Abstract
Orthodontic tooth movement (OTM) has been described as a bone remodeling process mediated by the expression of various inflammatory cytokines, including tumor necrosis factor-α (TNF-α). Necroptosis is a form of regulated cell death that is mainly induced by TNF-α, leading to the release of damage-associated molecular patterns (DAMPs) that cause inflammation. However, the role of osteocyte necroptosis in regulating osteoclastogenesis during OTM remains unclear. Here, we investigated the effects of osteocyte necroptosis on osteoclastogenesis in a mouse model of OTM. In wild-type mice, osteocyte death was remarkably increased on day 6 after OTM. Transmission electron microscopy identified apoptotic osteocytes, necrotic osteocytes, and empty lacunae based on morphological characteristics. TNF receptor type 1- and 2-deficient (TNFRsKO) mice showed a reduction in osteocyte death on day 6 after OTM. Immunofluorescence staining detected necroptosis markers in osteocytes on the compression side in wild-type OTM mice, whereas such osteocytes were almost undetectable in TNFRsKO OTM mice. Furthermore, the conditioned medium from primary osteocytes undergoing necroptosis significantly enhanced osteoclastogenesis. These findings suggest that TNF-α-induced osteocyte necroptosis enhances osteoclastogenesis and alveolar bone resorption on the compression side during OTM, involving the release of inflammatory factors including DAMPs.
Similar content being viewed by others
Introduction
Orthodontic treatment is the main method to correct malocclusions by applying force to teeth. The resulting orthodontic tooth movement (OTM) is controlled by a dynamic balance between osteoclastic bone resorption on the compression side and osteoblastic bone formation on the tension side1. Osteoclasts are bone-resorbing cells derived from hematopoietic stem cells. Macrophage-colony stimulation factor (M-CSF) and receptor-activator of nuclear factor-κB ligand (RANKL) are essential factors for osteoclast differentiation2. Moreover, the proinflammatory cytokine tumor necrosis factor-α (TNF-α) induces osteoclast differentiation both in vitro3 and in vivo.4 In humans, various cytokines, including TNF-α, are expressed in the periodontal ligament during OTM5. Previously, we have shown that osteoclast formation and tooth movement are decreased in TNF receptor type 1- and 2-deficient (TNFRsKO) mice during OTM6. Therefore, TNF-α is an important cytokine for tooth movement.
Osteocytes act as orchestrators of bone remodeling and coordinate osteoclasts and osteoblasts7. For decades, osteoblasts have been considered to be responsible for inducing osteoclast formation by expressing RANKL8. As osteocytes, similar to osteoblasts, also express RANKL and induce osteoclast formation, osteocytes came into the focus of bone metabolism research9. OTM is disturbed in osteocyte-ablated mice10 and mice lacking RANKL in osteocytes11. In our previous studies, TNF-α enhanced RANKL expression in osteocytes, inducing osteoclast formation during OTM12,13. Therefore, understanding the role of osteocytes is essential for elucidating the detailed mechanisms underlying alveolar bone resorption during tooth movement.
Previously, cell death was classified based on the morphological appearance into three different forms: apoptosis, autophagic cell death, and necrosis14. Currently, cell death is according to the Nomenclature Committee on Cell Death (NCDD) broadly divided into regulated cell death and accidental cell death, and various types of cell death are distinguished based on genetic, biochemical, pharmacological, and functional evidence15. Recent studies have identified novel types of regulated cell death, including pyroptosis, ferroptosis, and necroptosis15. Necroptosis resembles necrosis in morphology and is induced mainly by TNF-α stimulation with inhibited apoptosis16. Necroptosis research began with the discovery of TNF-α-induced necrotic cell death17. Necroptosis was identified in 2005 following the inhibition of TNF-α-dependent necrotic cell death by necrostatin-1 (Nec-1), an inhibitor of receptor-interacting protein 1 (RIP1)18. Additionally, RIP3 and mixed lineage kinase domain-like protein (MLKL) were identified as proteins essential for inducing necroptosis19,20. Thus, it is generally accepted that TNF-α-induced sequential phosphorylation of RIP1, RIP3, and MLKL induces necroptosis15,16. Necroptosis involves the rupture of the plasma membrane and the subsequent release of damage-associated molecular patterns (DAMPs)21. This release of DAMPs from necroptotic cells mediates various pathological conditions16,21. In bone diseases, necroptosis is, for instance, associated with rheumatoid arthritis22 and osteoarthritis23. Additionally, the recognition of DAMPs induces sterile inflammation, leading to inflammatory diseases such as metabolic disorders, neurodegenerative diseases, autoimmune diseases, and cancer24. Although alveolar bone resorption during OTM is perceived as sterile inflammation, the effects of DAMPs on tooth movement have not yet been investigated. Several studies observed during OTM osteocyte death in the alveolar bone on the compression side25,26,27. A recent report suggested that osteocyte necroptosis may be associated with bone loss in postmenopausal osteoporosis28. However, the effects of osteocyte necroptosis on osteoclastogenesis during OTM remain unclear.
This study aimed to investigate the effects of TNF-α-induced osteocyte necroptosis on osteoclastogenesis during OTM. We hypothesized that osteocyte necroptosis involves TNF-α expressed in periodontal ligaments during OTM and that DAMPs released from necroptotic osteocytes affect osteoclastogenesis. Using a mouse model of OTM, we detected TNF-α-induced osteocyte necroptosis in the alveolar bone on the compression side. In vitro, necroptosis of primary osteocytes enhanced osteoclastogenesis. Collectively, this study provides insights into the mechanism of tooth movement and suggests the importance of osteocyte necroptosis in the release of DAMPs to enhance osteoclastogenesis.
