Abstract
Adherence to plant-based diets has significantly increased in popularity recently, with claims that they reduce the risk of non-communicable diseases. This study investigated whether high adherence to plant-based diets can reduce the risk of hepatic steatosis and fibrosis. In this study, 8516 participants from the Ravansar Noncommunicable Disease cohort completed a validated food frequency questionnaire (FFQ) to assess their plant-based diet scores. The study used the fatty liver index and fibrosis-4 index to predict hepatic steatosis and fibrosis. The plant-based diet index (PDI) was used to measure the overall quality of diets from healthy and unhealthy plant-derived foods and animal-derived foods. Associations were determined using binary logistic regression, considering potential confounders. Participants in the highest tertiles of plant-based diet scores had higher energy-adjusted intakes of fructose than those in the lowest tertiles (16.09 ± 12.11 vs. 26.65 ± 12; P-value < 0.001). In multivariable-adjusted models, participants in the highest tertile of PDI had lower odds of hepatic fibrosis than those in the lowest tertile (OR: 0.59; 95%CI: 0.43–0.81). There was no significant association between adherence to PDI and hepatic steatosis after adjustment for potential confounders (OR: 0.989; 95%CI 0.78 − 1.25). The odds of hepatic fibrosis decreased by 6% for each unit increase in healthy plant-based foods (OR: 0.94; 95%CI: 0.91–0.97). The odds of hepatic steatosis increased by 14% for each 1 SD increase in fructose intake (OR: 1.14; 95% CI: 1.02–1.28). This study highlights the potential benefits of high adherence to plant-based diets in reducing the risk of hepatic fibrosis, but high fructose content in some plant-based foods may have an unfavorable role in hepatic steatosis. These findings highlight the importance of selecting whole, fiber-rich plant foods and minimizing intake of fructose-dense products in plant-based diets to promote liver health. Therefore, selecting low-fructose food items in plant-based diets is recommended, though further research is needed to confirm these findings.
Similar content being viewed by others
Introduction
Non-alcoholic fatty liver disease (NAFLD) is characterized by an excessive buildup of fat in the liver when triglyceride content is higher than 5% of liver weight. This condition includes simple steatosis and non-alcoholic steatohepatitis (NASH), which can lead to hepatic fibrosis in some subjects1. In recent decades, the prevalence of NAFLD has risen markedly, from 25 % in 1990–2006 to 38 % in 2016–20192. Based on this trend, the disease is predicted to become the leading cause of liver transplantation worldwide. A recent systematic review and meta-analysis revealed that over half of patients who underwent liver transplantation experienced recurrent NAFLD within one year3. Hence, lifestyle modification is the cornerstone of NAFLD management. Diet is one of the important lifestyle factors related to NAFLD, which can directly influence the flux of lipids to the liver3. Adherence to healthy, balanced diets can help to impede NAFLD contributors by achieving and maintaining a healthy weight, modulating inflammatory mediators, improving insulin sensitivity, and promoting a healthy gut microbiome4. These diets include all food groups in appropriate proportions (low, moderate, or high) and are nutritionally adequate, facilitating hepatocyte regeneration during damage. The Korea National Health and Nutrition Examination Survey (KNHANES) indicated that adherence to high-quality diets can reduce the risk of NAFLD5. Furthermore, high-quality diets can reduce the risk of metabolic dysfunctions associated with NAFLD6.
Despite robust evidence supporting high-quality diets for NAFLD patients, preliminary studies suggest that some restricted diets, especially plant-based ones, might offer additional benefits7. Plant-based diets are hypothesized to improve liver health due to their abundance of antioxidants, phytochemicals, fiber, and unsaturated fats (omega-3 fatty acids). These components help lower calorie density, modulate inflammatory mediators, boost antioxidant defenses, and alter gut microbiota composition8. Unlike animal-based diets, plant-based diets contain lower levels of saturated fats and cholesterol, which are linked to hepatic fat accumulation, metabolic dysfunctions, and lipotoxicity9. However, plant-based diets with a high emphasis on fruit consumption can increase fructose intake. Fructose promotes de novo lipogenesis in hepatocytes by bypassing the main regulatory enzyme of glycolysis. High intake of fructose is associated with abdominal obesity, NAFLD, and hepatic fibrosis10. Additionally, plant-based diets may lead to nutrient deficiencies such as B12, eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and high-quality proteins, likely impairing liver regeneration.
Despite increasing interest in plant-based nutrition in clinical care—as discussed in the review by Arslan et al. (2024), which highlights the health benefits and strategies for plant-based diets— there is a lack of evidence particularly linking these diets to liver health outcomes. While existing literature comprehensively covers cardiovascular, metabolic, and oncologic benefits, a focused investigation into the relationship between plant-based diet quality and liver-related outcomes such as steatosis and fibrosis is still limited11.
By evaluating the relationship between adherence to both healthy and harmful plant-based dietary patterns and hepatic steatosis and fibrosis in a population-based cohort, this study aims to address this crucial gap. This study provides significant insights that can guide dietary strategies for the prevention and management of NAFLD.
Methods
Study design and participants
This is a cross-sectional study of the Ravansar Non-Communicable Disease (RaNCD) cohort. The RaNCD cohort was conducted among the Kurdish population in Kermanshah Province, Iran. It is part of the Prospective Epidemiological Research Studies in Iran (PERSIAN) project.
