Abstract
Portal vein thrombosis (PVT) is an important complication of liver cirrhosis, especially in patients with chronic hepatitis B (CHB). Previous studies have revealed that hepatitis B virus (HBV) infection and several serological indicators are associated with PVT; however, no predictive model including virological indicators of PVT in cirrhosis patients with CHB has been established. A retrospective cohort study including 252 CHB patients at the Affiliated Hospital of Qingdao University between January 1, 2012, and December 31, 2023, was performed. Baseline data were collected. PVT outcomes and antiviral virological responses were subsequently evaluated. We recognized risk factors and their hazard ratios. Propensity score matching (PSM) analysis was used to control for confounding factors. A clinical predictive model of PVT in CHB patients was established and verified. During a median follow-up time of 2568 days, 28 patients (11.11%) developed PVT. There were significant differences in age, portal vein diameter, spleen major axis, antiviral therapy time, prothrombin time (PT), blood platelet count, aspartate aminotransferase (AST), gamma-glutamyl transpeptidase (GGT), alkaline phosphatase (ALP), alpha-feto protein (AFP), D-dimer, international normalized ratio (INR), low density lipoprotein (LDL), high density lipoprotein (HDL), total cholesterol (TC), serum hepatitis B virus deoxyribonucleic acid (HBV DNA) loads and levels after antiviral therapy, complications associated with hepatocellular cell cancer cirrhosis level, and antiviral responses between PVT patients and non-PVT patients. The effect of antiviral therapy was worse in more severe cirrhosis. There were greater PVT risks in patients with VBT antiviral responses, larger major axes in the spleen, and higher hepatitis B surface antigen (HBsAg) and hepatitis B surface antibody (HBsAb) levels. After controlling for confounding factors with PSM analysis, there was still a higher PVT risk in patients with adverse virological responses. A predictive nomogram for PVT in CHB patients was established and validated. PVT is a common complication in cirrhosis patients with CHB. Favorable virological responses are beneficial for lowering the risk of PVT in CHB patients. HCC, virological response, age, portal vein diameter, splenic major axis, PT, PLT, HBsAg, and HBsAb can be used to predict PVT in CHB patients.
Similar content being viewed by others
Introduction
Portal vein thrombosis (PVT) is a severe complication that can occur in patients with chronic hepatitis B (CHB), especially those with cirrhosis. Previous studies have established several predictive models for all cirrhosis patients1 and after-surgery patients2. Some studies have shown that not only HBV infection3,4 but also positive HBsAg and HBeAg5 are associated with PVT. Potential mechanisms of PVT in CHB patients include the inflammatory response6,7,8, vascular endothelial injury9,10,11, coagulation and anticoagulation disturbances12,13,14, and cardiolipin antibody production15,16,17. However, there is still no predictive model for PVT in CHB patients, who also have a high incidence of PVT. There are no clear predictors of PVT associated with HBV infection, such as HBV DNA and HBsAg.
In this study, we analyzed the baseline data, serological indicators, virological indicators, and sonographic data of CHB patients receiving long-term antiviral therapy; assessed their different PVT outcomes; identified risk indicators; and established and verified a predictive model for PVT.
Methods
Study cohort
A longitudinal retrospective study was conducted on an adult cohort with CHB. The data for the study were collected from patients who had been first diagnosed with CHB at the Affiliated Hospital of Qingdao University between January 1, 2012, and December 31, 2023. The patients were HBsAg-positive, and their serum HBV DNA was detected. For patients with HBV-related cirrhosis treated with nucleoside analogues (NAs), if HBV DNA was still detectable (HBV DNA > 100 IU/mL) after 24 weeks, it was recommended to adjust NA therapy (for entecavir (ETV) and lamivudine (LAM) resistance, switch to tenofovir (TDF); for adefovir dipicoxil (ADV) resistance, switch to LAM if LAM-naïve, switch to TDF if LAM resistant, and add ETV or switch to ETV if HBV DNA plateaued; for TDF resistance, switch to ETV if LAM naïve, and add ETV if LAM resistant; for multidrug resistance, switch to ETV plus TDF or tenofovir alafenamide (TAF) combination, or combine with of IFN-α and pegylated interferon-α (Peg-IFN))18.
