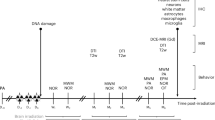
Abstract
Throughout their lives, individuals are exposed to various pollutants, potentially including co-exposure to radiological and chemical stressors. Yet, existing literature about these combinations is scarce. We selected tungsten and ionizing radiations. Tungsten is an emerging contaminant present as aerosolized particles in several scenarios, potentially concurrently with low-dose irradiation, causing a co-exposure. The cerebral toxicity of this co-exposure was studied after 24 h and 28 days in the frontal cortex and olfactory bulb of male Sprague–Dawley rats exposed to gamma irradiation (50 mGy) and/or inhalation of tungsten particles aerosol (80 mg.m−3). Co-exposure triggered significant effects more frequently than single stressors. Observed effects were associated with oxidative status changes, notably via NRF2 nuclear translocation, and modulation of pro-inflammatory cytokines (IL1β, TNFα). A reduction in cortical microglial density suggested a cellular migration toward the olfactory bulb and could contribute to the occurrence of a neuronal suffering phenotype. The effects persisted at 28 days and were brain structure specific. Biodistribution of tungsten showed that both local and systemic effects might be involved. Our results suggest interaction between our stressors, causing cerebral toxicity, and prove the importance of multi-stressor studies to improve risks evaluation in toxicology and radiation protection, as single stressors might wrongly be deemed safe.
Similar content being viewed by others
Introduction
Multiple factors, such as environment, life habits, and professional activity, define the nature of the stressors we are exposed to as well as their level of exposure. In the case of multiple exposure to these stressors, interactions between them can occur, and these are referred to as combined biological effects. Defining every stressor, and the level of exposure, to which an individual is exposed to, allows to characterize their exposome and assess the toxicity they might experience1,2. However, evaluating the toxicity using this approach is challenging due to knowledge gaps with respect to interactions of various stressors, whereas relevant studies suffer from complex experimental designs. This issue might, one day, be resolved using robust in silico methods which are currently being developed and which will be capable of modelling the exposome using data generated by experimental studies examining multiple stressors.
In the meantime, creating realistic experimental designs using co-exposure patterns is an innovative and efficient way to study combined effects of two or more selected stressors of interest. In this study, we proposed and used such an approach focusing on the occupational exposome of nuclear industry workers and their emerging exposures in relation to nuclear fusion reactors. We chose two relevant stressors they will encounter, the combined effects of which have not been previously studied to our knowledge: tungsten particle inhalation3,4 and low dose gamma radiation5,6.
Tungsten (W) has long been considered biologically inert, but recent studies have shown its dissolution in biological media, thus unravelling a potential for bioaccumulation, and biological effects such as oxidative stress7. Additionally, W has proved to be more environmentally mobile than previously thought, with elevated concentrations measured in soil, plants, and water around mining sites8 or military bases9. W is therefore considered an emerging contaminant, which has attracted the attention of various national public and environmental health agencies in recent years4,10. A recent paper by Wang et al.11 presented current knowledge on the source terms for tungsten particles, which could be activated in fusion reactors, as well as their behavior in the environment. At the cerebral level in rats, sodium tungstate was shown to increase reactive oxygen species (ROS) levels12, metallic W increased microglial apoptosis in the olfactory bulb and produced a phenotype of suffering cortical neurons3, and tungsten carbide was found to reduce the opening of voltage-gated potassium channels13. Various studies such as those by Li et al.14 and Naqvi et al.15 have demonstrated that W toxicity increases in the presence of other contaminants, such as uranium, barium, and cobalt, raising questions about its interaction and/or synergies with other stressors.
Every individual is exposed to ionizing radiation in everyday life, whether from natural or anthropogenic sources. Except in the event of an accident or during medical procedures, the dose of radiation received by the general population or even by nuclear workers is generally in the low dose range, which is defined by the UNSCEAR16 to be below 100 mGy. Even at such doses, a linear no-threshold model is still used and advocated in the radioprotection field, implying that cancer risks are proportional to dose, even at extremely low doses, probably through non-mutational events and processes17. Experimentally, irradiation has been linked to disruption of cellular redox balance by radiolysis of water, resulting in increased in ROS18 and DNA double-strand breaks19. Furthermore, Kempf et al.20 observed an increase in cerebral cell death and inflammation markers, and Mineyeva et al.21 found an interruption in neurogenesis following irradiation.
The number of experimental studies involving the combination of a chemical and radiological exposure is limited but do exist even if the available literature on this subject is still extremely scarce. Co-exposure paradigms such as: low-dose gamma ray and paraquat in adult mice22 or during neonatal development23, low-dose radiation and ketamine in neonatal mice24, and gamma rays and methyl mercury in neonatal mice25 have been investigated.
The ubiquity of low-dose radiation exposures in the population, combined with W being recognized as an emerging contaminant, means that the likelihood of co-exposure might be higher than previously anticipated, even among the general population. It is therefore of significant interest to understand the effects of this co-exposure and the mechanisms involved, as this will add valuable insight to the relatively sparse literature on chemical and radiological co-exposure.
As the two selected stressors have been associated with oxidative stress, cell death and inflammatory responses in the brain, our study focused on these biological processes. We report here the results of histological and molecular analyses on various brain structures in male Sprague–Dawley rats exposed to W particle inhalation and whole-body low-dose gamma-radiation, carried out at early (24 h) and late (28 days) time points. We extend our data with results on the kinetics of W excretion in various brain structures and organs with or without irradiation to assess the impact of co-exposure on this parameter.
Methods
This study is reported in accordance with ARRIVE guidelines.
This “Methods” section has been condensed, but a detailed version is available in the Supplementary Material.
Animals
Adult twelve-week-old male Sprague–Dawley rats (weighing 350 g ± 15 g) were used (Janvier Labs, France). Males were chosen for this study as they are more representative of the population exposed to our two stressors, such as nuclear industry workers who constitute 87% of the INWORKS epidemiological cohort26. We used 20 animals per group and per time point in total for different kinds of experiments (5 for histology, 10 for biomolecular assays, 5 for analytical chemistry). These numbers were decided upon use of statistical tools to optimise the statistical power of our study and after careful review of existing literature and past projects results. Animal experiments took place in the specific pathogen free ASNR animal facility (PARADIS Platform) in controlled humidity (55% ± 10%) and temperature (22 °C ± 2 °C) with a 12-h day/night cycle. For identification rats were randomly assigned a number written at the base of their tail. They were housed in individually ventilated cages (Double Decker Cages, Tecniplast, Italy) in groups of two or three animals with ad libitum access to food (Rod16R, LASVendi) and autoclaved tap water. All cages were enriched with an internal platform, a red plastic tunnel and a wooden stick. The animals were randomly assigned to four conditions: control, tungsten, irradiated, and co-exposed; and three post-exposure time points: 4 h, 24 h or 28 days. All animal work within this study was authorized by the French Ministry of Superior Education and Research under the animal protocol number APAFIS#33361-2021100608276923 v1, after ethical evaluation performed by the registered ASNR Ethic Committee on Animal Experimentation (C2EA-ASNR N°81). All experiments were conducted in compliance with European and French regulations on animal use for scientific purposes (EC Directive 2010/63/EU and French Decret 2013-118).
Tungsten particles inhalation and gamma radiation exposure
Upon arrival, the animals were allowed to acclimate to their new environment for one week, followed by two weeks of habituation to nose-only inhalation contention tubes (Ysebaert) for increasing durations, up to 60 min.
Upon completion of acclimatation and habituation, all rats were placed in nose-only inhalation contention tubes. Tungsten-only and co-exposed animals were transferred to a glovebox, connected to the inhalation chamber for 30 min and exposed to 80 mg m−3 of tungsten metal (W) particles (A-20, A.L.M.T. Corp.) as fully described in Macé et al., 20243. This concentration was based on the French Occupational Exposure Limit (OEL) for alveolar dust concentration (i.e. particles with diameters mainly below 4 µm) in an inside workspace (5 mg m−3 until 2022) for an 8-h exposure during a workday. After exposure, the rats had their nose wiped to minimize ingestion from grooming and were returned to their cages. Control and irradiated-only rats were sham exposed by being placed in nose-only inhalation tubes and transferred to a clean glovebox. The metrology of the W aerosol generation and design of the inhalation set-up were detailed by Macé et al.28. Irradiation-only and co-exposed rats were irradiated in a GSR D1 irradiator (Gamma Service Medical GmbH) using a Caesium-137 source, which radioactive decay was taken into account, to deliver a single dose of 50 mGy of gamma-rays at a dose-rate of 50 mGy min−1. The chosen dose represented a median value within the low-dose range16. Control and tungsten-only animals stayed in the irradiation boxes for the same duration. All animals were then returned to their cages.