Results
During OTM, osteocytes undergo cell death on the compression side
To understand the dynamics of tooth movement, we performed OTM in wild-type (WT) mice using a nickel-titanium (Ni-Ti) coil spring and measured the distance of tooth movement (Fig. 1a, b, Supplementary Fig. S1a). The body weight of the mice was properly monitored and maintained throughout the period of OTM (Fig. S1b). After the initial displacement, tooth movement stagnated until day 6 but restarted on day 8 (Fig. 1c). Histological analyses using tartrate-resistant acid phosphatase (TRAP) staining revealed that osteoclasts were induced on the compression side, resulting in alveolar bone resorption (Fig. 1d). The number of osteoclasts on the compression side significantly increased from day 6 to day 12 of OTM (Fig. 1e).
Time-course analysis of osteoclast formation in the mouse model of orthodontic tooth movement (OTM). a Left image: schematic representation of the experimental method in the mouse model of OTM. A Ni-Ti coil spring is ligated to the maxillary left first molar (M1) and the hole near the incisors. Right image: hematoxylin and eosin (H&E) staining of the sagittal section of the maxillary left molars on day 6 after OTM. Scale bar = 200 μm. b Representative images of maxillary left molars and dental impressions on day 0, 6, and 12 after OTM. c Distance of tooth movement from day 0 to day 12 (n = 6). d Tartrate-resistant acid phosphatase (TRAP) staining of horizontal sections with periodontal tissue and distobuccal root of the maxillary left first molar after OTM in wild-type (WT) mice. Scale bar = 100 μm. e Quantification of TRAP-positive cells on the compression side after OTM (n = 6). Black arrows indicate the direction of force. M1, first molar; M2, second molar; M3, third molar; M, mesial side; D, distal side; a, alveolar bone; p, periodontal ligament; r, root. **p < 0.01, ***p < 0.001, ****p < 0.0001.
To assess when osteocyte death was observed in OTM mice, osteocyte death was analyzed using hematoxylin and eosin (H&E) staining at seven time points for up to 12 days of OTM. Many dying osteocytes and empty lacunae were observed in the alveolar bone on the compression side compared to fewer on the tension side (Fig. 2a). The percentage of osteocyte death was the highest on the compression side on day 6 of OTM (Fig. 2b). To further characterize osteocyte death, transmission electron microscopy (TEM) images were analyzed on day 6 of OTM. Apoptotic osteocytes with nuclear fragmentation and condensation, necrotic osteocytes with loss of the plasma membrane, and empty lacunae were observed (Fig. 2c).
Time-course analysis of osteocyte death in the mouse model of orthodontic tooth movement (OTM). a Hematoxylin and eosin (H&E) staining of horizontal sections with periodontal tissue and distobuccal root of the maxillary left first molar after OTM in WT mice. Upper left image: the rectangles of 400 μm × 200 μm indicate the region of interest (ROI) on the compression and tension sides. Black arrows indicate the direction of force. Scale bar = 100 μm. Other images: high-magnification images of ROIs on the compression and tension sides. White arrowheads indicate normal osteocytes, orange arrowheads indicate dying osteocytes, and red arrowheads indicate empty lacunae. Scale bar = 50 μm. b Percentage of normal osteocytes (white), dying osteocytes (orange), and empty lacunae (red) (n = 6). c Transmission electron microscopy (TEM) analysis on days 0 and 6 after OTM in WT mice. Yellow arrows indicate empty lacunae. Scale bar = 2 μm. M, mesial side; D, distal side; a, alveolar bone; p, periodontal ligament; r, root.
TNF-α induces osteocyte necroptosis on the compression side during OTM
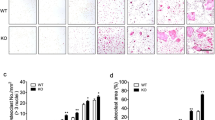
We next examined whether TNF-α induced osteocyte death during OTM. Because TNF-α is expressed in the periodontal ligament during OTM5we induced OTM using WT and TNFRsKO mice. According to our analysis of osteocyte death on the compression side on day 6 after OTM, the percentage of dying osteocytes and empty lacunae in TNFRsKO mice was significantly lower than that in WT mice (Fig. 3a, b). To examine the presence of osteocyte necroptosis, we used immunofluorescence staining to detect the necroptosis markers phosphorylated (p)-RIP3 and p-MLKL in osteocytes of WT and TNFRsKO OTM mice. p-RIP3-positive osteocytes were detected on the compression side of WT mice on day 6 (Fig. 3c, d). On day 6 of OTM, WT mice had the highest percentage of p-RIP3-positive osteocytes in the alveolar bone on the compression side, whereas p-RIP3-positive osteocytes were almost undetectable in TNFRsKO mice (Fig. 3e). Similarly, p-MLKL-positive osteocytes were observed on the compression side on day 6 after OTM in WT mice (Fig. 3f, g). The highest percentage of p-MLKL-positive osteocytes was also found on the compression side in WT mice on day 6, whereas these cells were rarely detected on the compression side in TNFRsKO mice or on the tension side in either mouse model (Fig. 3h). These results suggest that TNF-α induces osteocyte necroptosis on the compression side during OTM.