Every patient underwent annual serum HBV DNA testing until the end of the follow-up and underwent annual ultrasound examination at least three times. When patients developed PVT, treatment measures, including anticoagulation and interventional therapy, were used, and the follow-up period ended. For patients without PVT, the follow-up period ended on December 31, 2023.
Diagnosis of CHB
The diagnosis of CHB required the patient to be HBsAg-positive for at least 6 months. Our laboratory used a two-step immunoassay, using Chemiflex technology, a combination of chemiluminescence microparticle immunoassays (CMIA) and flexible detection modes, to quantitatively determine HBsAg and HBsAb, and qualitatively determine hepatitis B virus e antigen (HBeAg), hepatitis B virus e antibody (HBeAb), and hepatitis B core antibody (HBcAb). According to the test standard, when HBsAg concentration was < 0.05 IU/mL, the sample was considered nonreactive and was marked negative; when HBsAg concentration was > 250.00 IU/mL, the sample was labeled as reaching the upper threshold and was marked “ > 250.00 IU/mL.” Similarly, when HBsAb concentration was > 1000 mIU/mL, the sample was labeled as reaching the upper threshold. For samples that reached the upper threshold, specific concentration quantification was obtained by dilution calibration in accordance with the instructions. However, the dilution calibration procedure was not adopted for all samples, and the vast majority of the samples did not have a specific concentration. Therefore, the quantitative values of HBsAg and HBsAb for all samples that met the detection upper threshold were uniformly identified as the above upper threshold.
For HBeAg, HBeAb, and HBcAb, the system calculated the Cutoff Relative optical unit (RLU) value based on the average RLU value obtained from the three test calibrators, saved the result, and calculated the sample/cut off (S/CO) using the following formula: “S/CO = Sample-RLU/Cutoff-RLU.” When HBeAb detection was < 1.00 S/CO, the sample was considered reactive. When HBeAg and HBcAb detection was > 1.00 S/CO, the sample was considered reactive.
Inclusion criteria
The patients were enrolled in the cohort if they met all of the following criteria:
-
a.
Presence of HBsAg in serum for more than 6 months, along with at least one recorded detectable serum HBV DNA test (≥ 100 IU/mL);
-
b.
After stable HBV DNA levels were achieved, regular liver color Doppler ultrasonography was performed once a year at least three times before the end of the follow-up.
Exclusion criteria
Patients that met any of the following criteria were excluded from the cohort:
-
a.
Antiviral treatment before enrollment;
-
b.
Age under 18 years;
-
c.
Administration of anticoagulant drugs, including heparin, low-molecular-weight heparin, and warfarin, during the follow-up;
-
d.
Positive markers of HCV, hepatitis D virus, and HIV;
-
e.
Diagnosis of HCC before enrollment;
-
f.
Clear history of liver surgery or interventional liver surgery during the follow-up;
-
g.
Splenectomy history before or during the follow-up;
-
h.
Occurrence of PVT before virological response.
Virological responses to antiviral therapy
The virological treatment outcomes in our cohort were divided into five categories in line with the EASL 2017 Clinical Practice Guidelines18 as follows:
-
a.
Virological response (VR) was defined as undetectable serum HBV DNA by sensitive polymerase chain reaction (PCR) assay with a 10 IU/mL detection limit;
-
b.
Primary nonresponse (PNR) was defined as a decrease in serum HBV DNA of less than one log10 after 3 months of therapy;
-
c.
Partial VR (PVR) was defined as a decrease in HBV DNA of more than one log10 IU/mL but detectable HBV DNA after at least 12 months of therapy in compliant patients;
-
d.
Virological breakthrough (VBT) was defined as a confirmed increase in the HBV DNA level of more than one log10 IU/mL compared with the lowest value of the HBV DNA level;
-
e.
Sustained off-therapy VR (SOVT) was defined as a serum HBV DNA level < 2000 IU/mL for at least 12 months after the end of therapy.
Follow-up and survival outcomes
The primary outcome was the occurrence of PVT. Follow-up was started at the time of diagnosis of CHB and the beginning of antiviral therapy (at the same time), and ended when the patients achieved a PVT outcome or on December 31, 2023. Overall survival time was defined as the time from the start of the follow-up to the end of the follow-up.