Following these procedures, no animal showed any sign of suffering requiring special care or anticipated euthanasia, and all animals were included in this study.
Dosimetry
Prior to irradiating the animals, a dosimetry was performed in the exact conditions of the animal exposure. A cylindrical ionizing chamber (31010, PTW) was used following the recommendations of the American Association of Physicists in Medicine’s TG-6129. The ionizing chamber was calibrated in water with Caesium-137 in the Physikalisch-Technische Bundesanstalt.
Euthanasia, tissue collection and treatment
Two post-exposure time points were chosen for their recurrence in inhalation toxicological studies: 24 h (24 h) and 28 days (28d). A third time point was added for inductively coupled plasma mass spectrometry (ICP-MS) analysis at 4 h (4 h) post-exposure to measure early transfer of W and biodistribution among different target organs.
At the designated time point, the animals were injected subcutaneously with Buprenorphine (0.05 mg/kg s.c.) 30 min before being exposed to a mixture of 5% isoflurane (Aérane, Baxter) and 95% air to reach unconsciousness. The rest of the procedure differed depending on whether the animals were destined for histology or biochemical/molecular assay.
For histology, unconscious animals were injected intraperitoneally with a mix of ketamine (75 mg/kg) and xylazine (10 mg/kg) and removed from isoflurane. Once anaesthesia was confirmed by loss of pedal withdrawal reflex, the animals were perfused intracardially with 200 mL of a 4% paraformaldehyde (VWR Chemicals) solution over 10 min. Whole brains were collected and placed in a 30% sucrose solution at 4 °C overnight. Using a brain matrix (BioSeb), brains were then cut into 2 mm thick longitudinal slices. The brain slices were embedded in Cryomatrix (6769006, Epredia) in cassettes and frozen with a FlashFreeze (MicromMicrotech). Cassettes were stored at − 80 °C until use. Using a cryostat (NX50, Epredia), 14 µm thick sagittal sections were cut using a AccuEdge® 4690 blade (Sakura) and placed on SuperFrost Plus™ slides (Thermo Scientific™) which were stored at − 80 °C until use. To ensure consistency in neuroanatomical level across all slides, each slide used had to include at least a partial rostral migratory stream.
For biochemical or molecular biology assays, the rats were euthanized by terminal exsanguination through cardiac puncture using a heparinized needle (Héparine Choay®, CHEPLAFARM) while being maintained under isoflurane. Blood was collected, centrifugated at 3000g for 15 min for future plasma analysis and stored at − 80 °C. The brain was then extracted and dissected into substructures: olfactory bulb (OB), frontal cortex (FC), striatum (ST), hippocampus (HIP), cerebellum (CER) and brain stem (BrSt) as detailed by Macé et al. 20243. The ethmoid turbinates (ET) were also collected. Tissues were flash frozen in liquid nitrogen immediately after sampling and stored at − 80 °C until use.
Animals designated for ICP-MS quantification were euthanized similarly. The brain was extracted and dissected into the same substructures; the ET was also sampled. Additionally, the whole lung and trachea compartment, as well as the left kidney, were collected. All samples were weighted before being stored at -20 °C processed as described by Macé et al., 2024.
Immunochemistry
Slides were rehydrated and blocked for 1 h at room temperature using 5% donkey serum (S30-100ML, MERCK) diluted in 1X PBS prepared from tablets (18912-154, GIBCO) with 0.1% Triton (X100-100ML, Sigma-Aldrich). The blocking solution was then removed and 150 µL of primary antibodies at a 1:200 dilution in PBS-Triton 0.1% were added on the slides. Slides were left to incubate overnight in a humid chamber at 4 °C. The next day, the slides were washed 3 times for 10 min in PBS 1X under agitation. Then, 150µL of secondary antibody solution prepared identically to the primary antibody solution were added on the slides, which were then left to incubate in the dark in a humid chamber at room temperature for 2 h. Slides were then washed 3 times in PBS 1X for 10 min and mounted using 2 drops of Vectashield Mounting solution with DAPI (Vector) and a glass coverslip. Once dried, the slides were scanned using a Nanozoomer S60 (Hamamatsu) and quantified using Histolab 12.2 (Microvision Instruments).
The primary and secondary antibodies used are detailed in Table 1 in the Supplementary Material.
Microglial activation and density scoring
Given the very high microglial density in the OB, we developed a double-blind density scoring method with scores ranging from 0 to 5. Two persons trained on this scoring method independently compared each blind-coded slide to established standards and assigned scores.
Microglial activation was scored on a scale from 0 to 3 based on morphological criteria as described in the literature30,31. This scoring was performed both in the FC and in the OB. These scores were blindly and individually attributed by two persons trained in microglial activation stages identification.
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)
TUNEL labelling was combined with microglial marker staining (IBA1) conducted as described above with the following modification. After primary IBA1 and secondary antibody staining and prior to applying Vectashield, the TUNEL labelling was carried out using the Clickit™ Plus TUNEL assay kit (C10618, Invitrogen). Slides were then mounted and scanned as described previously using Vectashield and a Nanozoomer S60.
RNA extraction and RT-qPCR
We used the RNeasy® Lipid Tissue Mini Kit (74408, QIAGEN) for RNA extraction and scrupulously followed the manufacturer’s protocol. 30 mg of frozen OB or FC were homogenized in lysing matrix D tubes (MP biomedicals) a liquid nitrogen cooled Precellys® 24 (Ozyme®) with a single 20 s cycle at 5500 rpm. The RNA concentrations were measured using a NanoDrop One (ThermoFisher) and RNA quality was verified using an RNA 6000 Nano Kit, a 2100 Bioanalyzer and its 2100 Expert software (all manufactured by Agilent).
A sample volume containing 1 µg of RNA was transferred into each tube which contained 2 µL of reverse transcriptase buffer, 2 µL of primers at 1 nmol µL−1, 0.8 µL of dNTP and 1 µM of multiscribe RT (High-Capacity cDNA Reverse Transcription Kit, Life Technologies). The volume was then adjusted with RNAse-free water to reach 20 µL in total. Reverse transcription was performed in a thermocycler (SimpliAmp, Applied Biosystem) following the kit’s temperature cycle recommendations. The cDNA produced were then stored at − 20 °C until use.
MicroAmp® Optical 384-well plates (Applied biosystems) were filled with 4 µL of 1/50th diluted cDNA and 6 µL of a SYBR green (Applied Biosystem), RNAse free water and primers (see Table 2 in the supplementary material for details) mixture by an EpMotion 5073 (Eppendorf). The qPCR was then carried out on a QuantStudio 12 K Flex Real-Time PCR System (ThermoFisher). . Two housekeeping genes were systematically used: GAPDH and β-actin. Results were analysed using the double delta Ct method.
Protein extraction and Western Blot
50 mg of frozen OB and FC were placed in lysing matrix D tubes (MP biomedicals) containing 600 µL of RIPA lysis buffer (Thermo Fisher Scientific®) with protease inhibitors (cOmplete Tablets Easypack, Roche). Tissues were homogenized in Precellys® (Ozyme®) with a single 20 s cycle at 5500 rpm. A 150 µL aliquot was taken and labelled “total lysate”. The remaining content underwent differential centrifugation at 10 000 g for 15 min. The supernatantwas transferred to a tube labelled “supernatant”. The pellet was resuspended in 100 µL of RIPA with protease inhibitors and labelled “pellet”. All samples were then stored at -80 °C.
Protein concentration was assessed using a Pierce™ Rapid Gold BCA Protein Assay Kit (Thermo Fisher Scientific®, USA). At 480 nm on an Infinite M200 pro (TECAN®).
