Abstract
Oxidative stress is known to be associated with epilepsy, and antiseizure medication treatment, albeit with limited consensus on the specific oxidative stress pathways/proteins involved. Identifying these can reveal novel therapeutic targets for epilepsy management. This study utilized network pharmacology to identify potential protein targets of carbamazepine and valproic-acid that are implicated in oxidative stress and epilepsy, thereby highlighting their therapeutic potential. Drug targets for carbamazepine and valproic-acid were predicted using SuperPred/SwissTargetPrediction, while genes associated with epilepsy and oxidative stress were obtained from DisGeNET and GeneCards. Common proteins were identified, and a protein–protein interaction network was constructed using STRING, followed by analysis via Cytoscape. Hub proteins identified were EGFR, GSK3B, and STAT3 for carbamazepine, and PTGS2, mTOR, and TLR4 for valproic-acid. Molecular docking revealed a strong binding affinity of carbamazepine to its targets (ΔGbind > − 5 kcal/mol) and binding of valproic-acid to its targets (ΔGbind > − 3 kcal/mol), with PTGS2 showing the strongest interaction with valproic-acid (− 5.06 kcal/mol). These findings underscore EGFR, GSK3B, and STAT3, for carbamazepine and PTGS2, mTOR, and TLR4 for valproic-acid as pivotal therapeutic targets in oxidative stress-associated epilepsy. These identified proteins can be targeted by add on antioxidants to alleviate oxidative stress generated by chronic antiseizure medication.
Similar content being viewed by others
Introduction
Epilepsy is the world’s fourth most widespread neurological illness, with approximately 50 million global1 and 12 million Indian patients2. It is estimated that almost 75% of the patients suffering from this chronic, non-contagious disease remain untreated3. Many epileptic patients are from developing or low-income countries, where they may be ostracized due to this condition. With developmental issues in children like depression, social withdrawal and parental dependence, and impediments in gaining education, employment, and even matrimonial partners for adults, epilepsy can be a hindrance throughout a patient’s entire life4,5. A multitude of psychosocial problems along with a considerable mortality rate among epileptic patients, makes it evident that there is a lack of an efficient treatment for epilepsy, establishing it as an international medical problem. Usually, the first preferred method of treatment for epilepsy is the use of antiseizure medicines (ASMs) to control symptomatic seizures since ASMs are drugs that can reduce either the frequency, intensity, or both of seizures in epileptic patients6. Their mode of action generally targets three key mechanisms-potentiation of Gamma-aminobutyric acid (GABA), decreasing the excitatory events related to glutamate, or blocking of voltage-gated sodium/calcium channels7. Two of the commonly prescribed ASMs in India are carbamazepine (CBZ) and valproic acid (VPA)8. These are first-generation ASMs and are widely prescribed because of factors like their relatively low cost and established safety profile. The main mode of action of CBZ is via the inhibition of sodium channels, although it is contested9, since CBZ has also been shown to bind to voltage-gated calcium channels10, decreasing the flux of Ca+2 across the neuronal membrane, and also increase the effect of GABA11,12. It is hypothesized that CBZ limits the firing of action potentials by binding to sodium-operated channels, leading to a decrease in the stimulatory effects on neurons13. On the other hand, it is assumed that VPA primarily attenuates seizures by indirectly inhibiting GABA metabolism, preventing the breakdown of GABA, leading to an increase in GABA quantity, allowing it to exert more effect as an inhibitory neurotransmitter, which thus reduces neuronal activity14. Although not inherently toxic, extended treatment with ASMs—which is common for patients suffering from a chronic disease like epilepsy—can lead to the production and accumulation of toxic secondary metabolites inside the cells, leading to side effects like allergic reactions, cell death and organ damage in severe cases6.
There are various mechanisms/pathways through which drugs like ASMs can cause cell damage, inflammation, hormonal stress, oxidative stress, etc. Recently, it was observed that VPA administration in Huh7 cells caused cell damage through the process of endoplasmic reticulum stress—caused due to the oxidation of glutathione (GSH) to glutathione disulfide (GSSG) as a consequence of GSH involvement in oxidative protein folding15 and lipid peroxidation. Treatment with Obeticholic acid (OCA) led to the activation of the Farnesoid X receptor (FXR), inducing various oxidative stress pathways, which in turn led to the alleviation of oxidative stress induced due to VPA16. Similarly, a study performed using cortical neurons from rats showed that VPA treatment led to alterations in antioxidant defenses like GSSG and GSH and increases in the production of reactive oxygen species (ROS), mitochondrial damage, and lipid peroxidation, with an overall decrease in cell viability17. In another study performed to check the effectiveness of selenium (Se) and CBZ treatment using adult rats with pentylenetetrazol (PTZ) induced seizures, it was observed that simultaneous treatment with Se and CBZ alleviated PTZ-induced effects by diminishing epileptic spikes and markers of oxidative stress except lipid peroxidation, which was increased18. Another study investigated the effect of CBZ-induced renal toxicity in rat models. They observed that compared to the controls, the total antioxidant status was decreased, total oxidant status was increased, and markers of apoptosis—like PARP-1 and Caspase-3 were elevated in the CBZ-treated group, leading to their conclusion that renal toxicity due to CBZ treatment occurs through oxidative stress-mediated apoptosis19. These studies show that both CBZ and VPA are highly involved in causing oxidative stress-mediated side effects. In contrast, other first-line ASMs such as lamotrigine, not only avoid triggering oxidative stress but may even reduce it20,21, thus making CBZ and VPA prime targets for our study. With the large number of interconnected pathways, along with the sheer number of proteins involved with oxidative stress, it is a herculean task to identify the involvement of specific proteins in response to specific drugs. Therefore, in this paper, we propose an in silico methodology using network pharmacology to identify specific proteins that could be involved with CBZ and VPA-induced oxidative stress in the case of epilepsy. Furthermore, exogenous antioxidants can be used to target the identified targets as an add-on in epilepsy therapy.
