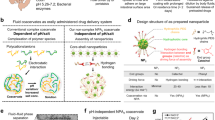
Abstract
Oral administration of protein-based biologics faces challenges such as low bioavailability and a short half-life in the gastrointestinal tract. This study focuses on developing a novel encapsulation technique using sodium alginate and chitosan to create nanospheres that efficiently deliver bovine serum albumin, a model protein. Through a combination of single-factor and response surface experiments, optimal preparation conditions were identified, yielding stable nanospheres with high encapsulation rates and small particle sizes. The release of the protein was pH-dependent, with more substantial release under alkaline conditions, resembling the environment of the colon. In vitro safety testing confirmed that the nanospheres had low toxicity and did not induce significant hemolysis or cell death. This approach demonstrates significant potential for enhancing the oral bioavailability of protein-based drugs, overcoming the typical challenges faced by oral drug delivery systems. The developed formulation offers a simple yet effective method for targeted colonic delivery, representing a promising strategy for improving the clinical efficiency of protein biologics.
Similar content being viewed by others
Introduction
Since the approval of recombinant human insulin and thyroxine by the U.S. Food and Drug Administration (FDA) in 1982, the biopharmaceutical industry has experienced significant growth. It has established a prominent position in the global pharmaceutical market1. This rapid expansion has been driven by the development of various innovative biopharmaceuticals, including PD-1/PD-L1 inhibitors, HER2 inhibitors, EGFR inhibitors, CAR-T cell therapies, and mRNA vaccines targeting COVID-192,3,4,5,6. The substantial size of this market underscores the medical, social, and economic significance of biopharmaceuticals for human health.
Because of their high specificity and biosafety, protein biologics have shown significant advantages in treating various diseases. These advantages have made developing protein drugs a hot spot in research7. However, despite the therapeutic potential of biopharmaceuticals, they are primarily administered via injection, a method that can cause patient discomfort, including pain at the injection site, risk of infection, and infusion reactions. More serious side effects may also occur, such as lupus-like syndrome, cardiovascular events, increased risk of tumors, and immunogenic reactions8,9,10. Oral administration, as a safe and highly compliant route of administration, has gradually become the focus of research. However, the gastrointestinal tract’s structural organization and physiological functions make polypeptide and protein drugs have low bioavailability and short half-lives after oral administration. Thus, the absorption of biological products and drugs has become a “bottleneck” in developing oral administration routes for such drugs.
An oral colon-specific drug delivery system (OCDDS) is a drug delivery system that allows drugs to be mainly delivered to the colon after oral administration through appropriate methods before they begin to be released, thereby exerting a colon or systemic therapeutic effect11. Compared with traditional drug delivery systems, this drug delivery system has the following advantages: it avoids the degradation and destruction of drugs, especially protein drugs, by substances such as gastric acid and enzymes in the stomach and small intestine; for the treatment of inflammatory intestinal diseases such as colitis, ulcerative colitis, and segmental colitis, the direct release of drugs in the lesion area is more conducive to the effectiveness of the drug; it avoids the stimulation of some drugs on the gastrointestinal tract and minimizes side effects12.
Microspheres are spherical particles made of suitable polymer materials used to encapsulate or adsorb drugs, usually made into suspensions for injection or oral use. Microspheres’ particle sizes generally range from 1 to 500 μm, of which those with a particle size of less than 500 nm are known as nanospheres13,14. Chitosan (CS), a deacetyl derivative of chitin, is one of the active polysaccharides with cationic properties in nature. It possesses a wide range of physiological activities and excellent biocompatibility, making CS highly desirable in the biomedical field15,16. A wide range of microorganisms can degrade it in the colon. In the presence of cross-linking agents such as glutaraldehyde and sodium tripolyphosphate (TPP), it can be reacted with various substances, making it an excellent material for formulations17,18. Different dosage forms of CS-based colon-targeted drug delivery systems have been developed, including capsules, tablets, nanoparticles, and membranes19,20. Sodium alginate (SA), a biopolymer derived from seaweed, is ideal for continuous drug delivery systems because of its excellent biocompatibility and biodegradability21. SA can bind to divalent cations such as Ca2+ to form stable nanospheres, and it can be complexed with other materials such as cellulose derivatives to prepare nanospheres that respond to specific environmental stimuli22. Moreover, some of the nanospheres can be degraded explicitly by microbial enzymes in the colon to achieve targeted drug release in the colon23. Bovine serum protein (BSA) is a major protein component in bovine serum, consisting of 583 amino acid residues with a molecular weight of approximately 66.5 kDa. In the biological and medical fields, BSA is primarily used as a carrier protein in drug delivery systems to enhance drug stability and bioavailability. Additionally, BSA serves as a standard protein in biochemical analysis, being used for protein quantification and structural studies.
Combining the advantages of these two materials, Sodium-Chitosan nanospheres (SA-CS) became an efficient colonic drug delivery system21. In the acidic environment of the gastrointestinal tract, these nanospheres can remain stable and undamaged. When the nanospheres reach the neutral or slightly alkaline environment of the colon, they are degraded by enzymes produced by colonic microorganisms, releasing the drug contained therein24. This property makes SA-CS particularly suitable for the treatment of localized diseases of the colon, such as inflammatory bowel disease. Therefore, SA-CS has essential applications in colon-targeted drug delivery systems25,26,27.
Currently, the primary methods for preparing SA-CS included traditional emulsification, spray drying, and phase separation techniques28,29. The traditional emulsification method was simple to operate and was suitable for large-scale production, but its effect on drug encapsulation and loading was not ideal. Spray drying and phase separation methods could prepare nanospheres with uniform particle size and high yield, but they required strict equipment and environmental conditions and were difficult to implement30,31. In response to the limitations of existing microsphere preparation methods, researchers have developed new drug-delivery technologies, such as liposomes, ethosomes, and hydrogels. These carrier systems showed significant advantages in enhancing drug bioavailability and achieving targeted drug delivery. However, these technologies still faced new challenges, such as insufficient stability and complex operational processes, in practical applications32,33.