Samples were prepared for electrophoresis by adjusting the protein concentration with MilliQ® water to obtain 20 µg of proteins in a volume of 14 µL to which 5 µL of Laemmli 4× buffer (Bio-Rad®) and 1 µL of Reducing Agent 20× (Bio-Rad®) were added. Samples were denaturated at 37 °C for 30 min, loaded in 10-well 10% acrylamide gels (Mini-Protean® TGX™, Bio-Rad®) and migrated at 70 V for 10 min followed by 120 V for 60 min approximately in a water and TGS 10× buffer (Bio-Rad®). Proteins were then transferred onto a membrane (Trans Blot Turbo™ Mini-PVDF Transfer Packs, Bio-Rad®, France) using a Trans-Blot Turbo (BioRad®). The membranes were blocked for at least 1 h in a 5% milk (whole milk powder, Régilait) solution made in PBS 1X and 0.1% of Tween®20 (Sigman-Aldrich). The blocking solution was removed and a 2.5% milk in PBS-Tween 0.1% containing the primary antibody was added and left to incubate overnight at 4 °C under agitation. The membranes were then washed in PBS-Tween 0.1%. The secondary HRP conjugated antibody, prepared in 2.5% milk in PBS-Tween 0.1% was added and allowed to incubate for 1.5 h. The detail of primary and secondary antibodies with their dilutions are available in Table 3 in the supplementary material. After washing the membranes in PBS-Tween 0.1%, the protein bands were revealed using chemiluminescence by following the manufacturers protocol (Supersignal West Pico Plus chemiluminescence substrat PIER34578, Thermo Fischer Scientific) and an ImageQuant™800 (Cytiva) imager. Acquired images were quantified using the ImageQuant™ software (ImageQuant TL 10.0.261, Cytiva). GAPDH (total lysate and supernatant) or β-actin (pellet) were used for normalization.
Statistical analysis
The statistical analyses were performed on Prism 8.4.2. (GraphPad). Normality was assessed using Shapiro–Wilk test, as recommended for datasets with n < 50. Our data were analysed either by a One-way ANOVA or a Kruskal–Wallis test, depending on normality. All tests were two-tailed with an ɑ significance level set at 0.05 for all tests. All the data included in the figures is presented ± Standard Error of the Mean (SEM).
Results
Tungsten levels remain elevated in the FC, lung, kidney, ET and plasma after 28 days
W concentration was measured in the brain structures (OB, FC, ST, HIP, CER, BrSt) (Fig. 1A), the ethmoid turbinate (ET) (Fig. 1B), the kidney (Fig. 1C) and the lung and trachea compartment (Fig. 1D) using ICP-MS. Tissues from irradiated-only animals were not used in these measurements as they were not exposed to W. We3,34 and others35 have previously observed rapid transportation of particulate materials along the nose-to-brain pathway. Therefore, to capture these rapid processes, we included an additional 4-h time point post-exposure, resulting in 3 treatment groups (control, tungsten-only, co-exposed) and 3 time points (4 h, 24 h, 28 days).
Dosage of tungsten in brain structures, ethmoid turbinates, lung and kidney. (A) to (E) show tungsten concentrations measured in different samples in control (unexposed), tungsten-only and co-exposed (tungsten + irradiation) groups at 4 h (4h), 24 h (24h) and 28 days (28d). The scale used are linear for (A) and (E), but in log10 for (B–D). This scaling allows for better visualization of differences between groups. (A) Tungsten concentrations in brain structures (olfactory bulb [OB], frontal cortex [FC], striatum [ST], hippocampus [HIP], cerebellum [CER] and brainstem [BrSt]) represented in ng of tungsten per g of tissue ± SEM. For readability, statistical differences were represented with letters for p-values thresholds when comparing each exposed group to the control group. n = 5–6 per group. Statistical differences are indicated by letters (a, b, c, d) corresponding to p-value thresholds (p-values: 0.05 > d > 0.01 > c > 0.001 > b > 0.0001 > a). The letter “e” was used to describe a statistical difference between the tungsten-only and co-exposed groups (p-value < 0.5). (B) Tungsten concentration in the ethmoid turbinates (ET) represented in log10 of ng of tungsten per g of tissue ± SEM. n = 5 per group. (C) Tungsten concentration in the kidney represented in log10 of ng of tungsten per g of tissue ± SEM. n = 5 per group. (D) Tungsten concentration in the lung represented in log10 of ng of tungsten per g of tissue ± SEM. n = 5 per group. (E) Tungsten concentrations in the plasma at 24h and 28d in ng of tungsten per L of plasma ± SEM. n = 4–5 per group. Plasma samples were not collected at the 4h time point. Tungsten concentration values for each tissue and time point are available in Supplementary table 1. CER, ET and plasma results did not pass the normality test and were analyzed using a Kruskal–Wallis test. The rest of the results were analyzed using a One-way ANOVA. Within each time point and structure, all groups were compared to each other. Significant differences between groups are indicated by asterisks (*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001).
Brain
In the brain at 4 h, in the OB, FC, ST, HIP, CER and BrSt, both tungsten-only and co-exposed groups were significantly elevated when compared to controls. At 24 h, results were more heterogeneous. In the BrSt, CER, HIP, and ST both the tungsten-only and co-exposed groups showed a significant increase in W concentration compared to the control group. In FC and OB, however, we found elevated W concentrations in the tungsten-only (p = 0.0004 in FC and p = 0.0051 in OB), but not in the co-exposed group. Additionally, the tungsten-only group at 24 h in OB contained significantly more W than the co-exposed group (p = 0.0229), suggesting that gamma-irradiation may affect W particle transportation and retention in this brain structure. At 28 days, none of the brain structures from either exposed group contained increased W concentrations compared to control, except for the FC, for both the tungsten-only (p = 0.0498) and the co-exposed groups (p = 0.0097).
Ethmoid turbinates
In the ET, at 4 h, 24 h, and 28 days, both exposed groups contained significantly more W than the controls and no statistically significant difference was observed between the tungsten-only and co-exposed groups.
Kidney
In the kidney, significantly increased concentrations of W were observed in both groups at 4 h, 24 h and 28 days. No significant difference was observed between the tungsten-only and co-exposed groups.
Lung compartment
In the lung, both tungsten-only and co-exposed groups showed significantly increased W concentrations when compared to control at 4 h, 24 h and 28 days. Interestingly, at 4 h and 24 h, but not 28 days, the W levels in the tissues of the co-exposed animals were lower than those in the tungsten-only treated group (p = 0.0196 and 0.0023 respectively).
Plasma
Plasma samples were collected from animals used for biomolecular assay and, therefore, did not include a 4 h time point (Fig. 1E). We observed elevated W concentrations in the tungsten-only (p = 0.0500) and co-exposed (p = 0.0019) groups compared to control at 24 h. At 28 days concentrations remained significantly elevated compared to control in both tungsten-only (p = 0.0037) and co-exposed (p = 0.0162) groups.
Early decrease and late increase in a suffering neuron phenotype in FC
A neuron suffering phenotype called “donut-like neurons” has been previously described in the literature and associated with oxidative stress, hypoxia and neuronal suffering in general3,36. A donut-like phenotype consists in the observation of what appears to be a nuclear pore or a lack of Dapi staining in the nucleus of the neuron as illustrated in Fig. 2B along with 2 healthy neurons exhibiting a homogeneous Dapi stained nuclei. The quantification of this phenotype in the FC (Fig. 2A) showed a statistically significant reduction in their density at 24 h post-exposure in the co-exposed group when compared to control (p = 0.0004), tungsten-only (p = 0.0139) and irradiated-only (p = 0.422) groups. After 28 days, a significant increase was observed when comparing the co-exposed group to the control group (p = 0.0120). No statistically significant differences were observed between the single stressor groups versus control at both time points. Neuronal marker (NeuN) staining confirmed that all the cells with this phenotype in our samples were neurons. We examined whether cell death was associated with this phenotype by co-staining with cleaved Caspase 3 (Fig. 2C) or TUNEL (Fig. 2D). None of the apoptotic markers showed co-labelling of donut-like cells suggesting a lack of association. Thus, our results indicate a significantly amplified effect of low-dose gamma-irradiation when combined with W particle inhalation, at 24 h in the FC, regarding the toxicity-associated neuronal phenotype. The reduction observed raises additional questions regarding the faith of these neurons, as cell death does not seem to be involved at this time point. After 28 days, single stressors do not cause any effect on their own but co-exposure triggers a significant increase compared to control, indicating once again amplified effects of co-exposure compared to single stressors, although the mechanisms involved seem different.