It follows participants to monitor outcomes of non-communicable diseases including cardiovascular diseases, cancers, diabetes mellitus, pulmonary diseases, kidney diseases, liver diseases, neurodegenerative diseases, and deaths12. This study was conducted in accordance with the Declaration of Helsinki and written informed consent was obtained from all participants. The current study was approved by the ethics committee of Kermanshah University of Medical Sciences (ID: IR.KUMS.REC.1401.426) and the members of the RaNCD steering committee.
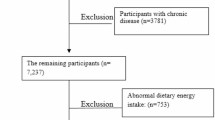
From March 2015 to February 2017, the RaNCD recruited 10,065 participants. In the current study, participants were excluded from the final analysis if they had implausible caloric intake (600–3500 kcal/day for females and 800–4200 kcal/day for males), missing relevant variables (biochemicals and anthropometrics), a history of alcohol consumption, or viral hepatitis (B and C).
Demographic assessment
Baseline data on age (years), sex (male, female), hypertension (yes, no), diabetes (yes, no), cardiovascular diseases (yes, no), smoking status (current smoker, former smoker, passive smoker, non-smoker), physical activity (low, medium, high), socioeconomic information (quantiles), dietary supplement use (yes, no), anti-hyperlipidemic drugs (yes, no), anti-lipidemic drugs (yes, no), and sleep duration (hours) were collected using self-administered questionnaires. Physical activity was measured based on metabolic equivalent rates (METs) by using the PERSIAN cohort questionnaire13, and categorized as low (24–36.5 METs), medium (36.6–44.9 METs), and high (≥ 45 METs).
Height was measured to the nearest 0.1 cm with a wall-mounted stadiometer. Weight was measured digitally using bioelectrical impedance analysis (Inbody 770, Biospace, South Korea). Body mass index (BMI) was calculated by dividing the weight (kilograms) by the squared height (meters). BMI was categorized into four levels: underweight (BMI < 18.5), normal weight (18.5 ≤ BMI < 25), overweight (25 ≤ BMI < 30), and obesity (30 ≤ BMI). Waist circumference (WC) was measured to the nearest 0.1 cm at the narrowest point between the lowest rib and above the iliac crest.
Hepatic steatosis and fibrosis
Hepatic steatosis and fibrosis were examined using non-invasive indices validated for use in epidemiological studies. One of the most common and non-invasive predictors of hepatic steatosis is the Fatty Liver Index (FLI), which is widely used in epidemiological studies14. It is calculated using both anthropometric data and biochemical tests, including triglyceride (TG), gamma-glutamyl transferase (GGT), waist circumference (WC), and body mass index (BMI). The formula for calculating FLI is:
FLI scores range from 0 to 100, with a recommended threshold score of ≥ 60 indicating hepatic steatosis, with an accuracy of 0.84 (95% confidence interval: 0.81–0.87)15.
On the other hand, the most frequently used non-invasive scoring system for hepatic fibrosis is the fibrosis-4 (FIB-4) index, which is applied in large-population studies16. This index is calculated using biochemical tests as follows:
The cut-off points for FIB-4 are categorized by age groups. 1.05 for individuals aged ≤ 49 years, 1.24 for those aged 50–59 years, and 1.88 for those aged 60–69 years. The area under the receiver operating characteristic curve (AUROC) for these age groups is 0.917, 0.849, and 0.855, respectively17.
Dietary intakes and Plant-Based dietary index
Dietary assessment was assessed using a validated semi-quantitative National Iranian Food Frequency Questionnaire (FFQ) comprising 118 food items to estimate nutrient consumption and to calculate PDI. The validity and reliability of this tool have been thoroughly evaluated and confirmed in a comprehensive study18. Participants reported their consumption of each food item over the past year. This instrument assessed the consumption and frequency of each food on a scale from “never or < 1 serving per month” to “≥ 6 servings per day”. The amount of each food item was converted to weight (grams/day). Using the Nutritionist IV software (First Databank Inc., Hearst Corp., San Bruno, CA, USA), the data obtained from the questionnaire were analyzed, and the average daily energy and nutrients consumed were calculated. Studies showed that the range of 600–3,500 kcal/day may be applied to data from women, and an allowable range of 800–4,200 kcal/day for men19,20. Participants with a daily energy intake outside of the predefined range were excluded from the final analysis.
The plant-based diet index (PDI) was developed by Martínez-González to measure the overall quality of a plant-based diet21. To calculate the PDI score, the frequency of consumption for each food item was converted into units consumed per day. Then, food items were summed and categorized into 18 food groups based on their similarities in nutrient content. These food groups include whole grains, refined grains, whole fruits, fruit juices, vegetables, potatoes, nuts, legumes, vegetable oils, animal fats, tea/coffee, sugar-sweetened beverages, desserts, dairy, eggs, fish/seafood, chicken/red meat, and miscellaneous animal foods. According to the PDI scoring system, a score of 5 was given to the highest quintile of consumption for each plant food group, while a score of 1 was assigned to the lowest quintile of consumption. Conversely, for animal food groups, a score of 1 was given to the highest quintile of consumption, and a score of 5 was assigned to the lowest. The PDI score for each participant will be between 12 (the lowest adherence to the plant-based diet) and 90 (the highest adherence to the plant-based diet)21.
To justify any association between the PDI score and the risk of hepatic steatosis, we also categorized plant food groups into the healthy plant-based food group and the unhealthy plant-based food group, which considers their impacts on overall health based on the literature22. More information is presented in Table 1.
