Abstract
Free-ranging female African lions maintain symmetrical social relationships by respecting each other’s “ownership” of valuable food items rather than by supplanting subordinates according to well-defined dominance hierarchies. However, captivity often skews relationships in captive carnivores, hence we investigated whether captive female lions demonstrate obvious dominance relationships. Oxytocin has been shown to elicit context-specific impacts that equalize dominant subordinate relationships, thus we hypothesized that oxytocin would reduce any asymmetries found between dominants and subordinates in captive lions. We designed two experimental protocols for investigating pairwise relationships. We first identified dominant individuals by performing neutral trials that allowed each female equal opportunity to possess the food item. Second, we performed non-neutral trials that biased the opportunity for subordinates to gain possession (“ownership”) of the food and thereby determined whether dominants would still gain access to the resource. The neutral tests revealed that pairs of captive females do display dominance relationships, with one individual possessing the resource more than the other. However, in non-neutral trials, subordinates behaved less submissively by increasing aggression and their possession of the resource after receiving oxytocin compared to receiving saline solution. Our study not only reaffirms the social dynamics altered by captivity, but also highlights the potential for oxytocin to mitigate these disturbances.
Similar content being viewed by others
Introduction
Captivity can serve as an important tool for education or population recovery of endangered species; however, captive environments drive changes in natural behavior, and it is important to understand and manage these changes1. Documented cases of natural behaviors altered in captivity range from foraging patterns2,3,4 to breeding and reproduction5,6 and social behavior7. Across species, these changes in social dynamics have been shown to impact levels of cooperation and the use of social information, leading to increased aggression, aberrant behaviors, morbidity, and mortality7,8,9,10,11,12.
One important impact of captivity on sociality is more disparate relationships in dominance8,13,14. Dominance, described as an asymmetrical relationship or distribution of behavior between two individuals15, is characterized by increased possession of a resource16,17. Furthermore, dominance status in a hierarchy is enforced by top-down intraspecific aggression between group members18,19. In captive carnivores, African wild dogs display a higher frequency of challenge for alpha than wild counterparts, resulting in increased levels of aggression between higher ranking individuals20, and dominant wolves display levels of intraspecific aggression towards pack members four times higher in captivity than in the wild19.
Unlike other carnivore species, wild prides of African lions are egalitarian, with highly symmetrical relationships between females17. Although feeding is considered the ‘most common context for aggressive competition in lions’17,21, wild female lions respect an “ownership” rule, and rarely supplant members of their same age-sex class from food, displaying no discernible dominance (increased possession/consumption of resource) or feeding hierarchy (top-down aggressive control). Lions possess formidable weaponry (teeth and claws) that ordinarily inflict costs that outweigh the advantage of gaining access to a single food source17. However, feeding habits of female lions in captivity have suggested the contrary, and dominant-subordinate relationships regarding food resources have been heavily observed by professionals, but are yet to be reported in the literature (professionals in the field: Hildegard Pirker, Dr Christine Steyrer, Kevin Richardson, Dr Jessica Burkhart). Hence, in the artificial confines of captivity, dominant-subordinate relationships and associated aggression arising in a naturally “egalitarian” species could potentially pose welfare and management issues17. Therefore, we formally tested whether unnatural dominance relationships are present in captive female lions, and, if so, investigated a potential ameliorative solution.
Administration of oxytocin, a neuropeptide highly implicated in social cognition22,23, has been shown to flatten dominance hierarchies in rodents and primates24,25,26. Intranasal oxytocin increases behavioral synchrony and efficiency in communicating dominance status, and reduces differences in related social behaviors between dominant and subordinate male macaques24, and, interestingly, oxytocin increases the social status of subordinate female macaques over males by increasing their (dominant) threatening and vocalization behaviors25. Previously, we have found that intranasal administration of oxytocin decreases vigilance and increases affiliative behavior in African lions in a non-feeding context27,28, however, the impacts of intranasal oxytocin have shown sex- and context-dependent effects (Ma et al. 2018). In the current study, we sought to further investigate the effects of oxytocin in the specific context of competition during feeding in female African lions to see whether intranasal administration of oxytocin could, in fact, restore symmetry to female lion relationships, reinstating the “ownership” rule. We hypothesized that: A) When presented with a food resource, a dominant-subordinate relationship between captive pairs of female African lions would present as consistent asymmetries in possession, and that B) Oxytocin would ameliorate the difference in pairwise dominance relationships by closing the gap between differences in resource possession, and reduce the subordinate lion’s tendency to behave submissively, consistent with the “ownership” rule.