Quantification and illustration of a neuron suffering phenotype in the frontal cortex, not linked to apoptosis or double-strand breaks. (A) Quantification of donut-like neurons in the apex of the FC at 24h and 28 days in number of marked cells per mm2 ± SEM. n = 5 per group. (B) Illustration of donut-like neurons in the apex of the FC with a close-up on two healthy neurons and a truncated one, cells were stained for a neuronal marker (NeuN, red) and DAPI (blue). (C) Illustration that donut-like neurons are not positive for cleaved Caspase 3, cells were stained for a neuronal marker (NeuN, green), cleaved Caspase 3 (red), and DAPI (blue). (D) Illustration that donut-like neurons (white arrows) are not stained by TUNEL marking (red arrows). The results did not pass normality test and were analyzed with a Kruskal–Wallis test. Within each time point, all groups were compared to each other. Significant differences between groups are indicated by asterisks (*p < 0.05; **p < 0.01; ***p < 0.001).
Increased inflammatory response at 24 h and modulated antioxidant and hypoxic response at 28 days following co-exposure in FC
Given that donut-like phenotypes have been associated with oxidative stress, hypoxia and cellular stress in general36, we investigated if these biological processes, as well as inflammation, could be contributing to our observations. We first measured the expression of the Sod1, Sod2, Catalase, IL-1β and Mcp1 genes in the FC, as these genes are involved in the regulation of the redox balance and proinflammatory response. No significant changes were observed 24 h after exposure in any of the groups for any of the 3 antioxidant genes (Fig. 3A). But in the co-exposed groups, a significant increase in IL-1β compared to control (p = 0.0069) was observed, as well as a similar trend for Mcp1, suggesting an inflammatory effect of co-exposure. At 28 days, a significant increase of Sod1 (p = 0.0013 and p = 0.0094), Sod2 (p = 0.0040 and p = 0.0005), and Catalase (p = 0.0125 and p = 0.0048) gene expression was observed in the co-exposed group compared to the control and tungsten-only groups (Fig. 3B).
Antioxidant, inflammation and hypoxia linked genes and proteins expression at 24h and 28 days in the FC. (A) Relative gene expression of Sod1, Sod2, Catalase, IL-1β and Mcp1 in the FC at 24h post-exposure ± SEM. Arbitrary units (a.u.). n = 7–8 per group. (B) Relative gene expression of Sod1, Sod2, Catalase, IL-1β and Mcp1 in the FC at 28 days post-exposure ± SEM. Arbitrary units (a.u.). n = 7–8 per group. (C) FC 24h post-exposure protein expression of NRF2 and HIF1ɑ, and pellet/supernatant ratio for NRF2 to assess nuclear translocation of NRF2 ± SEM. Arbitrary units (a.u.). n = 5–6 per group. (D) FC 28 days post-exposure protein expression of NRF2 and HIF1ɑ, and pellet/supernatant ratio for NRF2 to assess nuclear translocation of NRF2 ± SEM. Arbitrary units (a.u.). n = 5–6 per group. (E, F, G) Representative Western blot membranes used to quantify the protein expression of NRF2 and normalize with GAPDH (whole cell lysate or cytosolic fraction) or β-actin (nuclear fraction). (H) Representative Western Blot membrane for HIF1ɑ protein expression quantification. Western blot membranes images were cropped (Figure 3E to H) and original blots are presented in Supplementary Figure 1 (Sup. Figure 1E to H). Western blots were done in duplicates. All results passed normality test and were analyzed using a One-way ANOVA, except IL-1β which did not pass normality tests and was analyzed using a Kruskal–Wallis test. All groups were compared to each other within each time point. Significant differences between groups are indicated by asterisks (*p < 0.05; **p < 0.01).
Protein expression of HIF1ɑ and NRF2 was assessed using Western Blot. Since NRF2 is a transcription factor, it is inactive in the cytosol and needs to be translocated to the nucleus to exert its function. We therefore measured both its nuclear translocation, by dividing the quantified nuclear fraction of NRF2 by the cytosolic fraction, and its total expression. At 24 h (Fig. 3C), total NRF2 expression decreased significantly in the irradiated-only (p = 0.0202) and co-exposed (p = 0.0192) groups compared to control. HIF1ɑ protein expression and the nuclear translocation of NRF2 remained unchanged across all groups at 24 h. At 28 days (Fig. 3D), total NRF2 protein expression was unchanged across all groups, but its nuclear translocation was significantly reduced in all treated groups compared to control (p = 0.0026 for W-only, p = 0.0102 for irradiation-only, p = 0.0034 for the co-exposed group). Protein expression of HIF1ɑ increased significantly in the co-exposed group compared to the tungsten-only (p = 0.0416) and irradiation-only (p = 0.0045) groups, and showed an increasing trend compared to the control group. Representative Western blot images for the quantification of total, cytosolic and nuclear NRF2 and total HIF1ɑ are shown in Fig. 3E–H respectively (Fig. 3E–H). Altogether, we observed a trend towards potentiation of the effects of co-exposure to W particles and low-dose gamma-radiation on the expression of genes involved in the redox balance at 28 days post-treatment in the FC. Concomitantly, an increase in HIF1ɑ protein levels was observed in the co-exposed group.
Microglial density fluctuates in FC at 24 h and 28 days, and increases in the OB at 28 days
Neuroinflammation involving microglial cells is one of the main and most rapid responses triggered in the brain following exposure to stress37. We investigated the microglial response in OB and FC at two different levels: activation phenotypes and microglial cell density by immunostaining using a microglial marker (IBA1).
Microglial activation was scored according to the method published by Taetzsch et al.30 and described in Methods section and remained unchanged at both time points in the FC (data not shown). The microglial density (Fig. 4A) significantly increased at 24 h in the co-exposed group compared to the control (p = 0.0179) and irradiated-only (p = 0.0438) groups. At 28 days, the FC microglial density decreased significantly in the co-exposed group compared to tungsten-only (p = 0.0092) and had borderline significance compared to the controls (p = 0.0533). This poses a question whether the increased microglial density is due to migration of cells, their infiltration or proliferation of existing cells.
Microglial response in the FC and the OB at 24H and 28D. (A) Microglial density quantification in the FC at 24h and 28 days post-exposure in number of marked cells per mm2 ± SEM. n = 4–5 per group. (B) Illustration of microglia stained with a microglial marker (IBA1, green) and DAPI (blue) in the apex of the FC. (C) Illustration of microglia stained with a microglial marker with (IBA1, green) and DAPI (blue) in the granular layer of OB. (D) Microglial density scoring in the OB at 24h and 28 days post-exposure ± SEM. Arbitrary units (a.u.). n = 5 per group. (E) Microglial activation scoring in the OB 24h and 28 days post-exposure ± SEM. Arbitrary units (a.u.). n = 5 per group. Results passed normality tests for figures A and D and were analyzed using One-way ANOVA. Results for figure E did not pass the normality test and were analyzed using a Kruskal–Wallis test. All groups were compared to each other within each time point. Significant differences between groups are indicated by asterisks (*p < 0.05; **p < 0.01).
A different quantification approach was chosen for the OB microglial density due to its much higher basal density compared to the FC, resulting in a highly intricate cellular web. Representative images of microglia showing relative cell densities in the FC and OB are presented in Fig. 4B, C respectively. We have therefore implemented a scoring technique, based on a qualitative double blind scoring method detailed in the Methods section. No statistically significant changes were observed for the co-exposed group 24 h after exposure, but the tungsten-only group was elevated compared to control (p = 0.0448). After 28 days, the OB microglial density increased significantly in the co-exposed group compared to the controls (p = 0.00082) and tungsten-only groups (p = 0.0301) (Fig. 4D). The irradiated-only group was also significantly elevated compared to control (p = 0.0159).