To address these issues, this study proposed a novel emulsification method and optimized the traditional emulsification process. The first improvement involved the solvent system and emulsification conditions. Specifically, by selecting an appropriate combination of solvents and surfactants, we enhanced the interaction between SA and CS, thereby improving the drug encapsulation efficiency (EE, %). Additionally, the optimized emulsification process included controlling the emulsification temperature and stirring rate, effectively reducing particle aggregation and ensuring the uniformity and stability of the nanospheres. Traditional emulsification methods often struggled to achieve precise control over these parameters, leading to a broader particle size distribution and poorer nanosphere stability, particularly during storage and transportation, where phenomena such as phase separation or sedimentation were common. To further enhance the performance of SA-CS, we fine-tuned the process parameters based on the traditional emulsification method. For example, we achieved accurate control over the size of the nanospheres by precisely controlling the pH, ionic strength, and solvent evaporation rate during emulsification. It resulted in a more uniform particle size distribution and improved biocompatibility. It successfully formulated an oral colonic-targeted drug delivery system: sodium alginate-chitosan nanoparticles loaded with bovine serum albumin (SA-BSA-CS). Compared to the traditional method, the optimized emulsification process operated under milder conditions and exhibited reduced environmental sensitivity. This nanosphere system reduced production costs, simplified the process, and effectively addressed the human body’s low bioavailability of protein-based biopharmaceuticals, particularly in gastrointestinal pH fluctuations. This approach offered a simple and efficient method for the clinical delivery of protein biopharmaceuticals.
Materials and methods
Materials
Bovine serum protein (Batch No.: YB724801, Xinjiang Haize Ke Xin Biological Co., Ltd); CS (Batch No.: C8320, Beijing Solarbio Technology Co., Ltd.); SA (Batch No.: YY11151, Beijing Solarbio Technology Co., Ltd.); Anhydrous Calcium Chloride (Batch No.: C7250, Beijing Solarbio Technology Co., Ltd.); Liquid paraffin (Batch No.: M8040, Beijing Solarbio Technology Co., Ltd.); Ready-to-use dialysis bag (Batch No.:YA1031-0.5 m (MWCO 100–500 kDa), Beijing Solarbio Technology Co., Ltd.), BCA protein quantification kit (Batch No.: PC0020, Beijing Solarbio Technology Co., Ltd.); Caco-2 cells (ATCC® HTB-37 ™, Promocell, Hewlett Packard Co. ™, Promocell, Heidelberg, Germany); Trypsin-EDTA solution (Batch No. GA2407009, Wuhan Sevier Biotechnology Co., Ltd.), Fetal bovine serum (FBS) (Batch No. S020240620, Wuhan Sevier Biotechnology Co., Ltd.), Dulbecco’s Modified Eagle’s Medium (C11995500BT, Xinjiang Haize Ke Xin Biological Co., Ltd. (GIBCO)), Calcein-AM/PI live/dead cell double staining kit (Batch No.: PMK0989, Xinjiang Haize Ke Xin Biological Co., Ltd (PUMOKE)); CCK-8 kit (Batch No.: CPD001S-BR5, Xinjiang Haize Ke Xin Biological Co., Ltd (Cytosci)) The rest of the reagents were analytically pure.
Methods
Preparation of nanospheres and evaluation index
Preparation of SA-BSA-CS
An appropriate amount of sodium alginate (SA) powder was precisely weighed and was fully dissolved in 0.9% NaCl solution. This solution was then mixed with a specific quantity of bovine serum albumin (BSA) solution using an HJ-6 magnetic stirrer (Changzhou Box Scientific Instruments Co., Ltd., 600 rpm, 25 °C) for 1 h until complete dissolution. The solution was emulsified with liquid paraffin at a water-to-oil ratio of 2:3 (W: O) using a ZHWY-2102 C constant-temperature shaking incubator (Shanghai Zhicheng Analytical Instrument Manufacturing Co., Ltd., 37 °C, 220 rpm) for a specified period to achieve complete emulsification, resulting in an emulsion containing BSA. A certain amount of the emulsion was then mixed with a calcium chloride (CaCl2) solution at the appropriate mass fraction, and was crosslinked for 1 h at 37 °C and 220 rpm. The mixture was subsequently centrifuged at 12,000 rpm for 10 min using an H1750 tabletop high-speed centrifuge (Hunan Xiangyi Laboratory Instrument Development Co., Ltd.), and the precipitate was collected. The precipitate was washed three times with sodium acetate (pH = 4.0) buffer solution in a 1:3 ratio (5 mL buffer/g microspheres) at 12,000 rpm for 5 min. Afterward, the precipitate was dispersed in a chitosan (CS, degree of deacetylation ≥ 95%) solution at a 1:3 ratio, and was crosslinked for a second time at 37 °C and 220 rpm for 1 h. The final product was collected by centrifugation at 13,000 rpm for 10 min, was washed three times under the same conditions, and then was resuspended in an equal volume of 2% mannitol solution. The preparation was freeze-dried for storage using a Scientz-10 N freeze dryer (Ningbo Xinzhi Biotechnology Co., Ltd.).
Evaluation indexes
Particle size and polydispersity index(PDI): Take a certain amount of freeze-dried nanospheres, resuspend them in Phosphate-Buffered Saline (PBS), use probe sonication (power 200 W, sonication for 3 s, gap 2 s, sonication for 5 min) to make the nanospheres uniformly dispersed, and then determine the particle size of the nanospheres and PDI with the help of Mastersizer 3000 Malvern Laser Particle Size Analyser (Malvern Ltd., UK).
EE: The EE was determined using a BCA protein quantification kit. Specifically, the supernatant was collected during the preparation of nanospheres, and the protein concentration was detected according to the instructions of the BCA protein quantification kit. The nanospheres EE was calculated using Eq. (1).
Single-factor experiment
In this study, five bars of SA mass fraction (1%, 1.5%, 2%, 2.5%, 3%), CaCl2 mass fraction (1%, 2%, 3%, 4%, 5%), stirring time (20 min, 30 min, 40 min, 50 min, 60 min), and CS mass fraction (0.2%, 0.4%, 0.6%, 0.8%, 1%) were investigated as factorials. Nanospheres of EE, particle size, and particle distribution index (PDI) were used as the scaled indices for the single-factor experiment tests. The aim was to explore the optimal conditions for each factor and to lay the foundation for response surface experiments. Each set of experiments was repeated three times.
Response surface design
Based on the results of the one-factor test, the Box-Behnken design principle was applied to carry out a response surface optimization design with SA, CS, and calcium chloride mass fractions and mixing time as the independent variables, and encapsulation rate as the dependent variable. The factor-level design is shown in Table 1. Using Design-Expert 13.0.1.0 software, the Box-Behnken model was designed, and experiments were conducted. After collating and summarizing the data, multivariate linear regression and binomial equations were fitted.
Validation of the optimal formulation
Three dosage forms were prepared in parallel based on the optimal response surface simulation process (1.35% SA, 3.66% CaCl2, 0.93% CS, and a stirring time of 47.34 min). Process validation was carried out by measuring the EE, particle size, PDI, and Zeta potential.
