd-DTPA was injected into the CM at a rate of 1.6 µl/min (total injection time = 50 min) through a retention catheter, and MRI acquisition was continuously performed for approximately 5 h.
MRI data preprocessing
The DCE-MRI data were preprocessed using standardized tools ANTs(Version 2.3.3; http://stnava.github.io/ANTs/); preprocessing included the creation of brain templates, signal intensity normalization, and smoothing processing25,26. First, the average DCE images of each rat were obtained for all time points, and then a brain mask was drawn using MRIcronGL( Version 1.2.20201102; https://www.nitrc.org/projects/mricrogl). The mask was applied to all DCE images, and the surrounding muscle tissue was removed. The last DCE image of all subjects in the control group was used to construct a group-level template. The average DCE image of each rat was registered to the group-level template to obtain the bidirectional deformation relationship between individual images and the template. The mean signal within this region of interest (ROI) in DCE images for each subject from all time points was normalized by dividing by the mean to convert the original signal into a percentage. In this study, the selected ROI was a region with a high signal intensity, which was then smoothed, and a whole-brain mask was applied to the smoothed DCE image again.
MRI data analysis
Ben-Nejma’s treatment method was adopted24. First, the spatially smoothed and normalized time series of the two groups were analysed by principal component analysis (PCA). Since more than 99% of the data variability could be explained by the three largest components, we use them to reconstruct PCA-based time series, effectively reducing high-frequency noise in the data. The reconstructed time series of the two animal groups were then subjected to hierarchical clustering (ward linkage method, up to 15 classes), which was used to identify voxel clusters with similar temporal changes and allows the pattern of voxel clustering in each group to be determined. We also performed an analysis of seven predefined hypothesis-driven brain ROIs, namely, the aqueduct, cerebellum, cortex, hippocampus, medulla, olfactory bulb and pons. Mask images of each brain region were obtained using MRIcroGL(Version 1.2.20201102; https://www.nitrc.org/projects/mricrogl) based on the Paxinos Brain Atlas. Then, for each subject, the PCA-based average time series was calculated for each ROI, the area under the curve (AUC) of the time series was calculated, and the calculated AUC values were statistically compared between the two groups (t-test).
Ex vivo imaging of the efflux of ISF
The objective of this study was to observe the efflux of ISF in the brain into the deep cervical lymph nodes (dCLNs) in SHRs27,28. A total of 80 µL of Alexa Fluor 647 (OVA-647; 0.5 mg/mL artificial cerebrospinal fluid; Thermo Fisher Scientific, O34782) was stereotactically injected into the brain parenchyma at 1.6 mm anterior/posterior (AP), 2.0 mm medial/lateral (ML), and − 2.5 mm dorsal/ventral (DV) from bregma within 50 min. Two hours after injection, the dCLNs and brain tissue samples were dissected out and fixed. After fixation, the brain tissue samples were cut into 10 μm thick slices by a vibratome, and the tissue sections were placed on slides. A PANNORAMIC MIDI II instrument (3D Histech, Hungary) was used for fluorescence staining and image acquisition, and the images were analysed using ImageJ software (Version 1.53t; National Institutes of Health, USA; https://imagej.nih.gov/ij).
Immunofluorescence imaging
The rats were euthanized and perfused transcardially with phosphate-buffered saline (PBS), after which their brains were fixed with paraformaldehyde tissue fixative for 24 h and incubated in PBS containing 20% and 30% sucrose for washing and dehydration. From 1.60 mm rostral and 10.80 mm caudal to bregma, the brain tissues were cut into coronal Sect. (10 μm thick) on a cryostat microtome (Leica RM2235). For immunofluorescence staining, the sections were mounted on slides, permeabilized with 0.3% Triton X-100, and blocked with 10% goat serum. Then, they were incubated with the following primary antibodies: rabbit anti-AQP4 (Abcam ab128906, 1:100) and rabbit anti-glial fibrillary acidic protein (GFAP) (Abcam ab33922, 1:100). After washing off the primary antibody, the sections were incubated with secondary anti-goat anti-rabbit IgG H&L (Alexa Fluor 594; 1:200). Finally, the sections were placed on slides and covered in mounting medium containing DAPI (Sigma, D9542). Immunofluorescence staining of AQP4/lectin and GFAP/lectin image acquisition were performed using a PANNORAMIC MIDI II instrument (3D Histech, Hungary). The acquired images were visually assessed by at least 3 colleagues to ensure that representative images were selected. ImageJ(Version 1.53t; National Institutes of Health, USA; https://imagej.nih.gov/ij) was used to analyse the expression of AQP4 and astrocyte markers in a blinded manner.