Microglial activation in the OB was assessed using the same method as in the FC (Fig. 4E). The results show a statistically significant increase in the tungsten-only group at 24 h compared to control (p = 0.0055) and co-exposed groups (p = 0.0108). At 28 days, the microglial activation was significantly increased in the co-exposed group compared to control (p = 0.0163) and tungsten-only (p = 0.0013) groups. These results suggest that the microglial cell density in both the FC and OB is more likely to be affected by the combination of the two stressors rather than individual exposures, with the effects varying depending on the time elapsed after exposure. The same applies to microglial activation in the OB at 28 days, but not 24 h, indicating that the interaction between these stressors produces a nuanced and time-dependent impact on microglial responses in the brain.
Gene and protein expression of antioxidant and inflammatory regulators decreases in OB at 28 days
The expression of pro-inflammatory gene markers, Tnfa and Mcp1, was assessed, along with the gene expression of Sod2 and Catalase and the protein expression of NRF2 and its translocation to the nucleus in the OB.
Regarding antioxidant response at 24 h, a decrease in the irradiated-only group compared to tungsten-only (p = 0.0293) and control (p = 0.0282) was observed for Sod2 at 24 h (Fig. 5A) and while the total expression of NRF2 remained stable, an increase of its nuclear translocation, measured as the pellet-supernatant ratio, was observed in the co-exposed group compared to control (p = 0.0405) (Fig. 5C). After 28 days, all exposed groups showed a significantly reduced gene expression compared to control for Sod2 (p = 0.0063 for tungsten-only, 0.0019 for irradiated-only and 0.0038 for co-exposed) and Catalase (p = 0.0313 for tungsten-only, 0.0210 for irradiated-only and 0.0135 for co-exposed) (Fig. 5B) as well as a reduced nuclear translocation of NRF2 (p = 0.0026 for tungsten-only, 0.0102 for irradiated-only and 0.0034 for co-exposed), while its total expression remained stable (Fig. 5D). While significant, the reduction in gene and protein expression were low, suggesting a completion of the initial response to oxidative stress by 28 days which is reflected in the pattern of induction of the genes and translocation of NRF2 to the nucleus and subsequent reduction in both.
Inflammation and antioxidant response linked gene and protein expression in OB at 24h and 28 days post-exposure. (A) Gene expression levels of Sod2, Catalase, Tnfα and Mcp1 in the OB at 24h post-exposure ± SEM. Arbitrary units (a.u.). n = 7–8 per group. (B) Gene expression levels of Sod2, Catalase, Tnfα and Mcp1 in the OB at 28 days post-exposure ± SEM. Arbitrary units (a.u.). n = 7–8 per group. (C) OB 24h post-exposure protein expression levels of NRF2 and pellet/supernatant ratio of NRF2 to assess nuclear translocation ± SEM. Arbitrary units (a.u.). n = 5–6 per group. (D) OB 28 days post-exposure protein expression levels of NRF2 and pellet/supernatant ratio of NRF2 to assess nuclear translocation ± SEM. Arbitrary units (a.u.). n = 6 per group. (E, F, G) Representative Western blot membranes used to quantify the protein expression of NRF2 and normalize with GAPDH (whole cell lysate or cytosolic fraction) or β-actin (nuclear fraction). Western blot membranes images were cropped (E to G) and original blots are presented in Supplementary Figure 2 (Sup. Figure 2E to G). Western blots were done in duplicates. All results passed normality tests were analyzed using a One-way ANOVA. All groups were compared to each other within each time point. Significant differences between groups are indicated by asterisks (*p < 0.05; **p < 0.01).
At 24 h, the irradiated-only group showed a significantly decreased Mcp1 gene expression compared to tungsten-only (p = 0.0335) and control (p = 0.0499) while Tnfɑ expression remained unchanged across the groups (Fig. 5A). After 28 days, Mcp1 expression was back to control levels across all groups, suggesting normalization of Mcp1 gene expression with time after irradiation. But the co-exposed group showed a reduced Tnfɑ expression compared to control (p = 0.0322) (Fig. 5B), pointing to a lasting influence of the combined stressors, but not single exposures.
Representative Western blot images used for the quantification of total, cytosolic and nuclear NRF2 levels are displayed in Fig. 5E–G respectively (Fig. 5E–G). These findings, similar to those observed in the FC, suggest that the redox balance and inflammatory regulators respond differently to combined exposure to tungsten and low-dose radiation compared to individual exposures. However, the pattern of this response is complex, and the overall effects are subtle.
Lack of proliferation or peripheral macrophages infiltration at 24 h and 28 days
To address the lingering questions raised by the microglial density increase observed in Fig. 4D, we measured proliferative activity in the OB using the Ki67 marker and assessed the infiltration of peripheral macrophages using CD11b/c which also stains a sub-population of microglia.
The fraction of Ki67-positive cells in the OB was low (Fig. 6B) and showed little variation across experimental groups at both 24 h and 28 days (Fig. 6A). The only exception was for the irradiated-only group at 24 h, which had a significantly lower density of Ki67 + cells compared to the control group (p = 0.0254). Similar to microglia, the density of CD11b/c-positive cells was very high in the OB (Fig. 6D), and therefore, we used the same scoring methodology as for IBA1 staining to assess these cells’ density. No significant changes were observed at 24 h or 28 days across the different group (Fig. 6C), indicating that neither infiltration of CD11b/c-positive cells nor cell proliferation contribute to the observed alterations in the OB in response to the stressors and their combination, pointing to microglial migration as a probable mechanism.
Quantification of Ki67 and CD11b/c to investigate microglial density increase. (A) Quantification of proliferating cells in OB at 24h or 28 days post-exposure using Ki67 in number of marked cell per mm2 ± SEM. n = 4–5 per group. (B) Illustration of two proliferating cells at the border of the glomerular layer in OB stained with Ki67 (red) and DAPI (blue). (C) Density scoring of cells positive for CD11b/c in OB at 24h or 28 days post-exposure ± SEM. Arbitrary units (u.a.). n = 3–5 per group. (D) Illustration of CD11b/c (green) staining and DAPI (blue) in OB at the border of the glomerular layer. All results were analyzed using a One-way ANOVA. All groups were compared to each other within each time point. Significant differences between groups are indicated by asterisks (*p < 0.05).
Discussion
Our goal was to determine whether chemical and radiological exposures may interact when combined to produce a distinct toxic effect in the brain of exposed rats and to assess the effect over a medium-term duration. We chose two stressors which cerebral toxicity is either debated upon (low dose irradiation) or unclear due to limited research (Tungsten W). To achieve this, we focused on a range of physiological parameters and processes commonly measured in toxicological studies, which are known to be impacted by one or both of these stressors.
A previous study by Macé et al.3 observed donut-like neurons following W particles inhalation and linked it to oxidative stress, prompting us to measure this phenotype under co-exposure condition. Our results showed a decrease in donut-like neuron density at 24 h in the co-exposed group compared to both control and single exposed groups, indicating amplified effects when combining our two stressors. This observation does not seem to be caused by cell death, as observed by the lack of both caspase 3 and TUNEL signal in donut-like neurons. Although this result might seem counterintuitive at first, a shift to a more advanced suffering phenotype or a neurodegeneration-like phenotype could cause a loss of the neuronal marker staining (NeuN). It should also be considered that, as donut-like neurons are estimated to be an already fragilized cell population, we could be missing an early apoptotic response, as apoptosis has been demonstrated to happen as early as two hours post-exposure38. This will need to be investigated in future studies. Although of lower magnitude, similar indentations and intranuclear structure formations have been observed in Huntington’s disease39, reinforcing the case for further neurodegeneration studies. At 28 days, only co-exposure caused an increase in donut-like neurons density compared to control, implying a sustained imbalance that increases neuronal suffering in the FC for at least 28 days. While the impact of hypoxia, apoptosis, necrosis, and other stresses on the chromatin structure has been investigated and documented40, the resulting phenotypes do not resemble donut-like neurons. Truncated or donut-like neurons are less documented. In the context of metal exposure, only our previous study examined this phenotype in the brains of exposed rats3, with no studies to date investigating this in the context of radiation exposure. Some have linked this phenotype to hypoxia, oxidative stress, poor tissue fixation of the mouse brain36, or general neuronal suffering in a rat model3. To date, only hypotheses can be formulated on the mechanisms involved in the occurrence of these neurons. These hypotheses are based on the involvement of microtubules but would require experiments to provide a link with our observations. Recently, a study by Naso et al.41 observed donut-like cells in the human retinal cell line hTERT RPE-1, demonstrating that a nuclear pore was formed due to tubulin filaments crossing the nuclei following mitotic aberrations caused by TPX2 overexpression. As mature cortical neurons are post-mitotic, this cause is unlikely in our case. However, the implication of β-tubulin should be studied further if we make the hypothesis that our exposures could affect the organization of microtubules outside of any cell division by looking for cytoskeletal-like structures within the “pore”. Our results show that even control animals present a basal proportion of donut-like neurons. This proportion is higher at 24 h than 28 days in the control and might reflect an increased basal level caused by the contention of our sham exposures and would also indicate a reversibility in this phenotype. This might outline the existence of two phenotypes: a reversible “early-stage” of donut-like neuron and a more advanced phenotype challenging both its reversibility and the ability of neurons to maintain their function.
