Abstract
The increasing global demand for sustainable food production, coupled with the micronutrient limitations of aquaponic systems, calls for innovative fertilization strategies that enhance crop yield and nutritional quality while preserving environmental integrity. This study evaluates the effectiveness of zinc-functionalized calcium carbonate microparticles (Zn-Alg-CaCO₃ MPs) as a smart foliar fertilizer in aquaponics. Tomato plants (Solanum lycopersicum L. cv. Principe Borghese) were grown in a recirculating aquaponic system and treated with three concentrations of Zn-MPs (10, 50, and 250 ppm) via foliar spray, using a control group treated with water. Each treatment was applied to 15 plants under controlled greenhouse conditions. Yield parameters, antioxidant content, and nutritional quality were assessed. The 250 ppm treatment significantly increased yield per plant (+ 74.9%), number of fruits (+ 44.4%), and average fruit weight (+ 22.1%) compared to control. Antioxidant activity, phenolic, flavonoid, and lycopene content were also enhanced, particularly at the highest dose. Importantly, no increase in zinc was detected in recirculating water, highlighting the environmental safety of foliar application. These findings underscore the potential of Zn-Alg-CaCO₃ MPs as a sustainable solution for precision fertilization in aquaponic agriculture. Further molecular and environmental studies are recommended to optimize and scale this technology.
Similar content being viewed by others
Introduction
Aquaponics is an efficient, sustainable and alternative system of producing vegetables and raising fish in a symbiotic environment1. Aquaponics is receiving increasing attention from both the scientific community and the agri-tech industry, as evidenced by the growing number of publications and technological applications in recent years. The numerous positive aspects of aquaponics include water saving, the possibility of growing products out of seasonality, greater control, product safety, faster vegetal growth, and the spirit of sustainability that characterizes this type of cultivation method2. Despite the beneficial sides, one of the problems that every type of aquaponic system has to face is the depletion of micronutrients over time3. In fact, while macroelements are provided by fish waste, micronutrients must be introduced into the system to allow plants to reach optimal growth and production levels. This research has focused attention on zinc (Zn), a key element that performs numerous functions in plants. At the cellular level, Zn is in fact involved in processes including metabolic and physiological activities, enzymatic activation, and ionic homeostasis4,5. Zn deficiencies lead to phenotypic effects like chlorosis, necrotic spots, plants stunting, and dwarf or malformed leaves5,6. All these effects result in suboptimal growth of the plant which can lead to reduced fruit production. Zn, along with other micronutrients, can be administered through precision agriculture techniques. Among the various possible applications, these techniques involve the use of micro and nanotechnologies, with the advantage of delivering the necessary dose of smart fertilizer to individual plants7. Precision agriculture that utilizes micro and nanotechnologies is still a relatively unexplored field in the world of aquaponics8,9. The demand for developing new methods in agriculture arises from the fact that the global population is constantly increasing, and the resulting higher demand for food is a direct consequence10,11,12. Moreover, climate change necessitates the exploration and adoption of sustainable alternatives to traditional agricultural practices13,14. Therefore, adopting smart cultivation techniques that leverage biotechnologies could be one of the possible solutions to address the challenges of feeding an ever-growing population15 and counteracting climate changes16,17. From these premises, the present research was developed. In our experiment, various concentrations (10, 50, and 250 ppm) of Zn-Alg-CaCO3 MPs were investigated as a smart fertilizer on tomato plants (Solanum lycopersicum L. cv. Principe Borghese) grown in aquaponics. This research provides new information about the application of biotechnologies that exploit micro and nanomaterials in the field of aquaponics and precision agriculture. These types of interdisciplinary studies allow to add value and give credit to sustainability and circular economy, specific to the cultivation methodology in aquaponics.
Results
Recirculating water composition
The results relating to the system water through spectrophotometric tests (Table 1) are reported as micro- and macroelements concentration.
Zn-Alg-CaCO3 MPs characterization
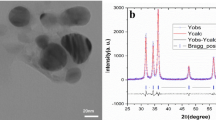
The Zn-Alg-CaCO₃ microparticles were subjected to detailed morphological and chemical characterization by Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray Spectroscopy (EDX) (Fig. 1). SEM imaging revealed that the microparticles exhibited a spherical morphology, with diameters ranging between 3 and 5 μm. High-magnification observations showed that the surface of the microparticles was rough and irregular, characterized by the aggregation of smaller subunits with a minimum diameter of approximately 100 nm. The microparticles appeared organized as hollow structures, with a distinct shell enclosing an internal cavity, suggesting a core–shell-like architecture likely resulting from the synthesis process. The chemical composition of the microparticles was investigated by EDX microanalysis. The corresponding spectra (Fig. 1A) confirmed the presence of calcium (Ca), carbon (C), oxygen (O), and zinc (Zn) as the main constituents of the microparticles, consistent with their expected formulation based on the use of calcium carbonate (CaCO₃) and zinc ions in the synthesis. Additionally, platinum (Pt) signals were detected; this element was introduced during the sputter-coating step performed to enhance sample conductivity and is indicated in the spectrum with an asterisk (*).
The uniform distribution of the detected elements further supported the effective incorporation of calcium and zinc within the microparticles’ structure. Overall, the SEM-EDX analysis demonstrated that the Zn-Alg-CaCO₃ microparticles possessed a well-defined morphological structure and a chemical composition compatible with the intended design for applications as smart foliar fertilizer in aquaponics.