Methods
Subjects
All trials were performed at Lionsrock Sanctuary FOURPAWS, in South Africa between February and October 2023. Procedures were approved by the Institutional Animal Care and Use Committee of the University of Minnesota, and in accordance with ARRIVE guidelines. All subjects (n = 20 females, 10 pairs) were captive born, healthy female adults in prime condition (age 4–18), housed in greater than 1 hectare, species appropriate enclosures at their current location for greater than one year. Participants were selected by sanctuary staff based on health and pair availability. All 20 female lions were included equally across trials and analyses. No studies were performed on females who were lactating, pregnant, or in estrus, and no animals in this study are used for breeding purposes. Pairs were selected for the study that had been housed amicably together (outside of a feeding context) since early adolescence or birth (direct levels of relatedness are unclear due to the nature of rescues), and all individuals were similar in age and condition within each pair. Pairs of females came from mixed group compositions and were separated into pairs for the study only on the days trials took place. Pairs from groups containing multiple females were selected randomly based on the animals that the handler separated together from the first baseline trial, and these were maintained throughout the trial period. All animals in the study were pre-trained to take meat blocks (approximately 0.1 kg per block) from the researcher at the gate (in the same manner they are accustomed to being fed meat blocks by the sanctuary staff) prior to study; any animals that showed hesitancy to approach individually were excluded from the study, and all animals participated spontaneously. Two baseline trials were performed to habituate the lions to the researcher and the experimental protocol. The lions are fed twice a week, and all trials were performed on non-feeding days, with no enrichment given prior to trial to ensure the animals were motivated by the resource and not satiated by feeding earlier in the day.
Trial types
Dominance can be characterized as monopolization over a resource17. In this study, we use food in the form of blocks of raw beef as a resource (meat was provided by the sanctuary and was within feeding guidelines for each animal). Two baseline trials were performed to habituate the lions to the researcher and the study procedure. The lions are fed twice a week, and all trials were performed on non-feeding days, with no enrichment given prior to trial to ensure the animals were interested in the resource and not full from feeding or distracted. Asymmetry in resource possession and changes in aggression were measured.
Each trial day consisted of two tests (neutral and non-neutral). First, the neutral test determined whether a dominant individual presented within the pair of females (determined by possession of resource). This was directly followed by the second test, the non-neutral test, to determine if dominant individuals would respect the ownership rule or if they would overtake the resource from the subordinate. The set of tests was administered consecutively on a trial day, and repeated on a total of four days (two days with oxytocin treatment and two days with saline control, in alternating order), with 10–14 days between (differences based on weather conditions). The possessor of the resource was identified as the animal that ultimately consumed the 0.1 kg piece of meat.
Trial protocol
Treatment (10 IU Oxytocin) or vehicle (saline, as control) was administered intranasally 45 min prior to trial commencement (presentation of resource), and both individuals in the pair received the same treatment at the same time. Oxytocin or saline was administered in alternating order across the four treatment days, with half of the pairs receiving oxytocin first, and the other half receiving saline first in order to control for order bias.
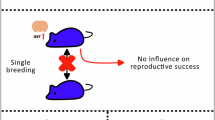
To commence the trial, the female pair was brought to the gate being lured by meat (Fig. 1). For the neutral test, the resource (meat) was placed on a stick against the fence at an equal distance between the two females, giving them equal opportunity to approach and possess the “resource.” The meat block was placed on the stick to make it difficult to pull off and consume (possess), therefore giving each lion time to supplant the first individual that tried to take “ownership” by biting the meat with approximately 30 s between each round to allow for a reset. For each treatment for each pair, meat was presented a total of 10 times occurring on two separate days (except in rare cases when one female was reluctant to return, usually after receiving excessive aggression, then only repeated 6 times as a humane endpoint). A lioness was considered “dominant” if she monopolized (consumed) the resource at least 70% of the time [note: in all but one pair the ratio was 90–100%]. Neutral tests were directly followed by the non-neutral tests in which an additional set of 5 rounds of meat presentation was administered per treatment day (total of 10 rounds). In non-neutral tests, the meat was held closer to the subordinate, as determined by the neutral test, giving the subordinate a chance to reach the meat first and claim “ownership.” It was then observed whether the dominant female allowed the subordinate to maintain possession, or if she overtook the resource regardless of the “ownership” rule normally observed in female lions in the wild.