Network pharmacology is a cost-effective and relatively easy-to-pick-up approach which shows considerable promise in the field of drug development, because of its reliability due to its basis in bioinformatics, polypharmacology, and systems biology22. It describes disease-related processes as networks by mapping the disease-related genes/proteins and drug targets to biomolecular networks, then performing analysis on the interactions between the proteins, biological systems, drug compounds, and diseases23. To our knowledge, this is the first study of its kind to utilize network pharmacology to identify genes which are associated with epilepsy, are potential targets of the ASMs CBZ and VPA, and are also involved with oxidative stress, to identify proteins of which specific oxidative stress pathways are induced due to CBZ and VPA treatment in epilepsy therapy.
Results
We devised an in silico pipeline to achieve our objective, which has been illustrated (Fig. 1), the results of which we have described below.
In silico methodology for identifying the key proteins involved with epilepsy, antiseizure medications, and oxidative stress, using network pharmacology †ASM – Antiseizure medication; CBZ – Carbamazepine; VPA – Valproic acid; PPI – Protein–protein interaction network, (Figure created using MS PowerPoint Version 2501).
Identification of putative targets of CBZ and VPA
103 potential targets of VPA were identified using SuperPred, with 103 UniProt IDs. 100 potential targets of VPA were identified using SwissTargetPrediction, with 106 UniProt IDs. In the case of CBZ, 94 proteins with 94 UniProt IDs were identified as potential targets from SuperPred, and 100 potential targets with 110 UniProt IDs were identified from SwissTargetPrediction. A total of 1140 genes with 1146 UniProt IDs associated with epilepsy were obtained from the DisGeNET database. A total of 10,816 genes involved with oxidative stress with 7561 UniProt IDs were retrieved from the GeneCards database. 52 proteins were found to be common between epilepsy, oxidative stress and VPA targets (Fig. 2A), and 50 proteins were found to be common between epilepsy, oxidative stress and CBZ targets (Fig. 2B). The full list of all the identified targets is available in Supplementary File 1.
Protein–protein interaction (PPI) network
In the case of VPA, for the 52 common proteins, STRING was able to generate a PPI network for 51 proteins. For CBZ, out of the 50 common proteins, STRING was able to construct a PPI network for 49 proteins. Visualization of the PPI network obtained from STRING was performed using Cytoscape 3.10.2 (Fig. 3), which showed that the PPI created for VPA had 51 nodes (proteins) and 187 edges (interactions), while the PPI created for CBZ had 49 nodes and 190 edges.
Protein–protein Interaction (PPI) network created using the proteins common between epilepsy, oxidative stress, and (A) VPA targets and (B) CBZ targets. Each circular “node” represents a protein, and the lines connecting the nodes are called “edges” which represent the interactions—either direct or indirect—between the proteins. (Figure created using Cytoscape 3.10.1).
Identification of hub proteins
The Network analyzer and cytohubba plugins were used to calculate the Degree and Betweenness Centrality (BC) scores of the PPI networks. The most relevant protein targets of VPA based on degree were found to be PTGS2, mTOR and TLR4 with a degree of 22, 20 and 19 respectively. Based on the BC score, the most relevant targets of VPA were PTGS2, mTOR and CNR1 with a BC score of 0.1583205, 0.1500786, and 0.1229429 respectively. The three most relevant protein targets of CBZ based on degree were EGFR, GSK3B, and STAT3 with a degree of 27, 24, and 20 respectively, and the three most relevant CBZ targets based on BC score were EGFR, GSK3B, and GRM5 with BC scores of 0.204467, 0.1688258, and 0.1061541 respectively. The top 10 proteins based on degree and BC score for both drugs are listed in Supplementary Tables S2-S5 (Supplementary File 2) while the full ranking of the proteins is listed in Supplementary File 1.
Molecular docking analysis
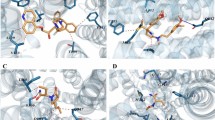
We evaluated the binding affinities of the VPA and CBZ compounds with the selected targets using AutoDock. The co-crystallized ligands were removed from the active sites of two proteins (MTOR and PTGS2) for VPA targets and three proteins (EGFR, GSK3B and STAT3) for CBZ. As there is no co-crystallized ligand for TLR4, we considered blind docking for TLR4 with VPA. The co-crystallized ligands were considered as the reference molecule to evaluate the binding mode of the drug molecules. The docking results revealed that the compounds were bound well in the active site of the targets. As depicted in Fig. 4 the drug molecules were bound well in the active site of the proteins.
The protein–ligand interaction of compound A) Co-crystallized ligand in the active site of EGFR B) CBZ with EGFR C) Co-crystallized ligand in the active site of GSK3B D) CBZ with GSK3B E) Co-crystallized ligand in the active site of STAT3 F) CBZ with STAT3 G) Co-crystallized ligand in the active site of MTOR H) VPA with MTOR I) Co-crystallized ligand in the active site of PTGS2 J) VPA with PTGS2 and K) VPA with TLR4 †ΔG = Binding energy (in kcal/mol); CBZ-Carbamazepine; EGFR-Epidermal Growth Factor Receptor; GSK3B-Glycogen Synthase 3 Kinase beta; STAT3-Signal Transducer and Activator of Transcription 3; VPA-valproic acid; PTGS2-Prostaglandin-Endoperoxide Synthase 2; MTOR-Mammalian Target of Rapamycin; TLR4-Toll Like Receptor 4 (Figure created using PyMOL V. 2.5.3).