(A) EDX spectrum of the Zn-Alg-CaCO₃ microparticles showing the identified elements. Calcium, carbon, oxygen, and zinc were detected, along with platinum, which is attributed to the sample coating and is indicated by an asterisk (*). (B) SEM micrograph acquired at 15,000× magnification, illustrating a representative microparticle. The image highlights the rough surface morphology and the aggregation of smaller subunits forming the shell. The internal cavity is marked with a red circle.
FTIR structural characterization study of the Zn-Alg-CaCO3 composite material, has been highlighted on Fig. 2 and the main spectral adsorption molecular bands have been also summarized on Table 2 (also containing the attributions of alginate, calcium carbonate and zinc oxide, as the reference compounds, for comparison).
The infrared (IR) spectrum clearly confirms the formation of a bond between ZnO and the alginate-decorated CaCO₃ microparticles. This is evidenced by the shift of vibrational frequencies to higher wavenumbers, corresponding to higher energy levels, compared to those observed for the individual ZnO and alginate/Alg compounds, as reported in Table 2. The nature of this new interaction within the Zn-Alg-CaCO₃ composite, as revealed by the IR spectrum, may originate from both dative coordination bonds and intermolecular hydrogen bonding.
The first type of interaction likely involves coordination between Zn²⁺ ions and the functional groups of the alginate ligands, forming dative bonds. These interactions, which are stronger than hydrogen bonds, can reasonably account for the higher vibrational frequencies observed in the composite. This hypothesis is supported by the presence of IR absorption bands characteristic of chelated species28, such as those observed at 2367 cm⁻¹ and 2500 cm⁻¹ (marked with * in Table 2). At the same time, intermolecular hydrogen bonding between the –OH groups of alginate/Alg physically adsorbed onto the ZnO surface may also contribute to the observed spectral features. This interaction likely explains the broad absorption band centered at 3417 cm⁻¹, which is shifted to lower frequencies compared to the typical –OH stretching band (~ 3600 cm⁻¹) of free, non-hydrogen-bonded hydroxyl groups, consistent with previous reports28.
Yield parameters
The data on fruit yield revealed significant variations across the different treatments (Fig. 3). Overall, all groups treated with 250 ppm Zn-Alg-CaCO3 MPs showed a marked increase in all analyzed fruit yield parameters compared to the CT.
Specifically, the 250 ppm Zn-Alg-CaCO3 MPs treatment led to a + 44.40% and + 48.52% increase in the number of fruits produced per plant compared to the CT and the 10 ppm Zn-Alg-CaCO3 MPs treatment, respectively (Fig. 4A). Likewise, the highest average fruit weight (AFW) was recorded in the 250 ppm Zn-Alg-CaCO3 MPs treatment, demonstrating a significant + 22.10% increase compared to CT; no significant differences were observed among the other treatments (Fig. 4B). To assess the difference in tomato fruit yield across the various treatments, yield per plant (Yield/Plant) was calculated. Therefore, the 250 ppm Zn-Alg-CaCO3 MPs treatment exhibited a significant increase compared to all other groups: +74.90% higher than the CT, + 63.70% higher than the 10 ppm Zn-Alg-CaCO3 MPs treatment, and + 31.90% higher than the 50 ppm Zn-Alg-CaCO3 MPs treatment (Fig. 4C).
Yield parameters. (A) Fruits number produced per plant. (B) Average fruit weight. (C) Yield per plant. The x-axis denotes the treatments, while the y-axis represents the units of measurement. The significances resulting from the comparisons between the various treatments are indicated by asterisks: * p < 0.05; ** p < 0.005; *** p < 0.001.
Quality traits
Soluble solids content (SSC) showed no statistical differences among the treatments (Fig. 5A). The evaluation of phenolic content (PHE) demonstrated that the application of different concentrations had a positive impact compared to the CT. Specifically, the 10 ppm concentration resulted in an enhancement of PHE by approximately + 19.80% relative to the CT; a further increase was observed at the 50 ppm concentration, which improved PHE by + 21.86% compared to the CT (Fig. 5B). The analysis of flavonoid content (FLA) revealed a significant improvement at the highest tested concentration. The 250 ppm treatment group exhibited a significant increase of + 55.02% in FLA compared to the CT (Fig. 5C). The FRAP assay data indicated statistically significant improvements in antioxidant activity across all tested concentrations. The 10 ppm treatment exhibited an increase of + 15.03% in FRAP values compared to the CT; similarly, the 50 ppm concentration led to a + 15.27% enhancement, while the 250 ppm group achieved the highest increase, showing a + 17.45% improvement relative to the CT (Fig. 5D). The lycopene (LYC) data revealed a + 176.51% increase at 10 ppm and a + 143.10% increase at 250 ppm, both relative to the CT (Fig. 6A). For α-carotene (α-CAR) and β-carotene (β-CAR) no statistical differences were observed (Fig. 6B and C).
(A) Soluble solids content. (B) Total phenolic content. (C) Total flavonoids content. (D) Ferric reducing antioxidant power. The x-axis denotes the treatments, while the y-axis represents the units of measurement. The significances resulting from the comparisons between the various treatments are indicated by asterisks: * p < 0.05; ** p < 0.005.