Dominance in female African lions as determined by possession of the food resource. A high value resource (meat) is held at the fence allowing two females to compete for the resource. A) one female behaves submissively while the other dominates the resource. B) the possessor of the resource defends herself, behaving less submissively than in (A) to maintain possession of the resource.
Pairs were selected randomly to receive oxytocin or saline in alternating order for a total of 4 trial days. The dose was 10 international units (IUs) of oxytocin administered intranasally via a DeVilbiss atomizer. Intranasal administration was achieved by placing the tip of the atomizer 1 cm into the subject’s nostril27. One spritz from the atomizer equals one international unit. Intranasally administered oxytocin bypasses the blood–brain barrier, peaking at 45 min and lasting ≈ 4 h29,30. Trials therefore commenced 45 min post oxytocin administration and lasted approximately 15 min. Treatments were prepared by research assistant, Dubois, so that the researcher, Burkhart, was blinded to the treatment in order to control for biasing behavior, interpretation, or interaction during trials. All trials were scored live by two researchers and also recorded using a Gopro Hero 5 and Samsung s20 5G + for later reference.
Behavior was measured during each round to determine dominance as follows: who possessed/consumed the resource, as well as aggressive vocalizations and physical actions (activation of weaponry: teeth or claws). The occurrence of vocal and physical aggression was scored as either 0 (absent) or 1 (present). Aggressive encounters were then scored and categorized by severity, 1 = brief non-contact, 2 = brief contact, 3 = prolonged contact. This number was considered the “aggression level”. A total aggression score was calculated by summing the 0/1 vocal score + the 0/1 physical score + the 1–3 level of aggression. The total aggression score could therefore range between 0–5. Also measured was how many body lengths apart the individuals were while the resource was consumed.
Statistical analysis
Differences in resource possession
Successful possession of the food resource by dominant vs subordinate lions was our primary indicator for the presence of dominance relationships within pairs. We therefore compared the ratio of within-pair resource possession across trials. Binomial generalized linear mixed models (GLMMS) were employed using “glmer” (R-package lme4) to model the likelihood of dominant and subordinate individuals to successfully possess the food resource. This “possession” likelihood was modeled as a function of treatment (saline or oxytocin), dominance (dominant or subordinate individual), and the interaction between treatment and dominance. We considered neutral vs non-neutral trials as separate tests and ran these data in separate models. Pair identity was added as a random effect to our mixed models to account for repeated measures of lion pairs across trials.
Differences in aggressive behavior
We explored differences in aggressive behavior and intensity across trials using 4 aggression metrics, as changes in aggression for both dominant and subordinate individuals was a likely mechanism for changes in pair-wise resource possession. We first modeled the presence/absence of vocal aggression (1) and physical aggression (2) using binomial GLM models, and then modeled physical aggression intensity level (3) and total aggression count (4) using poisson GLM models. Data from neutral vs non-neutral trials were again modeled separately, as was dominance status for easier interpretation of effect sizes. All aggression metrics were modeled as a function of treatment (saline or oxytocin), with pair identity used as a random effect to allow for pair and individual differences in displayed aggression.
Results
Asymmetrical relationship pairings in female African lions were first determined, then tested, in a sanctuary setting after administration of oxytocin or vehicle. Two types of trials were designed; first, a neutral trial with equal opportunity to possess the meat resource, in order to establish dominance, and second, a non-neutral trial where the subordinate animal had first right of possession to test oxytocin’s effect on the “ownership” rule.