Examination of the morphology of SA-BSA-CS
A certain amount of purified nanospheres was taken under the best formula and dropped on a copper mesh. Filter paper was used to absorb excess liquid, and the solution was allowed to stand for 1 min until it dried. Then, 2% Phosphotungstic Acid (PTA) was used for negative staining. After standing in the dark for 2–3 min, excess staining solution was removed using filter paper, and the sample was allowed to dry naturally. Microstructural images of the samples were obtained using a JEM-2100 transmission electron microscope (Nippon Electron Co., Ltd.) to observe the nanosphere’s structure.
Fourier infrared spectroscopy
The infrared spectra of CS, SA, BSA SA-BSA-CS, and SA-CS were recorded using an IR-Prestige21 Fourier Transform Infrared Spectrometer (Shimadzu Corporate Management (China) Co., Ltd.) with a KBr slice as the background. The scanned wavelengths were in the range of 4000–350 cm−1.
Examination of the in vitro release degree of SA-BSA-CS
The dialysis method was used for the in vitro release study of SA-BSA-CS. Separately, 2 mL of BSA standard solution (1 mg/mL) and SA-BSA-CS solution were placed in an activated dialysis bag (MWCO 100–500 kDa), respectively, placed in a centrifuge tube containing 40 mL of isotonic PBS buffer solution (pH 9.0, 7.4, and 2.0), and then shaken at 220 ± 5 rpm, 37 ± 0.5 ℃. The release was performed at 30 min, 1.0 h, 2.0 h, 4.0 h, 6.0 h, 8.0 h, 10 h, 12 h, 24 h, and 48 h, respectively. 2 mL of samples were taken and immediately replenished with isotonic PBS at the same temperature. The concentration of BSA was determined using the BCA method. The cumulative release rate was calculated using Eq. (2), and the test was repeated thrice for the average value. Meanwhile, five different dissolution models (mono-exponential model, zero-order kinetics, first-order kinetics, Weibull, Higuchi, and Ritger-Peppas models) were used to evaluate the drug release kinetics of SA-BSA-CS.
.
Evaluation of the biosafety of the best prescription
Normal Saline (NS) was used as the negative control, and ddH2O was used as the positive control. Ten milliliters of each SA-BSA-CS solution and BSA solution with BSA concentrations of 20 µg/mL, 15 µg/mL, and 5 µg/mL were prepared in NS, warmed up in a 37℃ water bath for 30 min, and then added to freshly diluted anticoagulated blood (0.2 mL). The mixture was mixed homogeneously, warmed up in a 37℃ water bath for 60 min, and centrifuged at 3000 rpm for 10 min. The supernatant was extracted and measured by a Multiskan GO full-wavelength enzyme marker (Thermo Fisher Scientific) at 545 nm. Another 0.2 mL of freshly diluted anticoagulated blood was added, mixed well, warmed up at 37℃ for 60 min, and centrifuged at 3000 rpm for 10 min. The supernatant was then taken, and the OD value was measured at 545 nm using the Multiskan GO full-wavelength enzyme labeler (Thermo Fisher Scientific). The assay was performed in triplicate. The qualitative evaluation of hemolysis was performed using Eq. (3).
.
Cytotoxicity experiment
Caco-2 cells were used as a model to simulate intestinal epithelial cells. Caco-2 cells were cultured in DMEM medium containing 10% fetal bovine serum and 1% penicillin-streptomycin double antibiotics. After Caco-2 cells had been cultured to the logarithmic growth phase, they were seeded into a 96-well plate at a rate of 5 × 10³ cells/well, cultured at 37℃, 5% CO₂, and adapted to saturated humidity for 24 h. SA-BSA-CS and BSA solutions were added (the BSA concentrations were 0 µg/mL, 0.312 µg/mL, 0.625 µg/mL, 1.25 µg/mL, 2.5 µg/mL, 5 µg/mL, 10 µg/mL, and 20 µg/mL, with negative controls set at the same time), and after exposure for 24 h and 48 h, 100 µL of DMEM medium containing 10% CCK-8 solution was added. The mixture was incubated for 2 h, and the absorbance was measured at 450 nm with a microplate reader. The cell survival rate was calculated according to Eq. (4).
.
10. Staining of viable cells
Caco-2 cells in the logarithmic growth phase were inoculated into a 24-well plate at 1 × 10⁴ cells/well, cultured for 24 h at 37℃, 5% CO₂, and adapted to saturated humidity. SA-BSA-CS and BSA solutions (BSA concentrations were 20 µg/mL, 1.25 µg/mL, and 0.31 µg/mL, with negative controls set) were taken and incubated with Caco-2 cells for 24 and 48 h. After the incubation, the original culture medium was discarded, and the cells were washed with PBS three times. Two hundred microliters of Calcein AM/PI working solution was added to each well, incubated at 37℃ in the dark for 30 min, and cell activity was observed using an IX71 fluorescence microscope (Japan) at the excitation wavelength of 490 nm. This procedure was repeated three times.
Results
Single-factor experiment
SA mass fraction inspection
The experimental results are shown in Fig. 1a. When the SA mass fraction was greater than 2%, the particle size and PDI of the nanospheres increased significantly, especially when the PDI approached 1, indicating that the stability of the nanospheres was poor. When the SA mass fraction was 1.5%, the maximum EE of the nanospheres was 96.99%, and the particle size and PDI were both small. Based on the EE, particle size, and PDI results, the SA mass fraction was determined to be 1.5%.
Examination of CaCl2 mass fraction
The experimental results are shown in Fig. 1b. When the CaCl2 mass fraction was greater than 2%, the particle size of the nanospheres decreased significantly, and when the CaCl2 mass fraction was 4%, the encapsulation rate of the nanospheres reached the highest value of 98.37%, with a particle size of 38.31 nm. When the CaCl2 mass fraction exceeded 4%, the encapsulation decreased to below 98.37%, and the particle size increased. Therefore, the CaCl2 mass fraction of 4% was finally determined.
Investigation of stirring time
The experimental results are shown in Fig. 1c. The stirring time had a significant impact on SA-BSA-CS’s EE and particle size. When the stirring time was 50 min, the minimum particle size of the nanospheres was 10.89 nm, the high EE was 96.67%, the PDI met the requirements, and the properties of the nanospheres were the most stable. The nanosphere’s particle size and EE decreased when the stirring time was less than 50 min. When the stirring time exceeded 50 min, the EE was lower than 96.67%, and the particle size increased. Therefore, 50 min was chosen as the best mixing time.
CS mass fraction investigation
The experimental results are shown in Fig. 1d. When the CS mass fraction was 1%, the particle size and PDI of the nanospheres were the smallest, 3.83 nm and 0.36, respectively. The maximum EE was 99.9%, which was the optimal condition. Therefore, the CS mass fraction was determined to be 1%.
