Abstract
Chronic low back pain (cLBP) is a leading cause of disability worldwide, however, the influence of age on electromyography (EMG) during lifting tasks is not well understood. This study examined the effects of age and pain on EMG and kinematics in 102 participants. They were divided into no low back pain (no-BP) (n = 42; mean age: 41.86) and cLBP groups (n = 60; mean age: 43.41) and further categorized by age: 44 under 40 years (mean age: 31.14) and 58 over 40 years (mean age: 51.38). Two lifting tasks from the ground to the hip height were performed: lifting a 10 kg box in front of the body (Task 1) and two 5 kg dumbbells beside the body (Task 2), with EMG and flexion angles recorded. Older participants showed significantly higher EMG amplitudes (p < 0.05), particularly in Task 1 while holding the weight at hip height. No significant EMG differences were found between cLBP and no-BP groups after adjusting for age, sex, and body mass index (BMI) (p > 0.05). Task 1 showed higher back muscle activation than Task 2 (p < 0.05). These findings suggest that age, rather than pain, may play a more critical role in muscle activation, highlighting the need for age-specific interventions in cLBP rehabilitation.
Similar content being viewed by others
Introduction
Chronic Low Back Pain (cLBP) is a major contributor to global disability, substantially affecting individuals’ quality of life and imposing significant economic burdens due to healthcare expenditures and lost productivity from work absenteeism1,2. Manual material handling, such as lifting and carrying, has been identified as a key physical risk factor for the development of cLBP3,4,5,6. These tasks increase the mechanical stress and strain placed on the spine, raising the risk of injury6,7. Studies have shown that lifting weights beside the body, rather than in front, considerably reduces low back loading, which may help in preventing both injury and re-injury to the lumbar region8,9,10. For instance, Subramaniyam et al.8 demonstrated that lifting loads beside the body significantly decreases back muscle activity by as much as 38–91%, highlighting the importance of load positioning in minimizing muscular strain during lifting tasks. This reduction in muscular effort is particularly beneficial in lowering the burden on trunk muscles. However, while these findings offer valuable insights into back muscle mechanics in individuals without low back pain (no-BP), the electromyographic (EMG) activity of abdominal muscles and the specific effects on cLBP patients remain insufficiently explored. Understanding these factors is crucial for developing more effective rehabilitation protocols for cLBP patients.
Lifting tasks are widely utilized in musculoskeletal assessments to evaluate functional capacity, especially in individuals with cLBP, as these tasks closely simulate daily activities that frequently cause or worsen pain7,10,11,12,13,14,15,16,17,18. Numerous studies have investigated differences in trunk muscle activations during lifting tasks, with a focus on muscle activation timing and EMG amplitude19,20,21,22. Several studies have shown that cLBP individuals demonstrate delayed muscle activation compared to no-BP group19,23. In contrast, Ferguson et al.18 found that the cLBP group demonstrated significantly earlier bilateral activation of the erector spinae (ES) muscles, with no differences in peak activation between groups. Similarly, Marras et al.17,24 observed increased activity in all back muscles in cLBP patients, while Courbalay et al.25 found higher ES activation during the lifting and lowering of heavy weights (20 lbs), though no significant differences in kinematics were found between the groups. Larivière et al.16 further reported that cLBP individuals exhibited reduced lumbar erector spinae (ESL) activity during the lowering phase and increased thoracic erector spinae (EST) activity during both lifting and lowering phases, again with no notable kinematic differences between groups. These findings suggest that variations in muscle recruitment patterns, rather than movement mechanics, may play a critical role in the pain and functional limitations observed in cLBP patients.
Most studies have predominantly focused on younger populations, often overlooking the impact of age on EMG patterns. Age-related musculoskeletal changes, including sarcopenia, reduced muscle strength, diminished flexibility, and decreased endurance, significantly influence functional capacity in older adults26. These changes can alter muscle recruitment strategies during physical tasks such as lifting. Additionally, as individuals age, the occurrence of cLBP becomes more common, with significant reductions in lumbar flexibility, particularly in flexion and extension, typically beginning around the age of 40 27,28. This highlights the importance of considering age as a crucial factor in EMG analyses and musculoskeletal assessments.
However, current research lacks a thorough understanding of how age affects trunk muscle activity and kinematics during lifting tasks in individuals with cLBP. This study aims to address this gap by examining the effects of age and pain on EMG and kinematic patterns, while also exploring how lifting loads beside the body versus in front of the body impacts these variables. We hypothesize that: (1) individuals with cLBP will exhibit significant alterations in EMG patterns and trunk flexion angles compared to those without cLBP; (2) age will significantly modulate these EMG and kinematic changes; and (3) different lifting tasks will yield distinct EMG activation patterns.
Method
Study participants and ethics approval
This study was conducted at the Julius Wolff Institute, Berlin Institute of Health at Charité – Universitätsmedizin Berlin, Germany, from January 2022 to June 2024 as part of a four-year prospective project investigating factors affecting the development of cLBP. Ethical approval was obtained from the Ethics Committee of Charité – Universitätsmedizin Berlin (registry numbers: EA4/011/10, EA1/162/13), and the study was prospectively registered (DRKS-ID: DRKS00027907). All methods were performed in accordance with the Declaration of Helsinki.
