Abstract
Cyclin-dependent kinase 5 (CDK5) plays a critical role in the inflammatory response. Macrophages are pivotal orchestrators of inflammation, fibrosis, and wound repair. However, the effectiveness of CDK5 in macrophages on cutaneous wound healing remains inadequately characterized. We determined the role of CDK5 signaling pathway in macrophages in mouse cutaneous wound healing through the established macrophage-specific deletion of CDK5 (myeCDK5−/−) mice and the pharmacological CDK5 inhibitor Roscovitine. Phosphorylated proteomics, western blotting, Masson staining, and dualimmunofluorescence staining were performed to investigate the potential mechanisms underlying CDK5-mediated inflammatory regulation in macrophages in wound healing. CDK5 expression and phosphorylation were both elevated significantly in cutaneous wound healing process in mice. Moreover, an accelerated wound healing in myeCDK5−/− mice was exhibited with the reduced pro-inflammatory mediators (IL-1β and iNOS) and the elevated anti-inflammatory markers (IL-10 and CD163) expression significantly. CDK5 deficiency in macrophages enhanced tissue remodeling, evidenced by increased collagen deposition and capillary density (CD31+ cells). Consistently, Roscovitine-treated mice also showed accelerated wound healing, accompanied by decreased pro-inflammatory factors and increased anti-inflammatory markers at the wound site. Mechanistically, the decreased phosphorylation of SIRT1 at the Ser14 and Ser47 sites, as a substrate of CDK5, was confirmed in myeCDK5−/− mice. These data are the first to indicate that CDK5 signaling-dependent regulation of SIRT1 phosphorylation in macrophage-mediated inflammation is required for the wound healing process, warranting consideration of the CDK5-SIRT1 pathway as a therapeutic target for cutaneous wound healing.
Similar content being viewed by others
Introduction
Skin wounds heal in larger injuries producing fibrotic scar and chronic wounds is a common clinical issue, involving the tight orchestration of cell migration, proliferation, matrix deposition, inflammation, angiogenesis, and remodeling in cell lineages1. A comprehensive molecular mechanism driving wound healing is still required for the development of therapeutics2. The complex physiological characteristics of cutaneous wound healing include three stages: inflammation, cell proliferation, and tissue remodeling. During the early inflammatory phase, inflammatory cells infiltrate to combat infection and clean the wound, providing a clean environment for tissue repair3. Macrophages play a pivotal role in the inflammatory and proliferative phases, as well as in the formation of granulation tissue4. Macrophage polarization is often associated with the physiological processes of cutaneous wound healing, mainly divided into pro-inflammatory M1 (expressing inflammatory factors such as IL-1β, TNF-α, etc.) and anti-inflammatory tissue repair-type M2 (expressing IL-10, CD163, etc.). When macrophages switch from the M1 to the M2 phenotype, they suppress the inflammatory response and promote tissue repair5,6. Recent evidences show macrophage polarization as a therapeutic target to promote wound healing7,8.
Serine/threonine cyclin-dependent kinase 5 (CDK5) is an atypical member of the cyclin-dependent kinase family9, primarily involved in the development, function, and associated diseases of the central nervous system10,11. Recent studies have found that CDK5 expression in macrophages mediates LPS-induced NF-κB activation, participating in inflammatory responses12. Concurrently, CDK5 also plays a role in promoting cell proliferation in various tumor cells13,14. Silent information regulator sirtuin 1 (SIRT1) is an enzyme that catalyzes the deacetylation of protein substrates and plays multiple roles in aging and disease. Studies have shown that SIRT1 in the epidermis regulates cell migration, redox response, inflammation, epidermis re-epithelialization, granulation formation and other processes, which are required for efficient wound healing in mice15. Although previous studies have shown that CDK5 as an SIRT1 kinase modulating Ser47 phosphorylation plays a key role in regulating SIRT1 phosphorylation16, whether disturbance of CDK5-SIRT1 signaling during cutaneous wound healing influences macrophage inflammation and polarization remains unclear. Therefore, this study established myeCDK5−/− mice to construct a cutaneous wound model and employed a CDK5 kinase-specific inhibitor (Roscovitine) for intervention to explore the effectiveness of CDK5 expression and activity on the phenotypic transformation of macrophages and inflammation, providing a therapeutic target for clinical treatment.
Results
CDK5 signaling is elevated in mouse cutaneous wound healing
To explore whether CDK5 signaling is associated with the process of cutaneous wound healing, we initially established full-thickness excisional wounds on the dorsal skin of mice and observed the wound healing progression. As shown in Fig. 1A, the wound bed area of WT mice naturally healed to approximately 80% around 7 days after injury. Skin tissues collected at different time points after injury (days 0, 3, 5, and 7) were subjected to western blot analysis to detect CDK5 protein and phosphorylation levels. The results showed that both CDK5 expression and its phosphorylation levels were significantly elevated during cutaneous wound healing, peaking on day 5 and then slightly declining (Fig. 1B), indicating that CDK5 signaling activation is involved in mouse wound healing.
CDK5 expression and phosphorylation modifications are involved in the cutaneous wound healing process in mice. (A) Representative images of full-thickness cutaneous wound model of mouse dorsal skin at different time points (days 0, 3, 5, 7). Scale bar = 0.2 cm. Right panel: the quantitative analysis of wound closure percentage (n = 5). (B) Expression of p-CDK5 and CDK5 protein in cutaneous wound tissues on days 0, 3, 5, 7 by western blotting. Right panel: the quantitative analysis (n = 5). *P < 0.05; **P < 0.01.
