Abstract
At least 60% of adults over 40 experience symptoms related to the storage passage of urine that negatively affect their health and quality of life. Our research team is developing a device to deliver noninvasive long-term urine measures at the patient’s home or clinic. We employed a participatory-design approach to guide development in understanding end-user needs. We gathered data from 45 stakeholders through focus groups with clinicians (N = 40, including medical doctors (urologists), pelvic-floor physical therapists, and nurses) and semi-structured interviews with patients and caregivers (N = 5). Our results showed similarities (e.g., collecting urine storage and voiding data) and differences (e.g., the importance of pelvic-floor function for physical therapists, and the associated workload of using the device for nurses) among the three clinician groups. There were differences between clinicians and patients, the latter emphasizing the shame and discomfort associated with current diagnostic procedures. We gathered participants’ views on the advantages and disadvantages of the proposed device, considerations for use, and design suggestions. Our results emphasize the importance of including the end users in early design stages of a novel technology tailored to the target population.
Similar content being viewed by others
Introduction
At least 60% of adults over the age of 40 experience one or more symptoms associated with the storage or passing of urine1,2,3,4. These symptoms include urinary urgency, increased frequency, nocturia (waking at night to void), weak stream, hesitancy, straining to void, incomplete emptying, and incontinence—all of which can significantly impair daily function, emotional wellbeing, and social participation5,6. Many of the underlying conditions occur slowly; producing first mild, tolerable symptoms and only more significant, highly impactful symptoms after many years. These symptomatic changes are accompanied by changes to the composition of the bladder wall that include the development of new blood vessels (angiogenesis), nerves (neurogenesis) and connective tissue (fibrosis)7,8. These changes to the composition of the bladder wall often affect the function of the bladder9 and increase the sensitivity to urine volume, perceived as urinary urgency. Changes to the expression of molecular receptors (e.g.10) affect bladder signalling and limit the extent to which available treatments can be effective.
Urinary symptoms associated with chronic pathologies are highly impactful; causing reduced overall health, higher levels of depression, poorer sleep quality and lower levels of overall health-related quality of life5,6. Urinary symptoms also lead to morbidities (fractures from falls as patients rush to the bathroom; urinary tract infection; incontinence-associated dermatitis) and mortality (from falls)11.
The early diagnosis of chronic diseases enables treatment and leads to improvements in quality of life12. It is evident from diseases such as Acute Kidney Injury that accurate early detection requires long-term monitoring, in order to establish a baseline and natural variability of a biomarker / biomarker combination, providing the means to identify departures that could indicate pathology13.
Current methods, such as cystometry and uroflowmetry, do not lend themselves to long-term monitoring, as they are typically only available in tertiary (specialist referral) practice and are therefore not directly accessible to many practitioners, furthermore, they are mostly available in specialist centers and require in-clinic procedures14. Beyond limited access, additional barriers include reliance on self-reported data (e.g., bladder diaries), which are often incomplete due to poor adherence and recall bias14,15. There is therefore an unmet need for a tool to allow patients, carers and healthcare practitioners to accurately determine key clinical measures in order to diagnose and monitor a number of diseases that affect the urinary tract.
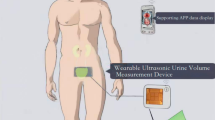
Our team is working on the development of a device that can provide these measures in the patient’s home or in the clinic. In all sessions, participants were presented with a conceptual description of a compact urinary monitoring system. The proposed device, still in early development, was described as a toilet-mounted sensor—attached like a toilet freshener—that uses an eye-safe laser and two cameras to measure stream width, speed, frequency, and volume. The system would wirelessly transmit data to a connected mobile application. Importantly, participants were informed that this was not a prototype but a conceptual idea used to explore needs and preferences. As part of the development process, the aim of this study is to use a participatory-design approach to better understand the key considerations of end users in the design of a device capable of measuring urine flow, volume and voiding patterns for the early detection and long-term monitoring of symptoms associated with the storage or passing of urine.
Results
Strand 1: clinicians in Israel
We grouped the topics that came up in the focus group discussion into two main categories: (1) issues related to clinical needs, and (2) issues specifically related to the device. Below we describe key themes along with interpretive reflections.
Clinical needs: Two key themes came up: (1) Medical history—clinicians highlighted various urinary parameters they prioritize when taking history. Table 1 lists the parameters which clinicians indicated are important to them when taking a medical history of patients with conditions related to the urinary system.