All participants gave written informed consent after being fully briefed on the study procedures, and informed consent was also obtained for the online open-access publication of identifiable images. The inclusion criteria required individuals aged 19 to 64 years with a body mass index (BMI) under 29 kg/m². We conducted our study based on previous research that considered 40 years as a threshold for age sub-grouping29,30. Participants in the no-BP group had no history of back or pelvic pain and had not undergone any surgery on the spine or lower limbs. The cLBP group included patients with chronic low back pain lasting more than 3 months, with pain levels measured using the Numeric Rating Scale (NRS) ranging from 0 (no pain) to 10 (worst pain). Exclusion criteria for the cLBP group included a history of vertebral fractures, radiculopathy with muscle weakness, prior spinal surgery, and other conditions that limit daily activity, such as chronic obstructive pulmonary disease (COPD), heart failure, neurological disorders, or cancer30.
Measurement devices and instrumentation
A Vicon Motion Capture System (Vicon Motion Systems, Inc., Oxford, UK) was utilized to capture 3D motion at a sampling frequency of 200 Hz. Twelve infrared cameras recorded the movement of 41 retro-reflective markers (14 mm in diameter) placed on key anatomical points, following the Vicon plug-in gait full-body marker protocol31.
Additionally, muscle activity was measured using a wireless EMG system (Myon Aktos, Schwarzenberg, Switzerland) with a sampling rate of 1000 Hz. Prior to placing the electrodes, the skin was shaved, cleaned, and disinfected with alcohol. EMG data were integrated into the Vicon Nexus system and synchronized with the Vicon data. Twelve surface EMG sensors were used to record muscle activity from the left and right multifidus (L/RMF) (~ 2 cm lateral to the midline at L5), left and right lumbar erector spinae (L/RESL) (~ 3 cm lateral to the midline at L3), and left and right thoracic erector spinae (L/REST) (~ 5 cm lateral to the midline at T9)30left and right external obliques (L/REO) (~ 10 cm lateral to the midline above the umbilicus, aligned with the muscle fibers), left and right internal obliques (L/RIO) (below the external oblique sensors, just above the inguinal ligament), and left and right rectus abdominis (L/RRA) (~ 3 cm lateral to the midline above the umbilicus)7,10. To reduce noise and artifacts, a band-pass filter (30–450 Hz) with a fourth-order Butterworth design was applied, and a notch filter was used to eliminate 50 Hz interference. After filtering, the EMG signals were full wave rectified, and the root-mean-square (RMS) envelopes were calculated using a 150-ms moving window. The RMS values were then normalized to the peak values from the maximal voluntary contraction (MVC)7,30.
Study protocol
Participants underwent a clinical examination by an experienced orthopedic and trauma specialist and completed the following questionnaires: the Roland-Morris Disability Questionnaire (RMDQ)32 to evaluate the disability, the Tampa Scale for Kinesiophobia (TSK)33and the Fear-Avoidance Beliefs Questionnaire (FABQ)34 to assess fear of movement and avoidance beliefs (Table 1).
All participants performed a series of MVC tests and lifting tasks. For the MVC assessments of the three back muscles, subjects were positioned prone with their upper body extending over the edge of the table, while their legs remained straight and securely fixed. Participants were instructed to raise their head, shoulders, and elbows off the table, while the examiner, positioned at the subject’s head, applied symmetrical manual resistance to the shoulders, prompting maximal exertion35. For the rectus abdominis (RA) and internal oblique (IO) muscles, participants lay supine with their knees flexed at 90 degrees and hands placed on their chest. They performed a resisted curl-up, during which the experimenter, standing at the head of the bed, applied maximal isometric resistance symmetrically through the shoulders35. For external oblique (EO) evaluation, subjects lay on their side with legs straight and fixed and hands on the chest. They performed a resisted lateral curl-up with the investigator applying resistance through the shoulder36.
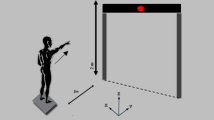
Participants took part in a practice session before the trials to ensure smoother movement during the tasks. Then, the participants were instructed to perform two symmetric lifting tasks from floor to hip height in the sagittal plane (Fig. 1): lifting a 10 kg box in front of the body to hip height (Task 1) and lifting two 5 kg dumbbells beside the body to hip height (Task 2)7. All tasks were initiated from a relaxed standing position, with the feet al.igned at shoulder width and the knees straight. From this starting position, participants bent forward, lifted the weight with self-selected velocity, returned to the upright standing position, held this position for 3 s, and then placed the box back down on the floor, returning to the initial position. Each task was repeated three times, with a one-minute rest period separating the trials7,10.
Data processing
For kinematic analysis, flexion angles of the lumbar, thoracic, and pelvic regions at maximum trunk inclination (MaxP/L/T) were computed. The Vicon Nexus 2.8.1 system with the plug-in gait model was utilized to identify relevant frames and calculate the segmental angles. The lumbar flexion angle was determined by the intersection of the sagittal thoracic and pelvic axes, using the fixed transverse axis of the pelvis as the reference point. The thoracic flexion angle was defined as the angle between the projected sagittal thoracic axis and the sagittal laboratory axis. Similarly, the pelvic angle was defined as the angle between the projected sagittal pelvic axis and the sagittal laboratory axis30,31.