Discussion
The number and weight of individual fruits are critical traits that significantly influence crop yield and hold substantial economic value in the trade of these products. This study highlights the positive effects of the 250 ppm Zn-Alg-CaCO3 MPs foliar spray on tomato fruit yield, showing notable improvements across various yield parameters. Scientific evidence supports the use of Zn-NPs (Zinc nanoparticles) as an effective tool for enhancing tomato yield through foliar applications29. In particular, the finding in this research showed that 250 ppm Zn-Alg-CaCO3 MPs treatment resulted in a substantial increase in the number of fruits per plant compared to both the CT and lower concentration treatments. Research indicates that foliar spraying of Zn on tomato plants, especially in nanoparticle form, enhances nutrient uptake and metabolite translocation to fruits by acting as a co-factor for enzymes14. This process facilitates the movement of metabolites, promoting fruit set, maturation, and overall yield improvement29,30. Additionally, the 250 ppm Zn-Alg-CaCO3 MPs treatment led to the highest AFW, significantly outperforming the CT group. This increase in fruit size may be attributed to improved plant vigor, driven by Zn role in critical cellular processes and growth regulation during fruit development31,32. The lack of significant differences among the other treatments further emphasizes the distinct benefits of the 250 ppm concentration, indicating its greater effectiveness in promoting tomato fruit growth under aquaponic cultivation conditions. The application of Zn-Alg-CaCO3 MPs through foliar spray significantly affected tomato productivity, in terms of yield per plant. It also had a marked influence on key yield-determining traits, including total number of fruits produced per plant and the AFW. When considering yield per plant, the 250 ppm Zn-Alg-CaCO3 MPs treatment again showed remarkable improvements, outstripping all other treatments in this category. This indicates that the highest concentration of Zn-Alg-CaCO3 MPs significantly boosted plant productivity, likely due to improved overall plant health and more robust fruit development. For SSC data, no significant differences were observed among the treatments. This could be attributed to the fact that sugar concentration is not directly influenced by zinc involvement33. Nevertheless, our findings align with those reported in other studies, as the concentrations of sugars and other soluble substances are consistent with values documented in the literature34,35,36. The data from this study on the concentrations of PHE and FLA are particularly intriguing. Since flavonoids belong to the phenolic compound family, the application of Zn-Alg-CaCO3 MPs at the highest tested concentration (250 ppm) appears to have specifically promoted flavonoid synthesis. Conversely, the 10 and 50 ppm concentrations primarily contributed to an overall increase in PHE. At a concentration of 250 ppm, zinc may have ensured sufficient availability to activate or enhance the activity of key enzymes involved in catalyzing the initial steps of the phenylpropanoid pathway, such as chalcone synthase (CHS)37,38 and phenylalanine ammonia-lyase (PAL), thereby promoting flavonoid production39,40. Also, high concentrations of zinc could act as a mild stressor, triggering a defense response in plants. This response often involves the upregulation of secondary metabolites like flavonoids, which function as antioxidants to protect the plant from oxidative stress caused by excess zinc or environmental conditions41,42. The FRAP test provided further evidence of the promotion of molecules with antioxidant properties when plants were treated with Zn-Alg-CaCO3 MPs, regardless of their concentration. The presence of antioxidant molecules highlights their potential beneficial effects on human health, such as mitigating the impacts of lipid peroxidation43. The data on LYC present a complex pattern that is challenging to interpret. LYC biosynthesis might exhibit a nonlinear response to zinc availability, with optimal enhancement occurring at low (10 ppm) and high (250 ppm) concentrations but being inhibited or unchanged at intermediate levels like 50 ppm. At 50 ppm, the metabolic flux might have been redirected toward other carotenoids or secondary metabolites, resulting in reduced LYC accumulation44,45. This diversion could be influenced by the plant’s specific nutrient demands or environmental conditions during the experiment.
This research allowed for the testing of a low-impact smart fertilizer applied within an aquaponic system. In this context, the fertilizer was administered selectively and precisely through foliar application, avoiding the generalized distribution in the substrate typical of traditional fertilizers. This approach offers significant environmental benefits by reducing the amount of fertilizer required compared to conventional methods, thus minimizing the risk of nutrient accumulation in the water. This helps maintain the ecological balance of the system, ensuring optimal conditions for both plants and aquatic organisms. Furthermore, the targeted application ensures greater efficiency in nutrient uptake by plants, optimizing resource use and reducing waste. This method represents a step forward towards more sustainable agricultural practices aligned with the principles of aquaponics. These fertilizers could represent a viable and sustainable alternative even for traditional soil-based agricultural systems. In conclusion, the findings suggest that the 250 ppm Zn-Alg-CaCO3 MPs treatment offers a significant advantage in enhancing tomato fruit yield across various parameters. These results support the potential of Zn-Alg-CaCO3 MPs as an effective tool for increasing tomato production, particularly when applied at 250 ppm concentration. Further research is needed to investigate the underlying mechanisms driving these improvements and to identify the optimal concentration for different growing conditions.