CDK5 null induces a phenotypic transition from M1 to M2 macrophages
Because CDK5 deletion in macrophage ameliorates sepsis-related inflammation associated with the increased expression of C-Maf and Il-1017, we bred macrophage-specific deletion mice for CDK5 (myeCDK5−/−) and then established full-thickness excisional wounds on the dorsal skin of mice to explore the role of macrophage-derived CDK5 in wound healing. The results uncovered that, compared with the wild-type (WT) group, the myeCDK5−/− group exhibited significantly an accelerated cutaneous wound healing (WT: 23% ± 3% vs. myeCDK5−/−: 8% ± 3%, P < 0.01, Fig. 2A). Subsequently, Western blot assay revealed a down-regulation of IL-1β and iNOS and up-regulation of IL-10 and CD163 expression in the wound healing tissues from myeCDK5−/− mice compared with WT mice (Fig. 2B). Immunofluorescence staining of CD45 (leukocyte marker) in day 5 wound tissues showed macrophage recruitment remained unchanged (Fig. 2C). In line with these results, the dual immunofluorescence staining and quantification of IL-1β (red) and IL-10 (green) positive cells also showing the same expression of IL-1β and IL-10 (Fig. 2D). To exclude potential confounding effects from different macrophage types in the wound bed, we performed dual immunofluorescence staining for F4/80 (macrophage marker) combined with iNOS or CD163. Compared to WT mice, wound tissues from myeCDK5−/− mice on day 5 exhibited a significant increase in CD163-positive macrophages and a marked reduction in iNOS-positive macrophages.(Fig. 2E). To investigate the temporal dynamics of M2 macrophage polarization, skin tissues were collected from WT and myeCDK5−/− mice at different time points post-injury (days 1, 3, 5, and 7). As previously described, CD11b + cells were isolated from wound tissues using CD11b MicroBeads and subjected to flow cytometric analysis18. As expected, the proportion of CD11b + CD206 + macrophages was significantly higher in myeCDK5−/− wounds than in WT controls at all examined time points (days 1, 3, 5, and 7) (Fig. 2F).These results suggest that CDK5 expression in macrophages plays a pro-inflammatory role during wound healing, targeting CDK5 expression mediating macrophage inflammation associated with transition from M1 to M2 polarization could be a potential therapeutic.
Deletion of CDK5 induces M2 macrophage polarization and promotes wound healing. (A) Representative images of full-thickness cutaneous wound models at 0, 3, 5, and 7 days after injury. Scale bar = 0.2 cm. Right panel: the quantitative analysis of wound closure percentage (n = 5). *P < 0.05. (B) Western blot analysis of iNOS, CD163, IL-1β and IL-10 protein expression in wound healing tissues on day 5. Right panel: quantitative protein levels (n = 5). **P < 0.01. (C) Dual immunofluorescence staining of CD45 (green) and DAPI (blue) in day 5 wound tissues. Scale bar = 50 μm. Right panel: CD45 + cell quantification (n = 5). ns, not significant. (D) Dual immunofluorescence staining to observe the in situ expression of IL-1β (red) and IL-10 (green) at the wound bed on day 5. Scale bar = 50 μm. Right panel: the quantitative analysis (n = 5). *P < 0.05; **P < 0.01. (E) Dual immunofluorescence staining to observe the in situ expression of F4/80 (green) with iNOS (red) or CD163 (red) in day 5 wound tissues. Scale bar = 50 μm. Right panel: the percentage of iNOS + F4/80-positive cells and CD163 + F4/80-positive cells (n = 5). **P < 0.01. (F) Flow cytometric analysis of CD11b and CD206 expression on macrophages isolated from wound tissues at days 1, 3, 5, and 7 post-injury. Right panel: percentage of CD11b + CD206 + cells (n = 5). *P < 0.05; **P < 0.01.
Macrophage-specific CDK5 deletion enhances angiogenesis and collagen deposition
To investigate the role of macrophage-derived CDK5 in angiogenesis and collagen remodeling during the three stages of skin wound healing: inflammatory, proliferative, and maturation, consecutive tissue sections from WT and myeCDK5−/− mice at different time points post-injury (days 1, 3, 5, and 7) wound tissues were analyzed via CD31 immunofluorescence and masson staining. The results revealed no statistically significant differences between myeCDK5−/− mice and wild-type (WT) controls on day 1. However, by day 3, myeCDK5−/−mice exhibited a modest increase in CD31-positive capillary density compared to WT controls. This enhancement became statistically significant on days 5 and 7 (Fig. 3A). Concurrently, Masson staining revealed no differences in fiber density between the two groups on day 1. As anticipated, myeCDK5−/− mice exhibited markedly enhanced collagen deposition and a higher collagen area percentage on days 3, 5, and 7 compared to WT controls (Fig. 3B). These findings indicate that macrophage-specific CDK5 deletion promotes angiogenesis and collagen accumulation, accelerating cutaneous wound healing.