Temporal relationship between antiviral therapy and PVT
To exclude the impact of an inverse temporal relationship between HBV DNA level and PVT, all patients on antiviral therapy were tested for HBV DNA every 6 months until they met the criteria for virological response described above. Patients who developed PVT before reaching a standard virological response were excluded from the statistical analysis.
Diagnosis and differential diagnosis of PVT
PVT can be diagnosed based on typical imaging findings from liver ultrasounds19. In our study, we identified the specific diagnostic imaging features of PVT as abnormal lumen echoes of the portal vein system on abdominal ultrasound. The following thrombosis locations were included in the diagnosis: the main portal vein, portal vein, left and right main stems, splenic vein, superior mesenteric vein (SMV), and inferior mesenteric vein (IMV). All patients with severe thrombosis were enrolled, regardless of whether it was minimally obstructive, obstructive, or occlusive20.
Although we excluded patients who had had HCC before the follow-up, cases of de novo HCC still emerged during the follow-up of this cohort. We used the A-VENA standard to distinguish PVT from portal vein tumor thrombosis (PVTT). PVTT was considered when a patient presented an abnormal lumen echo of the portal vein system and met three or more of the following five criteria21:
-
a.
Proximity to primary HCC;
-
b.
AFP > 1000 ng/mL;
-
c.
Portal vein diameter > 1.0 cm;
-
d.
Neovascularization of the portal vein system on computed tomography (CT) or ultrasound;
-
e.
Thrombus enhancement on CT (> 20 Hounsfield units).
Statistical analysis
Quantitative and qualitative difference analyses were used to determine the differences in baseline data between different groups. Survival curves were generated via the Kaplan–Meier method. Cox regression analysis was used to analyze the risk proportions associated with different risk factors. We used propensity score matching (PSM) analysis to control for confounding factors. In PSM, we divided all patients to the VR group and the No-VR group (including PVR, PNR, and VBT), identified the variables that showed significant differences between the groups with different virological response, set the caliper value at 0.1, and set the matching ratio at 3. A new data table was obtained after adjusting for significant confounding factors, and the same survival analysis as above was repeated. The training group and the internal validation group were randomly generated at a ratio of 7:3, and the difference analysis was carried out to ensure that there was no obvious difference between the two groups. Finally, there were 176 patients in the training group and 76 patients in the internal validation group, and there were no variables that exhibited significant differences between the two groups. The R packages ‘rms’ and ‘Hmisc’ were used to develop and verify the predictive model. The prediction efficiency of the model was verified internally. The area under the receiver operating characteristic curve (AUC), the calibration curve, and decision curve analysis (DCA) were used to verify and evaluate the accuracy of the model and the net clinical benefit. All of the above statistical analyses were performed in R software ver.4.3.0 (2023–04–21, R Foundation for Statistical Computing, Vienna, Austria). G*Power software ver.3.1.9.7 (Program written by Franz Faul, Universitat Kiel, Germany) and PASS 2021 software ver21.0.3, were used to perform post-hoc power analysis.
Ethics statement
The study was approved by the Ethics Review Committee of the Affiliated Hospital of Qingdao University on December 1, 2023 (QYFYWZLL28547), and was performed in accordance with the current version of the Declaration of Helsinki. Due to the retrospective nature of the study, the requirement of informed consent was waived by the Ethics Review Committee of the Affiliated Hospital of Qingdao University.
Results
Baseline characteristics
A total of 252 patients were included in the study and completed the follow-up. Among the patients enrolled, 32 chose not to receive antiviral therapy, whereas all of the other patients received regular and long-term antiviral therapy. Originally, a total of 183 patients received entecavir (ETV) only; 17 patients received tenofovir disoproxil fumarate (TDF) only; 10 patients received adefovir (ADF) only; 1 patient received lamivudine (LVD) only; 2 patients received ADF combined with LVD; and 7 patients received alpha interferon (Peg-IFN-α). The median overall survival time of PVT for the entire cohort was 2571 days. The average age of the cohort was 53.7 years. The proportion of males was 67.59%. The average baseline load of serum HBV DNA was 132,500.00 IU/mL (Q1 = 5587.50, Q3 = 3,737,500.00). The average load of HBV DNA after treatment was measured during the follow-up period, and 28 patients developed PVT (incidence rate: 11.11%). Among all of the patients, 109 (43.25%) were HBeAg-positive and 125 (49.60%) were HBeAb-positive patients. All of the patients were HBcAb-positive. There were significant differences between patients with and without PVT in terms of age (P < 0.001), portal vein diameter (P < 0.001), major axis of the spleen (P < 0.001), antiviral therapy time (P < 0.001), PT (P = 0.004), PLT (P < 0.001), AST (P = 0.049), GGT (P < 0.025), ALP (P < 0.001), AFP (P < 0.001), D-dimer (P < 0.001), INR (P < 0.001), LDL (P = 0.010), HDL (P < 0.015), TC (P = 0.014), HBV DNA loads and levels after antiviral therapy (P < 0.001), complications of HCC (P < 0.001), cirrhosis level (P < 0.001, Fisher’s exact), and antiviral responses (P < 0.001) (Table 1).