The contributions of hypoxia and oxidative stress in the occurrence of donut like neurons required further study36. But, as W presence in organs has also been associated with oxidative stress7, we first examined whether W was retained in various brain structures and other organs to determine if the effects were mediated locally or systemically. Tungsten has been observed to remain in the lung of dogs following inhalation for extended periods of time, up to 165 days42. A more recent study by Miller et al.43 measured significantly increased W concentration in the lung of mice 7 days after a 4-h inhalation of 1.7 mg.m−3 of micrometric metallic W particles. This finding indicates that rodents, like dogs, might also struggle to excrete W after inhalation, prompting us to measure W concentration in tissues using ICP-MS. We chose to measure W biokinetics in the lung, as Miller et al. and Aamodt did. We also broadened the study to include brain structures and the ethmoid turbinates (ET), as the hypothesis of a nose-to-brain passage of W was supported by findings of Macé et al.3 and Radcliffe et al.44 in rats. The kidneys were also included in the biokinetic study as W has been previously observed to be mostly excreted in urine in various human and animal models with diverse exposure modalities42,45,46. We also examined the plasma concentration to determine the persistence of circulating W.
We chose 24 h and 28 days as time points to link our biological observations to local accumulation of W in the brain or to systemic effects. Additionally, we wanted to confirm the observation made by Macé et al.3 of W cerebral passage as early as 4 h after inhalation, and investigate whether irradiation would affect W transport and/or excretion at this time point. In line with previous results3, high amounts of W were observed in all brain structures at 4 h post exposure and remained elevated at 24 h for both exposed groups. After 28 days, W was still detectable in the lung, kidney, ET and FC, a novel finding regarding ET and FC as it has not been measured before in these structures at similar time points for metallic W. Circulating plasma levels of W decreased over time but were still five to six times higher than control levels after 28 days, suggesting a long term whole body contamination following a single acute exposure. This finding is consistent with the elevated W levels observed in the kidney at 28 days. Irradiation did not seem to influence tissue levels of W. In terms of lung deposition and excretion kinetic, the residual activity of radiolabelled W observed in dogs by Aamodt42 7 days after inhalation was around 10% of the activity measured at 24 h and 3–5% at 28 days. In contrast, the study of Miller et al.43 measured lung concentrations of W in mice 7 days after inhalation that were around 70% of that at 24 h. The concentration we measured at 28 days was closer to 30–50% of the 24 h concentration, depending on the group, and is more coherent with the observations of Miller et al. than Aamodt. This underlines the fact that excretion parameters can differ significantly depending on the experimental model. Since W has been associated with oxidative stress and inflammation7, its slow excretion from the FC could be associated with functional deficits supported by the observed increase in suffering neuron phenotype47. Thus, additional time points should be studied to examine its effects. Similarly, the presence of W at 28 days in the ET could cause continuous diffusion of W into the brain over time through the nose-to-brain pathway, leading to long-lasting effects. If most brain structures show no significant increase in W levels at 28 days, we may simply be too close to the detection limit to measure it. Interestingly, irradiation induced a significant reduction in W concentration at 4 h and 24 h in the lung and 24 h in the OB. The main similarity between the OB and the lung is their high density in immune cells: microglia and alveolar macrophages, respectively. Since low doses of radiation have been shown to increase the activity of immune cells48,49, the question is raised as to the involvement of such a mechanism in the acceleration of W excretion. Possible mechanisms could involve increased phagocytosis, or lymphatic uptake. The latter was observed previously in relation to metal particles50.
Particulate matter and low-dose irradiation are known to cause oxidative stress in the brain51, even at doses as low as 20 mGy in mice52. Our results show that an early response to co-exposure in the FC is mediated through increased inflammation at 24 h when W had already entered the brain structures as measured via ICP-MS, while late effects involve antioxidant response mechanisms and a potential change in metabolism through the HIF pathway, as observed in other studies following low dose irradiation53,54. As mentioned above, these results can be put in relation to the increased density of donut-like neurons. This warrants further investigations into the kinetics of the transition from inflammation to antioxidant response in the FC between 24 h and 28 days, and the mechanisms involved.
Macé et al.3 detected that repeated W inhalation causes apoptosis of microglial cells in the OB and modulates microglial density in various selective areas of the OB, suggesting microglial cells migration between these different areas. These observations, in addition to the proinflammatory markers measured in the FC at 24 h prompted us to investigate the microglial response following the co-exposure.
At 24 h, microglial cellularity increased in the FC, which is in line with the increase in IL-1β and Mcp1 expression, but remained unaffected by the co-exposure in the OB, suggesting that compensatory mechanisms may have been at work before 24 h in this structure. However, at 28 days, microglia were impacted by the co-exposure in both the OB and FC compared not only to control but also to tungsten-only. The reduction in microglial cell density in the FC, without activation of cell death, was accompanied by a significant increase in the OB, without microglial proliferation or potential peripheral macrophages infiltration. These observations (absence of proliferation or infiltration of peripheral cells) are of major importance. Indeed, they reveal another ability of these microglial cells: that of migrating from one area to another, enabling the brain region concerned to respond to a particular stress. These data reinforce the hypothesis of a migration of microglial cells from the FC to the OB between 24 h and 28 days. This long-distance migration is consistent with previous observations, with measured speeds up to 10 µm.min−1 in the mouse brain55. In terms of activation, while microglial cells were overall weakly activated in the FC, they were significantly activated in the OB 28 days after co-exposure compared to control and tungsten-only, raising the question of whether a pro- or anti-inflammatory activation was in place. Only a decrease in Tnfɑ gene expression could be observed in the OB, suggesting an anti-inflammatory phenotype.
If we consider microglial results all together, we can hypothesize that variations in cell density in the cortex could contribute to a disturbance of the microenvironment. Microglial depletion in the cortex between 24 h and 28 days could then contribute to the increase of the neuronal suffering phenotype density and antioxidant response. On the contrary, in the OB at 28 days, we can make the hypothesis of a compensatory response with an increase in microglial density exhibiting an anti-inflammatory phenotype. This could help alleviate the stress caused by the co-exposure, as witnessed by a reduction in Sod2 gene expression and nuclear translocation of NRF2.
A complete overview of our results shows an interesting trend, in which the irradiation-only changes are usually very close to those seen in the co-exposure group, or at least follow the same trend, often significantly differing from control in the same way as the co-exposed group. We could identify only 3 occasions where the irradiation-only group differed from the co-exposed group. Whereas the tungsten-only group only differs from control in the same manner as the co-exposed group regarding the translocation of NRF2 in the FC and OB, and shows a completely opposite response for microglial cellularity and inflammation in the OB at 24 h. Additionally, the tungsten-only group significantly differs from the co-exposed group on 8 occasions, which is almost triple the number of such differences between the irradiation-only and co-exposed groups. This allows us to suggest that most observed effects of our co-exposure originate from the 50 mGy irradiation and tend to be potentiated by the addition of tungsten inhalation to the extent of reaching statistical significance. The mechanistic insights into the interactions between our two stressors can be supported by the fact that tungsten and gamma radiation produce effects on the same biological processes (inflammation, oxidative stress) and therefore could act on the same cell signalling pathways. This however is an overall trend, that cannot be generalized to every single observation because they demonstrate a complex behaviour.