Materials and methods
Aquaponic system characteristics
Between April and August 2024, experiments were conducted in the aquaponics greenhouse at the Botanical Gardens of Tor Vergata University of Rome (Rome, Italy). The aquaponics setup consisted of two coupled units, both for fish and plant tanks, and the cultivation method used was floating rafts, also called deep water culture (DWC). Two 4,000 L fish tanks with tilapias (Oreochromis niloticus L.), two 15,000 L cultivation tanks, a UV sterilizer, a reverse osmosis unit, a 5,000 L static biofilter filled with bio-media, and a bottom-up oxygenation system were the single components characterizing the system. The biofilter operated with a flow rate of 2.5 m³ h−1 and a retention time of 0.8 ± 0.75 h. It supplied nutrients to the two floating raft system units, each with a cultivation area of 27.5 m². The tilapias were fed a diet containing 35% protein (Skretting Italia S.p.A., Mozzecane, Italy).
Sensors and spectrophotometric assays
The environmental parameters (Table 3) were obtained using a thermohygrometer, which allowed obtaining data on maximum and minimum temperature (Tmax and Tmin) and maximum and minimum relative humidity (RHmax and RHmin). Water parameters were recorded over time using system sensors (Table 3); these included, water temperature entering the grow tanks (T1), temperature leaving the grow tanks (T2), pH, and dissolved oxygen (DO). Water analysis was conducted weekly to monitor the concentrations of various macro and micronutrients. Samples were taken from three different points in the plant cultivation tanks. These analyses were carried out using a spectrophotometer (model HI96800; Hanna Instruments, Woonsocket, RI, USA) and specific kits (Hanna Instruments, Woonsocket, RI, USA) designed for the respective elements being tested.
Synthesis of Zn-Alg-CaCO3 MPs
The biosynthetic strategy to obtain Zn-Alg-CaCO3 MPs was like the one provided in our previous work18. The only difference was the new molecule (prepared as 0.1 M aqueous solution of C10H12ZnN2O8*2H2O) used as Zn-element-precursor in the second step (Fig. 7), necessary to add the Zn element and include/entrap it in the final product. The summary scheme is shown below (Fig. 7). The present study confirms how the synthesis method of these microparticles is versatile and highly performing, since they can be functionalized with several different elements.
Zn-Alg-CaCO3 MPs characterization
For the morphological and structural characterization of the Zn-Alg-CaCO3 MPs, SEM/EDX and FTIR equipment have been applied, according to our previous paper18, respectively. Briefly, SEM/EDX measurements were carried out by Field Emission SEM (FE-SEM) LEO SUPRA™ 35 (CARL ZEISS, Oberkochen, Germany). The sample powder was dispersed in 100 mL of double-distilled water and ultrasonicated for 30 min. Afterwards, the aqueous nano dispersion was deposited on a Si (111) wafer, by drop casting, and the solvent was allowed to evaporate at room temperature. Then, the sample was metallized via sputtering (Angstrom Engineering Inc. sputter coater, Cambridge, Canada), for 10 min and 25 mA. Purified bi-distilled water, obtained with a milli-Q (Millipore, Darmstadt, Germany) water purification system, was used for sample preparation.
For the FTIR study, samples were assembled in KBr pellets and then, all measurements were performed by using a Shimadzu Model Prestige 21 (Kyoto, Japan) spectrophotometer model apparatus, according to our previous paper46,47.
Experimental design
The experiment was conducted on tomato plants, Solanum lycopersicum L. cv. Principe Borghese. Plants were purchased, certified, from “Orto Flora di Valter Finocchietti” (Rome, Italy) when they were approximately 30 days old. The soil was carefully removed, paying attention not to tear the roots, and they were placed in plastic baskets (PET) with red lapilli as a growth support. The baskets containing the substrate and plants were placed into custom made holes in closed cell expanded polystyrene floating panels. The experiment was designed to test three different concentrations of the smart fertilizer (10, 50, and 250 ppm) alongside a CT group that received sterile double-distilled water. Foliar applications were administered by spraying, ensuring that both the upper and lower leaf blades were thoroughly treated. For each treatment, 15 tomato plants were used, arranged across 3 panels and spaced approximately 30 cm apart. To prevent cross-contamination between treatments, a portable plexiglass panel was employed, allowing the treatment to be applied exclusively to the targeted plants without affecting those on neighboring panels. The volumes used were gradually increased as the plants grew, were measured using Gilson pipettes, and were administered with a portable spray device. In detail, the first application used 4 mL/plant, the second 8 mL/plant, the third 12 mL/plant, the fourth 14 mL/plant, both fifth and sixth 16 mL/plant, and, finally, both seventh and eight 18 mL/plant. The exclusive foliar application of the fertilizer studied offers significant advantages in aquaponic cultivation, as it avoids introducing substances into the recirculating water, thereby preventing potential negative interactions with the fish and/or beneficial bacteria in the system. Subsequent analyses focused on the collected fruits, specifically selecting the first four clusters that had formed for detailed investigation.
Fruit yield
To determine fruit production, the various tomatoes produced according to the various treatments were counted. Also, their AWF and Fruit/Yield were analyzed, always divided by the various treatments. A precision analytical balance was used for every weight measurement.
Soluble solids content
For the SSC analysis, the protocol established by Braglia et al. (2022) was followed34. In brief, 1.5 g of fresh weight (FW) from randomly selected samples, representing peel and pulp, was combined and homogenized in liquid nitrogen using a mortar and pestle. The resulting homogenate was centrifuged at 6,089 xg for 10 min. After centrifugation, 100 µL of the supernatant was collected and analyzed with a digital refractometer (model HI96800; Hanna, Woonsocket, RI, USA) to measure the sugar and other soluble solids content, expressed as Brix value.