Of all the patients who received antiviral therapy, 188 (74.60%) achieved VR, 30 (11.90%) achieved PVR, 5 (1.98%) achieved PNR, and 29 (11.51%) achieved VBT. Comparison of the groups of patients who achieved different virological responses showed significant differences of cirrhosis degree, age, and HCC (Supplemental Table 1).
Risk factors for PVT in cirrhosis patients with CHB
Univariate Cox regression analysis revealed that the risk of PVT was greater in patients with moderate HBV DNA levels after antiviral treatment (P < 0.001, hazard ratio [HR] = 5.21; 95% confidence interval [CI] = 2.18–12.45); PVR (P < 0.001, HR = 22.52, 95% CI = 4.54–111.66), PNR (P = 0.006, HR = 30.09, 95% CI = 2.70–335.92), and VBT (P < 0.001, HR = 65.60, 95% CI = 14.79–290.95) antiviral response; complications of de novo HCC (P < 0.001, HR = 14.29; 95% CI = 6.03–33.89); greater cirrhosis level (P < 0.001, HR = 2.37, 95% CI = 1.72–3.27); older age (P = 0.002, HR = 1.06, 95% CI = 1.02–1.10); greater portal vein diameter (P < 0.001, HR = 13.20; 95% CI = 5.32–32.75); and larger major axis of the spleen (P < 0.001, HR = 1.37; 95% CI = 1.26–1.49). After multivariate Cox regression, there was still a greater risk of PVT in patients with a VBT antiviral response (P < 0.001, HR = 42.48, 95% CI = 5.02–359.89), complications of de novo HCC (P < 0.001, HR = 30.69, 95% CI = 5.50–171.15), a larger major axis of the spleen (P < 0.001, HR = 1.49, 95% CI = 1.21–1.84), and higher HBsAg (P = 0.007, HR = 1.03, 95% CI = 1.01–1.05) and HBsAb (P = 0.004, HR = 1.02, 95% CI = 1.01–1.03) levels (Table 2).
To ensure the independence of different variables, a multicollinearity test was carried out (Table 2). Multivariate Cox regression analysis was performed after removing the variables with strong collinearity, which showed similar results (Supplemental Table 2).
The effect of antiviral therapy was greater in more severe cirrhosis. Univariate and multivariate ordered binary logistic regression revealed the correlation between the cirrhosis level and virological response. Decompensated stage of cirrhosis and advanced age were the independent risk factors for adverse virological response (Supplemental Table 3).
Higher PVT risk in different CHB patients
Kaplan‒Meier (KM) curves revealed increased PVT risk in patients with adverse virological responses to antiviral therapy (Fig. 1), increased spleen size (Supplemental figure 1), complications of HCC (Supplemental figure 2), and moderate HBV DNA levels after antiviral therapy (Supplemental figure 3). Next, we divided the patients into groups based on virological responses and analyzed the differences in their baseline data. The variables with significant differences in the baseline data were identified as possible confounding factors and were included in PSM analysis (Supplemental Table 1). The purpose of this analysis was to balance out the differences in these variables between the groups with different virological response as much as possible, so as to reduce the interference of these confounding factors on the outcome. PSM analysis substantially controlled the standardized mean differences (SMD) and probability densities of confounding factors to virological responses, including cirrhosis degree, age, HCC, PT, and PLT (Table 3, Fig. 2). KM curve after PSM of different virological responses to antiviral therapy showed a similar result as in Fig. 1 (Fig. 3).