Macrophage CDK5 deletion drives angiogenesis and collagen remodeling during wound healing. (A) Immunofluorescence staining of CD31 (green) and DAPI (blue) in days 1, 3, 5, and 7 wound tissues, showing capillary density. Scale bar = 50 μm. Right panels: quantitative analysis of CD31-positive capillaries (n = 5). *P < 0.05; **P < 0.01; ns, not significant. (B) Masson staining of days 1, 3, 5, and 7 wound tissues. Green: Collagen fibers. Scale bar = 100 μm. Right panels: quantitative analysis of collagen deposition (n = 5). **P < 0.01; ns, not significant.
CDK5 inhibitor roscovitine accelerates mouse cutaneous wound healing
To further validate the therapeutic of CDK5 signaling in wound healing, we applied Roscovitine sustained-release gel (0.5 mL at a final concentration of 2 g/L) to evenly cover the cutaneous wounds of mice (Fig. 4A), and observed the effects of CDK5 activity inhibition on the wound healing process. The results showed that compared to mice applied with saline, the accelerated wound healing in mice applied with Roscovitine was also exhibited (Vehicle: 38% ± 4% vs. Roscovitine: 24% ± 3%, P < 0.05, Fig. 4B). Meanwhile, the results of dual immunofluorescence staining with anti-IL-1β (green) and anti-IL-10 (red) antibodies revealed that, compared to Vehicle group, IL-1β expression in the wound healing tissue from mice applied with Roscovitine was reduced, while IL-10 expression was increased (Fig. 4C). Consistent with these findings, Roscovitine-treated mice exhibited a higher proportion of CD163-positive macrophages and fewer iNOS-positive macrophages compared to Vehicle controls (Fig. 4D). The findings suggest that pharmacological inhibition of CDK5 activity promotes wound healing with the same phenotypic transition from M1 to M2 macrophages as well.
Inhibition of CDK5 activity promotes wound healing and M2 phenotype transformation of macrophages. (A) Schematic diagram of the intervention with Roscovitine on the full-thickness cutaneous model on the dorsal skin of mice; (B) Representative images of full-thickness cutaneous wound models at different time points. Scale bar = 0.2 cm. Lower panel: quantitative analysis of wound closure percentage (n = 5). *P < 0.05; **P < 0.01. (C) Dual immunofluorescence staining to observe the in situ expression of IL-1β (red) and IL-10 (green) at the wound bed on day 5. Scale bar = 50 μm. Right panel: the quantitative analysis (n = 5). **P < 0.01. (D) Dual immunofluorescence staining to observe the in situ expression of F4/80 (green) with IL-1β (red) or IL-10 (red) in day 5 wound tissues. Scale bar = 50 μm. Right panel: the percentage of iNOS + F4/80-positive cells and CD163 + F4/80-positive cells (n = 5). **P < 0.01.
Phosphorylation of SIRT1 at Ser14, Ser47 by CDK5 regulates macrophage-mediated inflammation
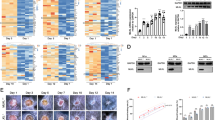
To delineate the molecular mechanism by which CDK5 signaling in macrophages impacts the wound healing, we employed phosphoproteomic approach to identify the CDK5-related substrates in cutaneous wound healing tissues from WT and myeCDK5−/− mice (Fig. 5A).Kinase substrate analysis uncovered that silent information regulator sirtuin 1 (SIRT1), a known element of inflammatory signaling regulator19, is a substrate of CDK5 kinase activity during the wound healing process (Fig. 5B). Moreover, compared to the WT mice, the phosphorylation levels at sites Ser14 and Ser47 of SIRT1 were significantly reduced in wound healing tissues from myeCDK5−/− mice (Fig. 5C). These phosphoproteomic results were further confirmed by Western blot using anti-SIRT1 (Ser47 site) antibody in wound tissues (Fig. 5D), which is consistent with previous research20. These findings suggest that inhibition of CDK5-SIRT1 signaling pathway alleviates inflammatory responses and facilitates wound healing.
Proteomic analysis of protein phosphorylation and kinase substrate analysis have identified SIRT1 as the target protein of CDK5 kinase phosphorylation. (A) Schematic diagram of the experimental workflow for protein phosphorylation and kinase substrate analysis. (B) Result graph of kinase substrate analysis. (C) The relative phosphorylation levels of serine residues at positions Ser14 and Ser47 of the histone deacetylase SIRT1. (D) Western blot validation of the phosphorylation at position Ser47 of the histone deacetylase SIRT1. Right panel: the quantitative analysis (n = 5). **P < 0.01.
Discussion
Excessive inflammatory reactions often lead to delayed wound healing or even exacerbation of cutaneous damage, which is associated with M1 macrophages involvement, conversely, M2 macrophages are anti-inflammatory and crucial for the skin damage repair and the maintenance of homeostasis21,22. This study showed that CDK5 signaling in macrophages plays an important role in the wound healing process of mouse cutaneous, with main results including: (1) increased expression and phosphorylation levels of CDK5 protein in cutaneous wound healing; (2) suppression of CDK5 expression in macrophages promotes mouse cutaneous wound healing associated with an increase in M2-type cells; (3) inhibition of CDK5 activity also promotes mouse cutaneous wound healing; (4) Phosphorylation of SIRT1 at Ser14 and Ser47 by CDK5 regulates the transition from M1 to M2 polarization of macrophages. Based on above points, it concludes that CDK5-dependent regulation of SIRT1 phosphorylation is required for inflammation and macrophage polarization during chronic wound healing, warranting consideration of the CDK5-SIRT1 pathway as a therapeutic target for cutaneous wound healing.