Secondary metabolites content and FRAP assay
As the fruits were gradually collected for subsequent investigations, they were stored at a controlled temperature of −80 °C. Approximately 24 h before the various analytical protocols the fruits were thawed at a controlled temperature of 4 °C. In order to observe the quality of the products obtained, always in relation to the treatment used, analyses were conducted to quantify the content of total PHE, total FLA, CAR, and, furthermore, the FRAP test was conducted to evaluate the antioxidant properties of the fruit extracts. Five tomatoes, randomly selected within each experimental treatment, were prepared and specific protocols were followed, both for the extraction and the analysis. For PHE and FLA, approximately 1 g of fresh material was ground using a mortar, pestle, and liquid nitrogen. Following this, 5 mL of ddH2O: MetOH (50:50 v/v) was added, and the samples were incubated in the dark on a rocking plate (60 rpm) for 48 h at 4 °C. After incubation, the samples were centrifuged at 3,500 xg for 10 min, and the supernatant was collected for further analysis. For PHE analysis, the protocols outlined by Heimler et al. (2005) and Meda et al. (2005) were followed48,49. In Eppendorf tubes, 200 µL of 1 M Na₂CO₃ and 250 µL of 10% Folin-Ciocalteu reagent were added to 50 µL of the sample. The reaction was allowed to proceed for 2 h in the dark at room temperature. After the incubation, the samples were measured at a wavelength of 760 nm. Data were expressed as µg of gallic acid equivalent per gram of FW sample (GAE g−1 FW). For FLA analysis, the protocol established by Arvouet-Grand et al. (1994) was used50. In Eppendorf tubes, 50 µL of sample were combined with 10 µL of 10% AlCl₃, 10 µL of 1 M CH₃CO₂K, 150 µL of methanol, and 280 µL of ddH₂O. The mixture was incubated in the dark at room temperature for 30 min. Absorbance readings were then taken at a wavelength of 415 nm. Data were expressed as µg of quercetin equivalent per gram of FW sample (QE g−1 FW). Both PHE and FLA absorbances were read using an ELISA microplate reader (Tecan Sunrise, Männedorf, Switzerland). To determine α-CAR, β-CAR, and LYC, the method outlined by De Sio et al. (2001) was employed51. 1 g sample of tomato was weighed and left to dissolve for 48 h in 2.5 mL of a Dichloromethane: Methanol mixture (2:1 v/v). After centrifugation (3,500 xg for 10 min), 2 mL of the supernatant was collected into an Eppendorf tube, dried, and resuspended in 1 mL of chloroform. The extract was analyzed using an HPLC-DAD (High-Performance Liquid Chromatography with photoDiode-Array Detection) system (Shimadzu, Kyoto, Japan), equipped with a CBM-20 A controller, an LC-20 AD pump, a SIL-20 A HT autosampler, an SPD-M20 A diode array detector, and a Luna 3 μm C18(2) chromatographic column (150 mm × 4.60 mm × 3 μm) (Phenomenex, Torrance, USA). A 20 µL aliquot of the extract was injected into the column, which was maintained at 40 °C. The analysis was performed under isocratic conditions with a mobile phase consisting of Acetonitrile: Methanol (30:70 v/v). Calibration curves for the standards were prepared across a concentration range of 0 to 25 µg for each carotenoid analyzed during the analytical phase. For FRAP analysis, the extraction protocol was identical to the one used for PHE and FLA. As reported in Benzie and Strain (1996), plant extract (50 µl) was mixed with 450 µl FRAP reagent (10 mM TPTZ in 40 mM HCl, 20 mM FeCl3, 0.3 M acetate buffer pH 3.6; 1:1:10 v/v/v) and placed in the dark, at 37 °C for 10 min52. This test evaluated the capacity of natural antioxidants to reduce the colorless Fe III - tripyridyltriazine compound (Merck, Darmstadt, Germany) - to the blue-colored Fe II form. The absorbance of the samples was read at 593 nm, using an ELISA microplate reader (Tecan Sunrise, Männedorf, Switzerland). Ascorbic acid was used as standard to obtain a calibration curve (0–1 mM, R2 = 0.9933) and results were expressed as molarity (M) of ascorbate equivalent (AE) per gram of plant FW (MAE g−1 FW).