MATLAB R2020b (The MathWorks, Inc.) was used to process the EMG data. The onset times of twelve muscles were recorded. Muscle activation onset was visually identified by two trained examiners as the first noticeable rise in EMG activity above baseline that persisted for at least 50 milliseconds37. Our method of determining EMG onset involved normalizing the lifting cycle from an upright standing posture through lifting and placing down the weight and back to the upright standing posture. Thereby accounting for variations in movement velocity and ensuring consistency across participants. Differences in muscle activation timing were compared using the percentage of the onset point relative to the entire movement. Additionally, the EMG amplitude was measured during three phases: (1) lifting phase: lifting the weight to hip height, (2) holding phase: holding the weight at hip height, and (3) lowering phase: lowering the weight back to the floor (Fig. 1).
Statistical analysis
Independent samples T-tests were performed to compare demographic and clinical variables between groups, ensuring comparability and providing descriptive statistics. Multivariate analysis of covariance (ANCOVA) was employed to evaluate the effects of age and pain status on EMG activity and flexion angles, with sex10,29and BMI38 included as covariates to control for potential confounding effects. When evaluating the impact of age on EMG activity and flexion angles, we controlled the pain status, sex, and BMI as covariates. In assessing the effect of pain status, we included age, sex, and BMI as covariates. To examine the interaction between age and pain status, we controlled sex and BMI as covariates. We applied logarithmic transformations to the data to stabilize variances and approximate a normal distribution. We conducted an Analysis of Variance (ANOVA) to compare the EMG differences between the two tasks, separately for the age subgroups and the pain subgroups, respectively.
All statistical analyses were performed using SPSS software (version 20, SPSS Inc., Chicago, USA). The statistical significance level was set at p < 0.05, and effect sizes were reported where applicable to quantify the practical significance of the findings. Partial eta squared (η²) was reported to quantify the effect sizes for significant effects identified in the ANCOVA. An η² value of 0.01 reflects a small effect size, 0.06 represents a moderate effect, and 0.14 signifies a large effect39. The sample size for this study was determined using G*Power 3.1.9.4, employing an a priori power analysis for an ANCOVA (fixed effects, main effects, and interactions) model. The calculation was based on an expected effect size (f) of 0.3, a significance level (α) of 0.05, and a statistical power (1-β) of 0.80. The analysis yielded a required total sample size of 90, with a denominator degree of freedom of 86 and an actual power of 0.803.
Result
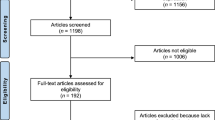
A total of 112 participants were measured, with four exclusions due to past cLBP. Of the 108 eligible participants, further exclusions were made for BMI > 29 and missing EMG data, resulting in a final sample of 102 participants who successfully completed all assessments (Fig. 2). They were categorized into no-BP (22 f/20 m; mean age: 41.86) and cLBP groups (29f/31 m; mean age: 43.41). Additionally, participants were further divided by age: 44 participants were under 40 years old (mean age: 31.14), and 58 were over 40 (mean age: 51.38). Participant demographics are outlined in Table 1. No significant differences were observed in height, weight, and BMI between the groups (all p > 0.05). Furthermore, pain intensity did not differ significantly between participants aged under and over 40 years (p > 0.05). Notably, individuals over 40 exhibited a significantly lower FABQ-W score compared to their younger counterparts (p = 0.03). Additionally, significant differences in flexion velocity were observed between the cLBP and no-BP groups, with the cLBP group performing lifting tasks at a slower rate (p = 0.01 for Task 1; p = 0.01 for Task 2) (Table 1).
Task1 results
EMG amplitude during the lifting phase
Significant EMG amplitude differences were found for LESL (p = 0.03, η² = 0.05) and LEO (p = 0.04, η² = 0.04) when assessing the effect of age, with pain controlled as a covariate. In contrast, no notable differences were observed when focusing solely on the effect of pain. However, considering the combined influence of both age and pain, significant differences were observed in RMF (p = 0.03, η² = 0.05) and LIO (p = 0.04, η² = 0.04) (Table 2).
EMG amplitude during the holding phase
For the EMG amplitude during the holding phase, when analyzing the effect of age and controlling the influence of pain, significant differences were observed in LMF (p < 0.01, η² = 0.10), RMF (p < 0.01, η² = 0.08), RESL (p = 0.02, η² = 0.06), LEST (p = 0.01, η² = 0.08), LEO(p = 0.04, η² = 0.04), and REO (p = 0.03, η² = 0.05). Conversely, when focusing solely on the effect of pain and excluding the influence of age, significant differences were observed only in RRA (p = 0.03, η² = 0.05). When considering the combined effects of age and pain, significant differences were observed only in the RMF (p = 0.04, η² = 0.04) (Table 3).
EMG amplitude during the lowering phase
During the lowering phase, significant differences were observed only in RMF (p = 0.02, η² = 0.05) when isolating age while controlling pain. Conversely, neither pain alone nor the combined effects of age and pain resulted in significant differences (Table 4).