(2) Desired technology—functions they wished existed to support diagnostics. For example, across all professions, urgency, frequency, and burning sensation were consistently prioritized, reflecting both diagnostic relevance and the fact that patients frequently report these symptoms during consultations. However, deeper discussion revealed tensions between holistic history-taking and technical data acquisition: some clinicians emphasized the importance of clinical intuition—that is, understanding the patient’s condition through their behavior and daily function (e.g., how often do they go to the bathroom), and overall context, not just through measurable data—while others prioritized quantifiable signs such as flow quality and residual volumes.
Across groups, there was strong interest in technologies that capture data objectively—particularly post-void residual, latency to urinate, and leakage quantification. Doctors also raised the issue of matching technology with diagnostic reasoning: ‘Knowing when the stream starts is just as important as how much comes out,’ one said, linking sensor input with broader clinical reasoning.
Device-related considerations: Participants discussed perceived advantages (objectivity, home use, non-invasiveness), disadvantages (lack of mobility, potential privacy issues), and conditions for adoption. While all valued ease-of-use and reliability, some voiced concern that “if it doesn’t give us what we need—especially for pelvic diagnostics—we won’t use it” (PT participant). The discussion often revealed diverging priorities: doctors emphasized integration with current medical records and system-wide benefit, while therapists cared more about body-centered parameters. Nurses highlighted workload implications and the need to limit use to home settings. These differing priorities reflect the diversity of clinical lenses brought to technology evaluation, which may ultimately effect it use.
Clinicians in Israel emphasized clinical integration and diagnostic accuracy, frequently referring to their experience with standard urodynamic testing. Themes such as ‘clinical feasibility’ and ‘data interpretation’ were discussed in detail, with disagreement emerging around which parameters are practical and useful. Some participants emphasized the importance of early detection through automated metrics such as stream initiation and post-void residual, while others expressed concern over overmedicalization and data overload. This variation reflects broader tensions between innovation and workload in hospital settings.
Strand 2: clinicians in the UK
UK clinicians focused predominantly on systemic barriers within the National Health Service (NHS), including delays, limited specialist access, and resource constraints. Their discussion of the device emphasized service-related impacts: reducing wait times, enabling remote monitoring, and improving quality of life. As one nurse noted, “we don’t always need precise data—we need tools that reduce the referral burden.”
The analysis revealed that perceived advantages were: promoting early detection, assisting those with mobility limitations, and lowering anxiety; and perceived disadvantages were: technical reliance, inequity in uptake, and professional capacity constraints. The question of who would or would not use the device raised issues of digital literacy, patient engagement, and fear of diagnosis.
The group applied a public-health logic: if the device reduces system load, its value increases—even without clinical specificity. Their focus on workflow and efficiency, rather than diagnostic precision, contrasted with the Israeli clinicians’ clinical concerns and reflected differing health system incentives. One participant summarized this view: “If we could screen at home, half these referrals wouldn’t even need to come through.”
Strand 3: patients in the UK
The main themes that emerged from the patient interviews included: (1) diagnostic discomfort and embarrassment; (2) quality of life limitations; and (3) reflections on the proposed device. All participants recounted emotional distress stemming from both the diagnostic process and living with urinary symptoms. Their accounts emphasized themes of visibility, vulnerability, and stigma.
Discomfort and embarrassment: Participants described urodynamic tests as violating and degrading. One patient noted: “The worst bit was sitting on the toilet to empty your bladder in front of everyone…” These moments created lasting emotional impact. Beyond the clinical setting, participants shared how they hid their condition and avoided social situations. One individual said she did not seek care for years because ‘admitting it would make it real.’
Quality of life: Daily life was characterized by fear of leaks, constant scanning for bathrooms, and fatigue from overthinking. The condition was not just limited to the medical realm—it affected their social participation and emotional wellbeing. Some avoided work, others stopped traveling. These insights point to how symptoms reshape identity and autonomy.
Device perspectives: Participants responded enthusiastically to the concept of such a device—especially due to its discretion and non-invasive nature. Many felt it would offer control, reduce shame, and help avoid repeated clinic visits or uncomfortable diagnostic procedures. However, they raised logistical concerns like how using shared toilets may affect measurements and difficulty with device portability. They viewed the device not only as a tool, but as a way to reclaim dignity.