2.10.Statistical analysis.
GraphPad Prism 8.0 (GraphPad Software, San Diego, CA, USA;https://www.graphpad.com) was used for statistical analysis. The Shapiro-Wilk method was used to test the normality of quantitative data. All measurement data consistent with normal distribution were expressed as mean ± standard deviation (SD), and non-normal distribution measurement data were expressed as median (interquartile range). Independent-samples T Test was used for comparison between the two groups, and P < 0.05 was considered statistically significant.Levels of significance are defined as* p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001; **** p ≤ 0.0001.
Results
Differences in the spatial distribution of Gd-DTPA between SHRs and WKY rats
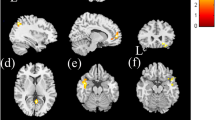
To determine the functional status of the glymphatic system in hypertension, we first evaluated the difference in Gd-DTPA transport via the glymphatic system between 18- to 20-week-old SHRs and WKY rats by assessing the dynamic distribution pattern of Gd-DTPA in specific anatomical regions of the brain. For this purpose, DCE images were obtained continuously from the beginning of Gd-DTPA injection into the cisterna pulposus for up to 5 h. Compared to that in the brains of WKY rats, the distribution of contrast agent in the brains of SHRs was more uniform, and contrast agent accumulation on the nasal side was significantly reduced. In addition, the contrast media exhibited a peak signal for longer and was cleared at a slower clearance rate in SHRs than in WKY rats (Fig. 3). Notably, significant differences in the signal distribution of the contrast agent were found in the hippocampus (P = 0.0076) and aqueduct (P = 0.0079) in the SHRs. However, no differences were found in the olfactory bulb (P = 0.0718), cortical (P = 0.5211), cerebellum (P = 0.5283), pons (P = 0.1594) or medulla (P = 0.4910) (Fig. 4). Therefore, these data suggest that glymphatic circulation in the SHRs had changed.
Cluster analysis of voxel time progression revealed different spatial patterns of Gd-DTPA between SHRs and WKY rats
In addition, the results of cluster analysis showed that the SHRs and WKY rats exhibited different voxel clustering patterns after Gd-DTPA injection. A progressive expansion of clusters was observed from the posterior part of the brain near the cisterna magna towards the anterior part of the brain, the olfactory bulb and the dorsal cortex (Fig. 5A). In addition, some voxel clusters showed a significant delay in reaching the peak signal intensity and a slower rate of signal reduction in SHRs compared with WKY rats (Fig. 5B).
ISF removal from the brain parenchyma is impaired in SHRs
To explore whether the efflux function of the glymphatic system is altered, we performed ex vivo imaging of ISF efflux from the brain parenchyma.Two hours after intraparenchymal injection of OVA-647, we observed the efflux of OVA-647 in the rat brain parenchyma and the accumulation of OVA-647 in dCLNs (Fig. 6). Compared to WKY rats, SHRs showed a significant increase in red fluorescence intensity in the brain parenchyma (p < 0.0001), indicating incomplete removal of OVA-647. Moreover, the red fluorescence intensity in the deep neck lymph nodes of SHRs was significantly reduced (Fig. 7) (p = 0.0023). These results indicate that ISF removal from the brain parenchyma is impaired in SHRs.
Alterations in glymphatic transport in SHRs are associated with brain pathology
To further clarify the observed differences in cerebral fluid circulation dynamics in the SHRs, we also evaluated the changes by immunohistochemistry. We examined the expression of AQP4 and astrocyte markers in the cortex, hippocampus, cerebellum, brainstem, and olfactory bulb. The results showed that the expression of Aqp4 in the brainstem (P = 0.0261, n = 7) and olfactory bulb (P = 0.0308, n = 7) was significantly lower in SHRs than in WKY rats. However, there was no significant difference in the expression of astrocyte markers (Figs. 8 and 9).