Therefore, based on particle size, PDI, and EE results, the optimal conditions for nanospheres preparation were determined to be: SA solution mass fraction 1.5%, calcium chloride solution mass fraction 4%, stirring time 50 min, and CS solution mass fraction 1%.
Response surface curve experimental results
Through the design in Table 1, we used DesignExpert 13 software to design 29 groups of experiments to discuss the impact of various factors on our evaluation index encapsulation rate. We obtained the encapsulation rate of each factor at different levels (Table 2).
Regression equation fitting and analysis of variance: Quadratic response surface regression analysis was performed on the experimental data using DesignExpert 13 software, and a binary polynomial regression model of four factors on SA-BSA-CS encapsulation ratio (Y) was obtained:
Y = 98.46–0.3833 A-1.04B-1.61 C-0.5108D + 0.79 AB + 1.31 AC + 1.91 AD-0.585BC-0.29BD + 0.1275 CD-2.79 A²−1.42B²−2.57 C²−1.9D². (Notes: A:SA B: Cacl2 C: CS D: Stirring time)
The model showed a nonlinear relationship between the four factors and protein EE and a specific interaction between the independent variables.
The experimental results were analyzed using Design-Expert 13.0.1.0 software, and the results are shown in Table 3. The quadratic response surface regression model demonstrated statistically significant validity (F = 31.29, P < 0.0001), indicating its robust capability in explaining the experimental variance and optimizing the formulation parameters. Upon analyzing the data in the table, we found that the interaction term AC (P < 0.05) had a significant impact on the response value Y (Y: EE of SA-BSA-CS); at the same time, the primary terms B and C and the interaction term AD (P < 0.001) had a very significant impact on Y. In addition, the square terms A², B², C², and D² (P < 0.001) also had a significant impact on Y.
In summary, the order of influence of each factor on the EE of SA-BSA-CS was CS mass fraction (C) > calcium chloride concentration (B) > stirring time (D) > SA mass fraction (A). The P value of the misfit term of the model was greater than 0.05, indicating that the misfit term was not significant, and the coefficient of variation was less than 10%, which indicated that non-experimental factors had little interference with the final result, thus ensuring good experimental stability of the model.
In addition, the R² value of the overall model was 0.9690, indicating that the model had a credibility level of 96.90%. The corrected coefficient of determination Radj² = 0.9381 further proved that the model was accurate, universal, had good credibility, and showed a small experimental error. Therefore, we could use this model to analyze and accurately predict the encapsulation rate of SA-BSA-CS.
Response surface analysis for the interaction of various factors: Design-Expert 13.0.1.0 software was used to build a contour plot of the nanospheres encapsulation ratio and a 3D response surface plot. The results are shown in Fig. 2, which shows the correlation between response and variable levels and the interaction between variables. It can be observed from the figure that the color changed gradually from green to red, which symbolized the change in encapsulation ratio from low to high. Using the response surface contour plot, we were able to intuitively evaluate the impact of different factors on the response values and then determine the best process parameters and the interactions between the parameters.
It can be seen from the analysis of Fig. 2 that the interaction between SA and CaCl2 was relatively significant (P = 0.0210). The EE showed a trend of increasing first and then decreasing with the increase of CaCl2 mass fraction, and the maximum value appeared in the range of 3.5–4% CaCl2 mass fraction and 1.2–1.4% SA mass fraction. The interaction between SA and CS was also significant, and the EE reached the maximum when the CS mass fraction was 0.9–1% and the SA mass fraction was 1.2–1.4% (P = 0.0007). The interaction between SA and stirring time was the most significant (P = 0.0001), which showed that the curve slope was large, and the maximum EE was obtained when the stirring time was 45–50 min and the SA mass fraction was 1.2–1.4%. The three interactions of CaCl2 and CS (P = 0.0748), CaCl2 and stirring time (P = 0.3562), and CS and stirring time (P = 0.6812) had little influence on the EE. All P values were referenced in Table 3. It can also be seen from the figure that the slope of the surface was relatively gentle at this time, implying that the interaction reflected in these figures was weak. The contour plot was used to project the 3D response surface map onto a two-dimensional plane, showing in detail the effects of changes in four factors—three levels of SA, CS, CaCl2, and stirring time—on the EE of specific areas. We observed that the center value of the contour plot was the higher encapsulation ratio that could be produced under the action of two factors, and the contour shape of the contour plot was elliptical, confirming that there was a specific interaction between the two factors (Fig. 2b). The overall result was consistent with Fig. 2a.
A comprehensive analysis showed that the interaction between SA and CS, CaCl2, and stirring time significantly impacted the EE. In contrast, the interaction between CS and CaCl2 and stirring time was not apparent, and the interaction between CaCl2 and stirring time was not apparent. The optimal value of the EE should be within the range of SA solution mass fraction 1.2–1.4%, CaCl2 mass fraction 3.5–4%, CS 0.9–1%, and stirring time 45–50 min. These findings were consistent with the ANOVA results of the response surface in Tables 3 and 4.