Patients voiced strong concerns about the invasiveness and emotional impact of current diagnostic methods. Multiple participants described experiences of physical exposure during urodynamics as ‘humiliating’ or ‘degrading.’ Others discussed feelings of not being believed by clinicians, particularly early in their diagnostic journey. Thematic emphasis was placed on dignity, autonomy, and the desire for discretion. As one participant summarized, “I just want something that helps me without making me feel broken”. Their support for the proposed device was strongly tied to the potential for passive monitoring and reduced embarrassment.
Cross-strand synthesis
While clinicians across strands acknowledged the potential benefits of continuous monitoring, their priorities varied: UK clinicians focused on reducing waiting times and easing service burdens—likely reflecting systemic pressures within the NHS. In contrast, Israeli clinicians emphasized diagnostic precision and debated the clinical value of parameters such as post-void residual, flow curve, and initiation delay. Within the Israeli focus groups, active debate often emerged regarding which measures were clinically meaningful and feasible. Patients, meanwhile, consistently emphasized discretion, emotional comfort, and the psychological burden of current diagnostics. For example, participants described urodynamic testing as ‘mortifying’ (P002) and ‘horrible’ (P001), citing both exposure and discomfort. One participant noted, ‘if how we diagnose removes their dignity… how does that make a person feel?’ (P003). These differences suggest that each group brings unique priorities to device design, and that an inclusive development process must address usability, clinical utility, and emotional impact alike.
Discussion
The aim of this study was to take the first step to improve the detection of diseases and conditions that cause urinary symptoms, by using a participatory-design approach to better understand the key considerations of end users in the design of a device capable of measuring changes in urine flow, volume and voiding patterns indicative of pathology. This device was introduced as a design probe—a conceptual tool to elicit feedback, not a tested prototype. It helped uncover what stakeholders consider useful, feasible, or problematic at an early development stage.
Experiences of undergoing diagnostic procedures
We held semi-structured interviews with four UK-based patients and one family member, who were asked about their experiences of undergoing diagnostic procedures to understand the basis of their lower urinary tract symptoms. These interviews revealed negative feelings about their experiences: embarrassment about the tests they underwent, and discomfort caused by an invasive diagnostic procedure (cystometry). While the small (n = 4) pool was itself consistent in reporting negative experiences, previous studies are inconsistent (e.g. Roberts et al., 1994 cf. Yeung et al.16). Although the sample was small, the consistency and depth of these narratives suggest that emotional and psychological aspects of urological diagnostics are often under-acknowledged. Previous research highlights how feelings of embarrassment, vulnerability, or shame can significantly impact patients’ willingness to seek care and to comply with assessments or treatment. For instance, Suskind et al. (2015) reported that patients experienced significant physical and emotional discomfort during urodynamic testing, emphasizing the need for improved patient education and support. The strong positive response to the proposed concept—despite it being in an early, conceptual stage—seemed to stem not from its technical sophistication, but from its potential to preserve control, privacy, and dignity. These insights suggest that for many patients, the perceived value of a health technology may depend as much on how data is collected as on what data is collected..
Priorities for the diagnosis and monitoring of conditions and pathologies that affect the urinary tract
Focus groups with 29 Israel-based clinicians (16 medical doctors (urologists), eight pelvic floor physical therapists, five nurses) aimed to understand which clinical measures they thought ought to be assessed in order to diagnose and monitor conditions and pathologies that affect the urinary tract, as well as their considerations for and against using the device. There was an overlap in clinical measures prioritised by doctors and physical therapists, with both groups prioritising characteristics of urine storage and voiding, and abdominal muscle activation during urination, as well as a fluid balance log. In addition, physical therapists were interested in pelvic floor function and dysfunction. Nurses, on the other hand, noted the importance of urine color and catheter use in the diagnosis process. One potential explanation for the nurses’ perspectives being different from the other clinicians could be that they were all RNs and not NPs, working in a hospital setting, and as such, the majority of clinical evaluations and decisions are made by the MDs they work with. Ours is not the first study to have explored what parameters pertaining to urine storage and voiding need to be assessed in order to inform diagnosis. Jarvis et al., (2012) highlight key parameters being urine volume, flow and stream; start/stop urination; and the frequency and timing of voiding. However, Jarvis et al. (2012) focused on information regarding uroflowmetry, whereas we collected information from clinicians from a variety of clinical disciplines also regarding symptoms and behavior.