Spatial distribution maps of Gd-DTPA in the brains of SHRs and WKY rats demonstrated that the contrast agent exhibited a prolonged time-to-peak signal intensity and slower clearance rate in SHRs. The spatial distribution of the MRI contrast agent in WKY rats and SHRs is shown in the sagittal plane at 10 time points, i.e., 0, 30, 60, 90, 120, 150, 180, 210, and 240 and 270 min after intracerebral perfusion. The colour scale represents the average percent signal intensity change, with dark blue/red indicating low/high percent signal intensity change, respectively, n = 10.
Region-of-interest (ROI)-based analysis. Average signal intensity changes over time refecting Gd-DTPA contrast agent distribution in the aqueduct (A, P = 0.0079), cerebellum (B, P = 0.5283), cortex (C, P = 0.5211), hippocampus (D, P = 0.0076), medulla (E, P = 0.4910), olfactory bulb (F, P = 0.0718), and pons (G, P = 0.1594). *signifcant diference in the area under the curve (AUC) across the two groups indicates a diferential distribution of contrast agent. Independent-samples T Test was used for comparison between the two groups, and P < 0.05 was considered statistically significant.Levels of significance are defined as *p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001; ****p ≤ 0.0001, n = 10.
A.
Spatial pattern of the Gd-DTPA signal over time in SHRs. Time course of MRI voxel clustering, reflecting the change in the distribution of the contrast agent Gd-DTPA over time, in SHRs and WKY rats. (A) Visualization of the 15 clusters in the two groups, each indicated by a different colour, showing brain regions with similar contrast agent distributions. (B) The time course of the mean voxel signal intensity in each cluster (mean ± SD) is shown, with the colours of the clusters corresponding to those in (A) In both groups of rats, there was a large increase in signal intensity 30 min after Gd-DTPA injection (onset of MRI acquisition), with a decrease in signal intensity in most clusters (contrast clearance) over time, but an accumulation of contrast in several clusters, n = 10.
Interstitial fluid removal from the brain parenchyma is impaired in SHRs. (A) Representative images of residual OVA-647 within the brain parenchyma post-injection (5 brain sections). Whole-slide scanning was performed at 1× magnification. (B) Representative images of fluorescence intensity reflecting OVA-647 drainage to the deep cervical lymph nodes (dCLNs). Whole-slide scanning was performed at 2× magnification, n = 10.
Statistical analysis of the area ratio of OVA-647 fluorescence in the brain parenchyma (P < 0.0001) and dCLNs (P = 0.0023). Data are shown as the mean ± SD, independent-samples T Test was used for comparison between the two groups, and P < 0.05 was considered statistically significant. Levels of significance are defined as * p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001; **** p ≤ 0.0001, n = 10.
Representative images of AQP4 and astrocytic expression in five brain regions (cortex, hippocampus, cerebellum, brainstem and olfactory bulb) of SHRs and WKY rats. Immunofluorescence imaging: AQP4 (red), lectin (green, vascular marker), DAPI (blue, nuclei), and GFAP (purple, astrocytes). Astrocytes were identified by GFAP immunostaining (purple). Images were acquired at 20× magnification, n = 7.
Expression of AQP4 and astrocytes around blood vessels in five brain regions. Ex vivo assessment was performed in key brain regions of SHRs and control WKY rats, including the olfactorybulb (AQP4: P = 0.0308, GFAP: P = 0.6955), brainstem(AQP4: P = 0.0261, GFAP: P = 0.0600). Data are shown as the mean ± SD, independent-samples T Test was used for comparison between the two groups, and P < 0.05 was considered statistically significant. Levels of significance are defined as * p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001; **** p ≤ 0.0001, n = 7.