In case of EGFR, GLN791 and THR790 are the important amino acids (Fig. 4A,B). A mutation at the position of T790M is commonly associated with resistance to first-generation tyrosine kinase inhibitors (TKIs) such as gefitinib24. Glutamine 791 lies near the ATP-binding site of EGFR, and it plays a role in stabilizing the kinase’s interaction with ATP or inhibitors25. Interestingly, the CBZ compounds have interactions with these two amino acids. GSK3B is known for its role as a negative regulator for transcription factors, microtubules, and Wnt signalling, as well as in the hormonal control of glucose homeostasis as well as neurological disorders26,27 This crystal structure reveals that VAL 135 and ASP 133 are important, and interactions with these amino acids may enhance the inhibitor’s activity (Fig. 4C,D). The CBZ compound interacts with both the amino acids VAL 135 and ASP 133 of GSK3B. Docking reveals the presence of the amino acids SER636, ARG609, GLU612, GLU638 and SER613 in STAT3. Interestingly, CBZ interacts with one of the amino acids GLU638 (Fig. 4E,F). The residue GLU638 in STAT3 has been studied in the context of drug targeting because it resides in the SH2 (Src Homology 2) domain of STAT328.
For VPA, the targets are MTOR, PTGS2 and TLR4. MTOR has its role in central serine/threonine protein kinase, regulating cellular metabolism in response to growth factors and stress signals29. While VAL2240 and ASP2195 are the important amino acids of the crystal structures of MTOR, particularly in the kinase domain (Fig. 4G,H). Our results show VPA interacting with the amino acid ASP2357, which is also found within the kinase domain of MTOR. ASP2357 may often contribute to stabilizing interactions, enhancing the drug’s specificity and affinity. In the case of PTGS2, the key amino acids are ARG513, PHE518 and ILE617. VPA compounds interact with the active site residue ARG513, which is crucial for drug efficacy and also plays a crucial role in ligand binding and catalysis (Fig. 4I,J). TLR4 is known for mechanistically activating NFκB, leading to cytokine secretion and inflammatory response, which is significant since developing TLR4 modulators shows therapeutic promise for attenuating inflammation-related disorders30. Both the amino acids LYS230 and ARG257 are part of the highly conserved binding interface between TLR4 and VPA (Fig. 4K). Tables 1 and 2 indicate their binding score (kcal/mol), Ligand efficiency, Inhibitory constant, and amino acid interactions of VPA and CBZ with their targets. This shows the identified targets and the compounds VPA and CBZ interact with the specific amino acids and may act as potential therapeutic approaches.
Discussion
The main goal of this study was to employ network pharmacology to identify the connecting links between epilepsy, antiseizure medications (ASMs) and oxidative stress, in an attempt to pinpoint specific oxidative stress pathway components which may possess the potential to be targeted in epilepsy therapy. Oxidative stress has already been identified as a participant in both damages caused due to epilepsy and chronic ASM treatment31,32, making it a prime candidate for developing potential novel therapies for epilepsy.
The proposed in silico pipeline was used to identify the potential protein targets of the ASMs carbamazepine (CBZ) and valproic acid (VPA). 50 unique proteins were found to be potential targets of CBZ, in addition to being involved with epilepsy alongside oxidative stress which were then used to generate a protein–protein interaction (PPI) network, whose topological parameters—namely “Degree” and “Betweenness Centrality (BC)” —were considered to identify the hub proteins. In a PPI network, the proteins are called “nodes”, while the interactions between proteins are called “edges”. The degree of a node in a PPI tells us the number of edges directly connected to that node. A higher node degree means that a specific protein has direct interactions with a higher number of proteins in the network, marking it as a “hub” protein33. The BC of a node in a PPI determines how often a node acts as a link along the shortest path between two other nodes in the network. High BC nodes are essential for preserving effective network communication, and a high BC score shows how much control a particular node has over how the other nodes interact with one another33.
In our study, the top 3 hub proteins for the CBZ targets based on the degree were identified, namely Epidermal growth factor receptor (EGFR), Glycogen synthase kinase 3 beta (GSK3B) and Signal Transducer and Activator of Transcription 3 (STAT3), whereas EGFR, GSK3B, and Glutamate metabotropic receptor 5 (GRM5) were the ones identified based on BC score. EGFR is a tyrosine kinase receptor that binds to ligands of the EGF family and activates several signaling cascades34. It is known to activate major cascades like PI3 kinase-AKT, RAS-RAF-MEK-ERK, and STATs cascades35, and has been hypothesized that it also activates the NFκB signaling cascade36, which is a well-known inflammatory and oxidative stress pathway. The role of EGFR in suppressing oxidative stress markers in an animal model of Parkinson’s disease has also been clarified by a recent study, using Lapatinib (LAP), an anti-cancer drug that acts by mediating oxidative stress, with proven neuroprotective effects in case of epilepsy37.They observed that LAP restored glutathione/glutathione peroxidase (GSH/GPx) through the suppression of p-EGFR/c-SRC/PKCβII/PLC-γ/ACSL-4 and PTGS238. GSK3B is a constitutively active protein kinase that acts as a negative regulator for transcription factors, microtubules, Wnt signalling, as well as in the hormonal control of glucose homeostasis39,40. GSK3B inhibition can induce the cysteine/glutamate transporter (xCT), through Phosphoinositide-3 kinases (PI3Ks), which imports cysteine and exports glutamate (an excitatory neurotransmitter), leading to an increase in cerebellar glutamate, which may play a role in epilepsy. Using human hippocampal samples and mouse neuronal cells, one study discovered that GSK3B inhibition led PI3Ks to induce xCT, which is essential for the modulation of oxidative stress resistance of nerve cells by the PI3Ks. This pathway of xCT induction by neuronal activity was also prevalent in the hippocampi of epileptic patients, pointing to its potential role in epilepsy, probably via the upregulation of extracellular glutamate41. STAT3 is responsible for mediating cellular responses to growth factors, interleukins, etc., and plays a part in cellular processes like apoptosis42, while also acting as a regulator of inflammatory responses43. In one study, STAT3 expression was increased in hippocampal neurons of rats induced with epilepsy. Overexpression of miR-98-5p, an upstream regulator of STAT3, led to STAT3 inhibition, resulting in the repression of oxidative stress and neuronal apoptosis44. A brief overview of how the hub proteins identified from CBZ targets are involved with oxidative stress/epilepsy is provided in Fig. 5.