Detection of tumor necrosis factor-α (TNF-α)-induced osteocyte necroptosis in the mouse model of orthodontic tooth movement (OTM). a Hematoxylin and eosin (H&E) staining of the region of interest (ROI) on the compression side in wild-type (WT) mice and TNF receptor type 1- and 2-deficient (TNFRsKO) mice. Scale bar = 50 μm. b Percentage of dying osteocytes (orange) and empty lacunae (red) in WT and TNFRsKO mice (n = 6). c Immunofluorescence staining for phosphorylated (p)-RIP3 (red) and DAPI staining (blue) of ROIs on the compression and tension sides in WT and TNFRsKO mice. Scale bar = 50 μm. d High-magnification images of the compression side on day 6 after OTM in WT mice. White arrows indicate p-RIP3-positive osteocytes. Scale bar = 50 μm. e Percentage of p-RIP3-positive osteocytes on the compression and tension sides of WT and TNFRsKO mice (n = 6). f Immunofluorescence staining for p-MLKL (red) and DAPI staining (blue) of ROIs on the compression and tension sides in WT and TNFRsKO mice. Scale bar = 50 μm. g High-magnification images of the compression side on day 6 after OTM in WT mice. White arrows indicate p-MLKL-positive osteocytes. Scale bar = 50 μm. h Percentage of p-MLKL-positive osteocytes on the compression and tension sides in WT and TNFRsKO mice (n = 6). The dotted line indicates the border between the alveolar bone and periodontal ligament. a, alveolar bone; p, periodontal ligament. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
TNF-α induces necroptosis of primary osteocytes
To isolate primary osteocytes with high purity, Topaz-positive cells were collected from Dmp1-Topaz mice using fluorescence-activated cell sorting (FACS) (Fig. 4a). Topaz-positive cells expressed green fluorescent protein and had a stellate-shaped morphology with extended dendrites, characteristic of osteocytes (Fig. 4b). In our previous study, we showed that Topaz-positive cells express osteocyte-specific genes such as Dmp1 and SOST.12,29 Therefore, Topaz-positive osteocytes were used as primary osteocytes in the current study. To characterize TNF-α-induced necroptosis, primary osteocytes were stimulated with TNF-α, SM-164, and zVAD (TSZ), a well-established method for inducing necroptosis30, for up to 12 h. Immunofluorescence staining confirmed the presence of p-RIP3-positive osteocytes after the addition of TSZ (Fig. 4c). Nec-1 (a RIP1 inhibitor) and GSK872 (a RIP3 inhibitor) inhibited RIP3 phosphorylation, whereas necrosulfonamide (NSA) (an MLKL inhibitor) did not (Fig. 4d). The percentage of p-RIP3-positive osteocytes increased at approximately 6 h after TSZ treatment (Fig. 4e). The percentage of p-RIP3-positive osteocytes was decreased by Nec-1 and GSK872 treatment (Fig. 4f). Furthermore, we examined MLKL phosphorylation in primary osteocytes. We found that p-MLKL-positive osteocytes temporarily increased 6 h after the addition of TSZ (Fig. 4g). MLKL phosphorylation was inhibited by Nec-1, GSK872, and NSA (Fig. 4h). The percentage of p-MLKL-positive osteocytes was highest 6 h after the addition of TSZ (Fig. 4i). Treatment with Nec-1, GSK872, and NSA decreased the percentage of p-MLKL-positive osteocytes (Fig. 4j).
Necroptosis of primary osteocytes induced by tumor necrosis factor-α (TNF-α) stimulation. a Isolation of Topaz-positive cells with fluorescence-activated cell sorting (FACS). b Morphology of Topaz-positive cells under a fluorescence microscope. Scale bar = 50 μm. c Immunofluorescence staining for phosphorylated (p)-RIP3 (red) and DAPI staining (blue) in primary osteocytes treated with TNF-α, SM-164, and zVAD (TSZ) for the indicated time. White arrows indicate p-RIP3-positive osteocytes. Scale bar = 100 μm. d Immunofluorescence staining for p-RIP3 (red) and DAPI staining (blue) in primary osteocytes treated with TSZ, Nec-1, GSK872, and NSA for 6 h. White arrows indicate p-RIP3-positive osteocytes. Scale bar = 100 μm. e, f Percentage of p-RIP3-positive osteocytes (n = 3). g Immunofluorescence staining for p-MLKL (red) and DAPI staining (blue) in primary osteocytes treated with TSZ for the indicated time. White arrows indicate p-MLKL-positive osteocytes. Scale bar = 100 μm. h Immunofluorescence staining for p-MLKL (red) and DAPI staining (blue) in primary osteocytes treated with TSZ, Nec-1, GSK872, and NSA for 6 h. White arrows indicate p-MLKL-positive osteocytes. Scale bar = 100 μm. i, j Percentage of p-MLKL-positive osteocytes (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001.
TNF-α-induced osteocyte necroptosis enhances osteoclastogenesis
We hypothesized that factors, including DAMPs from osteocyte necroptosis, are involved in osteoclastogenesis. To confirm our hypothesis, osteoclast precursors obtained from TNFRsKO mice were cultured in medium conditioned by necroptotic osteocytes with M-CSF and RANKL (Fig. 5a). Osteoclast precursors were generated from TNFRsKO mice to eliminate the effects of TNF-α on osteoclastogenesis because the osteocyte-conditioned medium contained TNF-α to induce osteocyte necroptosis. The conditioned medium from living osteocytes had no effect on osteoclastogenesis, whereas the conditioned medium from osteocytes undergoing necroptosis enhanced osteoclastogenesis (Fig. 5b, c). This result indicates that factors released during TNF-α-induced osteocyte necroptosis enhance osteoclastogenesis, and these factors may include DAMPs that promote osteoclastogenesis.
Tumor necrosis factor-α (TNF-α)-induced osteocyte necroptosis enhances osteoclastogenesis. a Schematic diagram showing the treatment of osteoclast precursors derived from TNF receptor type 1- and 2-deficient (TNFRsKO) mice with medium conditioned by necroptotic primary osteocytes. Representative images (b) and quantification of TRAP-positive multinucleated (≥ 3 nuclei) cells (c) (n = 4). TSZ, TNF-α (T) + SM-164 (S) + zVAD (Z); c.m., conditioned medium. Scale bar = 200 μm. **p < 0.01, ***p < 0.001, ****p < 0.0001.