CDK5 plays a role not only in the central nervous system but also in physiological and pathological processes such as inflammation, neovascularization, migration of epithelial and cancer cells, myogenesis, glucose metabolism, and insulin secretion23. This study found that the expression and phosphorylation levels of CDK5 protein increased during the cutaneous wound healing in mice, peaking on day 5, suggesting that its signaling changes are related to the wound healing process. Since CDK5 kinase plays an important role in various types of tissue cells, and Pfänder P et al. reported that the absence of CDK5 in macrophages improves the inflammatory response caused by endotoxemia by inducing the expression of C-Maf and Il-10 17, it is suggested that CDK5 expression may influence macrophages and thereby affect cutaneous wound healing. Through constructing of a cutaneous wound model of myeCDK5−/− mice and observing the cutaneous wound healing process, which revealed that, compared to the WT group, the wound healing in myeCDK5−/− mice was accelerated. IL-1β as a pivotal pro-inflammatory cytokine, orchestrates cytokine cascades, immune cell activation, and apoptosis24. During the early inflammatory phase of wound healing, IL-1β is predominantly secreted by activated macrophages, driving initial inflammation through immune cell recruitment and M1 polarization via NF-κB/NLRP3 pathways, which is critical for pathogen clearance. However, excessive IL-1β disrupts macrophage transition to pro-repair M2 phenotypes, exacerbates inflammation, and impairs progression to the proliferative phase, ultimately contributing to impaired healing and chronic wounds25,26. IL-10 is a characteristic marker protein of M2-type macrophages. By detecting the expression of IL-1β and IL-10 in the cutaneous healing tissue and confirming with in situ fluorescent staining, we found that the pro-inflammatory factor IL-1β was significantly reduced compared to the control group, while the expression of M2-specific protein IL-10 was relatively increased, indicating an increase in M2 macrophages (phenotypic transition or polarization enhancement), which accelerates wound healing. This suggests that CDK5 deletion in macrophages promotes cutaneous wound healing. In line with these results, Pfänder P, et al. proposed that CDK5 deletion plays an anti-inflammatory role27.M2 macrophages promote collagen deposition and neovascularization during tissue repair by secreting factors such as TGF-β1, VEGF, and exosomal miRNAs, which synergistically activate fibroblast proliferation and differentiation, as well as endothelial cell angiogenic signaling pathways28,29,30. CDK5 may be a potential target for the treatment of cutaneous wound healing. Recent clinical studies have shown that the CDK5 activity inhibitor Roscovitine has various potential biological effects, such as promoting the bactericidal and anti-inflammatory functions of alveolar macrophages31, suggesting that specific small molecule intervention with Roscovitine may have potential therapeutic effects on cutaneous wound healing in mice. As expected, our study also found that the wound healing in mice applied with topical Roscovitine was significantly accelerated. In situ immunofluorescence double staining results of the cutaneous healing tissue also showed that, compared to the control group, IL-1β expression was also significantly reduced, whereas the expression of M2-specific protein IL-10 was relatively increased in the Roscovitine-treated group, suggesting an increase in M2 macrophages (anti-inflammation or polarization), accelerated cutaneous tissue repair function, and inhibiting CDK5 activity plays an important role in anti-inflammation and promoting the repair of traumatized cutaneous tissue.
To further explore molecular mechanism by which macrophage-derived CDK5 regulates macrophage polarization and wound healing, we employed phosphoproteomic and discovered that the level of phosphorylation modification of the histone deacetylase SIRT1 was significantly reduced in the tissues of myeCDK5−/− mice. Analysis of significantly differentially modified peptides revealed that CDK5 highly phosphorylates SIRT1 at residues Ser14 and Ser47. Previous research has elucidated many biological functions of SIRT1 post-translational modifications, among which the phosphorylation of SIRT1 is related to its deacetylase activity. It has been reported that mTOR-dependent phosphorylation of SIRT1 at serine 47 results in the inhibition of the deacetylase activity of SIRT132,33. Likewise, other evidence indicates that CDK5-mediated hyperphosphorylation of SIRT1 facilitates the development of endothelial senescence and atherosclerosis in mice16,20. Therefore, inhibition of the phosphorylation activity of CDK5 can reduce the substrate phosphorylation and increase the deacetylase function of SIRT1, which exhibits a polarization of M2 macrophages and reduced polarization of M1 macrophages, exerting anti-inflammatory functions34,35. In fact, the increased SIRT1 activity is potent in promoting wound healing36, the CDK5-SIRT1 pathway thus serves as a promising candidate for treatment.
There were several limitations to our study. Firstly, Roscovitine is indeed a“selective inhibitor of CDKs”. Studies indicate that a rather limited set of protein kinases are targeted by roscovitine, with CDK5, CDK1, CDK2, CDK7, and CDK9 being the most sensitive kinases at present37. Secondly, cell lineages are equally crucial for wound healing. Topical application of Roscovitine on mouse cutaneous wounds for intervention not only inhibits the expression of CDK5 in macrophages but may also affect the proliferation of other cells in the wound tissue, such as fibroblasts and endothelial cells. Third, phosphorylation by cell cycle-dependent kinases as a major mechanism controlling the level and function of SIRT1, and it has been found that 13 phosphorylated residues of SIRT1 match the target motifs of various kinases, including CDK5, CDK1, and MAPK38. Unexpectedly, our study discovered that CDK5 can also phosphorylate SIRT1 at residue Ser14 expect for Ser47. However, whether CDK5 affects other phosphorylation sites remains to be further explored. Additionally, although our study focused on macrophage-mediated inflammation, immune cell infiltration analysis was limited to pan-leukocyte and macrophage markers, omitting key subsets such as neutrophils, T cells, and dendritic cells.