The involvement of the identified hub proteins from CBZ targets— (A) EGFR, (B) GSK3B, and (C) STAT3 with epilepsy/oxidative stress. Since the identified hub proteins have been shown to be involved with oxidative stress/epilepsy through various mechanisms, we are presented with a variety of potential therapeutic targets. The hub proteins together with any other components of related pathways can be exploited therapeutically. †CBZ-Carbamazepine; EGFR-Epidermal Growth Factor Receptor; GSH-Reduced glutathione; GPx-Glutathione peroxidase; xCT-Cysteine/glutamate transporter; PI3K-Phosphoinositide-3 kinase; GSK3B-Glycogen Synthase 3 Kinase beta; STAT3-Signal Transducer and Activator of Transcription 3 (Figure created using MS PowerPoint Version 2501).
For VPA targets, the top 3 hub proteins based on degree were Prostaglandin-Endoperoxide Synthase 2 (PTGS2/COX2), Mammalian Target of Rapamycin (mTOR), and Toll Like Receptor 4 (TLR4), while the top 3 hub proteins based on BC score were PTGS2, mTOR, and Cannabinoid Receptor 1 (CNR1). PTGS2 is a dual peroxidase and cyclooxygenase enzyme which is involved in the inflammatory response45. Multiple studies have already proven the involvement of PTGS2 in the case of epilepsy. In a post-traumatic epilepsy rat model, an increase in PTGS2 protein and mRNA levels was noted after induction of seizures. Treatment with an antagonist of the EP2 receptor (a downstream regulator of PTGS2) caused a significant reduction in the seizure frequency, incidence and duration of seizures, alluding to the role of PTGS246. In another study evaluating the potential of Pinoresinol-4-O-β-d-glucopyranoside (Pgu) using the lithium/pilocarpine-induced rat model, seizure induction caused an increase in the levels of malondialdehyde (MDA) —a marker of oxidative lipid damage—and decreased catalase activity, while also increasing the expression levels of PTGS2, all of which was reversed by Pgu pretreatment, which also mitigated neurodegeneration47. Based on another study using kainate-induced rat models, PTGS2 expression was elevated in the CA3, cortex, and dentate gyrus regions 18–24 h after seizures. Administration of a selective PTGS2 inhibitor led to an increase in the survival rates of CA3 neurons compared to the controls48. mTOR is a central serine/threonine protein kinase which regulates cellular metabolism in response to growth factors, stress signals, etc. and is responsible for the direct/indirect phosphorylation of nearly 800 proteins49. In a model of temporal lobe epilepsy (TLE), rats with induced seizures showed elevated levels of MDA, TNFα, hippocampal apoptosis, and decreased superoxide dismutase (SOD) activity. Overexpression of a lncRNA MEG3 reversed these, and decreased apoptosis through the activation of PI3K/AKT/mTOR pathway50. In another study using HT22 cells, huperzine A (HupA) + wortmannin treatment stopped the downregulation of p-mTOR, p-Akt, and p-p70s6 kinase under oxidative glutamate toxicity, leading to the conclusion that HupA abrogates oxidative glutamate toxicity in mouse hippocampal cells via the activation of the BDNF/TrkB-dependent PI3K/Akt/mTOR signaling pathway51. TLR4 is a transmembrane receptor that recognizes damage and pathogen-associated molecular patterns, functioning as a pattern recognition receptor52. It mechanistically activates NFκB, leading to cytokine secretion and inflammatory response53. In one study using human SH-SY5Y neuronal cells, it was observed that oxidative stress due to H2O2 increased expression of TLR4 and PTGS2, with NFκB signaling being activated after upregulation of TLR454. In a PTZ-induced rat model of epilepsy, treatment using Rhein (cassic acid) reduced duration and frequency of seizures, showing anticonvulsant activity. TLR4 and NFκB levels, which were high in PTZ group were also reduced on treatment with Rhein, which also reversed the upregulation of the IKBα protein which is an activator of NFκB55. A brief overview of how the hub proteins identified from VPA targets are involved with oxidative stress/epilepsy is provided in Fig. 6.