Discussion
In 2013, Iliff et al.29 were the first to use DCE-MRI to visualize the exchange of CSF-ISF in the rat brain and showed CSF inflow around arteries. The glymphatic system can transport solutes and waste products (such as the neurotoxic soluble substance Aβ) to the PVS and then remove them8,9. The function of the glymphatic system is closely related to the occurrence and development of a variety of central nervous system diseases. Large-caliber vessels in the brain, such as the internal carotid artery, absorb most of the systolic pulse wave when blood pressure rises, so they are more susceptible to changes in intraluminal pressure30. However, continuous long-term contraction can lead to hypertrophy and eutrophication remodelling of the vessel wall, resulting in increased wall stiffness and a smaller vascular lumen31. Stiffening of the arterial wall may alter the pulsation of the vessel wall and reduce the efficiency of perivascular pumping32,33,34,35. Acute hypertension can cause changes in the arterial wall motion waveform and a large negative wall velocity, resulting in increased reflux. This impairs perivascular pumping function and reduces the net flow of CSF in the PVS. As a result, the function of the entire glymphatic system is suppressed, which affects the removal of waste from the brain parenchyma, such as amyloid β7. Another study revealed that vasogenic brain oedema occurs in an acute hypertension rat model, and perfusion imaging was used to reveal the dynamic changes in cerebral blood flow, BBB destruction, and permeability changes under acute hypertensive conditions36,37. Further exploration is needed to determine how long-term elevation of blood pressure affects PVS fluid transport and vascular remodelling. Therefore, we conducted this DCE-MRI study. DCE-MRI of the brain revealed significant differences in the distribution of contrast agents over time in the hippocampus and aqueduct in SHRs, the contrast agent (1) exhibited a peak signal intensity for longer and (2) was cleared at a slower rate. These findings suggest that dysfunction of the glymphatic system under hypertensive conditions. One of the most important functions of the glymphatic system is to clear brain metabolites such as Aβ and tau33. There have been several previous studies on the role of the hippocampus in the glymphatic system. Mortensen et al.17 reported that tracer uptake in the hippocampus tends to be decreased in SHRs. Bedussi et al.38 reported that, compared with WKY rats, elderly SHRs had increased fluorescence diffusion in the hippocampus after a small amount of fluorescently labelled dextran was injected stereotactically, indicating enhanced flow. We analysed the temporal changes in signal intensity over up to 5 h by DCE-MRI and showed that there were significant differences in the distribution of the contrast agent in the hippocampus of SHRs, which may be an important cause of hypertension-induced cognitive dysfunction.
AQP4 expression in astrocytes is essential for the central nervous syste39,40,41. Astrocytes in AQP4 knockout mice exhibit swollen endfeet, a significantly reduced endfoot space, interstitial extracellular space, and significantly reduced brain ISF transport and clearance13. Mestred et al. used four individually generated mouse models in which AQP4 expression was disrupted as well as one lacking perivascular AQP4 expression, and five independent experiments confirmed that transport via the glymphatic system is dependent on AQP4 expression42. Glia-specific AQP4 knockout mice were found to exhibit a 31% reduction in brain water uptake after systemic hypoosmotic stress and delayed postnatal resorption of brain water43, resulting in a greater incidence of spontaneous hydrocephalus44. It is noteworthy that AQP4 expression demonstrates not only distinct variations across different disease animal models but also marked regional specificity and heterogeneity at distinct pathological stages within the same disease. In hypertensive models, the marked downregulation of AQP4 expression in brainstem autonomic nuclei stands in sharp contrast to its upregulation observed in cortical regions and hippocampal areas associated with neuroinflammation and vasogenic edema45,46. In our study, the expression of AQP4 was significantly reduced in the brainstem and olfactory bulb of SHRs. Mortensen’s early study did not observe significant differences in AQP4 expression between SHR and WKY rats. Given the highly region-dependent heterogeneity of AQP4 distribution, this finding may be explained by their immunofluorescence analysis predominantly targeting cortical and perivascular regions47. AQP4 is also associated with multiple types of cognitive dysfunction48. As AQP4 binds to astrocytes, astrocytes also play an important role in the glymphatic system. following intraparenchymal injection of an equivalent dose of OVA-647 in SHRs, the clearance rate from the brain to the deep cervical lymph nodes was significantly reduced, indicating impaired interstitial fluid drainage in SHRs. In conclusion, dysfunction of the glymphatic system can hinder the clearance of ISF, impairing the clearance of related metabolic waste and promote the occurrence and development of cognitive dysfunction in hypertensive patients.