By conducting in vivo experiments to gain a more comprehensive understanding of CDK5’s regulatory role, these results provide a solid basis for confirming that macrophage CDK5 signaling serves as an effective molecular target for mouse cutaneous wound therapeutics.
Materials and methods
Experimental animals and grouping
By crossing CDK5-floxed mice with Lyz2-Cre mice carrying the Cre recombinase inserted in the Lysozyme-M (Lyz2) gene locus, we aimed to achieve the specific deletion of CDK5 in myeloid cells. And then CDK5 flox/flox; Lyz2-Cre mice show no alterations in the myeloid compartment except for macrophages were isolated and confirmed by PCR analysis.
Male C57BL/6J mice aged 6–8 weeks with macrophage-specific knockout of CDK5 (myeCDK5−/−) and their cage-matched wild-type (WT) controls were used to construct a full-thickness skin incision model on the back. Experimental mice were sourced from SaiYe Model Organism Research Center Co., Ltd. [SYXK(Su)2018-0003], and all procedures were in compliance with the ethical review of Hebei University of Chinese Medicine (DWLL202203068).The ARRIVE guidelines have been followed for conducting and reporting animal experiments. For euthanasia, mice were subjected to CO2 asphxiation.The experiment was divided into four groups: WT, myeCDK5−/−, Roscovitine, and Saline, n = 5 mice per group. Mice were anesthetized with 5% isoflurane, after which they were transferred to the stereotaxic surgery setup and maintained on 1.5–2% isoflurane, followed depilation, a full-thickness square skin excision of approximately 1 cm × 1 cm was performed to establish the cutaneous wound model. Wounds were photographed on days 0, 3, 5, and 7, and wound areas were measured using ImageJ software. On the 5th day post-injury, skin tissues were harvested for total protein extraction or paraffin embedding.
Western blot analysis
Twenty milligrams of mouse cutaneous wound healing tissue was homogenized in 200 µL of protein lysis buffer and 2 µL of protease inhibitor at 4 °C, and total protein was extracted. Protein concentration was determined using the BCA method. Forty micrograms of total protein was loaded onto a 10% SDS-PAGE gel for electrophoresis, semi-dry transferred to a PVDF membrane, blocked with 5% skim milk, and the membranes were incubated overnight with the following primary antibodies: p-CDK5 (Ser159)(sc-12919, Santa Cruz Biotechnology), CDK5 (10430-1-AP, Proteintech), IL-1β(16806-1-AP, Proteintech), IL-10(60269-1-Ig, Proteintech), iNOS (22226-1-AP, Proteintech), CD163 (16646-1-AP, Proteintech), CD45 (20103-1-AP, Proteintech), SIRT1 (13161-1-AP, Proteintech), p-SIRT1 (Ser47) (#2314, Cell Signaling Technology) and β-actin (20536-1-AP, Proteintech), all at a dilution of 1:1000. After washing, the membrane was incubated with secondary antibodies for 1 h at room temperature, washed again, and developed using an ECL chemiluminescence kit. Band density was analyzed using ImageJ software.
Flow cytometry
Skin tissues were cut and washed twice in the medium, followed by digestion using 5 mL enzyme digestion solution (0.3 mg/mL Collagenase I, 0.3 mg/mL Collagenase IV), and incubation 37 °C for 60 min. Following complete digestion, the cell suspension was filtered through a 70 μm cell strainer (BS-70-CS; Biosharp), centrifuged at 400 × g for 8 min, and the supernatant was discarded. The pellet was resuspended and sequentially filtered through a 40 μm cell strainer (BS-40-XBS; Biosharp). After centrifugation, cells were resuspended in PBS to obtain a single-cell suspension.CD11b + cells were isolated using CD11b ultrapure magnetic beads (8802-6860-74; Thermo Fisher Scientific) from single cell suspensions as previously described18. For surface marker staining, cells were incubated with FITC-conjugated anti-mouse CD11b (Biolegend, 101206) and PE-conjugated anti-mouse CD206 (Biolegend, 141717) antibodies in the dark at 4 °C for 30 min. After two washing cycles, stained cells were resuspended in PBS for analysis. The percentage of CD206-positive cells was quantified using a NovoCyte flow cytometer (Agilent Technologies).
Tissue dual immunofluorescence staining
Cutaneous healing tissues were fixed with 4% paraformaldehyde at 4 °C for 30 min, transferred to a 30% sucrose solution overnight, embedded in OCT compound, and sectioned at 5 μm. Sections were allowed to return to room temperature, and washed with PBS, blocked with 10% goat serum for 1 h at room temperature, and incubated with primary antibodies F4/80 (GB11027, Servicebio), CD31 (11265-1-AP, Proteintech) (at a dilution of 1:100) overnight at 4 °C. After washing with PBS five times, sections were incubated with secondary antibodies for 1 h at room temperature, washed with PBS, and mounted with a DAPI (4’,6-diamidino-2-phenylindole, DAPI)-containing mounting medium. Images were captured under a Leica fluorescence microscope at high magnification (×400), and the percentages of positive cells were analyzed using ImageJ software.