Statistical analysis
All statistical analyses were conducted using SPSS software version 16 (SPSS Inc., Chicago, USA). Demographic variables across the tertiles of plant-based diet scores were compared using one-way ANOVA for quantitative variables, chi-square for nominal variables, and Jonckheere-Terpstra test for ordinal variables. The dietary intake of participants across the tertiles of plant-based diet scores was analyzed using ANCOVA, adjusting for energy intake. The odds ratio (95% confidence interval) for hepatic steatosis and fibrosis across the tertiles of plant-based diet scores were obtained using binary logistic regression, with both crude and adjusted models. The adjusted model was defined as follows:
-
Adjusted Model 1 considered age and sex as confounders to account for inherent differences between individuals.
-
Adjusted Model 2 additionally included energy intake, physical activity, socioeconomic status, sleep duration, education, and smoking status as confounders to account for lifestyle and social disparities between individuals.
-
Adjusted Model 3 further included comorbidities, medication use, and dietary supplements as confounders to account for differences in medical conditions between individuals.
We also conducted sensitivity analyses to assess the possibility of misclassification within plant-based diet index categories. P-values less than 0.05 were considered statistically significant.
Results
Following the eligibility criteria, 1549 participants were excluded from the data analysis because of reporting implausible caloric intake (N:972), a history of alcohol consumption (N:391), the lack of relevant variables (N:172), and hepatitis B and C (N:14). A total of 8516 participants were included in the final analysis (Fig. 1).
The mean (± SD) age of the participants was 47.70 (± 8.32) years, and their BMI was 27.49 (± 4.62) kg/m2. A total of 3316 (38.9%) subjects had hepatic steatosis according to the FLI cut-off point and 1285 (15.1%) had hepatic fibrosis according to FIB-4 cut-off points. The demographic characteristics of the participants across the tertiles of plant-based diet scores are shown in Table 2. Significant differences were observed in the distribution of the participants by sex, hypertension (HTN), diabetes mellitus (DM), cardiovascular diseases (CVDs), medication usage, dietary supplement usage, smoking status, socioeconomic status, and physical activity levels across the tertiles of plant-based diet scores (P-value < 0.05). The participants in the highest tertile of plant-based diet scores were more likely to be male and current smokers and were less likely to have HTN, DM, and CVDs than those in the lowest tertile (P-value < 0.05). Additionally, participants in the highest tertile of plant-based diet scores had higher weight, BMI, and FLI scores and had lower age, sleep duration, socioeconomic status, and FIB-4 scores than those in the lowest tertile (P-value < 0.05).
The energy-adjusted dietary intakes of the participants across the tertiles of plant-based diet scores are presented in Table 3. A significant upward trend was observed in the intake of energy, carbohydrates, polyunsaturated fatty acids (PUFA), fiber, fructose, vitamin E, vitamin C, and caffeine across increasing tertiles of plant-based diet scores (P-trend < 0.001). Conversely, the energy-adjusted intake of protein, fat, saturated fatty acids (SFA), monounsaturated fatty acids (MUFA), polyunsaturated fatty acids (PUFA) cholesterol, EPA + DHA, calcium, zinc, iron, sodium, vitamin B12, and vitamin D significantly decreased across the tertiles of the plant-based diet scores (P-trend < 0.001).
The odds ratios for having hepatic steatosis and fibrosis across the tertiles of plant-based diet scores are shown in Table 4. A statistically significant upward trend in the odds of hepatic steatosis was observed across the tertiles of plant-based diet scores in the crude model. After adjusting for age and sex (Model 1), this association remained significant (P-trend < 0.001). However, this upward trend was attenuated and became non-significant after further adjusting for energy intake, physical activity levels, SES, sleep duration, education, smoking status (Model 2), accompanying diseases, medications, and dietary supplement use (Model 3). We also conducted sensitivity analyses to assess the possibility of misclassification within the plant-based diet index categories. The same results were observed for the association between odds of hepatic steatosis and plant-based diet scores (odds ratio: 1.025; 95% CI: 0.933–1.126). There was a statistically significant downward trend in the odds of hepatic fibrosis across the tertiles of plant-based diet scores in the crude model. This downward trend remained significant after adjusting for age and sex (P-trend < 0.001) and persisted even after adjusting for energy intake and physical activity levels. Further adjustments for lifestyle factors and medical conditions (Models 2 and 3) revealed that individuals with the highest adherence to plant-based diets had a 41% lower risk of hepatic fibrosis compared to those with the lowest adherence (odds ratio: 0.592; 95% CI: 0.430–0.815; P-value < 0.021). Sensitivity analyses showed that the odds ratio of hepatic fibrosis was decreased by 22% for each 1 SD increase in plant-based diet scores (odds ratio per SD: 0.779; 95% CI: 0.685–0.885).
Multivariable-adjusted odds ratios of hepatic steatosis and fibrosis with increasing the score of plant-based diet (PDI) components and fructose intake are shown in Table 5. Among these components, only the score of healthy plant-based foods was associated with the odds of hepatic fibrosis (P-value < 0.001). The odds ratio of hepatic fibrosis was decreased by 6% for each increase in healthy plant-based food scores (odds ratio per unit: 0.941; 95%CI: 0.916–0.966). Additionally, fructose intake showed a positive relationship with hepatic steatosis. The odds ratio of hepatic steatosis was increased by 14% for each 1 SD increase in fructose intake (odds ratio per SD: 1.142; 95%CI: 1.021–1.278).