In earlier studies, the volume of grey matter in the Ca1 subregion and dentate gyrus decreased, the number of neurons in the Ca1 subregion decreased, and the number of GFAP-immunoreactive astrocytes increased in the hippocampus of 6-month-old SHRs49,50. Bedussi et al.also reported that GFAP and AQP4 were expressed at the ISF-CSF boundary, around arteries, and in the lacunar molecular layer. Sabbatini et al.51showed that the number of astrocytes in the hippocampal CA1 subregion increased in 6-month-old SHRs, but the size of the astrocytes did not increase. Mignini et al.52 also found increases in the number of GFAP-immunoreactive astrocytes and the average immunoreactive area in two cortical regions. The present study revealed a widespread increase in the number of astrocytes in SHRs but a slight decrease in the number of astrocytes in the olfactory bulb. Based on its important role in inflammation of the glymphatic system and central nervous system, our next study will further investigate changes in cell size and other parameters in SHRs. Another study revealed that changes in astrocytes can mediate neuronal excitotoxicity through excessive [Ca2+]-mediated glutamate release, which affects synaptic function but may also have a regulatory effect on glymphatic system function53.
Although the role of global brain activity and disruption of CSF circulation in initiating the pathology of spontaneous hypertension remains uncertain, it is speculated that abnormal expression of AQP4 and the change of brain fluid dynamics jointly promote overall neuronal variability. Therefore, it is imperative to investigate how AQP4 expression modulates glymphatic system function, including through the use of AQP4-targeted pharmacological interventions. Furthermore, deeper exploration is required to determine whether antihypertensive therapy can ameliorate glymphatic dysfunction, and conversely, whether such dysfunction contributes to the development of hypertensive phenotypes. Based on the results of this study, glymphatic system dysfunction in patients with hypertension provides a window for studying the mechanisms underlying the occurrence of related neuroinflammation.
Data availability
Data availability All data generated and analyzed during this study are available from the corresponding author upon reasonable request.
References
Mills, K. T., Stefanescu, A. & He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 16(4), 223–237 (2020).
Feldstein, C. A. Association between chronic blood pressure changes and development of Alzheimer’s disease. J. Alzheimers Dis. 32(3), 753–763 (2012).
Iadecola, C. & Davisson, R. L. Hypertension and cerebrovascular dysfunction. Cell. Metab. 7(6), 476–484 (2008).
Iadecola, C. & Gottesman, R. F. Neurovascular and cognitive dysfunction in hypertension. Circ. Res. 124(7), 1025–1044 (2019).
Peters, R. et al. Incident dementia and blood pressure Lowering in the hypertension in the very elderly trial cognitive function assessment (HYVET-COG): A double-blind, placebo controlled trial. Lancet Neurol. 7(8), 683–689 (2008).
Fields, J. A. et al. Preeclampsia and cognitive impairment later in life. Am. J. Obstet. Gynecol. 217(1), 74e1–74e11 (2017).
Mestre, H. et al. Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat. Commun. 9(1), 4878 (2018).
Aspelund, A. et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 212(7), 991–999 (2015).
Louveau, A. et al. Structural and functional features of central nervous system lymphatic vessels. Nature 523(7560), 337–341 (2015).
Mathieu, E. et al. Evidence for cerebrospinal fluid entry into the optic nerve via a glymphatic pathway. Invest. Ophthalmol. Vis. Sci. 58(11), 4784–4791 (2017).
Hablitz, L. M. et al. Increased glymphatic influx is correlated with high EEG delta power and low heart rate in mice under anesthesia. Sci. Adv. 5(2), eaav5447 (2019).
Harrison, I. F. et al. Impaired glymphatic function and clearance of Tau in an Alzheimer’s disease model. Brain 143(8), 2576–2593 (2020).
Klostranec, J. M. et al. Current concepts in intracranial interstitial fluid transport and the glymphatic system: Part I-anatomy and physiology. Radiology 301(3), 502–514 (2021).
Iliff, J. J. et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid Β. Sci. Transl Med. 4(147), 147ra111 (2012).
Benveniste, H., Heerdt, P. M., Fontes, M., Rothman, D. L. & Volkow, N. D. Glymphatic system function in relation to anesthesia and sleep states. Anesth. Anal. 128(4), 747–758 (2019).
Kyrtsos, C. R. & Baras, J. S. Modeling the role of the glymphatic pathway and cerebral blood vessel properties in Alzheimer’s disease pathogenesis. PLoS One. 10(10), e0139574 (2015).