Onset time and flexion angles
We further analyzed the effects of age and pain on the onset time and flexion angles for the lifting task. Our findings indicated that none of the onset time and flexion angles demonstrated statistical significance (all p > 0.05) (Supplementary Table S1).
Task2 results
None of the onset times or flexion angles showed statistical significance (Supplementary Table S2). Across the three phases of the lifting task, when analyzing the effect of pain alone and controlling for age, no significant differences were found in EMG amplitudes. Similarly, the combined effects of age and pain were not statistically significant. However, significant differences emerged when focusing on the effect of age while controlling for pain: RMF (p = 0.03, η² = 0.05 during the lifting phase), LEST (p = 0.03, η² = 0.05 during the lifting phase), REO (p = 0.03, η² = 0.05 during the holding phase), and RMF (p < 0.01, η² = 0.09 during the lowering phase) (Supplementary Tables S3-5).
Differences between tasks 1 and 2
Task 1 resulted in higher EMG amplitudes in all back muscles across both pain (no-BP and cLBP) and age (< 40 and > 40) groups compared to Task 2, with significant differences observed during the lifting and holding phases (p < 0.05) (Tables 5 and 6). During the lifting phase, ESL muscle activity increased by up to 36% in the no-BP group and 27% in the cLBP group, while participants under 40 showed up to a 28% increase, and those over 40, up to 32%. In the holding phase, ESL activity rose by as much as 1.7 times in the no-BP group and 2 times in the cLBP group, with increases of 1.9 times and 1.8 times for participants under and over 40, respectively. For the lowering phase, EST muscle activity increased by up to 32% in the no-BP group and 25% in the cLBP group, with participants under 40 showing up to a 26% increase and those over 40 showing a 24% rise.
In contrast to the back muscles, the activation patterns of the abdominal muscles were less consistent. For some muscles, Task 2 demonstrated significantly higher activation during lifting and lowering phases (P < 0.05) across both pain (no-BP and cLBP) and age (< 40 and > 40) groups (Tables 5 and 6). Specifically, during the lifting phase, RA activity increased by up to 1.6 times and 92% in the no-BP and cLBP groups, respectively, and by up to 2 times and 67% in participants under and over 40 years of age, respectively. In the lowering phase, ESL activity increased as much as 3.7 times in the no-BP group and 99% in the cLBP group, with a rise of 3.3 times and 1.3 times in participants under and over 40, respectively.
Discussion
This study aimed to investigate the effect of age and cLBP on EMG patterns and flexion angles during various lifting tasks. Our findings underscore the significant influence of age on muscle activation, with distinct patterns emerging across different tasks. Furthermore, almost no significant differences were observed in the EMG activities and flexion angles between the no-BP and cLBP groups.
When we controlled for age, sex, and BMI to examine the effect of pain on EMG, we found no significant differences in muscle activation between the cLBP group and those without cLBP. This contrasts with previous studies17,24,25which have reported significantly higher EMG amplitudes in individuals with cLBP. One possible explanation for the increased EMG activity observed in prior research is muscle weakness, as documented by Cassisi et al.40who reported reduced strength in individuals with cLBP. Due to this back muscle weakness, individuals in the cLBP group required greater activation of the back muscles to maintain equilibrium and an upright trunk posture against the trunk flexion moment. Additionally, the overall increase in activation of both back and abdominal muscles may improve lumbopelvic stability, this co-contraction of back and abdominal muscles, observed in cLBP individuals41,42has been suggested as a motor control strategy for stabilizing the lumbopelvic region42. This explanation may also account for why, although our findings did not reach statistical significance, EMG amplitudes tended to be higher in most muscles in the cLBP group compared to the no-BP group, particularly in the abdominal muscles and some back muscles during the lowering phase.
Furthermore, in contrast to previous study7we did not observe significant differences in MF activation between the two groups. Some studies, including Wesselink et al.43have highlighted that the MF at L5 is particularly susceptible to degenerative structural and functional changes in cLBP. However, our results did not reveal EMG differences at this segment. Moreover, the study on the association between EMG activity and paraspinal muscle degeneration found no significant association between MF activation and muscle degeneration at L544. The absence of differences in MF activation in our study may reflect the heterogeneity of cLBP populations, methodological differences across studies, or compensatory mechanisms, such as increased co-contraction of other trunk muscles, which could obscure changes in MF activity. Additionally, the relatively mild to moderate pain intensity in our cLBP participants may have contributed to the lack of observed differences. Further research is needed to clarify the factors underlying these inconsistencies.
However, our study found that the cLBP group exhibited a significantly slower lifting velocity compared to the no-BP group. This finding is consistent with the results of other studies25,45which may be attributed to the weakness of the trunk extensor muscles46,47 and gluteus maximus48,49 in patients with cLBP. Additionally, cLBP patients experience anxiety and fear related to their pain (the cLBP group showed significantly higher values in TSK and FABQ-PA scores). Moreover, participants with higher RMDQ scores (the cLBP group showed significantly higher values in RMDQ scores) demonstrated greater global stiffness of the lumbar spine50. Consequently, patients with cLBP require more time to complete tasks involving lifting or moving objects.