Discussion
In this large cross-sectional study, adherence to plant-based diets, especially those emphasizing healthy plant-based foods, was associated with decreased odds of hepatic fibrosis. However, adherence to plant-based diets, whether emphasizing healthy or unhealthy plant-based foods, was not associated with hepatic steatosis, likely due to the high fructose content. We found that high fructose consumption from plant-based diets was associated with an increased risk of hepatic steatosis. The robustness of these findings was determined through sensitivity analysis. To our knowledge, this is the first large-scale study to assess the associations between plant-based diets and their components with both hepatic steatosis and fibrosis in the Iranian population.
The present study showed that high adherence to plant-based diets was not associated with hepatic steatosis after adjusting for potential confounding factors. The plant-based diet in our study emphasized high consumption of both healthy and unhealthy plant-based food groups. In recent decades, plant-based diets have dramatically grown in popularity because of claiming to reduce the risk of several non-communicable diseases such as cardiovascular diseases and cancers23. However, our study does not support the hypothesis that high adherence to plant-based diets can reduce the risk of hepatic steatosis. Few studies have highlighted the relationship between plant-based diets and hepatic steatosis, with controversial results. Some studies such as those by Mazidi et al.24, Li et al.25, and Lv et al.26 showed a lower risk of hepatic steatosis with adherence to plant-based diets rich in healthy plant-based food groups (including whole grain, fruit, vegetable, nut, legume, and vegetable oil), However, Ratjen et al.27 did not find any associations between these plant-based diets and hepatic steatosis. On the other hand, Mazidi et al.24 and Lv et al.26 showed a higher risk of hepatic steatosis with adherence to plant-based diets rich in unhealthy plant-based food groups (including fruit juice, sugar-sweetened beverages, refined grain, and potato), while Li et al.25 and Ratjen et al.27 did not find any associations. Furthermore, Mazidi et al.24 and Lv et al.26 showed that adherence to plant-based diets rich in both healthy and unhealthy plant-based food groups was significantly associated with a lower risk of hepatic steatosis, but Li et al.25 and Ratjen et al.27 did not find any associations, similar to our study.
Potential explanations for these discrepancies are largely due to differences in confounding factors and the components of each plant-based diet regarding micronutrient and macronutrient content. Among the above-mentioned studies, only Ratjen et al. conducted a comprehensive adjustment for potential confounders and then observed that none of the associations remained statistically significant27. Also, these studies did not provide any information on the differences between plant-based diets in terms of micronutrients and macronutrients, especially fructose content. But what is evident from these studies is that the plant-based diets that were associated with increased odds of hepatic steatosis are rich in sources containing high fructose such as sugar-sweetened beverages (SSBs) and fruit juices. Plant-based diets rich in healthy plant food groups are not necessarily low in fructose when fruit consumption is high. Our results showed that fructose intake increased the odds of hepatic steatosis by 14% for each standard deviation (SD) after adjusting for potential confounding factors. Therefore, high fructose consumption from plant-based diets may be responsible for the absence of a favorable association with hepatic steatosis.
This finding is consistent with a recent meta-analysis from epidemiological studies that indicated excessive fructose consumption increases the risk of hepatic steatosis28. Fructose is preferentially metabolized by hepatocytes. It increases intrahepatic metabolites for de novo lipogenesis (DNL) by bypassing key rate-limiting steps of glycolysis, including glucokinase/hexokinase and phosphofructokinase. Also, fructose up-regulates intrahepatic hepatic lipogenesis through the activation of key transcription factors related to DNL and hepatic steatosis such as carbohydrate response element-binding protein (ChREBP) and sterol regulatory element-binding protein 1c (SREBP1c)29. Nomura et al. found that excessive fructose intake can worsen triglyceride accumulation in the liver, a key feature of NAFLD30. This mechanism highlights the risk of consuming large amounts of fructose, even in the context of a generally health-promoting plant-based diet. High fructose consumption has been linked to the development of insulin resistance, another significant contributor to hepatic steatosis. Insulin resistance impairs the body’s ability to regulate glucose and fat metabolism, leading to increased fat accumulation in the liver. The origin of fructose significantly influences its metabolic effects. In fact, whole fruits contain not only fructose, but also provide fiber, polyphenols, and other bioactive compounds thereby slowing the process of absorption, support gastrointestinal health, especially gut health, and reduce glycemic load. On the other hand, fructose from SSBs and fruit juice sources are rapidly absorbed, bypasses normal satiety signaling, and provide excessive caloric intake and hepatic fat accumulation. This distinction between whole food fructose particularly fruits and added sugars is crucial when explaining the health impact of plant-based diets on liver outcomes31. Plant-based diets rich in high-fructose foods could therefore negate some of the protective effects typically associated with these diets. Also, plant foods, including whole grains, vegetables, and legumes, not only contain low levels of fructose but also have high dietary fiber and polyphenols both of which play an essential role in improving liver health by regulating inflammatory and metabolic pathways. In fact, dietary fibers increase the production of short–chain fatty acids like butyrate which helps maintain the integrity of gut barrier and decrease systemic endotoxemia which is a key factor of hepatic inflammation32. Additionally, polyphenols, via their antioxidant and anti-inflammatory properties, inhibit key transcription factors such as NF-κB and change the composition of gut microbiota in favor of anti-inflammatory taxa33,34. Eating plant-based diets that are high in fiber, polyphenols, and antioxidants can improve liver health and reduce hepatic steatosis. However, it’s important to prioritize whole plant foods like vegetables, legumes, and whole grains, and minimize the intake of processed foods and beverages that are high in fructose. These findings emphasize the clinical importance of guiding individuals to adopt plant-based diets that are rich in plants. It is also crucial that these diets are low in added sugars and refined carbohydrates. Prioritizing whole fruits over juices and limiting processed plant-based snacks and sweetened drinks should be part of dietary counseling for individuals at risk of NAFLD35. In fact, striking this balance between whole plant foods and limiting high-fructose processed foods is crucial36. Future research should carefully consider and control fructose intake when evaluating the effects of plant-based diets on NAFLD. This approach will provide clearer insights into the benefits and risks associated with such diets.