Discussion
Osteocytes are cells embedded in bone tissue and function as key components of bone remodeling by regulating osteoclasts and osteoblasts via signaling molecules such as the RANKL/OPG and Sost/Dkk1/Wnt axes7. In osteocyte-ablated mice exposed to OTM, the number of osteoclasts and the resorption of alveolar bone are significantly reduced on the compression side10. Immunohistochemical staining has shown that osteocytes express RANKL on the compression side during OTM13. Reduced tooth movement and decreased osteoclast formation in osteocyte-specific RANKL-deficient OTM mice demonstrated the crucial role of osteocyte-derived RANKL11. During OTM, osteocytes also promote osteoclastogenesis via autophagy-mediated RANKL secretion on the compression side31. As highly sensitive mechanosensory cells, osteocytes play a central role in coordinating bone remodeling processes32. These studies highlight the importance of osteocytes in the precise regulation of osteoclastogenesis during OTM. Further investigations on the role of osteocytes are required to elucidate the detailed mechanisms of tooth movement. In this study, we demonstrated that osteocyte necroptosis enhances osteoclastogenesis during OTM, providing novel concepts for biological responses to control tooth movement.
Osteocyte death can promote osteoclast-mediated bone resorption. Targeted ablation of osteocytes with diphtheria toxin in mice results in an osteoporotic bone phenotype33. A previous study showed that during OTM, osteocytes in the alveolar bone undergo cell death on the compression side25. Thus, osteocyte death due to orthodontic forces may promote alveolar bone resorption on the compression side. In the present study, our time-course analysis of OTM revealed that osteocyte death was observed from day 2, whereas osteoclast formation occurred from day 6. Thus, when orthodontic forces are applied to the tooth, osteocyte death occurs first, followed by the appearance of osteoclasts. Additionally, the percentage of osteocyte death had markedly decreased on day 12. This reduction is likely due to osteoclastic resorption of alveolar bone that contained dying osteocytes and empty lacunae, which may have led to the removal of these cells from the bone tissue. The patterns of dying osteocytes and empty lacunae were mainly consistent with our previous results and those of other previous reports25,26,27. Moreover, our TEM analysis morphologically identified various types of osteocyte death. In addition to apoptotic osteocytes with nuclear fragmentation and condensation, necrotic osteocytes with ruptured plasma membranes and empty lacunae were observed. Apoptosis is considered a weak inducer of inflammation and is non-immunogenic because the cell membrane is not ruptured but instead fragmented to form apoptotic bodies21. Therefore, necrotic rather than apoptotic osteocytes may be involved in osteoclastogenesis and alveolar bone resorption on the compression side during OTM.
TNF-α is a proinflammatory cytokine that is a key molecule for inflammatory osteoclastogenesis and bone destruction34. Our research group has found that TNF-α is an important cytokine for tooth movement, as the number of osteoclasts and the distance of tooth movement were reduced in TNFRsKO OTM mice compared to WT OTM mice6. TNF-α was also found to induce inflammatory responses by mediating various types of cell death35. In the present study, the percentages of dying osteocytes and empty lacunae were significantly reduced in TNFRsKO OTM mice. These findings suggest that TNF-α is related to osteocyte death on the compression side during OTM. Necroptosis is a type of TNF-α-induced cell death that can be confirmed by the phosphorylation of RIP3 and MLKL as specific biomarkers36. In the present study, p-RIP3 and p-MLKL were detected by immunofluorescence staining of osteocytes on the compression side of WT OTM mice, whereas these biomarkers were barely detectable in osteocytes on the compression side of TNFRsKO OTM mice. Morphologically, necroptosis resembles necrosis with ruptured plasma membranes and the release of DAMPs21,37. DAMPs promote sterile inflammation, which is important for tissue repair and regeneration24. Macrophages sense intercellular DAMPs to induce local sterile inflammation, promote cellular self-renewal, and recruit a large number of immune cells38. Collectively, these findings suggest that the DAMPs released by necroptotic osteocytes regulate sterile inflammation during OTM and control osteoclastogenesis on the compression side.
Regarding in vitro experiments with osteocytes, the use of osteocyte-like cell lines or primary osteocytes must be considered. Osteocyte-like cell lines, such as MLO-Y4 and IDG-SW3, are useful for analyzing biological responses and functions39,40. However, because osteocyte-like cell lines behave substantially differently from osteocytes in vivo, the reliability of results from cell line experiments must be carefully evaluated. Although various methods have been devised to isolate primary osteocytes, the low purity of isolated osteocytes remains an issue41. To isolate primary osteocytes with high purity, a new method was established using FACS from mice with osteocyte-specific expression of green fluorescent protein9. We successfully isolated primary osteocytes with high purity from Dmp1-Topaz mice using this method, with minor modifications12,29. In the current study, we induced necroptosis with TNF-α, SM-164, and zVAD (TSZ) in these primary osteocytes. Immunofluorescence staining revealed that the necroptosis biomarkers p-RIP3 and p-MLKL were detected approximately 6 h after TSZ stimulation. To verify necroptosis in primary osteocytes, the RIP1 inhibitor Nec-1, the RIP3 inhibitor GSK872, and the MLKL inhibitor NSA were used. Nec-1 and GSK872 inhibited RIP3 and MLKL phosphorylation. NSA did not inhibit RIP3 phosphorylation but inhibited MLKL phosphorylation because MLKL activation is downstream of RIP3 phosphorylation in the necroptotic signaling pathway. These data suggest that the three inhibitors suppressed necroptosis in primary osteocytes. Similar to our findings in primary osteocytes, TNF-α-induced necroptosis in MLO-Y4 cells is inhibited by Nec-142. Our results showed that TNF-α can induce necroptosis in osteocytes which may release intracellular contents as DAMPs.