Regarding our experimental design, when deciding to expose first to W microparticles and then immediately to low-dose irradiation, we were guided by the question of how W may affect radiological effects and risks. Since W microparticles can reach the brain structures within a few hours, the two exposures in our experiment can essentially be considered simultaneous. Besides, some studies have demonstrated neuroprotective effects following low-dose irradiation using various models5. Therefore, the possibility that low-dose radiation administered prior to W inhalation could activate adaptive or protective mechanisms in the brain, which may counteract W-induced toxicity, would have complicated the interpretation of results. This kind of phenomenon has been previously reported in various studies, such as Otsuka et al. in mice56, Porrazzo et al. in fruit fly57, and were reviewed by Betlazar et al.58. While such a possibility is interesting in general and for future studies, it may be deemed less appropriate for real-life scenarios and was thus outside the scope of this study. In radiobiology, the low-dose range (below 100 mGy) is characterized by uncertainty of biological effects. It therefore seems crucial to increase our knowledge in this dose range, hence our choice of 50 mGy. This choice is a strong one, since it is by definition in the low-dose range. But it also questions the effects we could have observed in our study using doses below or above 50 mGy.
In terms of perspectives, additional parameters known to be impacted by particle inhalation and irradiation, such as cerebrovascular integrity and cognitive function, should be investigated using behavioural tests. As mentioned earlier, including intermediate time points, such as 7 or 14 days, would provide valuable insights and should be considered in future studies to better understand the mechanisms at play and define the time window of the biological outcomes. Additionally, the persisting effects observed at 28 days raise questions about whether and when the observed changes return to baseline levels, if they do at all.
Conclusion
Overall, the results suggest increased toxicity when combining the effects of inhaled tungsten particles and low-dose gamma-irradiation on the brain. This is supported by several instances where stress markers were unaffected by single stressors but were significantly impacted by co-exposure. However, not all parameters explored were affected by co-exposure, indicating a complex pattern that varies depending on the time point and specific brain structures. The observed trends point towards the involvement of redox balance regulation, inflammation and hypoxic response.
These novel findings add to the limited literature on radiological and chemical co-exposure, enhancing the understanding of complex exposure scenarios and improving radiation risk assessment by emphasizing the importance of considering a wide range of co-exposure stress factors that humans may encounter in various situations. More widely, multi-stressor studies are of particular importance as single stressors might wrongly be deemed safe. In this regard, they will improve risk assessment by providing information that regulatory authorities could use to establish guidelines for co-exposure scenarios.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
Wild, C. P. Complementing the genome with an “exposome”: The outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol. Biomark. Prevent. 14, 1847–1850. https://doi.org/10.1158/1055-9965.EPI-05-0456 (2005).
Barouki, R. L’exposome, un concept holistique et utile. Bull. Acad. Natl. Med. 204, 299–305 (2020).
Mace, L. et al. Exposure to tungsten particles via inhalation triggers early toxicity marker expression in the rat brain. Inhalation Toxicol. 36, 261–274. https://doi.org/10.1080/08958378.2024.2349895 (2024).
Lemus, R. & Venezia, C. F. An update to the toxicological profile for water-soluble and sparingly soluble tungsten substances. Crit. Rev. Toxicol. 45, 388–411. https://doi.org/10.3109/10408444.2014.1003422 (2015).
Narasimhamurthy, R. K., Mumbrekar, K. D. & Satish Rao, B. S. Effects of low dose ionizing radiation on the brain—a functional, cellular, and molecular perspective. Toxicology 465, 153030. https://doi.org/10.1016/j.tox.2021.153030 (2022).
UNSCEAR. Sources, effects and risks of ionizing radiation. UNSCEAR 2020/2021 Report to the General Assembly, with Scientific Annexes. Volume III. Scientific Annex C. Biological mechanisms relevant for the inference of cancer risks from low-dose and low-dose-rate radiation. 122–124 (UN, 2020/2021).
Sobrido, M. S. et al. Oxidative transformation of Tungsten (W) nanoparticles potentially released in aqueous and biological media in case of Tokamak (nuclear fusion) Lost of Vacuum Accident (LOVA). C. R. Géosci. 352, 539–558 (2020).
Lin, C., Li, R., Cheng, H., Wang, J. & Shao, X. Tungsten distribution in soil and rice in the vicinity of the world’s largest and longest-operating tungsten mine in China. PLoS ONE 9, e91981. https://doi.org/10.1371/journal.pone.0091981 (2014).
Bostick, B. C., Sun, J., Landis, J. D. & Clausen, J. L. Tungsten Speciation and Solubility in Munitions-Impacted Soils. Environ. Sci. Technol. 52, 1045–1053. https://doi.org/10.1021/acs.est.7b05406 (2018).
ANSES. Avis de l’Agence nationale de sécurité sanitaire de l’alimentation, de l’environnement et du travail relatif à « l’identification, la catégorisation et la hiérarchisation de polluants actuellement non réglementés pour la surveillance de la qualité de l’air. Report No. Saisine n° « 2015_SA_0216 », (2018).
Wang, Y. et al. Emerging activated tungsten dust: Source, environmental behaviors, and health effects. Environ. Int. 188, 108774 (2024).
Sachdeva, S., Pant, S. C., Kushwaha, P., Bhargava, R. & Flora, S. J. Sodium tungstate induced neurological alterations in rat brain regions and their response to antioxidants. Food Chem. Toxicol. 82, 64–71. https://doi.org/10.1016/j.fct.2015.05.003 (2015).
Shan, D., Xie, Y., Ren, G. & Yang, Z. Inhibitory effect of tungsten carbide nanoparticles on voltage-gated potassium currents of hippocampal CA1 neurons. Toxicol. Lett. 209, 129–135. https://doi.org/10.1016/j.toxlet.2011.12.001 (2012).
Li, J. et al. Association of non-essential metals with Chinese schizophrenia: A case-control study. Early Interv. Psychiatry https://doi.org/10.1111/eip.13505 (2024).
Naqvi, A. H., Hunt, A., Burnett, B. R. & Abraham, J. L. Pathologic spectrum and lung dust burden in giant cell interstitial pneumonia (hard metal disease/cobalt pneumonitis): review of 100 cases. Arch. Environ. Occup. Health 63, 51–70. https://doi.org/10.3200/AEOH.63.2.51-70 (2008).
UNSCEAR. Sources, Effects and Risks of Ionizing Radiation: Biological Mechanisms Relevant for the Inference of Cancer Risks from Low-Dose and Low-Dose-Rate Radiation, Vol. III (United Nations, 2020/2021).
Laurier, D., Billarand, Y., Klokov, D. & Leuraud, K. The scientific basis for the use of the linear no-threshold (LNT) model at low doses and dose rates in radiological protection. J. Radiol. Prot. 43, 024003 (2023).
Zhao, W., Diz, D. & Robbins, M. Oxidative damage pathways in relation to normal tissue injury. Br. J. Radiol. 80, S23–S31 (2007).
Averbeck, D. & Rodriguez-Lafrasse, C. Role of mitochondria in radiation responses: Epigenetic, metabolic, and signaling impacts. Int. J. Mol. Sci. 22, 11047 (2021).
Kempf, S. J. et al. The cognitive defects of neonatally irradiated mice are accompanied by changed synaptic plasticity, adult neurogenesis and neuroinflammation. Mol. Neurodegener. 9, 57. https://doi.org/10.1186/1750-1326-9-57 (2014).
Mineyeva, O. A. et al. Radiation induces distinct changes in defined subpopulations of neural stem and progenitor cells in the adult hippocampus. Front. Neurosci. 12, 1013 (2019).
Philippot, G. et al. Developmental effects of neonatal fractionated co-exposure to low-dose gamma radiation and paraquat on behaviour in adult mice. J. Appl. Toxicol. JAT 39, 582–589. https://doi.org/10.1002/jat.3748 (2019).
Heredia, L., Belles, M., Llovet, M. I., Domingo, J. L. & Linares, V. Neurobehavioral effects of concurrent exposure to cesium-137 and paraquat during neonatal development in mice. Toxicology 329, 73–79. https://doi.org/10.1016/j.tox.2015.01.012 (2015).
Hladik, D. et al. Combined treatment with low-dose ionizing radiation and ketamine induces adverse changes in CA1 neuronal structure in male murine hippocampi. Int. J. Mol. Sci. https://doi.org/10.3390/ijms20236103 (2019).