Masson staining
The extent of dermal tissue injury was assessed by masson staining. Skin tissues were fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned into 4 μm slices. The staining procedures were carried out following the protocol provided with the legend masson staining kit (DC0034, Leagend, China). Collagen deposition was visualized as green areas in masson-stained sections, reflecting the degree of fibrosis. Dermal collagen levels were observed under an optical microscope (Olympus BX53, Japan).
Preparation of roscovitine sustained-release gel
A sustained-release gel of Roscovitine for topical application was prepared using Pluronic F127. Thirty grams of solid Pluronic F127 powder were dissolved in PBS and stirred at 4 °C until clear, and the volume was adjusted to 100 ml. Roscovitine (50 mg) was first dissolved in 1 ml of anhydrous ethanol and mixed with 24 ml of Pluronic F127 gel, stirred overnight at 4 °C, to prepare a Roscovitine sustained-release gel with a final concentration of 2 g/L for application to mouse cutaneous wounds. Wild - type C57BL/6J mice received topical application of Roscovitine pharmacological reagent (2 g/L, 0.5 mL) daily for 7 days post—injury. The vehicle control group received topical application of Pluronic F127 containing saline daily for 7 days post-injury.
Phosphoproteomic analysis
Protein extraction and digestion were carried out as previously described39. In brief, lysates from cutaneous wound healing tissues of WT and myeCDK5−/− were prepared, sonicated, and centrifuged to obtain the supernatant, which was measured by BCA Protein Assay Kits (Bio-Rad, USA), trypsin digested with peptides were desalted using an OASIS HLB Vac cartridge (Waters, Wat036820) and subjected to phosphoproteomics analysis. The peptides were fractionated by a high-pH reverse phase column as previously described using an XBridgeTM BEH130 C18 column (4.6 × 150 mm, 3.5 μm, Waters) with the Agilent 1260 series system (Santa-Clara). Each fraction was then dried for subsequent phosphopeptide enrichment using a Speedvac concentrator (Labconco).
Phosphopeptides were enriched by immobilized metal afnity chromatography (Ti4+-IMAC, JK Chemical) as previously described39. Next, the supernatant was transferred to a clean microtube, dried by Speedvac concentrator and stored at − 20℃ until MS analysis. The MS raw data for each sample were searched using the MASCOT engine (Matrix Science, London, UK; version 2.2) embedded into Proteome Discoverer 1.4 software for identification and quantitation analysis. For phosphoproteomic analysis, we thank Shanghai Applied Protein Technology Co., Ltd. for technological assistance.
Bioinformatics analysis
Several published phosphoproteome datasets with motif peptides corresponding to 13-mer amino acid sequences centered on each phosphorylation sites were utilized to compare phosphorylation sites, including PhosphoSitePlus 6.6.0.2 database and Gygi’s mouse multi-tissue phosphoproteome datasets. IGPS 1.0 was used to annotate the regulatory relationships between kinases and phosphorylated sites, and construct a KSPN, which was visualized by Cytoscape 3.8.240. Kinase enrichment analysis was performed by Fisher’s exact test against our data and PhosphoSitePlus database after iGPS annotation. Benjamini–Hochberg (BH) corrected p-value (p.adjust) was used for enrichment analyses, and a p.adjust < 0.05 was considered significant.
Statistical analysis
All statistical analyses were performed using GraphPad Prism 9.0. The data in all figures are presented as mean ± standard deviation. Differences between groups were compared using the student’s t-test, one-way analysis of variance (ANOVA), or two-factor ANOVA, as appropriate. A p-value < 0.05 (two-tailed) was considered statistically significant.
Data availability
All data generated or analyzed during this study are included in this article.
Change history
21 August 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41598-025-16072-8
References
Peña, O. A. & Martin, P. Cellular and molecular mechanisms of skin wound healing. Nat. Rev. Mol. Cell. Biol. 25, 599–616. https://doi.org/10.1038/s41580-024-00715-1 (2024).
Zhou, Z. Y. et al. Exosomes derived from dental pulp stem cells accelerate cutaneous wound healing by enhancing angiogenesis via the Cdc42/p38 MAPK pathway. Int. J. Mol. Med. 50, 15. https://doi.org/10.3892/ijmm.2022.5199 (2022).
Rodrigues, M., Kosaric, N., Bonham, C. A. & Gurtner, G. C. Wound healing: a cellular perspective. Physiol. Rev. 99, 665–706. https://doi.org/10.1152/physrev.00067.2017 (2019).
Kim, S. Y. & Nair, M. G. Macrophages in wound healing: Activation and plasticity. Immunol. Cell. Biol. 97, 258–267. https://doi.org/10.1111/imcb.12236 (2019).
Danon, D., Kowatch, M. A. & Roth, G. S. Promotion of wound repair in old mice by local injection of macrophages. Proc. Natl. Acad. Sci. USA. 86, 2018–2020. https://doi.org/10.1073/pnas.86.6.2018 (1989).
Sorg, H., Tilkorn, D. J., Hager, S., Hauser, J. & Mirastschijski, U. Skin wound healing: an update on the current knowledge and concepts. Eur. Surg. Res. 58, 81–94. https://doi.org/10.1159/000454919 (2017).