Statistical analysis
Measurements were conducted using five replicas, with results expressed as mean ± sd (standard deviation). Statistical analyses were carried out using GraphPad Prism 10.1.2 software (GraphPad Software Inc.; San Diego, USA). The normality of the data was assessed using the Shapiro-Wilk test, which showed that the data distributions tended toward normality (p < 0.05), allowing for the use of parametric tests (ANOVA) for further analysis and comparison. Additionally, Tukey’s post-hoc test was applied for more detailed comparisons. Differences were considered statistically significant at p < 0.05. Comparative analyses were conducted between the micro-fertilization treatments and the CT group, as well as among the different zinc nanoparticle administration groups.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- A:
-
Ascorbate
- Alg:
-
Sodium Alginate
- AFW:
-
Average Fresh Weight
- CAR:
-
Carotene
- CHS:
-
Chalcone Synthase
- CT:
-
Control
- DAD:
-
Photodiode-Array Detection
- DO:
-
Dissolved Oxygen
- DWC:
-
Deep Water Culture
- EPA:
-
Eicosapentaenoic Acid
- FLA:
-
Flavonoids
- FRAP:
-
Ferric Reducing Antioxidant Power
- FTIR:
-
Fourier Transform Infrared Spectroscopy
- FW:
-
Fresh Weight
- GAE:
-
Gallic Acid Equivalent
- HPLC:
-
High-Performance Liquid Chromatography
- LYC:
-
Lycopene
- MPs:
-
Microparticles
- NPs:
-
Nanoparticles
- PHE:
-
Phenols
- PAL:
-
Phenylalanine Ammonia-Lyase
- PAN:
-
1-(2-Pyridylazo)-2-Naphthol
- QE:
-
Quercetin Equivalent
- RH:
-
Relative Humidity
- SD:
-
Standard Deviation
- SEM/EDX:
-
Scanning Electron Microscopy/Energy Dispersive X-Ray
- SSC:
-
Soluble Solids Content
- T:
-
Temperature
- TPTZ:
-
2,4,6-Tripyridyl-S-Triazine
References
Goddek, S. et al. Challenges of sustainable and commercial aquaponics. Sustainability 7, 4199–4224 (2015).
Ibrahim, L. A., Shaghaleh, H., El-Kassar, G. M., Abu-Hashim, M. & Elsadek, E. A. Alhaj Hamoud, Y. Aquaponics: a sustainable path to food sovereignty and enhanced water use efficiency. Water 15, 4310. https://doi.org/10.3390/w15244310 (2023).
Seawright, D. E., Stickney, R. R. & Walker, R. B. Nutrient dynamics in integrated aquaculture–hydroponics systems. Aquaculture 160, 215–237 (1998).
Kambe, T., Tsuji, T., Hashimoto, A. & Itsumura, N. The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol. Rev. 95, 749–784 (2015).
Fariduddin, Q., Saleem, M., Khan, T. A. & Hayat, S. Zinc as a versatile element in plants: an overview on its uptake, translocation, assimilatory roles, deficiency and toxicity symptoms in Microbial Biofertilizers and Micronutrient Availability: the Role of Zinc in Agriculture and Human Health (eds Khan, S. T. & Malik, A.) 137–158 (Springer, (2021).
Srivastava, A. K. & Singh, S. Zinc nutrition and citrus decline–a review. Agric. Rev. 25, 173–188 (2004).
Mikula, K. et al. Controlled release micronutrient fertilizers for precision agriculture–a review. Sci. Total Environ. 712, 136365. https://doi.org/10.1016/j.scitotenv.2019.136365 (2020).
Padhan, B., Ray, H., Dutta, A., Maji, S. & Das, J. Nanotechnology innovations in soilless microgreen farming for sustainable agriculture in Recent Trends and Applications of Leguminous Microgreens as Functional Foods (eds Mathur, P. & Gupta, A.) 449–465 (Springer, (2025).
Yadav, P., Shukla, R. & Gupta, A. Enhancing shelf life and nutritional quality of leguminous microgreens: insights into the application of nanotechnology, hydroponics, and genetic engineering approaches in Recent Trends and Applications of Leguminous Microgreens as Functional Foods (eds Mathur, P. & Gupta, A.) 421–448 (Springer, (2025).
Molotoks, A., Smith, P. & Dawson, T. P. Impacts of land use, population, and climate change on global food security. Food Energy Secur. 10, e261. https://doi.org/10.1002/fes3.261 (2021).
Van Dijk, M., Morley, T., Rau, M. L. & Saghai, Y. A meta-analysis of projected global food demand and population at risk of hunger for the period 2010–2050. Nat. Food. 2, 494–501 (2021).
Khatri, P., Kumar, P., Shakya, K. S., Kirlas, M. C. & Tiwari, K. K. Understanding the intertwined nature of rising multiple risks in modern agriculture and food system. Environ. Dev. Sustain. 26, 24107–24150 (2024).
Nair, C. S. et al. Recent advancements in aquaponics with special emphasis on its sustainability. J. World Aquac Soc. 56, e13116. https://doi.org/10.1111/jwas.13116 (2025).
Peña, L. E. et al. AQUAPONICS: A serious game to promote aquaponics systems for local community development. J. Clean. Prod. 492, 144905. https://doi.org/10.1016/j.jclepro.2025.144905 (2025).
Alemu, M. Trend of biotechnology applications in pest management: a review. Int. J. Appl. Sci. Biotechnol. 8, 108–131 (2020).
Chandra, A., McNamara, K. E. & Dargusch, P. Climate-smart agriculture: perspectives and framings. Clim. Policy. 18, 526–541 (2018).
Agrimonti, C., Lauro, M. & Visioli, G. Smart agriculture for food quality: facing climate change in the 21st century. Crit. Rev. Food Sci. Nutr. 61, 971–981 (2021).
Frassine, D. et al. Enhancing lettuce (Lactuca sativa) productivity: foliar sprayed Fe-Alg-CaCO3 MPs as fertilizers for aquaponics cultivation. Plants 13, 3416. https://doi.org/10.3390/plants13233416 (2024).