Eriksson, P., Fischer, C., Stenerlow, B., Fredriksson, A. & Sundell-Bergman, S. Interaction of gamma-radiation and methyl mercury during a critical phase of neonatal brain development in mice exacerbates developmental neurobehavioural effects. Neurotoxicology 31, 223–229. https://doi.org/10.1016/j.neuro.2010.01.002 (2010).
Richardson, D. B. et al. Cancer mortality after low dose exposure to ionising radiation in workers in France, the United Kingdom, and the United States (INWORKS): Cohort study. BMJ 382, e074520. https://doi.org/10.1136/bmj-2022-074520 (2023).
Française, R. Vol. (Articles R4222-10 à R4222-17) (Code du Travail).
Macé, L. et al. Design of an inhalation chamber and metrology assessment to study tungsten aerosol neurotoxic effects. Aerosol Air Qual. Res. 21, 200504 (2021).
Ma, C. M. et al. AAPM protocol for 40–300 kV X-ray beam dosimetry in radiotherapy and radiobiology. Med. Phys. 28, 868–893 (2001).
Taetzsch, T. et al. Redox regulation of NF-kappaB p50 and M1 polarization in microglia. Glia 63, 423–440. https://doi.org/10.1002/glia.22762 (2015).
Rawlinson, C., Jenkins, S., Thei, L., Dallas, M. L. & Chen, R. Post-ischaemic immunological response in the brain: Targeting microglia in ischaemic stroke therapy. Brain Sci. https://doi.org/10.3390/brainsci10030159 (2020).
Rothman, K. J. No adjustments are needed for multiple comparisons. Epidemiology 1, 43–46 (1990).
Althouse, A. D. Adjust for multiple comparisons? It’s not that simple. Ann. Thorac. Surg. 101, 1644–1645. https://doi.org/10.1016/j.athoracsur.2015.11.024 (2016).
Ibanez, C. et al. Evaluation of the nose-to-brain transport of different physicochemical forms of uranium after exposure via inhalation of a UO4 aerosol in the rat. Environ. Health Perspect. 127, 97010. https://doi.org/10.1289/EHP4927 (2019).
Oberdorster, G. et al. Translocation of inhaled ultrafine particles to the brain. Inhalation Toxicol. 16, 437–445. https://doi.org/10.1080/08958370490439597 (2004).
Dehghani, A. et al. Nuclear expansion and pore opening are instant signs of neuronal hypoxia and can identify poorly fixed brains. Sci. Rep. 8, 14770. https://doi.org/10.1038/s41598-018-32878-1 (2018).
Davalos, D. et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 8, 752–758. https://doi.org/10.1038/nn1472 (2005).
Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 35, 495–516. https://doi.org/10.1080/01926230701320337 (2007).
Davies, S. W. et al. From neuronal inclusions to neurodegeneration: Neuropathological investigation of a transgenic mouse model of Huntington’s disease. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 354, 971–979 (1999).
Johnson, A. B. & Barton, M. C. Hypoxia-induced and stress-specific changes in chromatin structure and function. Mutat. Res. 618, 149–162. https://doi.org/10.1016/j.mrfmmm.2006.10.007 (2007).
Naso, F. D. et al. Excess TPX2 interferes with microtubule disassembly and nuclei reformation at mitotic exit. Cells https://doi.org/10.3390/cells9020374 (2020).
Aamodt, R. L. Inhalation of 181W labeled tungstic oxide by six beagle dogs. Health Phys. 28, 733–742 (1975).
Miller, K. et al. Inhalation of tungsten metal particulates alters the lung and bone microenvironments following acute exposure. Toxicol. Sci. https://doi.org/10.1093/toxsci/kfab109 (2021).
Radcliffe, P. M. et al. Acute sodium tungstate inhalation is associated with minimal olfactory transport of tungsten (188W) to the rat brain. Neurotoxicology 30, 445–450. https://doi.org/10.1016/j.neuro.2009.02.004 (2009).
Radcliffe, P. M. et al. Pharmacokinetics of radiolabeled tungsten ((188)W) in male Sprague–Dawley rats following acute sodium tungstate inhalation. Inhalation Toxicol. 22, 69–76. https://doi.org/10.3109/08958370902913237 (2010).
Bolt, A. M. & Mann, K. K. Tungsten: An emerging toxicant, alone or in combination. Curr. Environ. Health Reports 3, 405–415. https://doi.org/10.1007/s40572-016-0106-z (2016).
Zhang, W., Xiao, D., Mao, Q. & Xia, H. Role of neuroinflammation in neurodegeneration development. Signal Transduct. Target. Ther. 8, 267 (2023).
Meziani, L. et al. Low doses of radiation increase the immunosuppressive profile of lung macrophages during viral infection and pneumonia. Int. J. Radiat. Oncol. Biol. Phys. 110, 1283–1294. https://doi.org/10.1016/j.ijrobp.2021.03.022 (2021).
Wu, Q. et al. Macrophage biology plays a central role during ionizing radiation-elicited tumor response. Biomed. J. 40, 200–211. https://doi.org/10.1016/j.bj.2017.06.003 (2017).
Davies, A. M., Cooper, S. A., Mangham, D. C. & Grimer, R. J. Metal-containing lymph nodes following prosthetic replacement of osseous malignancy: Potential role of MR imaging in characterisation. Eur. Radiol. 11, 841–844. https://doi.org/10.1007/s003300000666 (2001).
Jayaraj, R. L., Rodriguez, E. A., Wang, Y. & Block, M. L. Outdoor ambient air pollution and neurodegenerative diseases: The neuroinflammation hypothesis. Curr. Environ. Health Reports 4, 166–179. https://doi.org/10.1007/s40572-017-0142-3 (2017).
Veeraraghavan, J., Natarajan, M., Herman, T. S. & Aravindan, N. Low-dose gamma-radiation-induced oxidative stress response in mouse brain and gut: Regulation by NFkappaB-MnSOD cross-signaling. Mutat. Res. 718, 44–55. https://doi.org/10.1016/j.mrgentox.2010.10.006 (2011).
Lall, R. et al. Low-dose radiation exposure induces a HIF-1-mediated adaptive and protective metabolic response. Cell Death Differ. 21, 836–844. https://doi.org/10.1038/cdd.2014.24 (2014).
Chandel, N. S. et al. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: A mechanism of O2 sensing. J. Biol. Chem. 275, 25130–25138. https://doi.org/10.1074/jbc.M001914200 (2000).
Carbonell, W. S., Murase, S., Horwitz, A. F. & Mandell, J. W. Migration of perilesional microglia after focal brain injury and modulation by CC chemokine receptor 5: An in situ time-lapse confocal imaging study. J. Neurosci. 25, 7040–7047. https://doi.org/10.1523/JNEUROSCI.5171-04.2005 (2005).
Otsuka, K., Koana, T., Tauchi, H. & Sakai, K. Activation of antioxidative enzymes induced by low-dose-rate whole-body γ irradiation: Adaptive response in terms of initial DNA damage. Radiat. Res. 166, 474–478 (2006).
Porrazzo, A. et al. Low dose rate γ-irradiation protects fruit fly chromosomes from double strand breaks and telomere fusions by reducing the esi-RNA biogenesis factor Loquacious. Commun. Biol. 5, 905 (2022).
Betlazar, C., Middleton, R. J., Banati, R. B. & Liu, G. J. The impact of high and low dose ionising radiation on the central nervous system. Redox Biol. 9, 144–156. https://doi.org/10.1016/j.redox.2016.08.002 (2016).
Acknowledgements
This work was fully supported by the Autorité de Sûreté Nucléaire et de Radioprotection.
Author information
Authors and Affiliations
Contributions
Conception of the project and study design was conducted by CI. Acquisition and/or analysis of the results involved the participation of TF, FB, CB, NCG, TT, LM, CE, CH, CG, FV, VM, FG, IG and CI. TF and CI interpreted the data. TF drafted the work and created the figures. CI and DK thoroughly revised the manuscript and provided advice regarding its structure and content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Fréchard, T., Bachelot, F., Ménard, V. et al. Co-exposure to inhaled tungsten particles and low-dose gamma rays: neurotoxicological outcome in rats. Sci Rep 15, 18307 (2025). https://doi.org/10.1038/s41598-025-02278-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-02278-3