The present study also showed that adherence to a plant-based diet was associated with a lower risk of hepatic fibrosis even after the adjustment for potential confounders. Consistent with our findings, Li et al. demonstrated that adherence to vegetarian diets was associated with a reduced risk of hepatic fibrosis, as measured by FIB-4, among participants in the National Health and Nutrition Examination Survey (NHANES)37. Similarly, a few dietary patterns that look somewhat similar to plant-based diets have reported these associations. Soleimani et al. showed that adherence to a healthy dietary pattern that is rich in fruits, vegetables, nuts, vegetable oils, white meats, and low-fat dairy significantly reduced the risk of hepatic fibrosis38. Also, Sayegh et al. reported that adherence to a traditional diet that is rich in vegetables, legumes, vegetable oils/olives, nuts, cooked rice, red wine, and fish is reversely associated with a risk of hepatic fibrosis39. While the FIB-4 index is a non-invasive substitute for liver fibrosis, its changes are often reflective of early alterations in extracellular matrix turnover and hepatocellular injury. Lower FIB-4 scores in individuals adhering to plant-based diets may reflect decreased hepatic stellate cell activation and collagen deposition, hallmarks of fibrogenesis40,41. This explanation is consistent with anti-inflammatory and antioxidant effects of polyphenols, which suppress TGF-β/Smad and NF-κB pathways central to fibrosis progression42,43. Indeed, hepatic fibrosis is characterized by the accumulation of extracellular matrix (ECM) components in response to liver injury. Hepatic stellate cells (HSCs) store vitamin A and regulate ECM turnover in the normal liver. In pathological conditions, HSCs are activated, migrate, and subsequently transdifferentiate to myofibroblast-like cells in the injured sites of the liver. Activated HSCs also express alpha-smooth muscle actin (α-SMA) and secrete large amounts of collagen type I and other ECM proteins44. In fact, activated HSCs proliferate and secrete extracellular matrix proteins, leading to scarring of liver tissue45. Moreover, oxidative stress and inflammation trigger the activation of HSCs46. Recently, antioxidant and anti-inflammatory compounds found in plant-based foods such as polyphenols (e.g., resveratrol, curcumin) have been considered a promising approach for the management of hepatic fibrosis in subjects with NAFLD47. Furthermore, a review study revealed that natural compounds derived from plants, such as phenolic, flavonoid, and sulfur-containing compounds, have been shown to have effective antifibrotic effects with minimal side effects48. Additionally, Arslan et al. (2022) showed an association between increased adherence to the Mediterranean diet, a kind of plant-based dietary approach, and decreased frailty in older adults. This finding suggests that such dietary patterns offer systemic health advantages extending beyond specific organ systems like the liver49. The findings indicate that the potential of plant-based diets to reduce chronic disease incidence and promote healthy aging, underscoring the significance of dietary interventions, specifically in aging populations susceptible to fibrosis-associated liver conditions.
Some plant-based foods such as grapes, berries, tea, coffee, chocolates, whole grains, legumes, and spices contain numerous polyphenolic compounds that exert anti-inflammatory and antioxidant properties through modulating key transcription factors involved in cellular protection against oxidative stress and inflammation such as nuclear factor erythroid 2-related factor 2 (Nrf2)50. Evidence indicates a protective role of coffee and tea on hepatic fibrosis38,51. Our study also showed that following plant-based diets with higher caffeine content had a protective effect against hepatic fibrosis. Also, β-caryophyllene, a component of essential oils in various plants, can inhibit oxidative stress and reduce lipid peroxidation, both of which contribute to hepatic fibrosis. This compound also inhibits the activity of enzymes such as 5-lipoxygenase, which is involved in fibrogenesis52. Moreover, plant-based diets can modulate key signaling pathways involved in fibrogenesis, such as the transforming growth factor-beta (TGF-β)/Smad pathway. Compounds from plants like Curcuma wenyujin have been found to block the TGF-β/Smad signaling pathway, which is central to HSC activation and fibrosis progression. Also, plant-based diets help maintain a balance between matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs), which regulate extracellular matrix turnover and prevent excessive scarring53. In addition, plant-based diets promote a healthy gut microbiome, which plays a critical role in liver health. Fiber-rich plant-based foods encourage the growth of beneficial gut bacteria, such as Bifidobacterium and Coprococcus, which produce short-chain fatty acids like butyrate. These compounds reduce liver inflammation and fibrosis by enhancing gut barrier function and reducing endotoxin leakage that would otherwise promote liver inflammation54,55,56. In conclusion, the anti-fibrotic effects of plant-based diets are mediated through several mechanisms, including inhibition of hepatic stellate cell activation, reduction of oxidative stress and inflammation, modulation of fibrogenic signaling pathways, and interaction with the gut microbiome. These factors collectively contribute to the reduction of hepatic fibrosis and underscore the therapeutic potential of plant-based diets for liver health.