Chu, P. M., Guenther, F. R., Rhoderick, G. C. & Lafferty, W. J. The NIST quantitative infrared database. J. Res. Natl. Inst. Stand. Technol. 104, 59–81 (1999).
Leng, P. L., Naseri, M. G., Saion, E., Shaari, A. H. & Kamaruddin, M. A. Synthesis and characterization of Ni-Zn ferrite nanoparticles (Ni0.25 Zn0.75 Fe2O4) by thermal treatment method. ANP 2, 378; (2013). https://doi.org/10.4236/anp.2013.24052
Haq, S. et al. Phytogenic synthesis and characterization of NiO-ZnO nanocomposite for the photodegradation of brilliant green and 4‐nitrophenol. J. Chem. 3475036; (2021). https://doi.org/10.1155/2021/3475036 (2021).
Jayarambabu, N., Kumari, S., Venkateswara Rao, B., Prabhu, Y. T. & K. & Germination and growth characteristics of Mungbean seeds (Vigna radiata L.) affected by synthesized zinc oxide nanoparticles. Int. J. Curr. Eng. Technol. 4, 3411–3416 (2014).
Nie, B. et al. Preparation and characterization of sodium alginate/phosphate-stabilized amorphous calcium carbonate nanocarriers and their application in the release of Curcumin. Nanotechnology 32 (375712). https://doi.org/10.1088/1361-6528/ac05ea (2021).
Ma, Y. & Feng, Q. Alginate hydrogel-mediated crystallization of calcium carbonate. J. Solid State Chem. 184, 1008–1015 (2011).
Maensiri, S., Laokul, P. & Promarak, V. Synthesis and optical properties of nanocrystalline ZnO powders by a simple method using zinc acetate dihydrate and poly(vinyl pyrrolidone). J. Cryst. Growth. 289, 102–106 (2006).
Bashir, S. et al. In-vivo (Albino Mice) and in-vitro assimilation and toxicity of zinc oxide nanoparticles in food materials. Int. J. Nanomed. 17, 4073–4085 (2022).
Rahemi, A., Rouhaghdam, A. & Nazari, M. N-doped ZnO–CuO nanocomposite prepared by one-step ultrasonic spray pyrolysis and its photocatalytic activity. Chem. Phys. Lett. 705, 19–22 (2018).
Silverstein, R. M., Webster, F. X., Kiemle, D. J. & Bryce, D. L. Spectrometric Identification of Organic Compounds, 8th ednWiley,. ; ISBN: 978-0-470-61637-6. (2014).
Ahmed, R., Yusoff Abd Samad, M., Uddin, M. K., Quddus, M. A. & Hossain, M. M. Recent trends in the foliar spraying of zinc nutrient and zinc oxide nanoparticles in tomato production. Agronomy 11, ; (2074). https://doi.org/10.3390/agronomy11102074 (2021).
Raliya, R., Nair, R., Chavalmane, S., Wang, W. N. & Biswas, P. Mechanistic evaluation of translocation and physiological impact of titanium dioxide and zinc oxide nanoparticles on the tomato (Solanum lycopersicum L.) plant. Metallomics 7, 1584–1594 (2015).
Pejam, F., Ardebili, Z. O., Ladan-Moghadam, A. & Danaee, E. Zinc oxide nanoparticles mediated substantial physiological and molecular changes in tomato. Plos One. 16, e0248778. https://doi.org/10.1371/journal.pone.0248778 (2021).
Hamzah Saleem, M., Usman, K., Rizwan, M., Jabri, A., Alsafran, M. & H., & Functions and strategies for enhancing zinc availability in plants for sustainable agriculture. Front. Plant. Sci. 13, 1033092. https://doi.org/10.3389/fpls.2022.1033092 (2022).
Stanton, C., Sanders, D., Krämer, U. & Podar, D. Zinc in plants: integrating homeostasis and biofortification. Mol. Plant. 15, 65–85 (2022).
Braglia, R. et al. Phytochemicals and quality level of food plants grown in an aquaponics system. J. Sci. Food Agric. 102, 844–850 (2022).
Yang, Y. et al. Online soluble solids content (SSC) assessment of multi-variety tomatoes using Vis/NIRS diffuse transmission. Infrared Phys. Technol. 125, 104312. https://doi.org/10.1016/j.infrared.2022.104312 (2022).
Ruizendaal, J., Polder, G. & Kootstra, G. Automated and non-destructive Estimation of soluble solid content of tomatoes on the plant under variable light conditions. Biosyst Eng. 242, 80–90 (2024).
Koç, E., İşlek, C. & Hatice, N. B. Comparison of phenylalanine ammonia lyase response to lead and zinc stress in different wheat genotypes. Commun. Fac. Sci. Univ. Ankara Ser. C Biol. 27, 37–44 (2018).
Gu, Z. et al. Chalcone synthase is ubiquitinated and degraded via interactions with a RING-H2 protein in petals of Paeonia ‘he Xie’. J. Exp. Bot. 70, 4749–4762 (2019).
Seff, A. L., Pilbák, S., Silaghi-Dumitrescu, I. & Poppe, L. Computational investigation of the histidine ammonia-lyase reaction: a modified loop conformation and the role of the zinc (II) ion. J. Mol. Model. 17, 1551–1563 (2011).