The involvement of the identified hub proteins from VPA targets— (A) PTGS2, (B) mTOR, and (C) TLR4 with epilepsy/oxidative stress. Since the identified hub proteins have been shown to be involved with oxidative stress/epilepsy through various mechanisms, we are presented with a variety of potential therapeutic targets. Upstream regulators of mTOR which can cause its overexpression, inhibitors of inflammatory pathway components like NFκB, PTGS2, TLR4 etc., can all be targeted by designing epilepsy therapy accordingly. † VPA-valproic acid; PTGS2-Prostaglandin-Endoperoxide Synthase 2; mTOR-Mammalian Target of Rapamycin; TLR4-Toll Like Receptor 4; NFκB-Nuclear factor kappa B (Figure created using MS PowerPoint Version 2501).
From the studies discussed here, it is apparent that these genes may be directly or indirectly targeted in epileptic patients by developing an auxiliary treatment in adjunct to ASM treatment. However, the feasibility of this study needs to be verified. The expression of the identified targets—at both the RNA and protein levels—needs to be measured between groups with induced excitotoxicity, excitotoxicity + ASM, and excitotoxicity + ASM + antioxidants. Other markers of neuronal excitation like calcium influx and electrophysiological alterations also need to be compared between the treatment groups. Experiments need to be designed where the inhibitors/activators of the identified genes are administered simultaneously with various ASMs to evaluate the effects of their co-treatment. For example, comparing the markers of neuronal excitation between groups treated with only CBZ, CBZ + staurosporine (GSK3B activator)56 and CBZ + lithium (GSK3B inhibitor)57 will shed light on the practicality of utilizing GSK3B as a target in patients which are prescribed CBZ therapy. Similarly, experiments can be designed to observe the effect of treatment using antioxidants like ascorbic acid, α-tocopherol, α-lipoic acid, etc. on the expression of genes and proteins like TLR4 and PTGS2, and compare the outcomes of groups treated with and without antioxidants. Empirical evidence is necessary to ensure the practicality of targeting these identified genes as therapy for epileptic patients. Additional experiments also need to be performed to eliminate the possibility of contraindications of any possible adjuvants with the ASMs.
Utilizing a network pharmacology approach enables a systematic study of potential target proteins and pathways of drugs at the molecular level, allowing us a closer look at potential therapeutic targets. The molecular docking results revealed that the VPA and CBZ compounds could interact with the identified target’s active sites, and thus, the identified proteins may be suited for oxidative stress mediated therapy in epilepsy.
Methods
Identification of potential drug targets
The canonical SMILES (Simplified Molecular Input Line Entry System) of the drugs CBZ and VPA were obtained from PubChem58. These SMILES were then used as input for the tools SuperPred59 and SwissTargetPrediction (STP)60 to identify potential protein targets of the drugs. SuperPred is a web-based tool designed to predict the Anatomical Therapeutic Chemical (ATC) classification and potential targets of compounds, whereas STP uses multi-logistic regression models, leveraging 2D and 3D fingerprint similarity to forecast the likely targets of compounds. SuperPred targets with probability > 0, and STP targets possessing at least one known active site for our query molecule were selected.
Identification of the proteins involved with the disease epilepsy and oxidative stress
We retrieved the genes associated with epilepsy by using the keyword “epilepsy” (CUI: C0014544) from the DisGeNET database—a comprehensive collection of gene-disease associations from curated databases, experimental studies, inferred data, and existing literature, standardizing it through established ontologies61. On the other hand, genes associated with oxidative stress were obtained from the GeneCards database by using the keywords “oxidative stress”62. Genes were sourced from all 18 sections of the GeneCards database. Out of a total of seven, only three types of genes were selected i.e., Protein Coding, RNA gene, and Functional Elements. DisGeNET targets with gda score > 0 and GeneCards targets with Relevance score > 0 were selected.
Identification of the common proteins between drug targets, epilepsy, and oxidative stress
The list of UniProt IDs obtained in the previous three steps was used as input for the online tool Venny 2.1.063, which can identify common components and display them as Venn Diagrams. The list of drug targets was compiled after combining the outputs of SuperPred and SwissTargetPrediction and removing duplicates, and used as Set A, while epilepsy genes were used as Set B and oxidative stress-related genes were used as Set C. The common proteins between these three sets were considered as possible targets.
Creation of the protein–protein interaction (PPI) network
Protein–protein Interaction (PPI) networks were created with the identified common proteins from the targets of VPA and the targets of CBZ respectively using STRING (Search Tool for the Retrieval of Interacting Genes/Proteins)64. The default settings were used to create the PPIs, i.e. a full STRING network with medium confidence (0.400).
Identification of hub proteins
The PPI networks obtained from STRING were then further analyzed using the Cytoscape 3.10.2 software65. Analysis of the networks was performed using the “Network Analyzer”65 and the “cytohubba” plugins66, to obtain information on the topological parameters “Degree” and “Betweenness Centrality” (BC).
Targets and drugs preparation for molecular docking
The compounds VPA and CBZ were obtained from PubChem in .sdf format and converted to .pdb format using Open Babel67. The resulting .pdb files were processed in AutoDock Tools, where Gasteiger charges were applied, and the structures were converted to pdbqt format for docking studies68. The target proteins for VPA, namely MTOR (PDB ID: 4JT5), PTGS2 (PDB ID: 5KIR), TLR4 (PDB ID: 4G8A) and CBZ namely EGFR (PDB ID: 7KXZ), GSK3B (PDB ID: 5KPL), STAT3 (PDB ID: 6NJS) were retrieved from the RCSB Protein Data Bank. Protein preparation involved the removal of co-crystallized ligands, ions, and water molecules, followed by the addition of polar hydrogens and Kollman charges using AutoDock Tools (ADT). The finalized protein structures were then converted to pdbqt format using ADT. The 2D structures were converted to 3D structures and H atoms were added in the ligand preparation and converted to respective pdbqt format.