Resource possession in neutral trials
In neutral trials (designed to test dominance over equal opportunity possession), we observed during control (saline) treatments that across all pairs, one consistent individual (hereafter named the “dominant”) possessed (consumed) the resource on average 94% of the time (range of 70% – 100%) (Fig. 2). Results of binomial models show that dominant individuals were significantly more likely to possess the resource across neutral trials (Table 1; log odds: −5.3 ± 0.59, p < 0.0001).
Asymmetrical resources possession, demonstrating dominant-subordinate relationships, decreases with oxytocin (n = 10 pairs, 380 rounds). Data show mean within-pair proportion that dominant or subordinate individual possessed the resource under neutral (N) and non-neutral (NN) treatments. Error bars show standard error. In all treatments, the subordinate individual was less likely to possess the resource than the dominant (Binomial GLMM β: −5.3 ± 0.59, p < 0.0001 for neutral, β: −1.98 ± 0.3, p < 0.0001 for non-neutral). However, in non-neutral trials testing the “ownership” rule, the subordinate displayed a significant increase in maintaining possession after oxytocin (β: 1.2 ± 0.4, p = 0.01), decreasing asymmetry in pairwise dominance relationships.
Post oxytocin, dominant animals possessed the resource on average 89% of the time (range of 40% – 100%). Subordinate lions were 4.32 times more likely to maintain possession of the resource post-oxytocin compared to control, but this difference was only marginally significant (Table 1; log odds: 1.46 ± 0.75, p = 0.052).
Resource possession in non-neutral trials
In non-neutral trials (designed to bias possession toward the subordinate in order to test the “ownership” rule), during control treatments dominant individuals still possessed the resource on average 74% of trials (range of 30% – 100%), and dominant individuals were again significantly more likely to possess the resource (Fig. 2; Table 1; log odds: −1.98 ± 0.3, p < 0.0001). However, post-oxytocin, dominant individuals possessed the resource on average 61% of the time (range of 10% – 100%), and subordinate lions were 3.05 times more likely to maintain possession of the resource compared to saline trials, significantly increasing their ownership (Fig. 2; Table 1; log odds: 1.2 ± 0.4, p = 0.01).
Differences in aggressive behaviors across trials
Chi-sq tests were used to test for differences in body-length proximity score (BL score) of lion pairs during trials. No differences were found in BL score in either neutral (chi-sq test: df = 3, p = 0.42), or non-neutral trials (chi-sq test: df = 3, p = 0.17).
We found no significant differences in subordinate or dominant lion aggression across neutral trials (Table 2; Fig. 3). However, in non-neutral trials dominant lions were less likely to display vocal aggression post-oxytocin (Fig. 3a; log odds: −1.0 ± 0.37, p < 0.01), while subordinate individuals were more likely to display physical aggression (Fig. 3b; log odds: 1.1 ± 0.47, p = 0.02), displayed greater aggression intensity (Fig. 3c; odds: 0.36 ± 0.14, p = 0.01), and increased overall aggression count (Fig. 3d; odds: 0.93 ± 0.29, p = 0.001).
Subordinate female lions increase aggression in order to maintain possession of resources after oxytocin administration during non-neutral ownership trials. a-d) Changes in displayed aggression across trials. a-b) mean presence of vocal or physical aggression; c) mean level of physical aggression, and d) mean total aggression level. Error bars show standard error. e–f) effect sizes from Binomial and Poisson GLMM models indicating significant differences in both dominant and subordinate aggressive behavior post oxytocin (* p < 0.05, ** p < 0.005), subordinates increased occurrence of physical aggression, mean aggression level, and total aggression, while dominants decreased vocal aggression thus indicating context-dependent effects of oxytocin administration.
Discussion
Female lions in the wild are unique in their symmetrical, egalitarian behavior. However, here we observed that, in captivity, there are strong dominant-subordinate relationships between female African lions, as indicated by asymmetry in possession of a high-value resource. During neutral trials, where both members of a pair were offered equal opportunity for possession, a “dominant” individual possessed the resource an average of 94% of the time.