Kuninaka, Y. et al. Macrophage Polarity and wound age determination. Sci. Rep. 12, 11. https://doi.org/10.1038/s41598-022-24577-9 (2022).
Sharifiaghdam, M. et al. Macrophages as a therapeutic target to promote diabetic wound healing. Mol. Ther. 30, 2891–2908. https://doi.org/10.1016/j.ymthe.2022.07.016 (2022).
Dhariwala, F. A. & Rajadhyaksha, M. S. An unusual member of the Cdk family: Cdk5. Cell. Mol. Neurobiol. 28, 351–369. https://doi.org/10.1007/s10571-007-9242-1 (2008).
Gupta, K. K. & Singh, S. K. Cdk5: A main culprit in neurodegeneration. Int. J. Neurosci. 129, 1192–1197. https://doi.org/10.1080/00207454.2019.1645142 (2019).
Zhou, T. T. et al. The p35/CDK5 signaling is regulated by p75NTR in neuronal apoptosis after intracerebral hemorrhage. J. Cell. Physiol. 234, 15856–15871. https://doi.org/10.1002/jcp.28244 (2019).
Du, J. H. et al. Inhibition of CDKS by roscovitine suppressed LPS-induced < SUP>·NO production through inhibiting NFκB activation and bh < sub > 4 biosynthesis in macrophages. Am. J. Physiol. -Cell Physiol. 297, C742–C749. https://doi.org/10.1152/ajpcell.00138.2009 (2009).
Bhatia, R. et al. Malondialdehyde-Acetaldehyde extracellular matrix protein adducts attenuate unfolded protein response during alcohol and smoking-Induced pancreatitis. Gastroenterology 163, 1064–. https://doi.org/10.1053/j.gastro.2022.06.071 (2022).
Li, M. et al. Lnc-ATG9B-4 aggravates progress of hepatocellular carcinoma through cell proliferation and migration by upregulating CDK5. Exp. Biol. Med. 246, 177–186. https://doi.org/10.1177/1535370220963197 (2021).
Qiang, L., Sample, A., Liu, H., Wu, X. Y. & He, Y. Y. Epidermal SIRT1 regulates inflammation, cell migration, and wound healing. Sci. Rep. 7, 10. https://doi.org/10.1038/s41598-017-14371-3 (2017).
Bai, B. et al. Cyclin-Dependent kinase 5-Mediated hyperphosphorylation of Sirtuin-1 contributes to the development of endothelial senescene and atherosclerosis. Circulation 126, 729–. https://doi.org/10.1161/circulationaha.112.118778 (2012).
Pfänder, P., Eiers, A. K., Burret, U. & Vettorazzi, S. Deletion of < i > Cdk5 in macrophages ameliorates anti-inflammatory response during endotoxemia through induction of C-Maf and Il-10. Int. J. Mol. Sci. 22, 13. https://doi.org/10.3390/ijms22179648 (2021).
Mirza, R. E., Fang, M. M., Ennis, W. J. & Koh, T. J. Blocking interleukin-1β induces a healing-associated wound macrophage phenotype and improves healing in type 2 diabetes. Diabetes 62, 2579–2587. https://doi.org/10.2337/db12-1450 (2013).
Yang, Y. S. et al. Regulation of SIRT1 and its roles in inflammation. Front. Immunol. 13, 16. https://doi.org/10.3389/fimmu.2022.831168 (2022).
Bai, B., Vanhoutte, P. M. & Wang, Y. Loss-of-SIRT1 function during vascular ageing: hyperphosphorylation mediated by cyclin-dependent kinase 5. Trends Cardiovasc. Med. 24, 81–84. https://doi.org/10.1016/j.tcm.2013.07.001 (2014).
Royzman, D. et al. Soluble CD83 improves and accelerates wound healing by the induction of pro-resolving macrophages. Front. Immunol. 13, 15. https://doi.org/10.3389/fimmu.2022.1012647 (2022).
Lyu, L. et al. Exosomes derived from M2 macrophages induce angiogenesis to promote wound healing. Front. Mol. Biosci. 9, 19. https://doi.org/10.3389/fmolb.2022.1008802 (2022).
Roufayel, R. & Murshid, N. CDK5: Key regulator of apoptosis and cell survival. Biomedicines 7, 13. https://doi.org/10.3390/biomedicines7040088 (2019).
Dinarello, C. A. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 27, 519–550. https://doi.org/10.1146/annurev.immunol.021908.132612 (2009).
Eming, S. A., Krieg, T. & Davidson, J. M. Inflammation in wound repair: Molecular and cellular mechanisms. J. Invest. Dermatology. 127, 514–525. https://doi.org/10.1038/sj.jid.5700701 (2007).
Landen, N. X., Li, D. & Stahle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell. Mol. Life Sci. 73, 3861–3885. https://doi.org/10.1007/s00018-016-2268-0 (2016).
Pfänder, P., Fidan, M., Burret, U., Lipinski, L. & Vettorazzi, S. Cdk5 deletion enhances the anti-inflammatory potential of GC-Mediated GR activation during inflammation. Front. Immunol. 10, 13. https://doi.org/10.3389/fimmu.2019.01554 (2019).
Wynn, T. A., Barron, L. & Macrophages Master regulators of inflammation and fibrosis. Semin. Liver Dis. 30, 245–257. https://doi.org/10.1055/s-0030-1255354 (2010).