DAMPs are endogenous molecules produced during cellular damage or stress that play an important role in inflammation and inflammatory diseases43. It has been previously suggested that DAMPs released during osteocyte necrosis reach the bone surface through canaliculi, thereby promoting there osteoclastogenesis and bone resorption44. In bone diseases associated with osteocyte death, such as bone fracture, arthritis, and osteonecrosis, DAMPs released from necrotic osteocytes induce osteoclast-mediated bone loss45. In the present study, we cultured osteoclast precursors in conditioned medium from necroptotic osteocytes to assess the effect of DAMPs on osteoclastogenesis. To eliminate the effects of TNF-α on osteoclastogenesis, TNFRsKO mice-derived osteoclast precursors were used in this experiment. As expected, medium conditioned by necroptotic osteocytes enhanced osteoclastogenesis, suggesting that factors, including DAMPs, released during osteocyte necroptosis promote osteoclastogenesis.
A major limitation of this study is that we were unable to confirm that DAMPs are the strongest factors that enhance osteoclastogenesis following osteocyte necroptosis. Osteocytes escaping necroptosis may release other factors promoting osteoclastogenesis by TNF-α stimulation. The expression of cytokines such as CXCL8 and CXCL1 is upregulated in a cell-autonomous manner during necroptosis30. Additionally, we were unable to identify the DAMPs released during osteocyte necroptosis-regulated osteoclastogenesis. Some DAMPs have proinflammatory effects, whereas others have anti-inflammatory effects43. For example, upon cell death, the DAMP prostaglandin E2 negatively regulates the immune response46. Moreover, we reported that IL-33, one of the DAMPs, inhibits TNF-α-induced osteoclastogenesis and bone resorption47. To identify which DAMPs released from necroptotic osteocytes enhance osteoclastogenesis, comprehensive approaches such as proteomics are required. However, our method of isolating primary osteocytes by FACS resulted in low cellular yields, limiting the experiments and analyses that could be performed.
In summary, our data indicate that TNF-α induces osteocyte necroptosis on the compression side during OTM. Factors, including DAMPs, released from osteocytes undergoing TNF-α-induced necroptosis enhance osteoclastogenesis and contribute to alveolar bone resorption during OTM (Fig. 6). Because the regulation of inflammatory responses by DAMPs is important for bone remodeling, targeting the TNF signaling pathway and osteocyte necroptosis might be a novel treatment strategy for controlling tooth movement during orthodontic treatment.
Materials and methods
Mice
This study was approved by the Institutional Animal Care and Use Committee of the Tohoku University Environmental & Safety Committee (Approval Number: 2018DnA-028-06). All procedures were conducted in accordance with the Regulations for Animal Experiments and Related Activities at Tohoku University. We also complied with ARRIVE guidelines. All mice were housed in specific pathogen-free conditions under a 12-hour light/dark cycle with ad libitum access to feed (Labo MR Stock, Nosan Corporation, Kanagawa, Japan). The mouse OTM model was provided with powdered feed (CE-2, CLEA Japan, Tokyo, Japan) to make it easier to feed even with an attached orthodontic appliance. B6;129 S-Tnfrsf1a1 (p55, TNF receptor type 1-deficient) Tnfrsf1btm1Imx/J (p75, TNF receptor type 2-deficient) mice and C57BL/6-Tg (Dmp1-Topaz) 1lkal/J mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). C57BL/6J (WT) mice were purchased from CLEA Japan.
Reagents and antibodies
Recombinant mouse TNF-α was purchased from R&D Systems (Minneapolis, MN, USA). SM-164 and z-VAD were purchased from Selleck Chemicals (Houston, TX, USA). Nec-1, GSK872, and NSA were purchased from Sigma-Aldrich (St. Louis, MO, USA). Recombinant mouse RANKL was purchased from PeproTech (Rocky Hill, NJ, USA). Recombinant mouse M-CSF was obtained using the M-CSF-expressing CMG14-12 cell line48. The following antibodies were used in this study: phospho-RIP3 rabbit mAb (#91702), phospho-MLKL rabbit mAb (#37333), rabbit mAb IgG Isotype Control (#3900), Alexa Fluor 594 anti-rabbit IgG (#8889), and Alexa Fluor 555 anti-rabbit IgG (#4413; all Cell Signaling Technology, Danvers, MA, USA).
Orthodontic tooth movement
A mouse model of OTM using a Ni-Ti closed-coil spring was established as previously described6. Following the anesthesia via intraperitoneal injection of medetomidine, midazolam, and butorphanol of 8-week-old male WT and TNFRsKO mice, a Ni-Ti closed-coil spring (TOMY, Tokyo, Japan) was attached between the maxillary left first molar and incisor (Fig. 1a). To tightly fix the orthodontic appliance, a hole was created in the alveolar bone near the incisor by drilling with a round carbide bur; then the appliance was ligated with 0.1 mm stainless steel wire (Unique Medical, Tokyo, Japan) through this hole. According to the manufacturer’s instructions, a 10 g force was applied to the maxillary left first molar for mesial movement. Tooth movement was carried out for up to 12 days.
Distance of tooth movement
After tooth movement was completed at seven time points (days 0, 2, 4, 6, 8, 10, and 12), the mice were anesthetized via intraperitoneal injection of medetomidine, midazolam, and butorphanol, subsequently euthanized by cervical dislocation, and then the Ni-Ti closed-coil spring was removed. Dental impressions of the maxillary teeth were obtained using hydrophilic vinyl polysiloxane impression material (EXAMIXFINE, GC, Tokyo, Japan). The maxillary teeth and dental impressions were observed under a stereomicroscope (Leica M165 FC; Leica Microsystems, Wetzlar, Germany), and the distance of tooth movement was measured using cellSens imaging software (Olympus, Tokyo, Japan). The central fossae of the first and second molars in the dental impression were connected by a line, and the gap between the distal surface of the first molar and the mesial surface of the second molar was defined as the distance of tooth movement (Supplementary Fig. S1).