Molecular docking of VPA and CBZ with the identified proteins
The grid was generated around the co-crystallized ligand of the proteins MTOR, PTGS2, TLR4 EGFR, GSK3B and STAT3. The grid spacing was set to the default 0.375 Å. Centre box values were set to co-crystallized ligand. Supplementary Table S1 (Supplementary File 2) lists the centre box and grid box size values in each protein. The grid parameters file was saved to .gpf file. The .gpf file was given as input, and the grid box was generated using the Autogrid function. We considered the Lamarckian Genetic Algorithm for the molecular docking analysis using AutoDock with the following parameters viz, (i) the number of genetic algorithm (GA) runs 5; (ii) population size: 150; (iii) the number of energy evaluations: 2.5 million (2.0 Å clustered tolerance); and (iv) the maximum number of generations: 27, 000. The final output file contains the docking output in the form of top-scored conformers with binding energies. The interactions for the best conformers were analysed and have been discussed in the results and discussion section. All the online tools and databases used are mentioned in Supplementary Table S2 (Supplementary File 2).
Conclusion
The objective of this investigation was to employ network pharmacology to pinpoint the potential proteins which may be involved in the oxidative stress pathways in response to either epilepsy or epilepsy treatment using two antiseizure medications—carbamazepine and valproic acid. The top three hub proteins for carbamazepine targets were found to be EGFR, GSK3B, and STAT3, while the top three hub proteins for valproic acid targets were found to be PTGS2, mTOR, and TLR4. Both the drugs demonstrated a high affinity for their respectively identified protein targets based on molecular docking which indicate their potential to be targeted using antioxidants which can be administered as adjunct therapy to mitigate oxidative stress during epilepsy. The next step after this would be the validation of the identified hub proteins by conducting further in vitro as well as in vivo studies.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article (and/or) its Supplementary Materials.
References
Epilepsy: a public health imperative. https://www.who.int/publications/i/item/epilepsy-a-public-health-imperative.
Amudhan, S., Gururaj, G. & Satishchandra, P. Epilepsy in India I: Epidemiology and public health. Ann. Indian Acad. Neurol. 18, 263–277 (2015).
Saxena, S. & Li, S. Defeating epilepsy: A global public health commitment. Epilepsia Open 2, 153–155 (2017).
Wo, M. C. M., Lim, K. S., Choo, W. Y. & Tan, C. T. Employability among people with uncontrolled seizures: An interpretative phenomenological approach. Epilepsy Behav. 45, 21–30 (2015).
LaGrant, B., Marquis, B. O., Berg, A. T. & Grinspan, Z. M. Depression and anxiety in children with epilepsy and other chronic health conditions: National estimates of prevalence and risk factors. Epilepsy Behav. 103, 106828 (2020).
Bromfield, E. B., Cavazos, J. E. & Sirven, J. I. An Introduction to Epilepsy. An Introduction to Epilepsy 4 (2006).
Miziak, B. et al. Anti-epileptogenic effects of antiepileptic drugs. Int. J. Mol. Sci. 21, 2340 (2020).
Joshi, R., Tripathi, M., Gupta, P., Gulati, S. & Gupta, Y. K. Prescription pattern of antiepileptic drugs in a tertiary care center of India. Indian J. Pharmacol. 52, 283–289 (2020).
Ambrósio, A. F., Soares-da-Silva, P., Carvalho, C. M. & Carvalho, A. P. Mechanisms of action of carbamazepine and its derivatives, oxcarbazepine, BIA 2–093, and BIA 2–024. Neurochem. Res. 27, 121–130 (2002).
Gambeta, E., Chichorro, J. G. & Zamponi, G. W. Trigeminal neuralgia: An overview from pathophysiology to pharmacological treatments. Mol. Pain 16, 1744806920901890 (2020).
Flynn, S. & Babi, M. A. Anticonvulsants. Pharmacology and Therapeutics for Dentistry: Seventh Edition 176–192 (2017) https://doi.org/10.1016/B978-0-323-39307-2.00012-6.
Granger, P. et al. Modulation of the gamma-aminobutyric acid type A receptor by the antiepileptic drugs carbamazepine and phenytoin. Mol. Pharmacol. 47, 1189–1196 (1995).
Carbamazepine - StatPearls - NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK482455/.
Rosenberg, G. The mechanisms of action of valproate in neuropsychiatric disorders: Can we see the forest for the trees?. Cell. Mol. Life Sci. 64, 2090–2103 (2007).
Kaludercic, N., Deshwal, S. & Di Lisa, F. Reactive oxygen species and redox compartmentalization. Front Physiol. 5, 285 (2014).
Gai, Z., Krajnc, E., Samodelov, S. L., Visentin, M. & Kullak-Ublick, G. A. Obeticholic acid ameliorates valproic acid-induced hepatic steatosis and oxidative stress. Mol. Pharmacol. 97, 314–323 (2020).
Salimi, A., Alyan, N., Akbari, N., Jamali, Z. & Pourahmad, J. Selenium and L-carnitine protects from valproic acid-Induced oxidative stress and mitochondrial damages in rat cortical neurons. Drug Chem. Toxicol. 45, 1150–1157 (2022).
Mohammed, H. S., Aboul Ezz, H. S., Zedan, A. & Ali, M. A. Electrophysiological and neurochemical assessment of selenium alone or combined with carbamazepine in an animal model of epilepsy. Biol. Trace Elem. Res. 195, 579–590 (2020).