Awan, Z. A., Shoaib, A. & Khan, K. A. Crosstalk of Zn in combination with other fertilizers underpins interactive effects and induces resistance in tomato plant against early blight disease. Plant. Pathol. J. 35, 330. https://doi.org/10.5423/PPJ.OA.01.2019.0002 (2019).
Fini, A., Brunetti, C., Di Ferdinando, M., Ferrini, F. & Tattini, M. Stress-induced flavonoid biosynthesis and the antioxidant machinery of plants. Plant. Signal. Behav. 6, 709–711 (2011).
Di Ferdinando, M., Brunetti, C., Fini, A. & Tattini, M. Flavonoids as antioxidants in plants under abiotic stresses in Abiotic stress responses in plants: metabolism, productivity and sustainability (eds. Ahmad, J & Pasad, M. N. V.) 159–179 (Springer, 2012). (2012).
Gholami, F. et al. Tomato powder is more effective than lycopene to alleviate exercise-induced lipid peroxidation in well-trained male athletes: randomized, double-blinded cross-over study. JISSN 18, 1–7 (2021).
Rabbi, R. H. M. et al. Impact of different zinc concentrations on growth, yield, fruit quality, and nutrient acquisition traits of tomato (Lycopersicon esculentum L.) grown under salinity stress. Arch. Biol. Sci. 76, 71–82 (2024).
Vichi, E., Francini, A., Raffaelli, A. & Sebastiani, L. Effects of caffeine, zinc, and their combined treatments on the growth, yield, mineral elements, and polyphenols of Solanum lycopersicum L. Antioxidants 13, 1100. https://doi.org/10.3390/antiox13091100 (2024).
Bicchieri, M., Valentini, F., Calcaterra, A. & Talamo, M. Newly developed nano-calcium carbonate and nano‐calcium propanoate for the deacidification of library and archival materials. J. Anal. Methods Chem. 2017 (2372789). https://doi.org/10.1155/2017/2372789 (2017).
Valentini, F. et al. SiO2 nanoparticles as new repairing treatments toward the Pietraforte sandstone in Florence renaissance buildings. Crystals 12, 1182. https://doi.org/10.3390/cryst12091182 (2022).
Heimler, D., Vignolini, P., Dini, M. G. & Romani, A. Rapid tests to assess the antioxidant activity of Phaseolus vulgaris L. dry beans. J. Agric. Food Chem. 53, 3053–3056 (2005).
Meda, A., Lamien, C. E., Romito, M., Millogo, J. & Nacoulma, O. G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 91, 571–577 (2005).
Arvouet-Grand, A., Vennat, B., Pourrat, A. & Legret, P. J. J. B. Standardization of propolis extract and identification of principal constituents. J. Pharm. Belg. 49, 462–468 (1994).
De Sio, F., Servillo, L., Loiuduce, R. & Castaldo, D. A chromatographic procedure for the determination of carotenoids and chlorophylls in vegetable products. Acta Aliment. 30, 395–405 (2001).
Benzie, I. F. & Strain, J. J. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal. Biochem. 239, 70–76 (1996).
Acknowledgements
We would like to thank NANOBIOTECH s.r.l. for the support provided. Furthermore, we would like to thank the PhD program in “Evolutionary Biology and Ecology” of the Tor Vergata University of Rome (Rome, RM, Italy). The present study was funded by Regione Lazio, POR FESR LAZIO 2014–2020 (project name “ASTERIA”, project code E85 F21002340002); and by the Ministry of University and Research (MUR), FSE REACT-EU (project name “PON Ricerca e Innovazione 2014–2020”).
Funding
This research was funded by Regione Lazio, POR FESR LAZIO 2014–2020 (project name “ASTERIA”, project code E85 F21002340002); and by the Ministry of University and Research (MUR), FSE REACT-EU (project name “PON Ricerca e Innovazione 2014–2020”).
Author information
Authors and Affiliations
Contributions
Conceptualization, D.F., R.B., F.S., F.V. and A.C.; methodology, D.F., R.B., F.S., E.L.R., F.V., M.R., I.A.C., C.Z., A.M., I.A., A.G., G.D.M. and A.C.; validation, R.B., F.V. and A.C.; formal analysis, R.B., D.F., F.V. and A.C.; investigation, D.F., R.B., F.S., E.L.R., F.V., M.R., I.A.C., C.Z., A.M., I.A., A.G., G.D.M. and A.C.; resources, R.B. and A.C.; data curation, D.F., R.B., F.S. and F.V.; writing—original draft, D.F., R.B. and F.V.; writing—review and editing, D.F., R.B., F.S., E.L.R., F.V., M.R., I.A.C., C.Z., A.M., I.A., A.G., G.D.M. and A.C.; supervision, R.B. and A.C.; project administration, R.B. and A.C.; funding acquisition, R.B. and A.C. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics declaration
The present study was authorized for the use of experimental animals under protocol DE15B.173, issued by the Direzione Generale della Sanità Animale e dei Farmaci Veterinari, and carried out in compliance with Italian and European regulations (D.L. 26/14; European Directive 2010/63/EU). The study was conducted in accordance with local legislation and institutional requirements.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Frassine, D., Braglia, R., Scuderi, F. et al. Smart foliar fertilizer based on Zn-Alg-CaCO₃ microparticles improves Aquaponic tomato cultivation. Sci Rep 15, 18092 (2025). https://doi.org/10.1038/s41598-025-03136-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-03136-y