Histological staining
The dissected maxillae were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 24 h and decalcified with 14% ethylenediaminetetraacetic acid (EDTA, pH 7.2) for 1 month. After dehydration using an automatic tissue processor (Leica TP1020; Leica Biosystems, Wetzlar, Germany), the maxillary bone tissues were embedded in paraffin. Tissues were sectioned in the horizontal plane at a thickness of 4 μm. Histological analysis was performed using alveolar bone around the distobuccal root of the first molar as the region of interest (ROI; Supplementary Fig. S2a). To confirm osteoclast formation on the compression side, TRAP was stained at five section levels (100, 140, 180, 220, and 260 μm from the branch point of tooth roots) using a TRAP/ALP stain kit (Fujifilm, Osaka, Japan; Supplementary Fig. S2b). Hematoxylin was used for nuclear counterstaining. To evaluate osteocyte death, H&E staining was performed at sections approximately 150 μm from the branch point of the tooth roots. Rectangles of 400 μm × 200 μm on the compression and tension sides adjacent to the periodontal ligament were set up in the ROI to evaluate osteocyte death. These sections were observed under a light microscope (Leica DMRBE, Leica Microsystems).
Transmission electron microscopy (TEM)
After decalcification of the maxillae with 14% EDTA, the specimens were trimmed into small pieces containing only the molar region and post-fixed with 1% osmium tetroxide in PBS for 2 h. The specimens were dehydrated in graded concentrations of ethanol and propylene oxide and embedded in Epon 812 resin (TAAB Laboratories Equipment, Berks, UK). Semi-thin sections were prepared using an ultramicrotome (Leica EM UC7, Leica Microsystems). Semi-thin sections were cut with a glass knife in the horizontal plane until approximately 150 μm from the branch point of tooth roots. The observation area was confirmed by toluidine blue staining of the semi-thin sections. Thin sections were cut using diamond knives (Syntek, Tokyo, Japan) to a thickness of 70 nm and placed on reticular copper grids (JEOL, Tokyo, Japan). For TEM, sections were double-stained with uranyl acetate and lead citrate. These alveolar bone sections were imaged using a transmission electron microscope (JEM-1400, JEOL) at 80 kV.
Immunofluorescence staining of histological sections
To detect osteocyte necroptosis during OTM, immunofluorescence staining was performed using sections at approximately 150 µm from the branch point of the tooth roots. The paraffin sections were deparaffinized and rehydrated in xylene and graded concentrations of ethanol. The sections were treated in 0.2% Triton X-100 (Sigma-Aldrich) for 15 min at room temperature. After blocking with 10% goat serum (Abcam, Cambridge, UK) in PBS for 1 h at room temperature, the sections were incubated with phospho-RIP3 rabbit mAb (1:400 dilution) or phospho-MLKL rabbit mAb (1:1600 dilution) at 4°C overnight. As an isotype control, rabbit mAb IgG was diluted to the same concentration as the specific antibody. The sections were subsequently incubated with Alexa Fluor 594 anti-rabbit IgG (1:500 dilution) for 1 h at room temperature and mounted using ProLong™ Gold Antifade Mountant with 4’, 6-diamidino-2’-phenylindole (DAPI; Thermo Fisher Scientific, Waltham, MA, USA). Rectangles of 400 μm × 200 μm on the compression and tension sides adjacent to the periodontal ligament were set up in the ROI for evaluating osteocyte necroptosis (Supplementary Fig. S2c). The samples were observed under a confocal laser scanning microscope (LSM 780; Zeiss, Oberkochen, Germany).
Isolation of primary osteocytes
Primary osteocytes were isolated as described previously12,29. Briefly, calvariae were aseptically dissected from newborn Dmp1-Topaz mice whose osteocytes express the green fluorescent protein. The calvariae were incubated using 0.2% collagenase solution (Fujifilm) for 20 min and 5 mM EDTA solution for 15 min at 37 °C with agitation, then each digestion solution was collected as fraction F. Incubation and fractionation were performed in the order collagenase (F1), EDTA (F2), collagenase (F3), collagenase (F4), and EDTA (F5). F2–F5 were cultured in alpha-modified minimal essential medium (α-MEM, Fujifilm) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (P/S) at 37 °C and 5% CO2. After 24 h, non-attached cells were removed, and adherent cells were detached from the culture dishes with trypsin-EDTA (Thermo Fisher Scientific). The cell suspension was strained in preparation for FACS (FACSAria II, BD Biosciences, Franklin Lakes, NJ, USA) and analyzed using FlowJo software (BD Biosciences). Isolated Topaz-positive cells were used as primary osteocytes.
Immunofluorescence in primary osteocytes
Primary osteocytes were cultured in α-MEM supplemented with 10% FBS and 1% P/S at 37 °C and 5% CO2. Osteocytes were seeded at a density of 5.0 × 103 cells/well into multi-well glass bottom dishes (MATSUNAMI, Osaka, Japan). To induce necroptosis, osteocytes were treated with TNF-α (100 ng/mL), SM-164 (100 nM), and zVAD (20 µM; combined referred to as TSZ) for up to 12 h. The RIP1 inhibitor Nec-1 (20 µM), RIP3 inhibitor GSK872 (20 µM), and MLKL inhibitor NSA (20 µM) were used for necroptosis inhibition. The osteocytes were fixed with 4% paraformaldehyde for 15 min and permeabilized with 0.1% Triton X-100 (Sigma-Aldrich) in PBS for 15 min at room temperature. After blocking with 1% bovine serum albumin in PBS for 1 h at room temperature, osteocytes were incubated with phospho-RIP3 rabbit mAb (1:400 dilution) or phospho-MLKL rabbit mAb (1:1600 dilution) at 4 °C overnight. Rabbit mAb IgG was used as the isotype control. Antibodies were detected using Alexa Fluor 555 anti-rabbit IgG (1:500 dilution) for 1 h at room temperature. DAPI (Thermo Fisher Scientific) was used for nuclear counterstaining. The images were acquired using a confocal laser scanning microscope (LSM 780, Zeiss).