A limitation of the current study is related to cross-sectional design in which exposure and outcome are simultaneously measured, where it is not possible to assess causal relationships. Therefore, our observations need to be assessed in longitudinal or clinical trial studies. Another limitation is inevitable errors associated with self-reported dietary intake using the FFQ. However, the FFQ is a valid and reliable questionnaire for evaluating the relationship between diseases and overall dietary intakes in nutritional epidemiology studies with large sample sizes. A strength of this study is that it provides more precise estimates of existing relations due to the large sample size and control for numerous potential confounders. The findings of our present study should be interpreted cautiously, as they may not be broadly generalizable, especially when considering individuals with alcoholism and older adults.
This study emphasizes the intricate connection between plant-based diets and liver health, particularly focusing on the impact of fructose content on hepatic outcomes. To validate our findings, future research should prioritize long-term studies. These studies should aim to establish causal links between adherence to low-fructose plant-based diets and the development of hepatic steatosis and fibrosis. Furthermore, randomized clinical trials are crucial to assess the protective effects of these dietary patterns. These trials should concentrate on specific dietary interventions with varying fructose levels and other components to understand their influence on liver function. Additionally, it is important to investigate the underlying biological mechanisms through which low-fructose plant-based diets provide liver protection. Future studies should evaluate the quality of plant-based diets, including the role of fiber, antioxidants, and overall nutrient density. It is also important to include diverse populations in these studies to enhance the generalizability of the findings. Pursuing these research avenues will help gain a deeper understanding of the intricate relationship between plant-based diets and liver health. This understanding will ultimately help in developing more effective dietary recommendations for preventing hepatic steatosis and fibrosis.
This study illustrated that following plant-based diets is associated with a decreased risk of liver fibrosis. It also found that high fructose intake can increase the risk of hepatic steatosis. Therefore, it is important to differentiate between sources of fructose. Whole fruits, rich in fiber and polyphenols, might have protective effects, whereas refined or concentrated sources might promote liver fat accumulation. For optimal liver health, clinical advice should emphasize plant-based diets high in fiber, antioxidants, and minimally processed foods, while limiting fructose-dense products such as fruit juices, SSBs, and sweetened plant-based snacks. Furthermore, encouraging consumption of whole vegetables, legumes, whole grains, and moderate amounts of fruit might help enhance anti-inflammatory responses and support the beneficial modulation of gut microbiota. Future clinical trials are warranted to confirm these findings and to assess the mechanistic pathways mediating the effects of plant-based diets on liver outcomes.
Data availability
The data analyzed in this study is subject to the following licenses/restrictions: The data presented in this study are available on request from the steering committee of the RaNCD. The data are not publicly available as this is an ongoing cohort study. Requests to access these datasets should be directed to YP; Email: yahya.pasdar@kums.ac.ir.
References
Arab, J. P., Arrese, M. & Trauner, M. Recent insights into the pathogenesis of nonalcoholic fatty liver disease. Annu. Rev. Pathol. 13, 321–350 (2018).
Younossi, Z. M. et al. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology 77, 1335–1347 (2023).
Younossi, Z. M., Marchesini, G., Pinto-Cortez, H. & Petta, S. Epidemiology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: implications for liver transplantation. Transplantation 103, 22–27 (2019).
Guasch-Ferré, M. & Willett, W. The mediterranean diet and health: A comprehensive overview. J. Intern. Med. 290, 549–566 (2021).
Baek, S. U., Kim, T., Lee, Y. M., Won, J. U. & Yoon, J. H. Association between dietary quality and Non-Alcoholic fatty liver disease in Korean adults: A nationwide, Population-Based study using the Korean healthy eating index (2013–2021). Nutrients 16, 1516 (2024).
Zhang, W. et al. Healthy eating Index-2015 in relation to risk of metabolic dysfunction-associated fatty liver disease among US population: National health and nutrition examination survey 2017–2018. Front. Nutr. 9, 1043901 (2023).
Hu, Q. et al. The hepatoprotective effects of plant-based foods based on the gut–liver axis: A prospective review. Crit. Rev. Food Sci. Nutr. 63, 9136–9162 (2023).
Aziz, T., Hussain, N., Hameed, Z. & Lin, L. Elucidating the role of diet in maintaining gut health to reduce the risk of obesity, cardiovascular and other age-related inflammatory diseases: recent challenges and future recommendations. Gut Microbes. 16, 2297864 (2024).
Dinu, M., Abbate, R., Gensini, G. F., Casini, A. & Sofi, F. Vegetarian, vegan diets and multiple health outcomes: A systematic review with meta-analysis of observational studies. Crit. Rev. Food Sci. Nutr. 57, 3640–3649. https://doi.org/10.1080/10408398.2016.1138447 (2017).
Jensen, T. et al. Fructose and sugar: A major mediator of non-alcoholic fatty liver disease. J. Hepatol. 68, 1063–1075 (2018).
Arslan, S. et al. Innovative approaches to integrating plant-based nutrition in clinical care: a path to better patient outcomes. Clin. Sci. Nutr. 6, 175–190 (2024).
Pasdar, Y. et al. Cohort profile: Ravansar Non-Communicable disease cohort study: the first cohort study in a Kurdish population. Int. J. Epidemiol. 48, 682–683f (2019).
Kazemi Karyani, A. et al. Socioeconomic gradient in physical activity: findings from the PERSIAN cohort study. BMC Public. Health. 19, 1–11 (2019).