KM curves of different antiviral responses to PVT. Antiviral response = 0: VR; antiviral response = 1: PVR; antiviral response = 2: PNR; antiviral response = 3: VBT. When P < 0.05, the risk of PVT was considered significantly different among different groups. All PVT occurred after patients achieved virological response.
KM curve of different antiviral responses to PVT risk in CHB patients after PSM analysis. Antiviral response = 0: VR; antiviral response = 1: PVR; antiviral response = 2: PNR; antiviral response = 3: VBT. When P < 0.05, the risk of PVT was considered significantly different among different groups. All PVT occurred after patients achieved virological response.
Development and validation of the predictive model
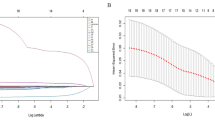
A nomogram was established on the basis of the significant risk factors recognized above (Fig. 4). The difference analysis showed no significant differences in any of the variables between the two groups (Supplemental Table 4). The AUC for the nomogram was 0.98 (95% CI = 0.96–1.00) in the training group and 0.97 (95% CI = 0.93–1.01) in the validation group (Fig. 5). The calibration curve for the probability of median survival showed a moderate level of consistency between the predictions and observations in the training group and the validation group (Fig. 6). DCA demonstrated that if the threshold probability of a patient was between 0.044 and 0.71, there would be favorable predictive effects for PVT (Fig. 7).
Decision curve analysis for the nomogram on the basis of clinical characteristics. The blue line represents the nomogram, the green line represents all negative samples, and the red line represents all positive samples. When the nomogram line is above the other two, it is considered to have a favorable prediction effect.
Discussion
PVT is associated with many serious complications, including gastroesophageal venous hemorrhage, mesenteric ischemia, chronic spongiosis of the portal system, and adverse prognostic outcomes. The incidence of PVT is relatively high in patients with cirrhosis and HCC.
The development of PVT in patients with cirrhosis can be attributed to various factors related to Virchow’s triad. These factors include changes in the flow of blood in the portal vein, alterations in coagulation, and local alterations in the portal vein. As for altered coagulation, HBV infection can cause coagulant‒procoagulant disorder14, which is called the coagulant side or a prothrombotic state. Thrombomodulin is a critical cofactor for the activable anticoagulant protein C system and subsequently thrombin-mediated activation of thrombin-activable fibrinolysis inhibitors22. As for local portal vein alterations, some studies have shown that HCV can directly cause damage to vascular endothelial cells10,23, but there have been no similar studies on HBV. Other factors, such as antiphospholipid antibodies and neutrophil extracellular traps (NETs), are increased in patients with chronic hepatitis B and C and are associated with venous thrombosis, but this remains controversial17.
This study established a CHB cohort and focused on the relationship between dynamic changes in HBV DNA levels after antiviral therapy and the occurrence of PVT. The correlation between the degree of cirrhosis and the effect of antiviral therapy was reported. Independent risk indicators of PVT, including VBT virological response, complications of de novo HCC, larger major axis of the spleen, and higher HBsAg and HBsAb levels, were reported in cirrhosis patients with CHB. A clinical predictive model of PVT for cirrhosis patients with CHB was established and verified. This means that we have more options, including virological indicators, to predict PVT risk in patients with CHB clinically, especially in those with long-term antiviral therapy. This information is helpful for the early identification and prevention of PVT during long-term management of CHB patients.
We performed a post-hoc power analysis for antiviral responses to PVT, which ultimately showed that while the sample size was small, it was sufficient to provide sufficient power.
Higher HBsAb and HBsAg were reported as risk factors for PVT in this study. Similar reports have emerged from previous studies5. HBsAb and HBsAg are commonly considered protective factors for HBV infection. This is actually somewhat controversial because higher level of HBsAb and HBsAg are considered to reflect a stronger immune response in vivo and a better antiviral therapy outcome. In fact, in our study, the correlations between HBsAb, HBsAg, and PVT were weak. The difference analysis did not show significant differences in HBsAb and HBsAg levels between the patients with PVT and those without PVT. This might have been caused by limitations and defects in our quantitative measurements of HBsAg and HBsAb. In this study, when concentration of HBsAg and HBsAb reached the upper threshold, dilution and calibration procedure was not adopted in all of the samples, which caused relatively narrow concentration ranges of HBsAg and HBsAb, especially for CHB patients with relatively higher baseline concentrations of HBsAg and HBeAg. These defects might have caused a confusion about the correlations among HBsAg, HBsAb, and PVT.