Interestingly, however, we found that these asymmetrical relationships decrease with intranasal administration of oxytocin. Post oxytocin, subordinates maintain possession of the resource significantly more often during non-neutral trials, which were designed to bias possession towards the subordinate in order to test the “ownership” rule observed in wild lions. Post oxytocin treatment, subordinate individuals also behaved less submissively by significantly increasing aggressive defense (level of physical aggression, total aggression level, and mean total aggression) in order to maintain the resource, while dominant individuals decreased in vocal aggression—their most common form of aggression, highlighting the potential for oxytocin to elicit differing context-specific effects.
Mech (1999) proposed that differences in social-dominance exist because free-roaming familial groups are able to disperse, where unrelated pairings or groups are forced to stay together for years in captivity. Captive environments hardly come close to mimicking lions’ natural home range or lifestyle5,31, and both size and quality of the physical captive environment play a crucial role in lion behavior. Less natural environments lead to increased abnormal behaviors5,11, and the inability to behave naturally, such as regulating social dynamics during carcass feeding, negatively impacts levels of aggression and relationship dynamics in captive lions21. Specifics of group dynamics, such as group size19 and sex ratios32 can also have a significant effect on the level of affiliative or aggressive behaviors displayed among captive group members. An array of factors likely leads to the unnatural formation of dominance relationships in female lions as we have observed here. While some species, such as primates, have been shown to employ protective strategies including tension-reduction and conflict-avoidance models to mitigate levels of aggression generated by the constraints of captivity7,33, the details of these models have not been well studied in captive carnivores.
The formation of unnatural asymmetrical dominant-subordinate relationships in captive female lions could, indeed, be a product of tension-reduction or conflict avoidance models brought on by captivity. We see here that oxytocin can be used as a potential tool to intervene. One possible explanation for this may be oxytocin’s ability to attenuate stress-induced social avoidance caused by dominance34, and regulate fear responses, promoting defense behaviors35,36. Specifically, oxytocin has been shown to attenuate response to conditioned fear37,38, which could be beneficial for managing continual forced subordination in a closed (captive) environment. However, the current study only analyzed relationships between pairs of individuals, which could change in relation to the remaining group members. Group level dynamics and the specific functions for why these social dominance relationships have developed in captivity should be further investigated before the implications of altering them with oxytocin can be fully understood.
Differences in group dynamics are further emphasized by the idea that specific personality traits drive behavioral variance and stress levels in captive big cats39,40. The difference in personality traits and affiliated stress levels likely impact the expression of dominance in captivity, as stress levels are directly linked to dominant-subordinate relationships34,41. Research has shown that the effects of oxytocin may differ among individuals with altered baseline states42, and here we show that oxytocin has contrasting behavioral impacts on dominant vs subordinate behavior. Importantly, these findings further our understanding of oxytocin’s specific effects and potential applications considering specific aspects of pairwise relationships and in varied contexts, helping to clarify the perception of oxytocin having a negative or “dark” side43, and emphasizing the importance of carefully considering differences in personality and related behavioral traits, as well as social-relationship dynamics, before administering oxytocin.
Furthermore, the social salience hypothesis emphasizes oxytocin’s role in enhancing attention to cooperative vs. competitive environments44. Oxytocin can both facilitate prosocial behavior in the presence of a positive social stimulus45 or increase protective responses towards adverse stimuli46. While oxytocin is mostly known for its ability to promote prosocial behavior, it has been shown to increase self-serving and defense-motivated behaviors when protecting against self-vulnerability in a competitive context4447,48;, which is in line with our current findings. While our previous work emphasized the cooperative aspects of the salience hypothesis by highlighting the importance and effectiveness of providing positive stimulation during socializations of African lions28, in the current study, we tested the competitive aspects by creating an environment of aggressive competition over a high-value resource, in order to obtain a more complete perspective of oxytocin’s potential to manage carnivore behavior across contexts.
In our previous work27, we also found that oxytocin reduced vigilance (number of roars) in response to out-group threats (roars) and increased in-group affiliative and tolerance behavior (proximity to each other and not play object). However, we did not find that oxytocin administration had an impact on observed social behaviors in response to a high-value food item. Those trials, which differed from the current study in several key parameters (group size, sexes, space allocation, controlled approach to resource), were not designed to elicit sufficient changes in ownership to allow us to identify the effects observed here. We consider the current results complementary to that prior work. Furthermore, we have previously found that repeated administration of oxytocin in pairs and groups of lions in non-feeding contexts lead to lasting prosocial effects28. While the current study revealed that, after a single dose, oxytocin administration had immediate, distinct impacts on dominant and subordinate counterparts during competition over a resource, dominance relationships were thereafter restored (as evident by following trials), and therefore, the long-term effects of continued administration of oxytocin on relationships within a competitive feeding context should be investigated further.