Our findings extend prior work by identifying how expectations vary across professional groups. Medical doctors emphasized integration with electronic records and quantifiable data; physical therapists prioritized muscle and postural aspects; nurses raised usability and workload concerns. This variation suggests that a one-size-fits-all approach may be inadequate, and a modular or adaptable system could better align with differing professional roles.
Perceptions of a device to measure changes in urine flow, volume and voiding patterns indicative of pathology
Both the Israel-based and UK-based clinicians and UK-based patients were presented with a concept of a device (Fig. 1) to be placed inside a toilet. The device would measure characteristics of urine volume, flow and stream; start/stop urination; and the frequency and timing of voiding over time. The core promise of this design probe lies in offering passive, objective, and non-invasive measurement in a familiar environment. However, responses from different stakeholder groups reflect distinct interpretations of what such data can or should be used for.
Main advantages of the concept device
Israel-based clinicians identified two main advantages associated with the data that the device would generate. First, the means to provide objective measures of bladder function was seen as a major advantage. While this is no different to tools used in specialist referral (uroflowmetry, cystometry), it is in contrast to methods such as a bladder diary and fluid balance log, used in initial assessment, which are subject to individual error. The importance of having objective indicators for testing the effectiveness of procedures was also indicated. Second, that the device provides the means to elucidate changing function over time was identified as something which is possible but not practically achievable with current diagnostic methods. Temporal trends in urine-flow patterns may permit early detection of chronic conditions, including overactive bladder, enlargement of the prostate and diabetes mellitus. Each of these conditions typically sees symptom severity sustained or progress over time3with only a very small proportion of individuals (e.g., 6.5% of 2526 women; Sutcliffe et al.17) maintain optimum bladder health. As such, early diagnosis and early intervention ought to be prioritised to improve patient outcomes.
Both Israel-based and UK-based clinicians identified perceived advantages for individuals that would be achieved because of home testing; addressing not only the issue raised by our patient participants of embarrassment of tests performed in a clinical setting, but providing a number of practical benefits including benefits for patients (convenience; reduced travel)18 and reduced demand on healthcare infrastructure. Physical therapists in Israel suggested that home testing may reduce their perceived psychological effects on measurements taken in clinical settings. UK-based community nurses identified further, associated benefits of the device including helping individuals to be proactive with their health and being of use for individuals with reduced mobility and / or those who are housebound. These themes align to the growing trend of supporting patients to be empowered in their healthcare (NHS Long Term Plan, 2019) and the role that technology in managing, improving and support health and wellbeing.
Main disadvantages of the concept device
Several potential disadvantages were raised by both clinicians and patients. A device located in a single toilet risks information being collected from multiple users. Although there are potential technical solutions to ensuring that data comes from only a single individual, there may be a residual risk of the trust of patients and practitioners in the data generated19. The issue of privacy was raised; both in terms of the data generated and, given the location of the device within a toilet, for the potential of sharing intimate data. Both issues can be overcome with existing mitigations. For example, full patient privacy can be achieved by a top-covering screen, to obfuscate anything above the device; limiting sensors to resolve only the stream of urine, and nothing more; and limiting the bandwidth of the camera so that, were the device to be inadvertently rotated upwards, anything above the device cannot be resolved.
There were also considerations mentioned by the clinicians in their response which might influence their decision on whether to use the device or not. The main considerations for all groups were the device’s ease of use and cost. These are common considerations that come up frequently when individuals are asked about new technologies20,21,22. The above considerations apply to various medical fields, including Parkinson’s disease treatment23stroke rehabilitation20and vestibular rehabilitation24. A further consideration raised by the nurses was whether the device would increase their workload. Considerations related to adding workload when using technology also arose when clinicians and experts were asked about integrating robots in rehabilitation21.
Since these considerations arise in multiple fields, technology developers should take them into account in the early stages of the technology development process.
The main contributions of this paper are the identification of: (1) the needs from different stakeholders (patients, PTs, MDs, RNs) regarding urine-related measurements; (2) the varying perspectives of those stakeholders on using a urine-measurement device for (e.g., the patients’ focus on shame in the evaluation procedures vs. the clinicians’ estimation of workload when considering whether a urine-measuring device would be used), including potential benefits and concerns; (3) insights and ideas for tools that would be helpful for these patients and clinicians (e.g., measuring urine sugar levels)—to be used by future developers of technologies for this sector.