It is necessary to further prove this correlation in future studies. In the future study, it will be necessary to expand the sample size. Prospective cohort study would be a better study design. Already-matched CHB patients with quantitative concentration of HBsAg and HBsAb will be included into the cohort and divided into different groups based on their concentration levels. The concentration changes will be followed up prospectively and dynamically during antiviral therapy, and the final outcome of PVT will be observed. During the follow-up, dilution and calibration procedure will be adopted to confirm specific quantitative concentrations of HBsAg and HBsAb. Finally, we will analyze the differences in PVT occurrence between different HBsAg and HBsAb concentration groups, at baseline and after therapy, to find the correlation between them and PVT.
In addition, in Cox regression analysis, portal vein diameter, which was a risk factor in the univariate analysis, turned to a protective factor in the multivariate analysis. We considered whether there was a phenomenon similar to Simpson’s paradox. This might have been caused by the small sample size and a large number of potential confounding factors. To avoid this situation as much as possible, we eliminated the variables with strong collinearity and re-performed multivariate Cox regression analysis, and then the paradox significantly diminished.
This was a small-sized (252 patients in total) retrospective study conducted at a single center. To confirm the robustness of the study results, Cox Regression was calculated using PASS 2021 software, and α = 0.05 (bilateral test) was selected to perform post-hoc power analysis. According to results, the correlation coefficient R2 between antiviral response and other independent variables was 0.237, the standard deviation of antiviral response was 0.99, and the power achieved 1.00. (Related Fig. 1, Related Table 1).
Although PSM was used to reduce the influence of confounders on the relationship between primary factors and outcomes, the inevitable loss of data during the matching process still weakened the strength of the conclusions drawn regarding the correlations. Second, because of the limitation of the retrospective study design, hematological disorders and diseases that developed during the follow-up could not be completely excluded. In the future, multicenter and prospective studies should be designed to further explore the relationship between antiviral therapy and PVT. Long-term dynamic observation and analysis of hemagglutination indexes are also necessary, which can exclude blood system–related diseases, but also dynamically observe the coagulation and procoagulant changes of patients during antiviral therapy.
Conclusion
This study revealed a significant incidence of PVT and differences in baseline data, sonographic indexes, and serological and virological indicators in cirrhosis patients with CHB. We followed up PVT outcomes and antiviral virological responses of the cohort. Independent risk factors for PVT were identified, and a prospective model of PVT for cirrhosis patients with CHB was established.
Data availability
All the data were collected from the clinical research platform of Affiliated Hospital of Qingdao University and were collected by the first author. If the data materials involved in the study are requested, the authors are willing to provide them upon request, by connecting corresponding author Jingxue (Jingxue@qdu.edu.cn).
References
Li, Y. et al. Diagnostic Prediction of portal vein thrombosis in chronic cirrhosis patients using data-driven precision medicine model. Brief Bioinform. https://doi.org/10.1093/bib/bbad478 (2023).
Hackert, T. et al. Portal vein resection in pancreatic cancer surgery: Risk of thrombosis and radicality determine survival. Ann. Surg. 277(6), e1291–e1298 (2023).
Lertpipopmetha, K. & Auewarakul, C. U. High incidence of hepatitis B infection-associated cirrhosis and hepatocellular carcinoma in the Southeast Asian patients with portal vein thrombosis. BMC Gastroenterol. 11, 66 (2011).
Chen, H. et al. A single hospital study on portal vein thrombosis in cirrhotic patients - clinical characteristics & risk factors. Indian J. Med. Res. 139(2), 260–266 (2014).
Yue-Meng, W. et al. Portal vein thrombosis in patients with cirrhosis undergoing elective transjugular intrahepatic portosystemic shunt: Risk factors, warfarin efficacy, and clinical outcomes. Clin. Appl. Thromb. Hemost. 24(3), 462–470 (2018).
Tichelaar, V. Y. et al. Active cytomegalovirus infection in patients with acute venous thrombosis: A case-control study. Am. J. Hematol. 86(6), 510–512 (2011).