Osteoclast differentiation
Bone marrow cells were isolated from 8-week-old male TNFRsKO mice by flushing their femora and tibiae. Bone marrow cells were seeded into Petri dishes and incubated in α-MEM containing 10% FBS, 1% P/S, and M-CSF (100 ng/mL) at 37 °C and 5% CO2 to obtain bone marrow macrophages as osteoclast precursors. After 3 days, non-attached cells were removed, and osteoclast precursors were collected from the culture dishes using trypsin-EDTA (Thermo Fisher Scientific). Subsequently, osteoclast precursors were cultured in 96-well plates at a density of 5.0 × 104 cells/well in osteoclast medium containing M-CSF (100 ng/mL) and RANKL (100 ng/mL). To examine the effects of factors released from osteocyte necroptosis on osteoclastogenesis, primary osteocytes were incubated with TNF-α (100 ng/mL), SM-164 (100 nM), and zVAD (20 µM; TSZ) at a density of 1.0 × 105 cells/well in 12-well plates for 24 h, and the supernatants were collected afterward. Subsequently, BMMs were stimulated with this supernatant as a conditioned medium. The medium was changed every 2 days. After 4 days, the differentiated osteoclasts were fixed with 4% paraformaldehyde for 15 min and permeabilized with 0.1% Triton X-100 (Sigma-Aldrich) in PBS for 15 min at room temperature. A TRAP/ALP staining kit (Fujifilm) was used to visualize osteoclasts. TRAP-positive cells with three or more nuclei were considered osteoclasts, and the number of osteoclasts was counted under a light microscope (IX71, Olympus).
Statistical analysis
One-way analysis of variance (ANOVA) with the Tukey–Kramer test was used for post-hoc analyses. All data were analyzed using JMP Pro 17 software (JMP Statistical Discovery, Cary, NC, USA). All data are expressed as the mean ± standard error of the mean (SEM). Statistical significance was set at p < 0.05.
Data availability
All data generated or analyzed during this study are included in this published article and its Supplementary information files.
References
King, G. J., Keeling, S. D. & Wronski, T. J. Histomorphometric study of alveolar bone turnover in orthodontic tooth movement. Bone 12, 401–409 (1991).
Teitelbaum, S. L. Bone resorption by osteoclasts. Science 289, 1504–1508 (2000).
Azuma, Y., Kaji, K., Katogi, R., Takeshita, S. & Kudo, A. Tumor necrosis Factor-α induces differentiation of and bone resorption by osteoclasts. J. Biol. Chem. 275, 4858–4864 (2000).
Kitaura, H. et al. Marrow stromal cells and osteoclast precursors differentially contribute to TNF-α-Induced osteoclastogenesis in vivo. J. Immunol. 173, 4838–4846 (2004).
Garlet, T. P., Coelho, U., Silva, J. S. & Garlet, G. P. Cytokine expression pattern in compression and tension sides of the periodontal ligament during orthodontic tooth movement in humans. Eur. J. Oral Sci. 115, 355–362 (2007).
Kitaura, H. et al. An Anti-c-Fms antibody inhibits orthodontic tooth movement. J. Dent. Res. 87, 396–400 (2008).
Robling, A. G. & Bonewald, L. F. The osteocyte: new insights. Annu. Rev. Physiol. 82, 485–506 (2020).
Yasuda, H. et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc. Natl. Acad. Sci. U.S.A. 95, 3597–3602 (1998).
Nakashima, T. et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 17, 1231–1234 (2011).
Matsumoto, T., Iimura, T., Ogura, K., Moriyama, K. & Yamaguchi, A. The role of osteocytes in bone resorption during orthodontic tooth movement. J. Dent. Res. 92, 340–345 (2013).
Shoji-Matsunaga, A. et al. Osteocyte regulation of orthodontic force-mediated tooth movement via RANKL expression. Sci. Rep. 7, 8753 (2017).
Marahleh, A. et al. TNF-α directly enhances osteocyte RANKL expression and promotes osteoclast formation. Front. Immunol. 10, 2925 (2019).
Marahleh, A. et al. Effect of TNF-α on osteocyte RANKL expression during orthodontic tooth movement. J. Dent. Sci. 16, 1191–1197 (2021).
Galluzzi, L. et al. Cell death modalities: classification and pathophysiological implications. Cell. Death Differ. 14, 1237–1243 (2007).
Galluzzi, L. et al. Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell. Death Differ. 25, 486–541 (2018).
Weinlich, R., Oberst, A., Beere, H. M. & Green, D. R. Necroptosis in development, inflammation and disease. Nat. Rev. Mol. Cell. Biol. 18, 127–136 (2017).
Laster, S. M., Wood, J. G. & Gooding, L. R. Tumor necrosis factor can induce both apoptic and necrotic forms of cell Lysis. J. Immunol. 141, 2629–2634 (1988).
Degterev, A. et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 1, 112–119 (2005).
He, S. et al. Receptor interacting protein Kinase-3 determines cellular necrotic response to TNF-α. Cell 137, 1100–1111 (2009).
Sun, L. et al. Mixed lineage kinase Domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148, 213–227 (2012).
Pasparakis, M. & Vandenabeele, P. Necroptosis and its role in inflammation. Nature 517, 311–320 (2015).
Lee, S. H., Kwon, J. Y., Kim, S. Y., Jung, K. & Cho, M. L. Interferon-gamma regulates inflammatory cell death by targeting necroptosis in experimental autoimmune arthritis. Sci. Rep. 7, 10133 (2017).
Riegger, J. & Brenner, R. E. Evidence of necroptosis in Osteoarthritic disease: investigation of blunt mechanical impact as possible trigger in regulated necrosis. Cell. Death Dis. 10, 683 (2019).
Gong, T., Liu, L., Jiang, W. & Zhou, R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 20, 95–112 (2020).