In addition, we excluded confounding factors such as pain, sex, and BMI and analyzed the effect of age on EMG. The results demonstrated that age significantly affects muscle activation, particularly during the holding phase of lifting tasks. Specifically, younger participants exhibited lower EMG amplitudes compared to older participants in several muscles, LMF, RMF, LEST, RESL, LEO, REO, and LRA. Notably, our study indicates no statistically significant differences when analyzing the effect of pain alone or the combined effects of age and pain; thus, our results suggest that age is an independent factor affecting EMG amplitude. Our study aligns with the findings of an earlier study29which reported significant differences in amplitudes recorded between the standing and maximum flexion positions across age groups, with older patients showing higher values compared to the younger group. These findings may be attributed to age-related changes in muscle mass, composition, contractile properties, and tendon function26. Such changes lead to reduced muscle power and strength26. For instance, a decline in tendon stiffness with age can lower the rate of force production during muscle contraction13,26. Consequently, older adults require greater muscle activation to partially compensate for these changes, thereby maintaining postural stability and motor function.
Additionally, in a study on anticipatory postural control in older adults51it was found that the activation of the ESL occurred later in older patients compared to younger individuals. This delay may be due to age-related impairments in neural structures responsible for detecting postural instability, such as the supplementary motor area and the foot area of the sensorimotor cortex52. In addition, the age-related decline in muscle mass is due to the loss of both slow and fast motor units, with a more rapid reduction in fast motor units26,53. These impairments likely contribute to increased postural instability in older adults, requiring them more time to prepare for movement. However, our study did not find significant differences in the onset time of EMG. The absence of significance may be due to the age range of our participants, as we only included patients aged 19–64, with the average age of our older group being 51.38 years.
The comparison in EMG amplitudes between the two tasks showed that Task 1 had significantly higher activation in most back muscles, with back muscle activity increasing by up to 2 times compared to Task 2. This pattern was observed across both pain groups (no-BP and cLBP) and age groups (< 40 and > 40), particularly during the lifting and holding phases (p < 0.05). This difference may be attributed to the following reason: Task 1 involved lifting a 10 kg box from the ground to hip height, which generated a significant moment arm due to the anterior position of the load relative to the body’s center of gravity. Task 2, on the other hand, involved lifting two 5 kg dumbbells from the ground and holding them at the sides of the body, where the moment arms are shorter, reducing the mechanical demand on the back muscles. Although the total load is the same (10 kg), its distribution between both hands in Task 2 minimizes the moment arm8 and, consequently, the load on individual muscle groups, particularly the back muscles9. The observation is supported by previous studies that show muscle activity increases with rising loads12. When the load is held in front of the body with both hands, the center of mass of the upper body shifts anteriorly9. This shift creates a significantly increased moment8requiring the back muscles to generate greater force9 and increased muscle activity8 for stabilization and lifting. Furthermore, spine stability depends on the relative activation of all trunk muscles, as no single muscle can be identified as the most crucial for lumbar spine stability54. These explained why in Task 1, nearly all back muscles exhibit significantly higher EMG amplitudes compared to Task 2. In contrast, Task 2 showed greater abdominal muscle contraction during both the lifting and lowering phases, with EMG in abdominal muscle increasing by up to 3.7 times compared to Task 1, showing significant differences across both pain and age groups. The varying activation levels of back and abdominal muscles across different tasks and phases suggest that interventions should be tailored to meet the specific demands on these muscle groups during various activities.
While our study provided important findings, several limitations should be noted. First, the cLBP group had relatively mild to moderate pain intensity, which may not cover the full range of cLBP severity. Second, aspects such as the duration of cLBP and occupational influences, which might affect EMG and flexion angles, were not thoroughly investigated. Additionally, surface EMG recordings were susceptible to cross-talk artifacts. Future research with larger and more diverse groups and comprehensive evaluations could offer more detailed insights.
Conclusions
The significant differences in EMG amplitudes observed in older participants suggest that age-related factors may contribute to altered neuromuscular responses, which could influence injury risk and rehabilitation outcomes. Additionally, The absence of significant differences in EMG between the cLBP and no-back pain groups, after controlling for confounding variables, highlights the complexity of pain perception and muscle activation in individuals with cLBP. Furthermore, the variation in EMG patterns between different lifting tasks emphasizes the importance of task specificity in both assessment and treatment planning. Given that some of the observed neuromuscular adaptations may be linked to muscle weakness, it is essential to integrate age-appropriate strengthening programs targeting both deep and superficial trunk muscles into treatment protocols. Such age and pain related interventions may help improve lumbopelvic stability, mitigate functional impairments, and enhance long-term clinical outcomes in individuals with cLBP. Additional investigation is required to get deeper into basic mechanisms and devise specific therapies for various age cohorts.
Data availability
The data presented in this study are available upon reasonable request from the corresponding author.
References
Traeger, A. C., Qaseem, A. & McAuley, J. H. Low Back Pain Jama 326, 286, doi:https://doi.org/10.1001/jama.2020.19715 (2021).