Mortensen, K. N. et al. Impaired glymphatic transport in spontaneously hypertensive rats. J. Neurosci. 39(32), 6365–6377 (2019).
Louis, W. J. & Howes, L. G. Genealogy of the spontaneously hypertensive rat and Wistar-Kyoto rat strains: implications for studies of inherited hypertension. J. Cardiovasc. Pharmacol. 16(Suppl 7), S1–5 (1990).
OKAMOTO, K. & AOKI, K. Development of a strain of spontaneously hypertensive rats. Jpn Circ. J. 27, 282–293 (1963).
Adams, M. A., Bobik, A. & Korner, P. I. Differential development of vascular and cardiac hypertrophy in genetic hypertension. Relation to sympathetic function. Hypertension 14(2), 191–202 (1989).
Ramos, M. et al. Cisterna magna injection in rats to study glymphatic function. Methods Mol. Biol. 1938, 97–104 (2019).
Taoka, T. & Naganawa, S. Glymphatic imaging using MRI. J. Magn. Reson. Imaging 51(1), 11–24 (2020).
Xavier, A. et al. Cannula implantation into the cisterna magna of rodents. J. Vis. Exp. 135, 57378 (2018).
Ben-Nejma, I. et al. Altered dynamics of glymphatic flow in a mature-onset tet-off APP mouse model of amyloidosis. Alzheimers Res. Ther. 15, 23 (2023).
Bai, Y. et al. Hypothermia reduces glymphatic transportation in traumatic edematous brain assessed by intrathecal dynamic contrast-enhanced MRI. Front. Neurol. 13, 957055 (2022).
Ben-Nejma, I. et al. Altered dynamics of glymphatic flow in a mature-onset Tet-off APP mouse model of amyloidosis. Alzheimers Res. Ther. 15(1), 23 (2023).
Chen, W. et al. Cocaine-induced structural and functional impairments of the glymphatic pathway in mice. Brain Behav. Immun. 88, 97–104 (2020).
Liu, K. et al. Attenuation of cerebral edema facilitates recovery of glymphatic system function after status epilepticus. JCI Insight. 6(17), e151835 (2021).
Iliff, J. J. et al. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J. Clin. Invest. 123(3), 1299–1309 (2013).
Hsu, C. Y. et al. Automatic recognition of subject-specific cerebrovascular trees. Magn. Reson. Med. 77(1), 398–410 (2017).
Lindesay, G., Ragonnet, C., Chimenti, S., Villeneuve, N. & Vayssettes-Courchay, C. Age and hypertension strongly induce aortic stiffening in rats at basal and matched blood pressure levels. Physiol. Rep. 4(10), e12805 (2016).
Hadaczek, P. et al. The perivascular pump driven by arterial pulsation is a powerful mechanism for the distribution of therapeutic molecules within the brain. Mol. Ther. 14(1), 69–78 (2006).
Iliff, J. J. et al. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J. Neurosci. 33(46), 18190–18199 (2013).
Kedarasetti, R. T., Drew, P. J. & Costanzo, F. Arterial pulsations drive oscillatory flow of CSF but not directional pumping. Sci. Rep. 10(1), 10102 (2020).
Kiviniemi, V. et al. Ultra-fast magnetic resonance encephalography of physiological brain activity—glymphatic pulsation mechanisms. J. Cereb. Blood Flow. Metab. 36(6), 1033–1045 (2016).
Gao, B., Lyu, C., Lerner, A. & McKinney, A. M. Controversy of posterior reversible encephalopathy syndrome: What have we learnt in the last 20 years. J. Neurol. Neurosurg. Psychiatry 89(1), 14–20 (2018).
Wang, Q. et al. Blood-Brain barrier disruption as a potential target for therapy in posterior reversible encephalopathy syndrome: Evidence from multimodal MRI in rats. Front. Neurol. 10, 1211 (2019).
Bedussi, B. et al. Enhanced interstitial fluid drainage in the hippocampus of spontaneously hypertensive rats. Sci. Rep. 7(1), 744 (2017).
Pedersen, T. J., Keil, S. A., Han, W., Wang, M. X. & Iliff, J. J. The effect of aquaporin-4 mis-localization on Aβ deposition in mice. Neurobiol. Dis. 181, 106100 (2023).