Considerations for using the device
The clinicians in Study 2 noted that the concept device would not be suitable for incontinent patients or patients with catheters. Being mindful of which patients are not likely to benefit from technology has been recommended as part of successful implementation of technology, as it reduces frustration21. They also noted the potential health inequity which the device may introduce, by being accessible to a part of the population (e.g., people who are better versed in technology). An example of health inequity due to the use of technology can be found in a variety of fields of medical technology, such as insulin pumps, where it is evident that there exists a level of inequality due to a lack of awareness of technology among patients, insufficient technical support, and difficulties with insurance companies25.
Design suggestions
Participants made the following suggestions for enhancing the utility of the concept device: (1) transferring the collected data directly to the patient’s computerized medical file; (2) using a wide laser beam to measure a split stream of urine; (3) measuring parameters such as ovulation stage, sugar levels in the urine, the presence and amount of blood or pathogens in the urine and the pH level of the urine; (4) providing feedback to the user through the associated phone application, e.g., on their need for increased fluid intake; and (5) using artificial intelligence (AI) or machine learning for the early detection of pathologies, based on urinary patterns.
Suggestions and next steps
Participants made several technical and conceptual suggestions, ranging from laser positioning to AI-driven alerts. Many of these—such as integration with hospital medical records— are immediately feasible. Others, such as chemical analysis or pathogen detection, are speculative and would require substantial investment.
We therefore propose a staged development model: beginning with flow analysis and remote tracking, and later expanding functionality based on user priorities and clinical validation. Importantly, the insights from this study provide not only guidance for this particular device but also a reusable framework for future user-centered design in digital health.
Limitations
This study had several limitations. For practical reasons, clinicians recruited to focus groups were both Israel-based and UK-based, while the patients were all UK-based. The UK-based patient group included only four participants, limiting the extent to which conclusions could be drawn. The clinicians in Israel and the UK came from different healthcare systems, and we believe it was valuable to capture both local and international perspectives. Yet, future research should recruit larger groups of both patients and clinicians in both countries, to permit comparison about perceptions between different cultures.
Conclusions
There are limitations in current methods to measure changes in urine flow, volume and voiding patterns indicative of diseases and conditions that cause urinary symptoms, with patients reporting embarrassment and discomfort from invasive procedures. A toilet-based sensor for the home-setting offers both advantages (objective measures; trends over time; practical advantages in home-testing) and disadvantages (potential influence of the data of other users; privacy of the data).
Methods
Overview
This qualitative study used a multi-strand design to gather perspectives from different stakeholder groups. Data were collected across three complementary strands: (1) clinicians in Israel, (2) clinicians in the UK, and (3) patients in the UK with urinary system disorders. These strands were designed to address a shared objective—to identify clinical needs and considerations relevant to the design and implementation of a novel urinary monitoring device. While each group provided distinct input, the overarching research questions and thematic framework were consistent across all strands.
Strand 1: focus groups with clinicians, Israel
Participants
Twenty-nine (29) clinicians participated in this study (15 men, 14 women): eight pelvic floor physical therapists, five registered nurses, 16 medical doctors—six seniors, nine residents, and one intern. The average years of experience of the clinicians ranged from 1 to 38 years (mean 10.2, SD = 7.0).
To participate in the study, participants had to meet the following criteria: (1) Qualifications and work experience as a: physical therapist/nurse/medical doctor specializing in urogynecology, gynecology, urology, geriatrics, neurology, or family medicine; (2) fluency in Hebrew. Participants with significant uncorrected visual impairments were excluded from the study.
Recruitment
We recruited a convenience sample through the distribution of information sheets via social media and direct contact with relevant clinicians. Sessions were held during January-February 2023. All participants signed an informed consent form, and the study was approved by the Ethics Committee of Ben-Gurion University of the Negev, Israel.
Procedure
We conducted a total of five focus groups, with 4–10 individuals participating in each group. The recruited group size is based on the existing literature regarding focus groups20,23,24,26,27,28,29,30.
The group composition was as follows:
-
Group 1 (n = 4) and Group 2 (n = 4): physical therapists specializing in the pelvic floor.
-
Group 3 (n = 10) and Group 4 (n = 5): medical doctors specializing in urology.
-
Group 5 (n = 5): registered nurses from a urology department.