Airaksinen, O. Et al. Chapter 4. European guidelines for the management of chronic nonspecific low back pain. Eur. Spine J. 15(Suppl 2), 192–300. https://doi.org/10.1007/s00586-006-1072-1 (2006).
Wai, E. K., Roffey, D. M., Bishop, P., Kwon, B. K. & Dagenais, S. Causal assessment of occupational lifting and low back pain: results of a systematic review. Spine J. 10, 554–566. https://doi.org/10.1016/j.spinee.2010.03.033 (2010).
Swain, C. T. V., Pan, F., Owen, P. J., Schmidt, H. & Belavy, D. L. No consensus on causality of spine postures or physical exposure and low back pain: A systematic review of systematic reviews. J. Biomech. 102, 109312. https://doi.org/10.1016/j.jbiomech.2019.08.006 (2020).
Hoy, D. et al. The global burden of low back pain: estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 73, 968–974. https://doi.org/10.1136/annrheumdis-2013-204428 (2014).
Coenen, P. et al. The effect of lifting during work on low back pain: a health impact assessment based on a meta-analysis. Occup. Environ. Med. 71, 871–877. https://doi.org/10.1136/oemed-2014-102346 (2014).
Firouzabadi, A., Arjmand, N., Zhang, T., Pumberger, M. & Schmidt, H. Effect of low back pain on the kinetics and kinematics of the lumbar spine - a combined in vivo and in Silico investigation. J. Biomech. 164, 111954. https://doi.org/10.1016/j.jbiomech.2024.111954 (2024).
Subramaniyam, M., Min, S. N., Park, S. J. & Park, S. Muscle activity and spinal loading in lifting symmetrical loads beside the body compared to in front of the body. J. Mech. Sci. Technol. 29, 5075–5081. https://doi.org/10.1007/s12206-015-1104-z (2015).
Rohlmann, A., Zander, T., Graichen, F., Schmidt, H. & Bergmann, G. How does the way a weight is carried affect spinal loads? Ergonomics 57, 262–270. https://doi.org/10.1080/00140139.2014.887789 (2014).
Firouzabadi, A., Arjmand, N., Pan, F., Zander, T. & Schmidt, H. Sex-Dependent Estimation of spinal loads during static manual material handling Activities-Combined in vivo and in Silico analyses. Front. Bioeng. Biotechnol. 9, 750862. https://doi.org/10.3389/fbioe.2021.750862 (2021).
Nolan, D., O’Sullivan, K., Newton, C., Singh, G. & Smith, B. E. Are there differences in lifting technique between those with and without low back pain? A systematic review. Scand. J. Pain. 20, 215–227. https://doi.org/10.1515/sjpain-2019-0089 (2020).
Mueller, J., Engel, T., Kopinski, S., Mayer, F. & Mueller, S. Neuromuscular trunk activation patterns in back pain patients during one-handed lifting. World J. Orthop. 8, 142–148. https://doi.org/10.5312/wjo.v8.i2.142 (2017).
Hubley-Kozey, C. L., Hanada, E. Y., Gordon, S., Kozey, J. & McKeon, M. Differences in abdominal muscle activation patterns of younger and older adults performing an asymmetric leg-loading task. Pm R. 1, 1004–1013. https://doi.org/10.1016/j.pmrj.2009.09.018 (2009).
Shojaei, I., Salt, E. G., Hooker, Q. & Bazrgari, B. Mechanical demands on the lower back in patients with non-chronic low back pain during a symmetric Lowering and lifting task. J. Biomech. 70, 255–261. https://doi.org/10.1016/j.jbiomech.2017.06.032 (2018).
Heidari, E., Arjmand, N. & Kahrizi, S. Comparisons of lumbar spine loads and kinematics in healthy and non-specific low back pain individuals during unstable lifting activities. J. Biomech. 144, 111344. https://doi.org/10.1016/j.jbiomech.2022.111344 (2022).
Larivière, C., Gagnon, D. & Loisel, P. A Biomechanical comparison of lifting techniques between subjects with and without chronic low back pain during freestyle lifting and Lowering tasks. Clin. Biomech. (Bristol Avon). 17, 89–98. https://doi.org/10.1016/s0268-0033(01)00106-1 (2002).
Marras, W. S., Ferguson, S. A., Burr, D., Davis, K. G. & Gupta, P. Spine loading in patients with low back pain during asymmetric lifting exertions. Spine J. 4, 64–75. https://doi.org/10.1016/s1529-9430(03)00424-8 (2004).
Ferguson, S. A., Marras, W. S., Burr, D. L., Davis, K. G. & Gupta, P. Differences in motor recruitment and resulting kinematics between low back pain patients and asymptomatic participants during lifting exertions. Clin. Biomech. (Bristol Avon). 19, 992–999. https://doi.org/10.1016/j.clinbiomech.2004.08.007 (2004).
Suehiro, T., Ishida, H., Kobara, K., Osaka, H. & Watanabe, S. Altered trunk muscle recruitment patterns during lifting in individuals in remission from recurrent low back pain. J. Electromyogr. Kinesiol. 39, 128–133. https://doi.org/10.1016/j.jelekin.2018.02.008 (2018).