Simon, M. et al. Loss of perivascular aquaporin-4 localization impairs glymphatic exchange and promotes amyloid Β plaque formation in mice. Alzheimers Res. Ther. 14(1), 59 (2022).
Zeppenfeld, D. M. et al. Association of perivascular localization of aquaporin-4 with cognition and alzheimer disease in aging brains. JAMA Neurol. 74(1), 91–99 (2017).
Mestre, H. et al. Aquaporin-4-dependent glymphatic solute transport in the rodent brain. Elife 7, e40070 (2018).
Haj-Yasein, N. N. et al. Glial-conditional deletion of aquaporin-4 (Aqp4) reduces blood-brain water uptake and confers barrier function on perivascular astrocyte endfeet. Proc. Natl. Acad. Sci. U S A 108(43), 17815–17820 (2011).
Verkman, A. S., Tradtrantip, L., Smith, A. J. & Yao, X. Aquaporin water channels and hydrocephalus. Pediatr. Neurosurg. 52(6), 409–416 (2017).
Tomassoni, D., Bramanti, V. & Amenta, F. Expression of aquaporins 1 and 4 in the brain of spontaneously hypertensive rats. Brain Res. 1325, 155–163 (2010).
González-Marrero, I. et al. Altered expression of AQP1 and AQP4 in brain barriers and cerebrospinal fluid may affect cerebral water balance during chronic hypertension. Int. J. Mol. Sci. 23, 12277 (2022).
Mortensen, K. N. et al. Impaired glymphatic transport in spontaneously hypertensive rats. J. Neurosci. 39, 6365–6377 (2019).
Wang, Y., Huang, C., Guo, Q. & Chu, H. Aquaporin-4 and cognitive disorders. Aging Dis. 13(1), 61–72 (2022).
Sabbatini, M., Strocchi, P., Vitaioli, L. & Amenta, F. The hippocampus in spontaneously hypertensive rats: a quantitative microanatomical study. Neuroscience 100(2), 251–258 (2000).
Sabbatini, M., Strocchi, P., Vitaioli, L. & Amenta, F. Changes of retinal neurons and glial fibrillary acid protein immunoreactive astrocytes in spontaneously hypertensive rats. J. Hypertens. 19(10), 1861–1869 (2001).
Sabbatini, M. et al. The hippocampus in spontaneously hypertensive rats: an animal model of vascular dementia. Mech. Ageing Dev. 123(5), 547–559 (2002).
Mignini, F., Vitaioli, L., Sabbatini, M., Tomassoni, D. & Amenta, F. The cerebral cortex of spontaneously hypertensive rats: a quantitative microanatomical study. Clin. Exp. Hypertens. 26(4), 287–303 (2004).
Wu, C. et al. Glutamate and γ-aminobutyric Acid Differentially Modulate Glymphatic Clearance of Amyloid β Through pulsation- and aquaporin-4 Dependent Mechanisms (Cold Spring Harbor Laboratory, 2020).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81871333, 82260340), Guizhou Province 7th Thousand Innovational and Enterprising Talents (GZQ202007086), the 2020 Innovation Group Project of Guizhou Province Educational Commission (KY[2021]017), the Guizhou Province Science & Technology Project ([2020]4Y159 and [2021]430), Guizhou Province Science & Technology Innovation Talent Team (CXTD[2022]006), and the Discipline Leading Talent of The Affiliated Hospital of Guizhou Medical University (gyfyxkrc-2023-04). Additionally, We acknowledge Kai Ai for his help in data handling and graphic assistance.
Author information
Authors and Affiliations
Contributions
Z.C., B.G.. Conceived the study design and oversaw all animal experiments. Y.X., C.L.. Collected experimental data and edited the manuscript. P.C., Y.J. Conducted statistical analyses, interpreting results to draw scientific conclusions. C.Q., X.L. Reviewed the manuscript for clarity and coherence, ensuring all sections adhered to journal guidelines.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xia, Y., Lyu, C., Chen, P. et al. The glymphatic system was impaired in spontaneously hypertensive rats. Sci Rep 15, 18321 (2025). https://doi.org/10.1038/s41598-025-02054-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-02054-3