The optimization analysis and verification results
The binary multiple regression equation model demonstrated some practical predictability (Table 4). Meanwhile, the perturbation diagram of the encapsulation rate was generated by changing one variable and keeping the other variables constant (Fig. 3a), in which the four-factor curve was steeper, suggesting that all the factors positively influenced the encapsulation rate. Regression simulation analysis was performed by applying Design-Expert 13.0.1.0 software, which resulted in the optimal theoretical prescription for nanospheres encapsulation rate of 1.35% SA, 3.66% CaCl2, 0.93% CS, and 47.34 min of agitation (Fig. 3a). Under this condition, SA-BSA-CS predicted an encapsulation rate of 99.05%. The nanospheres prepared under the optimal prescription had a measured microglobulin encapsulation rate of (98.77 ± 0.35)%, which was close to the expected value. The average particle size of the nanospheres was 21.17 ± 0.41 nm, the PDI was 0.328 ± 0.05, which was normally distributed, and the average Zeta potential was − 1.55 ± 0.31 mV as measured by Malvern laser particle sizing (Fig. 3b; Table 4). The nanospheres were observed to be a regular spherical shape under TEM, with a uniform size consistent with that determined by Malvern laser particle sizing (Fig. 3c). FT-IR analysis revealed in detail the characteristic absorption peaks of SA, CS, and BSA and their corresponding chemical functional groups. Characteristic absorption peaks of SA included: Hydroxy (-OH) absorption peak at 3541.30 cm−1, methyl and carbonyl (C = O) stretching vibration absorption peak at 1620.20 cm−1, carbon-oxygen (C-O) stretching vibration absorption peak at 894.97 cm−1, and ether bond (C-O-C) stretching vibration absorption peak at 1033.84 cm[−1 [34,35; The characteristic absorption peaks of CS appeared at 3502.72 cm−1 hydroxyl (-OH) absorption peak, 1658.78 cm−1 carbonyl (C = O) absorption peak, 1381.03 cm−1 methyl symmetric and asymmetric bending vibration (CH) absorption peak, and 894.97 cm−1 β-glycoside bond vibration absorption peak36,37,38,39; The characteristic absorption peaks of BSA included the 3572.16 cm−1 amino and hydroxyl groups (N-H and O-H) stretching vibration absorption peak, the 1242.15 cm−1 carbon-nitrogen (C-N) stretching vibration absorption peak, and the 1712.78 cm−1 carbonyl group (C = O) stretching vibration absorption peak in the peptide bond band of amide I. In addition, the absorption peak at 1064.70 cm−1 was associated with a carbon-nitrogen (C-N) bond and was often attributed to the stretching vibration of peptide bonds in proteins40,41,42. Through comparing the infrared spectra of SA-CS nanospheres and SA-BSA-CS nanospheres, we observed that the latter had characteristic absorption peaks of BSA at 3572.16 cm−1 and 1712.78 cm−1 and 1242.15 cm−1 and 1064.70 cm−1. However, these absorption peaks did not appear in the infrared spectra of SA-CS nanospheres without BSA coating. These specific absorption peaks indicated that BSA had been successfully coated on the surface of SA-CS nanospheres. Therefore, we can conclude that the presence of BSA in SA-BSA-CS nanospheres was confirmed by detecting their characteristic absorption peaks (Fig. 3d).
In vitro release experimental results
The sustained and stable release of drugs in nanoparticles is a key factor in achieving therapeutic effects. This experiment used the forward dynamic dialysis method to investigate in vitro release. It can be seen from the results that the release rates of free BSA solutions at different pH values (pH 2.0, 7.4, and 9.0) for 48 h were 65.67%, 63.67%, and 79.69%, respectively. It can be seen that it showed an apparent burst release phenomenon during 8–12 h (Fig. 4a). Compared with free BSA, SA-BSA-CS showed a very low release rate in the acidic environment of simulated gastric juice, and the cumulative release rate within 48 h was only 12.73%, showing a relatively significant sustained release effect. The release rate also revealed that the introduction of CS and SA made the prepared nanospheres relatively stable under acidic conditions; in a neutral environment, the release behavior of the nanospheres was little different from that of the free BSA group, and the cumulative release rate within 48 h was 50.58%. In contrast, under alkaline conditions simulating the intestinal environment, the release rate of the nanospheres was the highest. Compared with free BSA, after preparation of the nanospheres, the drug was released faster and more smoothly within 0–15 h, and the cumulative release rate within 48 h reached 79.24% (Fig. 4a).
In oral SA-CS nanosphere drug research, Ritger-Peppas, Weibull, and Higuchi models are essential in evaluating drug release kinetics. The Ritger-Peppas model is specially used to describe the release of oral drugs from polymer matrices, especially for non-Fickian release mechanisms43; the Weibull model is suitable for simulating random events and time dependence during the release process of oral drugs because it can adapt to a variety of nonlinear release curves, and is used to describe the dynamic changes of drug release44; The Higuchi model is based on the pseudo-steady state assumption and is suitable for the release of drugs in a perfect pool45. It describes the relationship between the amount of drug released and the square root of time. This study is suitable for calculating drug release from sustained-release nanoparticles. These models help predict and optimize the release characteristics of oral drugs, mainly to provide an in-depth understanding of the release mechanism of BSA release in this study and maximize the efficacy of the drug delivery system. Our study showed that the release curves of SA-BSA-CS nanospheres at pH 2.0 (Fig. 4b) and 7.4 (Fig. 4c) exhibited an excellent fit with Ritger-Peppas, with r values of 0.988 and 0.978, respectively. However, the release of free BSA under acidic conditions was more in line with the Higuchi model, with an r value of 0.964, and the release of free BSA under neutral conditions was more in line with the Ritger-Peppas model, with an r value of 0.980. The release curve of SA-BSA-CS nanospheres at pH 9.0 (Fig. 4d) showed a high fit with the Weibull distribution function model, with an r value of 0.989, while free BSA was more in line with the Higuchi model, with an r value of 0.967.
Safety evaluation experimental results
Hemolysis experiment
The experimental results showed no significant difference in the hemolysis rates of different concentrations of BSA solutions in the control group, and the hemolysis percentages were all lower than 5%. At the concentrations of 5 µg/mL, 15 µg/mL, and 20 µg/mL, the hemolysis percentages of nanospheres in the experimental group were 0.86%, 0.79%, and 1.10%, respectively, which were also lower than 5% (Fig. 5a). These results demonstrated that the biomaterial components of the nanospheres had minimal destructive effect on red blood cells in the circulation system and had high biological safety.
Cytotoxicity results
Through the CCK-8 cell viability assay, we evaluated the effect of SA-BSA-CS on the proliferation and survival of Caco-2 cells within 24 h and 48 h. The results indicated that SA-BSA-CS had no significant inhibitory effect on the Caco-2 cell line, and cell viability did not decrease significantly with the increase of nanosphere concentration, which further confirmed that SA-BSA-CS was not toxic to cells within the experimental concentration range (Fig. 5b). Cytotoxicity grading was carried out according to the cytotoxicity evaluation standards in the United States Pharmacopeia49, which suggested that the cytotoxicity of SA-BSA-CS was level 1, indicating that SA-BSA-CS provided an experimental basis for the safety of SA-BSA-CS as a drug delivery system in potential clinical applications. It also laid the foundation for subsequent drug release and targeted therapy research.
Live cell staining
The survival rate analysis of the SA-BSA-CS treated Caco-2 cell line was performed using the live cell staining method. The results showed that, compared with the control group, the experimental group did not show significant changes in cell survival rate after SA-BSA-CS treatment and did not affect normal cell proliferation (Fig. 5c), confirming that SA-BSA-CS had little cytotoxicity to cells and demonstrated high biological safety.
Discussion
There were many ways to administer biological drugs in clinical applications, but injection was the primary method. Although injection could deliver the drug directly into the body, long-term injection could bring allergies, infection risks, or other adverse reactions, and patient compliance was poor50. In contrast, oral administration had the advantages of convenience, safety, painlessness, and higher patient compliance, and it could utilize a larger absorption area to improve the treatment effect further51. In particular, the oral colon-targeted drug delivery system ensured that the drug was not released from the upper end of the gastrointestinal tract after ingestion through the mouth but was transported to the colon before it began to swell and release the drug, thereby exerting local or systemic treatment effects52,53.