Aspects driving social differentiation in group-living species are multifactorial15,49, and captivity adds an additional array of challenges with a multitude of issues which potentially contribute to the formation of hierarchical societies21,31,50,51,52. Further investigation is required to understand all the ways in which behavior is manipulated by an environment, and how oxytocin may directly impact these behaviors. Nonetheless, this study implicates the use of oxytocin as a recovery tool for the formation of unnatural behaviors in captivity, by ameliorating asymmetrical relationships and restoring the “ownership” rule in female African lions.
Data availability
References
Crates, R., Stojanovic, D. & Heinsohn, R. The phenotypic costs of captivity. Biol. Rev. 98(2), 434–449 (2023).
Schwitzer, C. & Kaumanns, W. Foraging patterns of free-ranging and captive primates-implications for captive feeding regimes. Zoo Anim. Nutr. 2, 247–265 (2003).
Hocking, D. P., Salverson, M. & Evans, A. R. Foraging-based enrichment promotes more varied behaviour in captive Australian fur seals (Arctocephalus pusillus doriferus). PLoS ONE 10(5), e0124615 (2015).
McGowan, R. T., Robbins, C. T., Alldredge, J. R. & Newberry, R. C. Contrafreeloading in grizzly bears: Implications for captive foraging enrichment. Zoo Biol. 29(4), 484–502 (2010).
Clubb, R. & Mason, G. Captivity effects on wide-ranging carnivores. Nature 425(6957), 473–474 (2003).
Farquharson, K. A., Hogg, C. J. & Grueber, C. E. A meta-analysis of birth-origin effects on reproduction in diverse captive environments. Nat. Commun. 9(1), 1055 (2018).
Palagi, E. & Bergman, T. J. Bridging captive and wild studies: Behavioral plasticity and social complexity in theropithecus gelada. Animals 11(10), 3003 (2021).
Mech, L. D. Alpha status, dominance, and division of labor in wolf packs. Can. J. Zool. 77(8), 1196–1203 (1999).
Forthman, D. L. (Ed.). An elephant in the room: the science and well-being of elephants in captivity. Tufts Center for Animals and Public Policy. (2009).
Pitsko, L.E. Wild tigers in captivity: A study of the effects of the captive environment on tiger behavior, PhD Thesis, Virginia Tech, 2003. Accessed: Aug. 13, 2024. [Online]. Available: https://vtechworks.lib.vt.edu/items/84695ef4-df91-435c-a79a-f4e84f9d79eb
Khan, B. N. et al. Impact of different captive environmental conditions on behavior of African lions and their welfare at Lahore Zoo and Safari Zoo Lahore. Pak. J. Zool. https://doi.org/10.17582/journal.pjz/2018.50.2.523.531 (2018).
Gutierrez, S., Canington, S. L., Eller, A. R., Herrelko, E. S. & Sholts, S. B. The intertwined history of non-human primate health and human medicine at the Smithsonian’s national Zoo and conservation Biology Institute. Notes Rec. 77(1), 73–96 (2023).
Creel, S. Social dominance and stress hormones. Trends Ecol. Evol. 16(9), 491–497 (2001).
Adams, N. Establishment of dominance in domestic Norway rats: Effects of the degree of captivity and social experience. Anim. Learn. Behav. 13(1), 93–97. https://doi.org/10.3758/BF03213371 (1985).
van Hooff, J. A. & Wensing, J. A. 11. Dominance and its behavioral measures in a captive wolf pack. Man Wolf Adv. Iss. Probl. Captive Wolf Res. 4, 219 (1987).
Archie, E. A., Morrison, T. A., Foley, C. A., Moss, C. J. & Alberts, S. C. Dominance rank relationships among wild female African elephants, Loxodonta africana. Anim. Behav. 71(1), 117–127 (2006).