Han, A. L. Validation of fatty liver index as a marker for metabolic dysfunction-associated fatty liver disease. Diabetol. Metab. Syndr. 14, 44 (2022).
Bedogni, G. et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 6, 1–7 (2006).
Eren, F., Kaya, E. & Yilmaz, Y. Accuracy of Fibrosis-4 index and non-alcoholic fatty liver disease fibrosis scores in metabolic (dysfunction) associated fatty liver disease according to body mass index: failure in the prediction of advanced fibrosis in lean and morbidly obese individuals. Eur. J. Gastroenterol. Hepatol. 34, 98–103 (2022).
Ishiba, H. et al. The novel cutoff points for the FIB4 index categorized by age increase the diagnostic accuracy in NAFLD: a multi-center study. J. Gastroenterol. 53, 1216–1224. https://doi.org/10.1007/s00535-018-1474-y (2018).
Eghtesad, S. et al. Validity and reproducibility of a food frequency questionnaire assessing food group intake in the PERSIAN cohort study. Front. Nutr. 10 https://doi.org/10.3389/fnut.2023.1059870 (2023).
Tedeschi, S. K. et al. Relationship between fish consumption and disease activity in rheumatoid arthritis. Arthritis Care Res. 70, 327–332 (2018).
Willett, W. Nutritional Epidemiology (Oxford University Press, 2012).
Martínez-González, M. A. et al. A provegetarian food pattern and reduction in total mortality in the prevención Con Dieta mediterránea (PREDIMED) study. Am. J. Clin. Nutr. 100, 320S–328S (2014).
Turati, F. et al. Indices of healthy and unhealthy plant-based diets and the risk of selected digestive cancers. Clin. Nutr. 44, 76–85. https://doi.org/10.1016/j.clnu.2024.11.039 (2025).
Trautwein, E. A. & McKay, S. The role of specific components of a plant-based diet in management of dyslipidemia and the impact on cardiovascular risk. Nutrients 12, 2671 (2020).
Mazidi, M. & Kengne, A. P. Higher adherence to plant-based diets are associated with lower likelihood of fatty liver. Clin. Nutr. 38, 1672–1677. https://doi.org/10.1016/j.clnu.2018.08.010 (2019).
Li, X. et al. A healthful Plant-Based diet is associated with lower odds of nonalcoholic fatty liver disease. Nutrients 14 https://doi.org/10.3390/nu14194099 (2022).
Lv, Y. et al. Plant-based diets, genetic predisposition and risk of non-alcoholic fatty liver disease. BMC Med. 21, 351. https://doi.org/10.1186/s12916-023-03028-w (2023).
Ratjen, I. et al. Adherence to a plant-based diet in relation to adipose tissue volumes and liver fat content. Am. J. Clin. Nutr. 112, 354–363. https://doi.org/10.1093/ajcn/nqaa119 (2020).
Liu, W. et al. Meta-analysis of the association between major foods with added Fructose and non-alcoholic fatty liver disease. Food Funct. 14, 5551–5561. https://doi.org/10.1039/d3fo00882g (2023).
Ter Horst, K. W. & Serlie, M. J. Fructose Consumption, Lipogenesis, and Non-Alcoholic Fatty Liver Disease. Nutrients 9, (2017). https://doi.org/10.3390/nu9090981
Nomura, K. & Yamanouchi, T. The role of fructose-enriched diets in mechanisms of nonalcoholic fatty liver disease. J. Nutr. Biochem. 23, 203–208 (2012).
Tappy, L. & Lê, K. A. Metabolic effects of fructose and the worldwide increase in obesity. Physiological reviews (2010).
Bäckhed, F., Manchester, J. K., Semenkovich, C. F. & Gordon, J. I. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proceedings of the National Academy of Sciences 104, 979–984 (2007).
Del Rio, D., Costa, L. G., Lean, M. & Crozier, A. Polyphenols and health: what compounds are involved? Nutr. Metabolism Cardiovasc. Dis. 20, 1–6 (2010).
Tomova, A. et al. The effects of vegetarian and vegan diets on gut microbiota. Front. Nutr. 6, 447652 (2019).
Chiu, S. et al. Effect of Fructose on markers of non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of controlled feeding trials. Eur. J. Clin. Nutr. 68, 416–423 (2014).
Li, H. Y. et al. Plant-Based foods and their bioactive compounds on fatty liver disease: effects, mechanisms, and clinical application. Oxid. Med. Cell. Longev. 2021 (6621644). https://doi.org/10.1155/2021/6621644 (2021).
3 Li, R., Li, M., Fly, A. D., Bidulescu, A. & Luo, J. Vegetarian diets and risk of nonalcoholic fatty liver disease: An observational study of National Health and Nutrition Examination Survey 2005-2018 using propensity score methods. J. Hum. Nutr. Diet. 37, 643–654. https://doi.org/10.1111/jhn.13290 (2024).
Soleimani, D. et al. Dietary patterns in relation to hepatic fibrosis among patients with nonalcoholic fatty liver disease. Diabetes Metab. Syndr. Obes. 12, 315–324. https://doi.org/10.2147/dmso.S198744 (2019).
Sayegh, N. F. et al. Relation of dietary patterns and nutritional profile to hepatic fibrosis in a sample of Lebanese Non-Alcoholic fatty liver disease patients. Nutrients 14 https://doi.org/10.3390/nu14122554 (2022).
Hernandez-Gea, V. & Friedman, S. L. Pathogenesis of liver fibrosis. Annu. Rev. Pathol. 6, 425–456 (2011).