Hamaya, M., Mizoguchi, I., Sakakura, Y., Yajima, T. & Abiko, Y. Cell death of osteocytes occurs in rat alveolar bone during experimental tooth movement. Calcif Tissue Int. 70, 117–126 (2002).
Sakai, Y. et al. CTGF and apoptosis in mouse osteocytes induced by tooth movement. J. Dent. Res. 88, 345–350 (2009).
Moin, S. et al. Osteocyte death during orthodontic tooth movement in mice. Angle Orthod. 84, 1086–1092 (2014).
Cui, H. et al. TNF-α promotes osteocyte necroptosis by upregulating TLR4 in postmenopausal osteoporosis. Bone 182, 117050 (2024).
Marahleh, A. et al. Obtaining primary osteocytes through murine calvarial fractionation of GFP-Expressing osteocytes. J. Vis. Exp. 2020, 61513. https://doi.org/10.3791/61513 (2020).
Zhu, K. et al. Necroptosis promotes cell-autonomous activation of Proinflammatory cytokine gene expression. Cell. Death Dis. 9, 500 (2018).
Li, W. et al. Osteocytes promote osteoclastogenesis via autophagy-mediated RANKL secretion under mechanical compressive force. Arch. Biochem. Biophys. 694, 108594 (2020).
Marahleh, A., Kitaura, H., Ohori, F., Noguchi, T. & Mizoguchi, I. The osteocyte and its osteoclastogenic potential. Front. Endocrinol. 14, 1121727 (2023).
Tatsumi, S. et al. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell. Metab. 5, 464–475 (2007).
Kitaura, H. et al. Immunological Reaction in TNF- α -Mediated Osteoclast Formation and Bone Resorption In Vitro and In Vivo. Clin. Dev. Immunol. 2013, 1–8 (2013).
Van Loo, G. & Bertrand, M. J. M. Death by TNF: a road to inflammation. Nat. Rev. Immunol. 23, 289–303 (2023).
Zhu, T. & Wu, B. W. Recognition of necroptosis: from molecular mechanisms to detection methods. Biomed. Pharmacother. 178, 117196 (2024).
Murai, S. et al. A FRET biosensor for necroptosis uncovers two different modes of the release of damps. Nat. Commun. 9, 4457 (2018).
Huang, Y., Jiang, W. & Zhou, R. DAMP sensing and sterile inflammation: intracellular, intercellular and inter-organ pathways. Nat. Rev. Immunol. 24, 703–719 (2024).
Kato, Y., Windle, J. J., Koop, B. A., Mundy, G. R. & Bonewald, L. F. Establishment of an Osteocyte-like cell line, MLO-Y4. J. Bone Min. Res. 12, 2014–2023 (1997).
Woo, S. M., Rosser, J., Dusevich, V., Kalajzic, I. & Bonewald, L. F. Cell line IDG-SW3 replicates osteoblast-to-late-osteocyte differentiation in vitro and accelerates bone formation in vivo. J. Bone Min. Res. 26, 2634–2646 (2011).
Gu, G., Nars, M., Hentunen, T. A., Metsikkö, K. & Väänänen, H. K. Isolated primary osteocytes express functional gap junctions in vitro. Cell. Tissue Res. 323, 263–271 (2006).
Cui, H. et al. Necrostatin-1 treatment inhibits osteocyte necroptosis and trabecular deterioration in ovariectomized rats. Sci. Rep. 6, 33803 (2016).
Ma, M., Jiang, W. & Zhou, R. DAMPs and DAMP-sensing receptors in inflammation and diseases. Immunity 57, 752–771 (2024).
Komori, T. Cell death in chondrocytes, osteoblasts, and osteocytes. Int. J. Mol. Sci. 17, 2045 (2016).
Andreev, D. et al. Osteocyte necrosis triggers osteoclast-mediated bone loss through macrophage-inducible C-type lectin. J. Clin. Invest. 130, 4811–4830 (2020).
Hangai, S. et al. PGE2 induced in and released by dying cells functions as an inhibitory DAMP. Proc. Natl. Acad. Sci. U S A. 113, 3844–3849 (2016).
Ohori, F. et al. IL-33 inhibits TNF-α-Induced osteoclastogenesis and bone resorption. Int. J. Mol. Sci. 21, 1130 (2020).
Takeshita, S., Kaji, K. & Kudo, A. Identification and characterization of the new osteoclast progenitor with macrophage phenotypes being able to differentiate into mature osteoclasts. J. Bone Min. Res. 15, 1477–1488 (2000).
Acknowledgements
Part of this study was supported by the support system for young researchers to use research equipment, instruments, and devices at Tohoku University. The TEM analysis was conducted using the transmission electron microscope at the Center of Common Research Laboratory, Graduate School of Dentistry, Tohoku University. We are also grateful to the Biomedical Research Core of Tohoku University Graduate School of Medicine for supporting the immunofluorescence and fluorescence-activated cell sorting experiments.
Funding
This work was supported by JSPS KAKENHI Grant Numbers JP20J14571, JP22K10236, JP22K17244 from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Contributions
F.O., H.K., and I.M. contributed to designing this study. F.O., A.M., J.M., M.M., J.R., K.N., Z.F., and A.L. performed experiments. F.O., H.K., A.M., and I.M. analyzed the data and confirmed the results. F.O., H.K., and J.M. drafted the manuscript. I.M. supervised the project. All authors approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All animal experiments were performed following the Regulations for Animal Experiments and Related Activities at Tohoku University (2018DnA-028-06) by the Institutional Animal Care and Use Committee of the Tohoku University Environmental & Safety Committee. This study was reported in accordance with ARRIVE guidelines.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ohori, F., Kitaura, H., Marahleh, A. et al. Osteocyte necroptosis drives osteoclastogenesis and alveolar bone resorption during orthodontic tooth movement. Sci Rep 15, 19413 (2025). https://doi.org/10.1038/s41598-025-04697-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-04697-8