Packer, C., Pusey, A. E. & Eberly, L. E. Egalitarianism in female African Lions. Science 293(5530), 690–693. https://doi.org/10.1126/science.1062320 (2001).
Glickman, S. E. et al. Social facilitation, affiliation, and dominance in the social life of spotted hyenas. Ann. N. Y. Acad. Sci.-Pap. Ed. 807, 175–184 (1997).
White, A. B. Wild and captive wolf (Canis lupus) aggression in relation to pack size and territory availability. Environ. Popul. Org. Biol. 1–38 (2001).
De Villiers, M. S., Richardson, P. R. & Van Jaarsveld, A. S. Patterns of coalition formation and spatial association in a social carnivore, the African wild dog (Lycaon pictus). J. Zool. 260(4), 377–389 (2003).
Höttges, N., Hjelm, M., Hård, T. & Laska, M. How does feeding regime affect behaviour and activity in captive African lions (Panthera leo)?. J. Zoo Aquar. Res. 7(3), 117–125 (2019).
Graustella, A. J. & MacLeod, C. A critical review of the influence of oxytocin nasal spray on social cognition in humans: Evidence and future directions. Horm. Behav. 61(3), 410–418 (2012).
Yao, S. & Kendrick, K. M. Effects of intranasal administration of oxytocin and vasopressin on social cognition and potential routes and mechanisms of action. Pharmaceutics 14(2), 323 (2022).
Jiang, Y. & Platt, M. L. Oxytocin and vasopressin flatten dominance hierarchy and enhance behavioral synchrony in part via anterior cingulate cortex. Sci. Rep. 8(1), 8201 (2018).
Jiang, Y. & Platt, M. L. Oxytocin and vasopressin increase male-directed threats and vocalizations in female macaques. Sci. Rep. 8(1), 18011 (2018).
Jing, P. & Shan, Q. Exogenous oxytocin microinjection into the nucleus accumbens shell attenuates social dominance in group-housed male mice. Physiol. Behav. 269, 114253 (2023).
Burkhart, J. C., Gupta, S., Borrego, N., Heilbronner, S. R. & Packer, C. Oxytocin promotes social proximity and decreases vigilance in groups of African lions. Iscience 25, 4 (2022).
Burkhart, J. C., Heilbronner, S. R. & Packer, C. Oxytocin administration is a potential tool for behavioral management in felids. Front. Mammal Sci. 2, 1148214 (2023).
Weisman, O., Zagoory-Sharon, O. & Feldman, R. Intranasal oxytocin administration is reflected in human saliva. Psychoneuroendocrinology 37(9), 1582–1586 (2012).
Lee, M. R. et al. Labeled oxytocin administered via the intranasal route reaches the brain in rhesus macaques. Nat. Commun. 11(1), 2783 (2020).
Altman, J. D., Gross, K. L. & Lowry, S. R. Nutritional and behavioral effects of gorge and fast feeding in captive lions. J. Appl. Anim. Welfare Sci. 8(1), 47–57 (2005).
Matoba, T., Kutsukake, N. & Hasegawa, T. Head rubbing and licking reinforce social bonds in a group of captive African lions. Panthera leo. PloS one 8(9), e73044 (2013).
Videan, E. N. & Fritz, J. Effects of short-and long-term changes in spatial density on the social behavior of captive chimpanzees (Pan troglodytes). Appl. Anim. Behav. Sci. 102(1–2), 95–105 (2007).
Šabanović, M., Liu, H., Mlambo, V., Aqel, H. & Chaudhury, D. What it takes to be at the top: The interrelationship between chronic social stress and social dominance. Brain Behavior 10(12), e01896 (2020).
Hale, L. H., Tickerhoof, M. C. & Smith, A. S. Chronic intranasal oxytocin reverses stress-induced social avoidance in female prairie voles. Neuropharmacology 198, 108770 (2021).
Olivera-Pasilio, V. & Dabrowska, J. Oxytocin promotes accurate fear discrimination and adaptive defensive behaviors. Front. Neurosci. 14, 583878 (2020).