Erdem Guzel, E., Kaya Tektemur, N., Tektemur, A. & Etem Önalan, E. Carbamazepine-induced renal toxicity may be associated with oxidative stress and apoptosis in male rat. Drug Chem. Toxicol. 46, 136–143 (2023).
Agarwal, N. B., Agarwal, N. K., Mediratta, P. K. & Sharma, K. K. Effect of lamotrigine, oxcarbazepine and topiramate on cognitive functions and oxidative stress in PTZ-kindled mice. Seizure 20, 257–262 (2011).
Eren, I., Naziroǧlu, M. & Demirdaş, A. Protective effects of lamotrigine, aripiprazole and escitalopram on depression-induced oxidative stress in rat brain. Neurochem. Res. 32, 1188–1195 (2007).
Wang, X., Wang, Z. Y., Zheng, J. H. & Li, S. TCM network pharmacology: A new trend towards combining computational, experimental and clinical approaches. Chin. J. Nat. Med. 19, 1–11 (2021).
Zhang, R., Zhu, X., Bai, H. & Ning, K. Network pharmacology databases for traditional Chinese medicine: Review and assessment. Front. Pharmacol. 10, 123 (2019).
Yun, C. H. et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc. Natl. Acad. Sci. USA 105, 2070–2075 (2008).
Cross, D. A. E. et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 4, 1046–1061 (2014).
McCubrey, J. A. et al. Multifaceted roles of GSK-3 and Wnt/β-catenin in hematopoiesis and leukemogenesis: Opportunities for therapeutic intervention. Leukemia 28, 15–33 (2014).
Singh, S. D., Bharali, P. & Nagamani, S. Exploring bacterial metabolites in microbe-human host dialogue and their therapeutic potential in Alzheimer’s diseases. Mol. Divers https://doi.org/10.1007/S11030-024-11028-Y (2024).
Geiger, J. L., Grandis, J. R. & Bauman, J. E. The STAT3 pathway as a therapeutic target in head and neck cancer: Barriers and innovations. Oral. Oncol. 56, 84–92 (2016).
Wu, Y. et al. The role of serine/threonine protein kinases in cardiovascular disease and potential therapeutic methods. Biomed. Pharmacother. 177, 117093 (2024).
Saleh, H. A., Yousef, M. H. & Abdelnaser, A. The anti-inflammatory properties of phytochemicals and their effects on epigenetic mechanisms involved in TLR4/NF-κB-mediated inflammation. Front. Immunol. 12, 606069 (2021).
Ersan, S., Cigdem, B., Bakir, D. & Dogan, H. O. Determination of levels of oxidative stress and nitrosative stress in patients with epilepsy. Epilepsy Res. 164, 106352 (2020).
Viana, C. E. et al. Lutein-loaded nanoparticles reverse oxidative stress, apoptosis, and autism spectrum disorder-like behaviors induced by prenatal valproic acid exposure in female rats. Neurotoxicology 94, 223–234 (2023).
Doncheva, N. T., Assenov, Y., Domingues, F. S. & Albrecht, M. Topological analysis and interactive visualization of biological networks and protein structures. Nat. Protoc. 7, 670–685 (2012).
Bu, J. et al. CD82 palmitoylation site mutations at Cys5+Cys74 affect EGFR internalization and metabolism through recycling pathway. Acta Biochim. Biophys. Sin. (Shanghai) 54, 400–408 (2022).
Runkle, K. B. et al. Inhibition of DHHC20-mediated EGFR palmitoylation creates a dependence on EGFR signaling. Mol. Cell 62, 385–396 (2016).
Habib, A. A. et al. The epidermal growth factor receptor engages receptor interacting protein and nuclear factor-kappa B (NF-kappa B)-inducing kinase to activate NF-kappa B. Identification of a novel receptor-tyrosine kinase signalosome. J. Biol. Chem. 276, 8865–8874 (2001).
Jia, J. N. et al. Neuroprotective effects of the anti-cancer drug lapatinib against epileptic seizures via suppressing glutathione peroxidase 4-dependent ferroptosis. Front. Pharmacol. 11, 601572 (2020).
Mansour, H. M., Mohamed, A. F., Khattab, M. M. & El-Khatib, A. S. Lapatinib ditosylate rescues motor deficits in rotenone-intoxicated rats: Potential repurposing of anti-cancer drug as a disease-modifying agent in Parkinson’s disease. Eur. J. Pharmacol. 954, 175875 (2023).
Cho, J. H. & Johnson, G. V. W. Primed phosphorylation of tau at Thr231 by glycogen synthase kinase 3beta (GSK3beta) plays a critical role in regulating tau’s ability to bind and stabilize microtubules. J. Neurochem. 88, 349–358 (2004).
Yin, L., Wang, J., Klein, P. S. & Lazar, M. A. Nuclear receptor Rev-erbalpha is a critical lithium-sensitive component of the circadian clock. Science 311, 1002–1005 (2006).
Lewerenz, J. et al. Phosphoinositide 3-kinases upregulate system x c-via eukaryotic initiation factor 2a and activating transcription factor 4-A pathway active in glioblastomas and epilepsy. Antioxid Redox Signal 20, 2907–2922 (2014).
Yuan, Z. et al. Central role of the threonine residue within the p+1 loop of receptor tyrosine kinase in STAT3 constitutive phosphorylation in metastatic cancer cells. Mol. Cell Biol. 24, 9390–9400 (2004).
Cheung, K. L. et al. Distinct roles of Brd2 and Brd4 in potentiating the transcriptional program for Th17 cell differentiation. Mol. Cell 65, 1068-1080.e5 (2017).