Groups 1–2 were held online via Zoom to allow for geographic diversity. Groups 3–5 were held in person in Be’er Sheva with clinicians from the urology department at Soroka Hospital.
Three groups had a moderator and an observer (Groups 1, 3, 4), and two had a moderator only (Groups 2, 5). The moderator in all groups, except Group 4, was author AK. The moderator in Group 4 was a female graduate student in Cognitive Science. Observers included a biotechnological engineer, a university professor (author SL), and an undergraduate student in Mechanical Engineering.
Before participation, a pilot group of healthy young adults provided feedback on question clarity.
To ensure that participants felt comfortable, the facilitator stated at the beginning of each session that there are no “right” or “wrong” answers and emphasized the importance of expressing a variety of viewpoints. The participants were also asked to indicate whether they agreed with the opinions and statements expressed throughout the session by other members of the group.
The list of six questions which the moderators presented to the participants is shown in Table 2. To keep participants as objective as possible, the moderators described the goals of the focus group discussion, without mentioning the conceptual device itself, prior to asking the first two questions. This was done so we can collect a wide range of desired functions of a potential technology. The specific conceptual device was not mentioned at this point so as not to limit the scope of their responses. Following the first two questions, we explained the goal of the planned device and showed an illustration of it to the participants (see Fig. 1). Group 1 received the explanation without the illustration. Once participants were confident that they understood how the device worked, they were presented with questions 3–6, which focused on the device itself. The questions focused on the potential uses, advantages, disadvantages, and improvements related to the device we presented. We used a specific conceptual device in the second part of each session as an anchor around which discussions may progress from overarching desired functions of a future device to specific form requirements.
An illustration of the device shown to the participants in two of the three focus groups held with clinicians (Study 1). The planned device is shown in orange. This visual served as a design probe during focus groups and interviews. It is not a functional prototype, but a conceptual rendering used to prompt discussion.
Data analysis
All sessions were audio-recorded for analysis, with the consent of the participants. All sessions were then transcribed. The author AK did the generalised inductive coding using the ATLAS.ti software (version 22). The themes were derived from the data and were not predetermined. To establish the themes, a joint discussion with the author SL was conducted.
Strand2: focus group with clinicians, UK
Participants
Eleven healthcare professionals participated in this strand: nine community nurses, one senior NHS manager, and one former community nurse.
Recruitment and procedure
All participants provided verbal consent, and the study was approved by the Ethics Committee of Teesside University, UK.
A single focus group was conducted, with 11 individuals participating. The session lasted for 60 min and took place at the National Horizons Centre, Teesside University, UK in March 2023. The focus group had a single moderator, who was author JSY, a university professor of Translational Healthcare. Following an introduction of the device, participants were presented with questions 3–6 (Table 2). Participants recorded their responses to each question on self-adhesive notes, which they attached to whiteboards labelled with the associated question.
Data analysis
The participants’ responses to each question were photographed and then transcribed. Analysis was undertaken by JSY. The themes were derived from the data and were not predetermined.
Strand 3: interviews with individuals with urinary system disorders
Participants
A total of five individuals (1 man, 4 women) participated in this study: four individuals with urinary system disorders (P1-4) and one caregiver (family member (wife); C1). The interviews were held in the United Kingdom (UK).
Recruitment and procedure
A semi-structured interview is the most common qualitative method of obtaining data in healthcare27. This method involves asking a series of predefined questions to collect information. However, if the researcher discovers something novel or interesting during the interview, he or she may ask further questions31. Because of the flexibility of this methodology, the participants can emphasize the most important issues from their perspective, thus allowing the researchers to gain insight into matters that may not have been central to the researchers’ considerations27.
We conducted four semi-structured interviews. Each interview lasted between 25 and 44 min. The interviews were conducted face-to-face or via a video call, according to the geographical proximity and availability of the participants. Participants were patients with urinary symptoms referred to Consultant Urological Surgeon (author MN) at the James Cook Hospital, Middlesbrough, UK or the Friarage Hospital, Northallerton, UK. They were approached by MN and, following a description of the study, were invited to take away the Participant Information Sheet to read and approach either MN or JSY, if they have any questions about the study. It was made clear that inclusion will in no way affect the treatment the patient will receive; nor will their decision, at any point, to withdraw from the study. Three interviews were held with a single interviewee, and one interview was held with two interviewees (patient and his spouse). All interviews were audio recorded and later transcribed.