Levi, M., Keller, T. T., van Gorp, E. & ten Cate, H. Infection and inflammation and the coagulation system. Cardiovasc. Res. 60(1), 26–39 (2003).
Lazzaroni, M. G. et al. Coagulation dysfunction in COVID-19: The interplay between inflammation, viral infection and the coagulation system. Blood Rev. 46, 100745 (2021).
Van Dam-Mieras, M. C., Bruggeman, C. A., Muller, A. D., Debie, W. H. & Zwaal, R. F. Induction of endothelial cell procoagulant activity by cytomegalovirus infection. Thromb. Res. 47(1), 69–75 (1987).
Span, A. H. et al. The effect of virus infection on the adherence of leukocytes or platelets to endothelial cells. Eur. J. Clin. Invest. 21(3), 331–338 (1991).
Dalpke, A. H., Thomssen, R. & Ritter, K. Oxidative injury to endothelial cells due to Epstein-Barr virus-induced autoantibodies against manganese superoxide dismutase. J. Med. Virol. 71(3), 408–416 (2003).
van Dam-Mieras, M. C. et al. The procoagulant response of cytomegalovirus infected endothelial cells. Thromb. Haemost. 68(3), 364–370 (1992).
Tripodi, A. et al. Detection of the imbalance of procoagulant versus anticoagulant factors in cirrhosis by a simple laboratory method. Hepatology 52(1), 249–255 (2010).
Tripodi, A. & Mannucci, P. M. The coagulopathy of chronic liver disease. N Engl J Med 365(2), 147–156 (2011).
Zignego, A. L., Ferri, C., Pileri, S. A., Caini, P. & Bianchi, F. B. Italian association of the study of liver commission on extrahepatic manifestations of HCVi. Extrahepatic manifestations of Hepatitis C virus infection: A general overview and guidelines for a clinical approach. Dig. Liver Dis. 39(1), 2–17 (2007).
Ambrosino, P. et al. Viral hepatitis and anti-phospholipid antibodies positivity: A systematic review and meta-analysis. Dig. Liver Dis. 47(6), 478–487 (2015).
Mangia, A. et al. Anticardiolipin antibodies in patients with liver disease. Am. J. Gastroenterol. 94(10), 2983–2987 (1999).
European Association for the Study of the Liver. Electronic address eee, European association for the study of the L. EASL 2017 Clinical practice guidelines on the management of hepatitis B virus infection. J. Hepatol. 67(2), 370–398 (2017).
Tessler, F. N. et al. Diagnosis of portal vein thrombosis: Value of color doppler imaging. AJR Am. J. Roentgenol. 157(2), 293–296 (1991).
Intagliata, N. M., Caldwell, S. H. & Tripodi, A. Diagnosis, development, and treatment of portal vein thrombosis in patients with and without cirrhosis. Gastroenterology 156(6), 1582–99 e1 (2019).
Sherman, C. B. et al. Distinguishing tumor from bland portal vein thrombus in liver transplant candidates with hepatocellular carcinoma: The A-VENA criteria. Liver Transpl. 25(2), 207–216 (2019).
Loghmani, H. & Conway, E. M. Exploring traditional and nontraditional roles for thrombomodulin. Blood 132(2), 148–158 (2018).
Rahbar, A. & Soderberg-Naucler, C. Human cytomegalovirus infection of endothelial cells triggers platelet adhesion and aggregation. J Virol 79(4), 2211–2220 (2005).
Acknowledgements
We thank LetPub (www.letpub.com.cn) for its linguistic assistance during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
As the first author, Zhanghao Li was responsible for data collection, data analysis, the production of pictures and tables, and manuscript writing. Jingxue and Xinjuan Kong were involved in study design, coordination of clinical work, and review of imaging data. Shijin Wang, Yingjun Jiang and Qiaoyi were involved in data collection and analysis. All authors listed in the article were involved in the review of the manuscript and the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
The consent for publication is not applicable in this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, Z., Jiang, Y., Wang, S. et al. Correlation between virological response and portal vein thrombosis in patients with chronic hepatitis B. Sci Rep 15, 20856 (2025). https://doi.org/10.1038/s41598-025-04316-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-04316-6