Hodges, P. W., Moseley, G. L., Gabrielsson, A. & Gandevia, S. C. Experimental muscle pain changes feedforward postural responses of the trunk muscles. Exp. Brain Res. 151, 262–271. https://doi.org/10.1007/s00221-003-1457-x (2003).
Kim, J. W., Kang, M. H. & Oh, J. S. Patients with low back pain demonstrate increased activity of the posterior oblique sling muscle during prone hip extension. Pm R. 6, 400–405. https://doi.org/10.1016/j.pmrj.2013.12.006 (2014).
Suehiro, T. et al. Individuals with chronic low back pain demonstrate delayed onset of the back muscle activity during prone hip extension. J. Electromyogr. Kinesiol. 25, 675–680. https://doi.org/10.1016/j.jelekin.2015.04.013 (2015).
MacDonald, D., Moseley, L. G. & Hodges, P. W. Why do some patients keep hurting their back? Evidence of ongoing back muscle dysfunction during remission from recurrent back pain. Pain 142, 183–188. https://doi.org/10.1016/j.pain.2008.12.002 (2009).
Marras, W. S., Davis, K. G., Ferguson, S. A., Lucas, B. R. & Gupta, P. Spine loading characteristics of patients with low back pain compared with asymptomatic individuals. Spine (Phila Pa) 26, 2566–2574. https://doi.org/10.1097/00007632-200112010-00009 (2001).
Courbalay, A. et al. Contribution of load expectations to neuromechanical adaptations during a freestyle lifting task: A pilot study. J. Manipulative Physiol. Ther. 40, 547–557. https://doi.org/10.1016/j.jmpt.2017.07.004 (2017).
Lang, T. et al. Sarcopenia: etiology, clinical consequences, intervention, and assessment. Osteoporos. Int. 21, 543–559. https://doi.org/10.1007/s00198-009-1059-y (2010).
Sullivan, M. S., Dickinson, C. E. & Troup, J. D. The influence of age and gender on lumbar spine sagittal plane range of motion. A study of 1126 healthy subjects. Spine (Phila Pa) 19, 682–686. https://doi.org/10.1097/00007632-199403001-00007 (1994).
Galbusera, F. et al. Ageing and degenerative changes of the intervertebral disc and their impact on spinal flexibility. Eur. Spine J. 23(Suppl 3), 324–332. https://doi.org/10.1007/s00586-014-3203-4 (2014).
Kienbacher, T. et al. Age and gender related neuromuscular pattern during trunk flexion-extension in chronic low back pain patients. J. Neuroeng. Rehabil. 13, 16. https://doi.org/10.1186/s12984-016-0121-1 (2016).
Zhang, T., Firouzabadi, A., Yang, D., Liu, S. & Schmidt, H. Age-dependent flexion relaxation phenomenon in chronic low back pain patients. Front. Bioeng. Biotechnol. 12, 1388229. https://doi.org/10.3389/fbioe.2024.1388229 (2024).
Nexus, V. Nexus Documentation, (2023). https://docs.vicon.com/display/Nexus216 (accessed 8.21.23)>
Stroud, M. W., McKnight, P. E. & Jensen, M. P. Assessment of self-reported physical activity in patients with chronic pain: development of an abbreviated Roland-Morris disability scale. J. Pain. 5, 257–263. https://doi.org/10.1016/j.jpain.2004.04.002 (2004).
Gregg, C. D. et al. The relationship between the Tampa scale of kinesiophobia and low back pain rehabilitation outcomes. Spine J. 15, 2466–2471. https://doi.org/10.1016/j.spinee.2015.08.018 (2015).
Waddell, G., Newton, M., Henderson, I., Somerville, D. & Main, C. J. A Fear-Avoidance beliefs questionnaire (FABQ) and the role of fear-avoidance beliefs in chronic low back pain and disability. Pain 52, 157–168. https://doi.org/10.1016/0304-3959(93)90127-b (1993).
Konrad, P. The ABC of EMG- A Practical Introduction to Kinsesiological Electromyography. (2006).
Dankaerts, W., O’Sullivan, P. B., Burnett, A. F., Straker, L. M. & Danneels, L. A. Reliability of EMG measurements for trunk muscles during maximal and sub-maximal voluntary isometric contractions in healthy controls and CLBP patients. J. Electromyogr. Kinesiol. 14, 333–342. https://doi.org/10.1016/j.jelekin.2003.07.001 (2004).
Massé-Alarie, H., Beaulieu, L. D., Preuss, R. & Schneider, C. Task-specificity of bilateral anticipatory activation of the deep abdominal muscles in healthy and chronic low back pain populations. Gait Posture. 41, 440–447. https://doi.org/10.1016/j.gaitpost.2014.11.006 (2015).
Boocock, M., Naudé, Y., Saywell, N. & Mawston, G. Obesity as a risk factor for musculoskeletal injury during manual handling tasks: A systematic review and meta-analysis. Saf. Sci. 176, 106548. https://doi.org/10.1016/j.ssci.2024.106548 (2024).
Richardson, J. T. E. Eta squared and partial Eta squared as measures of effect size in educational research. Educational Res. Rev. 6, 135–147. https://doi.org/10.1016/j.edurev.2010.12.001 (2011).