However, it was difficult to achieve the ideal localized drug release effect for colon-specific drug delivery preparations designed solely using time-lag effects or pH differences. For example, when using a time-lag effect to control drug release in the colon, the type of food had to be considered, and the drug release had to be individualized. Otherwise, the bioavailability of the drug could be affected. When developing pH-dependent drugs, it was necessary to consider that in addition to the low pH value in the stomach, the pH value between the small intestine and the colon was also low. Due to the action of bacteria in the colon and under pathological conditions, the pH value of the colon could be lower than that of the small intestine. Low, so the accuracy of targeted drug release was difficult to grasp.
To meet this challenge, this study optimized SA-CS based on the formation process of electrostatic interaction between anions and cations. This made its structure sensitive to pH change, wrinkling in acidic environments and dissolving in alkaline environments. Thus, it adapted to the pH change of the gastrointestinal tract in its physiological state and released encapsulated drugs into the colon environment54,55,56.
According to ShakibiR’s research, sodium alginate and chitosan could promote wound healing and enhance cell activity57,58, which was consistent with the results of our living cell staining and CCK-8 experiments. The preparation of SA-CS was affected by many factors. In their work in 2013, Liljana Makraduli et al. identified key factors affecting SA-CS, including the concentration of raw materials, the concentration of the cross-linking agent, stirring time, and the pH of the reaction. Zohri M et al. conducted further research on these factors. Generally, they believed that calcium chloride, CS, and SA concentrations were the main factors affecting the performance and EE of nanospheres59,60,61,62. This study verified the above factors through single-factor experiments and response surface methodology and evaluated the EE and particle size. The results were consistent with literature reports, confirming the importance of these factors in the preparation of nanospheres and providing precise process parameters for the preparation of nanospheres. By optimizing these factors, nanospheres’ EE and particle size uniformity could be improved, thereby improving their application potential in biologics delivery.
Conclusions
This study successfully developed a microencapsulation system based on SA and CS to deliver BSA efficiently. The preparation process was optimized through single-factor experiments and response surface methodology, resulting in SA-BSA-CS nanospheres with favorable particle size distribution, stability, and high encapsulation efficiency. The system demonstrated excellent biocompatibility, low hemolytic activity, and the capability to achieve targeted drug release under varying pH conditions, particularly within the colonic environment. These findings suggest that this microencapsulation system offered a simple and effective new strategy for oral colonic-targeted drug delivery, providing a novel direction for the efficient delivery of protein-based biopharmaceuticals in clinical applications.
With the continuous advancement of nanotechnology and biopharmaceutical research, the SA-BSA-CS system held significant potential for broader application in the treatment of various diseases. Although this study focused on oral delivery of protein-based biopharmaceuticals, extending the system to other therapeutic agents—such as nucleic acid drugs (e.g., small interfering RNA, siRNA) and hydrophobic anticancer compounds—could have provided new drug delivery platforms for the treatment of a range of complex diseases. As modern therapeutic modalities, nucleic acid drugs and anticancer agents gradually gained clinical relevance. However, one of the main challenges they faced was the effectiveness and stability of delivery systems. Due to these drugs’ inherent molecular complexity and biodegradability, traditional delivery methods often struggled to overcome biological barriers, resulting in low bioavailability. Therefore, applying the microencapsulation system from this study to nucleic acid and hydrophobic anticancer drugs could have significantly enhanced their stability, targeting capability, and bioavailability in vivo, thus improving their clinical therapeutic efficacy.
In the process of advancing this system toward clinical application, preclinical validation was crucial. While preliminary data on the system’s performance in vitro had been obtained, further research was needed to ensure its effectiveness and safety in vivo. These studies would have encompassed pharmacokinetics, targeting efficiency, drug release kinetics, and long-term toxicity assessments. Specifically, the in vivo behavior of SA-BSA-CS nanospheres—such as their transit through the gastrointestinal tract, drug release patterns, and interactions with biological systems—required validation through animal studies. The results from these experiments would have provided more comprehensive support for the system’s application in humans and laid the groundwork for future clinical trials.
Additionally, considering the complexities of colonic-targeted drug delivery, mainly due to the variability in the gastrointestinal tract’s acidic and alkaline environments, our study presented a solution based on pH-responsive materials to address these challenges. However, in practical application, differences in the gastrointestinal environment—such as inter-individual variability and the influence of food—might have still impacted drug release. Therefore, future research would explore optimizing drug bioavailability through personalized release mechanisms, ensuring that each patient received the maximum therapeutic benefit.
Moreover, with the continuous development of oral drug delivery systems, the preparation process and application scope of SA-BSA-CS nanospheres were expected to expand. By incorporating more functionalized materials and therapeutic agents coupled with precise targeting release mechanisms, our research not only provided a new delivery platform for protein-based biopharmaceuticals but might have also offered insights into the oral delivery of other drugs. For instance, employing multifunctional surface modification techniques could have enhanced the nanospheres’ targeting specificity for specific cell types, thereby improving the therapeutic efficacy of the system.
In conclusion, the SA-BSA-CS microencapsulation system proposed in this study provided a highly efficient, stable, and biocompatible novel carrier for oral colonic-targeted delivery of protein-based biopharmaceuticals. The successful development of this system addressed key challenges in existing drug delivery systems, such as low bioavailability and poor targeting efficiency. It provided a theoretical and experimental foundation for its future clinical application. However, further preclinical studies and clinical validation were necessary to achieve widespread application of this system. Additionally, exploring the potential applications of other drugs within this system would have further enhanced its versatility and efficacy in treating a wide range of diseases.
Data availability
Data Availability Statement:The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
SANDOW, J. et al. Equivalent Recombinant human insulin preparations and their place in Therapy[J]. Eur. Endocrinol. 11 (1), 10–16 (2015).
LIU D. Cancer biomarkers for targeted therapy[J]. Biomark. Res. 7 (1), 25 (2019).
STERGIOPOULOS, S. et al. Adverse drug reaction case safety practices in large biopharmaceutical organizations from 2007 to 2017: an industry Survey[J]. Pharm. Med. 33 (6), 499–510 (2019).
JIN, C. et al. Efficacy and safety of PD-1/PD-L1 and CTLA-4 immune checkpoint inhibitors in colorectal cancer: a meta-analysis[J]. J. Comp. Eff. Res. 11 (3), 203–212 (2022).
FREY, C. Adverse events of PD-1, PD-L1, CTLA-4, and LAG-3 immune checkpoint inhibitors: an analysis of the FDA adverse events Database[J]. Antibodies 13 (3), 59 (2024).