Chanmee, T., Ontong, P., Konno, K. & Itano, N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers 6, 1670–1690. https://doi.org/10.3390/cancers6031670 (2014).
Dai, S. et al. Hypoxia macrophage-derived Exosomal miR-26b-5p targeting PTEN promotes the development of keloids. Burns Trauma. 12 https://doi.org/10.1093/burnst/tkad036 (2024).
Meijer, L. et al. Safety and pharmacokinetics of roscovitine (Seliciclib) in cystic fibrosis patients chronically infected with < i > pseudomonas < i > aeruginosa, a randomized, placebo-controlled study. J. Cyst. Fibros. 21, 529–536. https://doi.org/10.1016/j.jcf.2021.10.013 (2022).
Gong, C. J. et al. IL-6-induced acetylation of E2F1 aggravates oxidative damage of retinal pigment epithelial cell line. Exp. Eye Res. 200 https://doi.org/10.1016/j.exer.2020.108219 (2020).
Back, J. H. et al. Cancer cell survival following DNA damage-mediated premature senescence is regulated by mammalian target of rapamycin (mTOR)-dependent inhibition of sirtuin 1. J. Biol. Chem. 286, 19100–19108. https://doi.org/10.1074/jbc.M111.240598 (2011).
Park, S. Y. et al. SIRT1/Adenosine Monophosphate-Activated protein kinase α signaling enhances macrophage polarization to an Anti-inflammatory phenotype in rheumatoid arthritis. Front. Immunol. 8, 11. https://doi.org/10.3389/fimmu.2017.01135 (2017).
Li, J. H. et al. Deacetylation of Notch1 by SIRT1 contributes to HBsAg- and HBeAg-mediated M2 macrophage polarization. Am. J. Physiol. -Gastroint Liver Physiol. 322, G459–G471. https://doi.org/10.1152/ajpgi.00338.2021 (2022).
Wahedi, H. M. et al. NED416, a novel synthetic Sirt1 activator, promotes cutaneous wound healing via the MAPK/Rho pathway. Int. J. Mol. Med. 46, 149–158. https://doi.org/10.3892/ijmm.2020.4564 (2020).
Bach, S. et al. Roscovitine targets, protein kinases and pyridoxal kinase. J. Biol. Chem. 280, 31208–31219. https://doi.org/10.1074/jbc.M500806200 (2005).
Sasaki, T. et al. Phosphorylation regulates SIRT1 function. PLoS One. 3, 13. https://doi.org/10.1371/journal.pone.0004020 (2008).
Fan, Y. et al. Phosphoproteomic analysis of neonatal regenerative myocardium revealed important roles of checkpoint kinase 1 via activating mammalian target of Rapamycin C1/Ribosomal protein S6 kinase b-1 pathway. Circulation 141, 1554–1569. https://doi.org/10.1161/circulationaha.119.040747 (2020).
Saito, R. et al. A travel guide to cytoscape plugins. Nat. Methods. 9, 1069–1076. https://doi.org/10.1038/nmeth.2212 (2012).
Acknowledgements
We would like to express our gratitude to all the medical staff, teachers, and students who participated in this research.
Institutional review board statement
The animal study protocol was approved by the ethical review of Hebei University of Chinese Medicine (DWLL202203068).
Funding
This research was funded by The Government-funded Project for Training Outstanding Clinical Medical Talents by the Health Commission of Hebei Province (No. 2021379) and Natural Science Foundation of Hebei Province of China (H2022423343).
Author information
Authors and Affiliations
Contributions
Conceptualization, Jingjing Wang, Lin Ji, Xiaobin Zhou, Yujia Ding, Zhenmu Lv and Dong Ma; Data curation, Jingjing Wang, Lin Ji, Xiaobin Zhou, Yujia Ding, Zihan Zhou and Xiaofan Guo; Formal analysis, Jingjing Wang, Lin Ji, Zihan Zhou and Xiaofan Guo; Funding acquisition, Zhenmu Lv and Dong Ma; Investigation, Jingjing Wang, Lin Ji, Zihan Zhou, Xiaofan Guo and Chao Liu; Methodology, Jingjing Wang, Lin Ji, Xiaobin Zhou, Yujia Ding, Yingbo Gao and Jingyu Sun; Project administration, Yujie Wang; Resources, Zhenmu Lv and Dong Ma; Software, Jingjing Wang, Lin Ji, Zihan Zhou and Xiaofan Guo; Supervision, Yujie Wang; Validation, Jingjing Wang and Lin Ji; Visualization, Jingjing Wang, Lin Ji, Zhenmu Lv and Dong Ma; Writing—original draft, Jingjing Wang, Lin Ji, Xiaobin Zhou, Yujia Ding, Zhenmu Lv and Dong Ma; Writing—review & editing, Qingfu Zhang, Haitao Zhao, Zhenmu Lv and Dong Ma. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: In the original version of this Article, Zhenmu Lv was omitted as a corresponding author. Correspondence and requests for materials should also be addressed to lvzhenmu@126.com.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, J., Ji, L., Gao, Y. et al. Inhibition of CDK5 signaling mediated inflammation in macrophages promotes cutaneous wound healing. Sci Rep 15, 18509 (2025). https://doi.org/10.1038/s41598-025-02488-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-02488-9