Eckstein, M. et al. Oxytocin facilitates the extinction of conditioned fear in humans. Biol. Psych. 78(3), 194–202 (2015).
Acheson, D. et al. The effect of intranasal oxytocin treatment on conditioned fear extinction and recall in a healthy human sample. Psychopharmacology 229, 199–208 (2013).
Gartner, M. C., Powell, D. M. & Weiss, A. Comparison of subjective well-being and personality assessments in the clouded leopard (Neofelis nebulosa), snow leopard (Panthera uncia), and African lion (Panthera leo). J. Appl. Anim. Welfare Sci. 19(3), 294–302 (2016).
Torgerson-White, L. L. & Bennett, C. Rating methodology personality axes and behavioral plasticity: A case study in African lions. Anim. Behav. Cogn. https://doi.org/10.12966/abc.08.02.2014 (2014).
Sands, J. L. Stress hormones and social behavior of wolves in Yellowstone National Park (Doctoral dissertation, Montana State University-Bozeman, College of Letters & Science). (2001).
Hecht, E. E., Robins, D. L., Gautam, P. & King, T. Z. Intranasal oxytocin reduces social perception in women: Neural activation and individual variation. Neuroimage 147, 314–329 (2017).
Zik, J. B. & Roberts, D. L. The many faces of oxytocin: Implications for psychiatry. Psych. Res. 226(1), 31–37 (2015).
Shamay-Tsoory, S. G. & Abu-Akel, A. The social salience hypothesis of oxytocin. Biol. Psych. 79(3), 194–202 (2016).
Ford, C. L. & Young, L. J. Refining oxytocin therapy for autism: Context is key. Nat. Rev. Neurol. 18(2), 67–68 (2022).
Striepens, N. et al. Oxytocin facilitates protective responses to aversive social stimuli in males. Proc. Natl. Acad. Sci. 109(44), 18144–18149 (2012).
Xu, X. et al. Oxytocin facilitates self-serving rather than altruistic tendencies in competitive social interactions via orbitofrontal cortex. Int. J. Neuropsychopharmacol. 22(8), 501–512 (2019).
De Dreu, C. K., Shalvi, S., Greer, L. L., Van Kleef, G. A. & Handgraaf, M. J. Oxytocin motivates non-cooperation in intergroup conflict to protect vulnerable in-group members. PLoS ONE 7(11), e46751 (2012).
Moran, G. Long-term patterns of agonistic interactions in a captive group of wolves (Canis lupus). Anim. Behav. 30(1), 75–83 (1982).
Morgan, K. N. & Tromborg, C. T. Sources of stress in captivity. Appl. Anim. Behav. Sci. 102(3–4), 262–302 (2007).
McPhee, M. E. Intact carcasses as enrichment for large felids: Effects on on-and off-exhibit behaviors. Zoo Biol.: Publ. Aff. Am. Zoo Aquarium Assoc. 21(1), 37–47 (2002).
Finch, K., Williams, L. & Holmes, L. Using longitudinal data to evaluate the behavioural impact of a switch to carcass feeding on an Asiatic lion (Panthera leo persica). J. Zoo Aquarium Res. 8(4), 283–287 (2020).
Acknowledgements
We would like to thank Lionsrock, FOURPAWS, for allowing us access to the animals to participate in this study. A special thank you to Hildegard Pirker for permission and cooperation, and to Dr. Christine Streyr for participating in the conceptual framework and contributing to the study design.
Funding
Funding was provided by the Grad Program in EEB, University of Minnesota, and from the UMN AHC seed Award to SH.
Author information
Authors and Affiliations
Contributions
JB designed and implemented all physical aspects of the study under advisement from SH and CP. ED assisted with implementing physical aspects of the study, including data recording. AG performed statistical analysis and provided relevant written portions of the manuscript. JB, ED, SH, and CP were all involved in the conception and design of the study. JB, SH, and CP all contributed to the writing and editing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests (financial or non-financial).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Burkhart, J.C., Guthmann, A., DuBois, E.M. et al. Oxytocin reduces asymmetries in dominance relationships between pairs of captive female lions. Sci Rep 15, 23366 (2025). https://doi.org/10.1038/s41598-025-04276-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-04276-x