MAKRADULI, L. et al. Factorial design analysis and optimisation of alginate-Ca-chitosan microspheres[J]. J. Microencapsul. 30 (1), 81–92 (2013).
MANNING M et al. Stability of protein pharmaceuticals: recent Advances[J]. Pharm. Res. 41 (7), 1301–1367 (2024).
SZKODNY A C, LEE, K. H. Biopharmaceutical manufacturing: historical perspectives and future Directions[J]. Annual Rev. Chem. Biomol. Eng. 13 (1), 141–165 (2022).
WALSH, G. Biopharmaceutical benchmarks 2022[J]. Nat. Biotechnol. 40 (12), 1722–1760 (2022).
THAPA MAGAR, K. et al. Injectable long-acting formulations (ILAFs) and manufacturing techniques[J]. Expert Opin. Drug Deliv. 21 (6), 881–904 (2024).
WANG, D. et al. Research progress of colon-targeted oral hydrogel system based on natural polysaccharides[J]. Int. J. Pharm. 643, 123222 (2023).
DONG, Q. et al. Exploring β-glucan as a micro-nano system for oral delivery targeted the colon[J]. Int. J. Biol. Macromol. 253 (Pt 6), 127360 (2023).
LIU, J. et al. Nano-Micron combined hydrogel microspheres: novel answer for minimal invasive biomedical Applications[J]. Macromol. Rapid Commun. 45 (11), e2300670 (2024).
WU, X. et al. Microfluidic synthesis of multifunctional Micro-/Nanomaterials from process intensification: structural engineering to high electrochemical energy Storage[J]. ACS Nano. 18 (32), 20957–20979 (2024).
DONG, X. & SHI, L. Chitin/Chitosan nanofibers toward a sustainable future: from hierarchical structural regulation to functionalization Applications[J]. Nano Lett. 24 (39), 12014–12026 (2024).
CARVALHO D N, GONÇALVES, C. et al. Extraction and purification of biopolymers from marine origin sources envisaging their use for biotechnological Applications[J]. Mar. Biotechnol. 26 (6), 1079–1119 (2024).
ANDRADE, D. et al. Nanoencapsulation of Maqui (Aristotelia chilensis) extract in Chitosan–Tripolyphosphate and Chenopodin-Based Systems[J]. Antioxidants 13 (3), 273 (2024).
LI, H. et al. Combining gut microbiota modulation and Enzymatic-Triggered colonic delivery by prebiotic nanoparticles improves mouse colitis Therapy[J]. Biomaterials Res. 28, 62 (2024).
BHIRUD, D., BHATTACHARYA, S. & PRAJAPATI, B. G. Bioengineered carbohydrate polymers for colon-specific drug release: current trends and future prospects[J]. J. Biomedical Mater. Res. Part. A. 112 (11), 1860–1872 (2024).
ARIF, A. et al. Lipase-copper complex/chitosan microspheres; loaded with attapulgite used for the treatment of E. coli-induced diarrhea[J]. Int. J. Biol. Macromol. 277 (Pt 4), 134167 (2024).
GENG, H. et al. Marine polysaccharides: biological activities and applications in drug delivery systems[J]. Carbohydr. Res. 538, 109071 (2024).
GUERRERO, R. & HENG P W S, TUMOLVA, T. P. Preparation of crosslinked Alginate-Cellulose derivative microparticles for protein Delivery[J]. Key Eng. Mater. 931, 69–75 (2022).
HU, Y. et al. Oral delivery of sodium alginate/chitosan bilayer microgels loaded with Lactobacillus rhamnosus GG for targeted therapy of ulcerative colitis[J]. Int. J. Biol. Macromol. 278 (Pt 3), 134785 (2024).
KHORSHIDIAN, N. et al. Chitosan-Coated alginate microcapsules loaded with herbal galactagogue extract: formulation optimization and Characterization[J]. Iran. J. Pharm. Res. 18 (3), 1180–1195 (2019).
WANG, X. et al. Preparation of a Lactobacillus rhamnosus ATCC 7469 microencapsulated-lactulose synbiotic and its effect on equol production[J]. Food Funct. 15 (18), 9471–9487 (2024).
ZHANG, M. et al. Dual targeting total saponins of Pulsatilla of natural polymer crosslinked gel beads with multiple therapeutic effects for ulcerative colitis[J]. Eur. J. Pharm. Biopharm. 199, 114309 (2024).
TANG, Z. et al. Berberine hydrochloride-loaded Dung beetle chitosan/sodium alginate microspheres ameliorate DSS-induced colitis and regulate gut microorganisms in mice[J]. Int. J. Biol. Macromol. 255, 128219 (2024).
NAGPAL, K., SINGH S K & MISHRA D N. Chitosan nanoparticles: a promising system in novel drug delivery[J]. Chem. Pharm. Bull. (Tokyo). 58 (11), 1423–1430 (2010).
SUNDAR, S. & KUNDU, J. KUNDU S C. Biopolymeric nanoparticles[J]. Sci. Technol. Adv. Mater. 11 (1), 14104 (2010).
LIU, S. & FANG, Z. Incorporating inulin and Chitosan in alginate-based microspheres for targeted delivery and release of Quercetin to colon[J]. Food Res. Int. 160, 111749 (2022).
SATHIYASEELAN, A. & ZHANG, X. Enhancing the antioxidant, antibacterial, and wound healing effects of Melaleuca alternifolia oil by microencapsulating it in Chitosan-Sodium alginate Microspheres[J]. Nutrients 15 (6), 1319 (2023).
SHAO, M. et al. Drug nanocarrier, the future of atopic diseases: advanced drug delivery systems and smart management of disease[J]. Colloids Surf., B. 147, 475–491 (2016).
Van, T. R. A. N. V. & LEE, M. O. O. N. J. Liposomes for delivery of antioxidants in cosmeceuticals: challenges and development strategies[J]. J. Controlled Release. 300, 114–140 (2019).
BI Y G, LIN Z T, DENG, S. T. Fabrication and characterization of hydroxyapatite/sodium alginate/chitosan composite microspheres for drug delivery and bone tissue engineering[J]. Mater. Sci. Eng. C Mater. Biol. Appl. 100, 576–583 (2019).
CHEN, J. et al. Preparation of chitosan/nano hydroxyapatite organic-inorganic hybrid microspheres for bone repair[J]. Colloids Surf. B Biointerfaces. 134, 401–407 (2015).
VARMA, R. Extraction, characterization, and antimicrobial activity of Chitosan from horse mussel Modiolus modiolus[J]. ACS Omega. 5 (32), 20224–20230 (2020).