Tacke, F. & Weiskirchen, R. Update on hepatic stellate cells: pathogenic role in liver fibrosis and novel isolation techniques. Expert Rev. Gastroenterol. Hepatol. 6, 67–80 (2012).
Calderon-Ospina, C. A. et al. Possible genetic determinants of response to phenytoin in a group of Colombian patients with epilepsy. Front. Pharmacol. 11, 555 (2020).
Niki, E. Antioxidant capacity: which capacity and how to assess it? J. Berry Res. 1, 169–176 (2011).
Ray, K. Hepatic stellate cells hold the key to liver fibrosis. Nat. Reviews Gastroenterol. Hepatol. 11, 74–74 (2014).
Duval, F., Moreno-Cuevas, J. E., González-Garza, M. T., Rodríguez-Montalvo, C. & Cruz-Vega, D. E. Protective mechanisms of medicinal plants targeting hepatic stellate cell activation and extracellular matrix deposition in liver fibrosis. Chin. Med. 9, 27–38. https://doi.org/10.1186/s13020-014-0027-4 (2014).
Ramos-Tovar, E. & Muriel, P. Molecular mechanisms that link oxidative stress, inflammation, and fibrosis in the liver. Antioxidants 9, 1279 (2020).
Soleimani, D. et al. Protective effects of propolis on hepatic steatosis and fibrosis among patients with nonalcoholic fatty liver disease (NAFLD) evaluated by real-time two‐dimensional shear wave elastography: A randomized clinical trial. Phytother. Res. 35, 1669–1679 (2021).
Xu, W. et al. A review of edible plant-derived natural compounds for the therapy of liver fibrosis. Eur. J. Gastroenterol. Hepatol. 35, 133–152. https://doi.org/10.1097/meg.0000000000002483 (2023).
Arslan, S., Bozkurt, C. & Bulut, H. The effect of the adherence to the mediterranean diet on frailty in older people with chronic obstructive pulmonary disease. Turk. J. Geriatr./Türk Geriatri Dergisi 25, 600–610 (2022).
Hussain, T. et al. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxidative medicine and cellular longevity 7432797, (2016). https://doi.org/10.1155/2016/7432797 (2016).
Hayat, U. et al. The effect of coffee consumption on the non-alcoholic fatty liver disease and liver fibrosis: A meta-analysis of 11 epidemiological studies. Ann. Hepatol. 20, 100254 (2021).
Calleja, M. A. et al. The antioxidant effect of β-caryophyllene protects rat liver from carbon tetrachloride-induced fibrosis by inhibiting hepatic stellate cell activation. Br. J. Nutr. 109, 394–401 (2013).
Xie, H. et al. Raw and vinegar processed Curcuma Wenyujin regulates hepatic fibrosis via bloking TGF-β/Smad signaling pathways and up-regulation of MMP-2/TIMP-1 ratio. J. Ethnopharmacol. 246, 111768 (2020).
Li, M., Zhou, Y., Zuo, L., Nie, D. & Li X.-a. Dietary fiber regulates intestinal flora and suppresses liver and systemic inflammation to alleviate liver fibrosis in mice. Nutrition 81, 110959 (2021).
Zhang, Q. S. et al. The influence of dietary patterns on gut Microbiome and its consequences for nonalcoholic fatty liver disease. Trends Food Sci. Technol. 96, 135–144 (2020).
Ralli, T., Neupane, Y. R., Saifi, Z. & Kohli, K. Gut microbiota as an emerging therapeutic avenue for the treatment of nonalcoholic fatty liver disease. Curr. Pharm. Design. 27, 4677–4685 (2021).
Acknowledgements
We sincerely appreciate all participants and the Student Research Committee, Kermanshah University of Medical Sciences, Kermanshah, Iran. This research was supported by Kermanshah University of Medical Sciences (grant number: 4020046). The Iranian Ministry of Health and Medical Education has contributed to the funding used in the PERSIAN Cohort through Grant no 700/534.
Funding
This work was supported by the Vice-Chancellor for Research at the Kermanshah University of Medical Sciences, Kermanshah, Iran (grant number: 4020046).
Author information
Authors and Affiliations
Contributions
Ali Azizi and Mahsa Miryan analyzed the data, drafted the manuscript, and prepared Tables. Yahya Pasdar and Mojgan Moradi collected the data. Ali Azizi, Mojgan Moradi, Yahya Pasdar, and Mahsa Miryan designed this work and revised the manuscript. All authors approved the final manuscript and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
Consent is available for publication.
Ethics approval and consent to participate
The study was approved by the ethics committee of Kermanshah University of Medical Sciences (IR.KUMS.REC.1401.426). All methods were carried out by relevant guidelines and regulations. All the participants were provided oral and written informed consent. All methods were carried out according to relevant guidelines and regulations. This study was conducted by the Declaration of Helsinki.
Ethics approval statement
The RaNCD cohort was conducted by the Declaration of Helsinki, and written informed consent was obtained from all participants. The current study was approved by the steering committee of the RaNCD and the ethics committee of Kermanshah University of Medical Sciences (IR.KUMS.REC.1402.403).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Miryan, M., Azizi, A., Pasdar, Y. et al. Adherence to plant based diets reduce the risk of hepatic fibrosis in nonalcoholic fatty liver disease. Sci Rep 15, 17403 (2025). https://doi.org/10.1038/s41598-025-02613-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-02613-8