Cassisi, J. E., Robinson, M. E., O’Conner, P. & MacMillan, M. Trunk strength and lumbar paraspinal muscle activity during isometric exercise in chronic low-back pain patients and controls. Spine (Phila Pa. 1976). 18, 245–251. https://doi.org/10.1097/00007632-199302000-00012 (1993).
van Dieën, J. H., Cholewicki, J. & Radebold, A. Trunk muscle recruitment patterns in patients with low back pain enhance the stability of the lumbar spine. Spine (Phila Pa. 1976). 28, 834–841 (2003).
D’Hooge, R. et al. Increased intramuscular fatty infiltration without differences in lumbar muscle cross-sectional area during remission of unilateral recurrent low back pain. Man. Ther. 17, 584–588. https://doi.org/10.1016/j.math.2012.06.007 (2012).
Wesselink, E. O. et al. Investigating the associations between lumbar paraspinal muscle health and age, BMI, sex, physical activity, and back pain using an automated computer-vision model: a UK biobank study. Spine J. 24, 1253–1266. https://doi.org/10.1016/j.spinee.2024.02.013 (2024).
Zhang, T. et al. Association between lumbar paraspinal muscle activities and quality in chronic low back pain: a cross-sectional analysis. Eur. Spine J. https://doi.org/10.1007/s00586-025-08727-x (2025).
Rudy, T. E., Boston, J. R., Lieber, S. J., Kubinski, J. A. & Stacey, B. R. Body motion during repetitive isodynamic lifting: a comparative study of normal subjects and low-back pain patients. Pain 105, 319–326. https://doi.org/10.1016/s0304-3959(03)00247-1 (2003).
Holmström, E., Moritz, U. & Andersson, M. Trunk muscle strength and back muscle endurance in construction workers with and without low back disorders. Scand. J. Rehabil Med. 24, 3–10 (1992).
Reid, S., Hazard, R. G. & Fenwick, J. W. Isokinetic trunk-strength deficits in people with and without low-back pain: a comparative study with consideration of effort. J. Spinal Disord. 4, 68–72 (1991).
Kankaanpää, M., Taimela, S., Laaksonen, D., Hänninen, O. & Airaksinen, O. Back and hip extensor fatigability in chronic low back pain patients and controls. Arch. Phys. Med. Rehabil. 79, 412–417. https://doi.org/10.1016/s0003-9993(98)90142-3 (1998).
Leinonen, V., Kankaanpää, M., Airaksinen, O. & Hänninen, O. Back and hip extensor activities during trunk flexion/extension: effects of low back pain and rehabilitation. Arch. Phys. Med. Rehabil. 81, 32–37. https://doi.org/10.1016/s0003-9993(00)90218-1 (2000).
Xia, T. et al. Association of lumbar spine stiffness and flexion-relaxation phenomenon with patient-reported outcomes in adults with chronic low back pain - a single-arm clinical trial investigating the effects of thrust spinal manipulation. BMC Complement. Altern. Med. 17, 303. https://doi.org/10.1186/s12906-017-1821-1 (2017).
Kanekar, N. & Aruin, A. S. The effect of aging on anticipatory postural control. Exp. Brain Res. 232, 1127–1136. https://doi.org/10.1007/s00221-014-3822-3 (2014).
Slobounov, S., Hallett, M., Stanhope, S. & Shibasaki, H. Role of cerebral cortex in human postural control: an EEG study. Clin. Neurophysiol. 116, 315–323. https://doi.org/10.1016/j.clinph.2004.09.007 (2005).
Lexell, J. & Downham, D. Y. The occurrence of fibre-type grouping in healthy human muscle: a quantitative study of cross-sections of whole Vastus lateralis from men between 15 and 83 years. Acta Neuropathol. 81, 377–381. https://doi.org/10.1007/bf00293457 (1991).
Cholewicki, J. & VanVliet, J. J. t. Relative contribution of trunk muscles to the stability of the lumbar spine during isometric exertions. Clin. Biomech. (Bristol Avon). 17, 99–105. https://doi.org/10.1016/s0268-0033(01)00118-8 (2002).
Funding
Open Access funding enabled and organized by Projekt DEAL. This study is part of the Research Unit 5177 and financed by the German Research Foundation (DFG, SCHM 2572/12 − 1). S.L., T.Z., D.Y. received China Scholarship Council (CSC, No. 202208080039, No. 202208080046, No. 202208080034).
Author information
Authors and Affiliations
Contributions
HS: Writing – review & editing, Project administration, Funding acquisition, Supervision; TZ: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Investigation, Methodology, Formal Analysis, Visualization; AF: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Methodology, Investigation, Formal Analysis; DY: Writing – review & editing, Formal Analysis; SL: Writing – review & editing, Formal Analysis; LM: Writing – review & editing, Formal Analysis — all authors have reviewed and approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Charité—Universitätsmedizin Berlin (EA1/059/21). All participants signed a written consent form.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, T., Firouzabadi, A., Yang, D. et al. Age-related variations in trunk muscle activation and kinematics during lifting in chronic low back pain: a cross-sectional study. Sci Rep 15, 20250 (2025). https://doi.org/10.1038/s41598-025-04780-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-04780-0