The interviewer in all the interviews was author JSY, a university professor of Translational Healthcare. The interviewer and the participants had no prior acquaintance.
In the interviews, participants were first asked about their diagnosis process. They then received an explanation of the device by the interviewer, and asked to give their opinion on it, including specific perceived advantages and disadvantages.
This study and all its procedures were approved by the London—West London & Gene Therapy Advisory Committee Research Ethics Committee (22/PR/1027). All methods were carried out in accordance with the guidelines and regulations of Teesside University. Written informed consent was obtained from all participants.
Data analysis
We used the ATLAS.ti software (version 22) for analysis and coding, which was performed by author AK. Analyzing the data was done using an inductive approach. According to this approach, themes were not predetermined; they were determined based on the data collected32. A joint discussion with the author SL was conducted in order to establish the themes.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Chapple, C. et al. Prevalence of lower urinary tract symptoms in china, taiwan, and South korea: results from a Cross-Sectional, Population-Based study. Adv. Ther. 34, 1953–1965 (2017).
Coyne, K. S. et al. The prevalence of lower urinary tract symptoms (LUTS) in the USA, the UK and sweden: results from the epidemiology of LUTS (EpiLUTS) study. BJU Int. 104, 352–360 (2009).
Irwin, D. E., Milsom, I., Chancellor, M. B., Kopp, Z. & Guan, Z. Dynamic progression of overactive bladder and urinary incontinence symptoms: a systematic review. Eur. Urol. 58, 532–543 (2010).
Przydacz, M., Golabek, T., Dudek, P., Lipinski, M. & Chlosta, P. Prevalence and bother of lower urinary tract symptoms and overactive bladder in poland, an Eastern European study. Sci. Rep. 10, 19819 (2020).
Coyne, K. S. et al. The impact of overactive bladder, incontinence and other lower urinary tract symptoms on quality of life, work productivity, sexuality and emotional well-being in men and women: results from the EPIC study. BJU Int. 101, 1388–1395 (2008).
Sinclair, A. J. & Ramsay, I. N. The psychosocial impact of urinary incontinence in women. Obstetric Gynaecologis. 13, 143–148 (2011).
Furuta, A. et al. Angiogenesis in bladder tissues is strongly correlated with urinary frequency and bladder pain in patients with interstitial cystitis/bladder pain syndrome. Int. J. Urol. 26 (Suppl 1), 35–40 (2019).
Peyronnet, B. et al. A comprehensive review of overactive bladder pathophysiology: on the way to tailored treatment. Eur. Urol. 75, 988–1000 (2019).
Firouzmand, S. et al. Investigating the associations of mucosal P2Y6 receptor expression and urinary ATP and ADP concentrations, with symptoms of overactive bladder. Neurourol. Urodyn. 39, 926–934 (2020).
Elneil, S., Skepper, J. N., Kidd, E. J., Williamson, J. G. & Ferguson, D. R. Distribution of P2X(1) and P2X(3) receptors in the rat and human urinary bladder. Pharmacology 63, 120–128 (2001).
Soliman, Y., Meyer, R. & Baum, N. Falls in the elderly secondary to urinary symptoms. Rev. Urol. 18, 28–32 (2016).
Megari, K. Quality of life in chronic disease patients. Health Psychol. Res. 1, e27 (2013).
Sawhney, S. et al. Acute kidney injury-how does automated detection perform? Nephrol. Dial Transpl. 30, 1853–1861 (2015).
Jarvis, T. R., Chan, L. & Tse, V. Practical uroflowmetry. BJU Int. 110 (Suppl 4), 28–29 (2012).
Funada, S. et al. Bladder training for treating overactive bladder in adults. Cochrane Database Syst. Rev. (2023).
Yeung, J. Y., Eschenbacher, M. A. & Pauls, R. N. Pain and embarrassment associated with urodynamic testing in women. Int. Urogynecol. J. 25, 645–650 (2014).
Sutcliffe, S. et al. Changes in bladder health over time: A longitudinal analysis of adult women in the Boston area community health survey. J. Urol. 207, 1086–1095 (2022).
Novara, G. et al. Telehealth in urology: A systematic review of the literature. How much can telemedicine be useful during and after the COVID-19 pandemic?? Eur. Urol. 78, 786–811 (2020).
Lie, M. L. S., Lindsay, S. & Brittain, K. Technology and trust: older people’s perspectives of a home monitoring system. Aging Soc. 36, 1501–1525 (2016).