DEVENDRAPANDI, G. et al. Direct sunlight induced room temperature synthesis of anticancer and catalytic silver nanoparticles by shrimp shell waste derived chitosan[J]. Int. J. Biol. Macromol. 252, 126205 (2023).
DRABCZYK, A. et al. Physicochemical investigations of Chitosan-Based hydrogels containing Aloe Vera designed for biomedical Use[J]. Mater. (Basel). 13 (14), 3073 (2020).
MOSTAFA E M, BADR, Y. et al. Reducing the effective dose of Doxycycline using Chitosan silver nanocomposite as a carriers on gram positive and gram-negative bacteria[J]. Sci. Rep. 14 (1), 27819 (2024).
TINCU I C, DARABA O M, J. E. R. O. M. E. C. et al. Albumin-Based hydrogel films covalently Cross-Linked with oxidized Gellan with encapsulated Curcumin for biomedical Applications[J]. Polym. (Basel). 16 (12), 1631 (2024).
DANFENG WANG N L & Y K. Biotin-modified bovine serum albumin nanoparticles as a potential drug delivery system for paclitaxel[J]. Mater. Life Sci. 54, 8613–8626 (2019).
RAGHU SOLANKI K P S P. Bovine serum albumin nanoparticles for the efficient delivery of berberine: preparation, characterization and in vitro biological studies[J]. Colloids Surf., A. 608, 125501 (2021).
SON G H, LEE B J, CHO C, W. Mechanisms of drug release from advanced drug formulations such as polymeric-based drug-delivery systems and lipid nanoparticles[J]. J. Pharm. Invest. 47, 287–296 (2017).
CORSARO, C. et al. Weibull modeling of controlled drug release from Ag-PMA Nanosystems[J]. Polymers 13 (17), 2897 (2021).
PAUL D R. Elaborations on the Higuchi model for drug delivery[J]. Int. J. Pharm. 418 (1), 13–17 (2010).
WANG, C. et al. Preparation and evaluation of chitosan/alginate porous microspheres/bletilla striata polysaccharide composite hemostatic sponges[J]. Carbohydr. Polym. 174, 432–442 (2017).
AHMED N M et al. Picoplatin (II)-loaded Chitosan nanocomposites as effective drug delivery systems: preparation, mechanistic investigation of BSA/5-GMP/GSH binding and biological evaluations[J]. Carbohydr. Res. 545, 109292 (2024).
CHEN, X. et al. Alginate/chitosan-based hemostatic microspheres loaded with doxorubicin liposomes for postoperative local drug delivery in solid tumor[J]. Int. J. Biol. Macromol. 282 (5), 137090 (2024).
PHARMACOPEIA U S. Biological reactivity tests, in vivo[M]. USP 42-NF 37, (2019).
WANG, D. et al. Efficacy, acceptability and side-effects of oral versus long-acting- injectables antipsychotics: systematic review and network meta-analysis[J]. Eur. Neuropsychopharmacol. 83, 11–18 (2024).
CHU, J. N. Foundations of gastrointestinal-based drug delivery and future developments[J]. Nat. Rev. Gastroenterol. Hepatol. 19 (4), 219–238 (2022).
LE, M. Colonic drug delivery: formulating the next generation of colon-targeted therapeutics.[J]. J. Controlled Release: Official J. Controlled Release Soc. 353, 1107–1126 (2023).
T I, R. K. Mucoadhesive carriers for oral drug delivery.[J]. J. Controlled Release: Official J. Controlled Release Soc. 351, 504–559 (2022).
M G, TE A. Polyionic hydrocolloids for the intestinal delivery of protein drugs: alginate and chitosan–a review.[J]. J. Controlled Release: Official J. Controlled Release Soc., 114(1): 1–14. (2006).
SWASTHA, D. & VARSHA, N. Alginate-based drug carrier systems to target inflammatory bowel disease: A review[J]. Int. J. Biol. Macromol. 244, 125472 (2023).
KURL, S. Challenges, opportunities, and future prospects of polysaccharide-based nanoparticles for colon targeting: A comprehensive review[J]. Carbohydr. Polym. Technol. Appl. 6, 100361 (2023).
SANG, F. & LIU, C. Polysaccharide- and protein-based hydrogel dressings that enhance wound healing: A review[J]. Int. J. Biol. Macromol. 280 (Pt 1), 135482 (2024).
SHAKIBI, R. et al. Enhancing cell activities through integration of polyanionic alginate or hyaluronic acid derivatives with triboelectric nanogenerators[J]. Carbohydr. Polym. 346, 122629 (2024).
ZOHRI, M. et al. Response surface methodology for statistical optimization of chitosan/alginate nanoparticles as a vehicle for Recombinant human bone morphogenetic Protein-2 Delivery[J]. Int. J. Nanomed. 15, 8345–8356 (2020).
CASTRO R I, MORALES-QUINTANA L, ALVARADO, N. et al. Design and Optimization of a Self-Assembling Complex Based on Microencapsulated Calcium Alginate and Glutathione (CAG) Using Response Surface Methodology[J]. Polymers (Basel), 13(13): 2080. (2021).
SABBAGH, N., TAHVILDARI, K. & MEHRDAD, S. A. Application of chitosan-alginate bio composite for adsorption of malathion from wastewater: characterization and response surface methodology[J]. J. Contam. Hydrol. 242, 103868 (2021).
PESSOA B, COLLADO-GONZALEZ M, SANDRI, G. et al. Chitosan/Albumin coating factorial optimization of alginate/dextran sulfate cores for oral delivery of Insulin[J]. Mar. Drugs. 21 (3), 179 (2023).
Funding
This work was supported by the National Natural Science Foundation of China (32260192,81760656), the Outstanding Youth Fund of Xinjiang Autonomous Region (2022D01E50), and the Natural Science Fund of Xinjiang Uygur Autonomous Region (2024D01 C90).
Author information
Authors and Affiliations
Contributions
Author Contributions: L.M.:Conceptualization, Software, Writing original draft, Writing reviewand editing.W.Z.: Methodology, Datacuration, Formal analysis.X.M.:Investigation, Validation.X.L: Investigation, Visualization. N.C.:Investigation, Methodology.W.M.:Software, Investigation. Q.Z.:Writing reviewand editing, Software, Resources.X.T.:Methodology, Data curation, Writing original draft, Supervision.X.Z.: Conceptualization, Funding acquisition, Supervision, Project administration.All authors reviewed manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ma, L., Zhou, W., Ma, X. et al. Preparation and property evaluation of oral colon targeted protein delivery system with sodium alginate and chitosan. Sci Rep 15, 21598 (2025). https://doi.org/10.1038/s41598-025-04983-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-04983-5