Guo, Z., Zhong, W. & Zou, Z. miR-98-5p prevents hippocampal neurons from oxidative stress and apoptosis by targeting STAT3 in epilepsy in vitro. Neuropsychiatr. Dis. Treat. 19, 2319–2329 (2023).
Kim, S. F., Huri, D. A. & Snyder, S. H. Inducible nitric oxide synthase binds, S-nitrosylates, and activates cyclooxygenase-2. Science 310, 1966–1970 (2005).
Rawat, V. et al. Temporal expression of neuroinflammatory and oxidative stress markers and prostaglandin E2 receptor EP2 antagonist effect in a rat model of epileptogenesis. ACS Pharmacol. Transl. Sci. 6, 128–138 (2023).
Youssef, F. S., Menze, E. T. & Ashour, M. L. A potent lignan from prunes alleviates inflammation and oxidative stress in lithium/pilocarpine-induced epileptic seizures in rats. Antioxidants (Basel) 9, 1–15 (2020).
Kawaguchi, K. et al. Cyclooxygenase-2 expression is induced in rat brain after kainate-induced seizures and promotes neuronal death in CA3 hippocampus. Brain Res 1050, 130–137 (2005).
Lipton, J. O. & Sahin, M. The neurology of mTOR. Neuron 84, 275–291 (2014).
Zhang, H., Tao, J., Zhang, S. & Lv, X. LncRNA MEG3 reduces hippocampal neuron apoptosis via the PI3K/AKT/mTOR pathway in a rat model of temporal lobe epilepsy. Neuropsychiatr. Dis. Treat. 16, 2519–2528 (2020).
Mao, X. Y., Zhou, H. H., Li, X. & Liu, Z. Q. Huperzine a alleviates oxidative glutamate toxicity in hippocampal HT22 cells via activating BDNF/TrkB-dependent PI3K/Akt/mTOR signaling pathway. Cell Mol Neurobiol. 36, 915–925 (2016).
Fang, H. et al. TLR4 is essential for dendritic cell activation and anti-tumor T-cell response enhancement by DAMPs released from chemically stressed cancer cells. Cell. Mol. Immunol. 11, 150–159 (2013).
Lee, H. et al. Recombinant human KAI1/CD82 attenuates M1 macrophage polarization on LPS-stimulated RAW264.7 cells via blocking TLR4/JNK/NF-κB signal pathway. BMB Rep. 56, 359–364 (2023).
Arena, A. et al. Oxidative stress and inflammation in a spectrum of epileptogenic cortical malformations: Molecular insights into their interdependence. Brain Pathol. 29, 351–365 (2019).
Yu, L., Yang, J., Yu, W., Cao, J. & Li, X. Rhein attenuates PTZ-induced epilepsy and exerts neuroprotective activity via inhibition of the TLR4-NFκB signaling pathway. Neurosci. Lett. 758, 136002 (2021).
Welz, B. et al. Activation of GSK3 prevents termination of TNF-induced signaling. J. Inflamm. Res. 14, 1717 (2021).
Jitendra Joshi, N. & Raja Sekhar Reddy, A. Navigating the GSK-3β inhibitors as versatile multi-target drug ligands in Alzheimer’s disease intervention—A comprehensive review. Results Chem. 7, 101500 (2024).
Kim, S. et al. PubChem 2023 update. Nucleic Acids Res. 51, D1373–D1380 (2023).
Nickel, J. et al. SuperPred: Update on drug classification and target prediction. Nucleic Acids Res. 42, W26 (2014).
Daina, A., Michielin, O. & Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 47, W357–W3664 (2019).
Piñero, J. et al. The DisGeNET knowledge platform for disease genomics: 2019 update. Nucleic Acids Res. 48, D845–D855 (2020).
Stelzer, G. et al. The GeneCards suite: From gene data mining to disease genome sequence analyses. Curr. Protoc. Bioinform. 2016, 1.30.1-1.30.33 (2016).
Venny 2.1.0. https://bioinfogp.cnb.csic.es/tools/venny/.
Szklarczyk, D. et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 51, D638–D646 (2023).
Shannon, P. et al. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 (2003).
Chin, C. H. et al. cytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 8, 1–7 (2014).
O’Boyle, N. M. et al. Open Babel: An open chemical toolbox. J. Cheminform. 3, 1–14 (2011).
Huey, R., Morris, G. M. & Forli, S. Using AutoDock 4 and AutoDock Vina with AutoDockTools: A Tutorial. http://autodock.scripps.edu. (2012).
Funding
Dr Gurpreet Kaur Grewal acknowledges Department of Science & Technology, India for the SERB TARE grant (TAR/2022/000636) from Anusandhan National Research Foundation (ANRF), Govt. of India under mentorship of Prof. (Dr) Shrikant Kukreti, Nucleic Acids Research Lab, Department of Chemistry, University of Delhi (North Campus), Delhi 110007, India. Funding agency had no role in the decision to submit the manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization: GKG and SK. Methodology and Software: MD, SDS, SN, SM, and GKG. Formal Analysis and Investigation: MD, SDS, SN, SM, and GKG. Writing, review, and editing: MD, SN, AS, RK, SK, and GKG. Figure creation (MS PowerPoint/Cytoscape/AutoDock Tools): MD, SDS and SN. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Desai, M., Singh, S.D., Nagamani, S. et al. Decoding the relationship between oxidative stress and antiseizure medications using network pharmacology and molecular docking. Sci Rep 15, 33294 (2025). https://doi.org/10.1038/s41598-025-02884-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-02884-1