Dembovski, A., Amitai, Y. & Levy-Tzedek, S. A. Socially assistive robot for stroke patients: acceptance, needs, and concerns of patients and informal caregivers. Front. Rehabilit Sci. 2, 793233 (2022).
Feingold-Polak, R., Weiss, P. L. & Levy-Tzedek, S. Chapter 13—Middle East region: Israel. In Rehabilitation Robots for Neurorehabilitation in High-, Low-, and Middle-Income Countries (eds. Johnson, M. J. & Mendonca, R. J.), 209–222 (Academic Press, 2024). https://doi.org/10.1016/B978-0-323-91931-9.00033-5
Kaplan, A., Barkan-Slater, S., Zlotnik, Y. & Levy-Tzedek, S. Robotic technology for parkinson’s disease: needs, attitudes and concerns of individuals with parkinson’s disease and their family members. A focus group study. Int. J. Hum. Comput. Stud. 181, 103148 (2024).
Bar-On, I., Mayo, G. & Levy-Tzedek, S. Socially assistive robots for parkinson’s disease: needs, attitudes and specific applications as identified by healthcare professionals. J. Hum. -Robot Interact. 12 (1–11), 25 (2023).
Kalderon, L., Kaplan, A., Wolfovitz, A., Levy-Tzedek, S. & Gimmon, Y. Barriers and facilitators of vestibular rehabilitation: patients and physiotherapists’ perspectives. J. Neurol. Phys. Ther. https://doi.org/10.1097/NPT.0000000000000470 (2024).
Agarwal, S., Simmonds, I. & Myers, A. K. The use of diabetes technology to address inequity in health outcomes: limitations and opportunities. Curr. Diab Rep. 22, 275–281 (2022).
Fruchter, D., Feingold Polak, R., Berman, S. & Levy-Tzedek, S. Hierarchy in Algorithm-Based feedback to patients working with a robotic rehabilitation system: toward User-Experience optimization. IEEE Trans. Human-Machine Syst. 52, 907–917 (2022).
Gill, P., Stewart, K., Treasure, E. & Chadwick, B. Methods of data collection in qualitative research: interviews and focus groups. Br. Dent. J. 204, 291–295 (2008).
Halliday, M., Mill, D., Johnson, J. & Lee, K. Let’s talk virtual! Online focus group facilitation for the modern researcher. Res. Social Administrative Pharm. 17, 2145–2150 (2021).
Kalderon, L., Kaplan, A., Wolfovitz, A., Gimmon, Y. & Levy-Tzedek, S. Do we really need this robot? Technology requirements for vestibular rehabilitation: input from patients and clinicians. Int. J. Hum. Comput. Stud. 192, 103356 (2024).
Polak, R. F., Bistritsky, A., Gozlan, Y. & Levy-Tzedek, S. Novel gamified system for post-stroke upper-limb rehabilitation using a social robot: Focus groups of expert clinicians. In International Conference on Virtual Rehabilitation (ICVR) 1–7. (2019). https://doi.org/10.1109/ICVR46560.2019.8994657
Young, J. C. et al. A methodological guide to using and reporting on interviews in conservation science research. Methods Ecol. Evol. 9, 10–19 (2018).
Thomas, D. R. A general inductive approach for analyzing qualitative evaluation data. Am. J. Evaluation. 27, 237–246 (2006).
Acknowledgements
The authors wish to thank Yuliya Berdichevsky, Shirel Barkan Slater, and Mary Spichakov for their help in collecting data in the focus group sessions and Merav Kaplan for her assistance in drawing the device illustration.
Author information
Authors and Affiliations
Contributions
Azriel Kaplan: Conceptualization, Methodology, Investigation, Writing—original draft, Writing—review & editing. Mehwash Nadeem: Methodology, Investigation, Writing—review & editing. John S. Young: Project administration, Methodology, Conceptualization, Writing—original draft, Writing—review & editing, Funding acquisition.Shelly Levy-Tzedek: Project administration, Methodology, Conceptualization, Writing—review & editing, Funding acquisition.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kaplan, A., Nadeem, M., Young, J.S. et al. Clinician and patient perspectives on a novel device for measuring urine flow volume and voiding patterns. Sci Rep 15, 19756 (2025). https://doi.org/10.1038/s41598-025-04655-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-04655-4