Abstract
Seaweeds are a valuable source of bioactive molecules widely discussed as having potential to reduce enteric methane (CH4) emissions from livestock. The brown seaweeds, Ascophyllum nodosum (Linnaeus) Le Jolis and Himanthalia elongata (Linnaeus) S. F. Gray are rich in polyphenols, specifically phlorotannins, with known antimicrobial and astringent bioactivities. Brown seaweeds can find use as animal feed additives if issues concerning their palatability and digestibility as well as the impact on protein absorption by the animal are overcome. Fermentation and hydrolysis are traditional processes used for preservation of biomass, but which may beneficially improve palatability and digestibility of feeds. The aim of this work was to assess the potential of fermented and hydrolysed brown seaweeds as agents for CH4 abatement. Ascophyllum nodosum and Himanthalia elongata of Irish origin were fermented and hydrolysed independently with commercially available lactic acid bacteria (LAB) and xylanase enzyme. Molecular weight cut off (MWCO) fractions generated from treated seaweed biomasses were assessed for their antimicrobial activities against the methanogens Methanococcus maripaludis and Methanobrevibacter ruminantium using 96-well plate and well diffusion assays. Batch experiments were carried out using anaerobic conditions at 37 °C for a period of 11 days. The A. nodosum fermentate and H. elongata hydrolysate were characterised for their polyphenol, phlorotannin, peptide, fatty acid methyl ester (FAME) and volatile compound contents using different mass spectrometry methods including GC–MS and LC–MS. Inhibition of M. maripaludis and M. ruminantium was observed in the presence of MWCO extracts generated from the treated seaweeds using the 96-well microtiter plate and well diffusion assays at concentrations of 1 mg/mL. The most antimicrobial A. nodosum MWCO fractions included the polyphenol fractions ≤ 100-kDa & 50-kDa in size, the A. nodosum phlorotannin extract ≤ 100-kDa in size and the H. elongata tannin fraction ≤ 100-kDa in size. 147 and 82 novel peptide sequences were identified in the 3 kDa MWCO fractions generated independently from the A. nodosum fermentate and H. elongata xylanase hydrolysate. Fifty volatile compounds were identified in the A. nodosum fermentate. The H. elongata hydrolysate and A. nodosum fermentate contained significant levels of palmitic acid −1536.37 µg fatty acid/g and 1139.37 µg fatty acid/g lipid, respectively.
Similar content being viewed by others
Introduction
Use of plant derived bioactive molecules in ruminants for health and sustainable food production is a growing research area, especially due to reforms concerning the administration of antibiotics in livestock and a need to produce traditional protein sources like dairy and meat in a sustainable manner. Previously documented benefits of bioactive use in animal feed include reduced protein degradation and increased nitrogen use by ruminants1; reduced emissions including methane (CH4) emissions2; antimicrobial, anti-oxidative, prebiotic and anti-helminthic benefits and increased polyunsaturated fatty acid (PUFA) content of feeds1,3,4. Seaweeds are rich in bioactive compounds including peptides, polyphenols and phlorotannins (found exclusively in brown seaweeds), lectins, alkaloids, PUFAs and prebiotic carbohydrates. Feeds for ruminants such as silage are necessary especially during the winter months or during times of drought when feeds like grass are in short supply1. During silage manufacture, plant proteins are broken-down by plant proteases to soluble nitrogen, amino acids and ammonia (NH3). Soluble nitrogen content increases to between 40–60% of total nitrogen and the pH drops to pH 4. Lactic acid bacteria (LAB) inoculants, chemicals and enzymes are useful during silage production as are plant bioactive compounds such as tannins5. Brown seaweeds including Himanthalia elongata (Linnaeus) S.F. Gray (common name Sea Spaghetti) and Ascophyllum nodosum (Linnaeus) Le Jolis as well as Fucus species are rich in phlorotannins and offer potential for use as feed additives to protect protein, reduce NH3 production and improve nutritive value of end feed formulations if included in the diet at the correct concentrations6,7.
Previously, tannins from terrestrial plants were shown to reduce proteolysis of high protein silage made from alfalfa, moringa and legumes8. Moreover, use of chestnut tannin extracts in moringa and indigofera silages reduced emissions of CH4, albeit in vitro9. Seaweeds have a long history of use as livestock feed especially in regions with harsh climates where fodder can be limited such as Iceland and Norway. However, inclusion of phlorotannin rich seaweeds in the diet of ruminants poses several challenges regarding palatability and digestibility10.
Fermentation and hydrolysis are traditional processes used for preservation of biomass, but which can also beneficially improve palatability and bioactivities of feeds and foods11. Fermentation involves microbial conversion of sugars to acids such as lactic acid, acetic acid and propionic acid or to ethanol, and lactic acid bacteria (LAB) are the most commonly used and characterised microorganisms12. Lactic acid fermentation can alter the nutritional and sensory properties of the product13. In nature, the most prevalent sugars in plant cell walls include xylans, mannans, arabans and galactans which may be hydrolysed by xylanases (EC: 3.2.1.8), mannanases (EC: 3.2.1.78), arabinases (EC: 3.2.1.99) and galacturonases (EC: 3.2.1.15). Xylanase enzymes are approved for use as dietary aids in monogastric animals in the EU 27. Additionally, several commercial xylanase preparations exist including Allzym PT®, Fibrozyme® produced by Alltech (Alltech Inc., USA) and Ecosane® produced by Biotec (Biotec, USA) to upgrade animal feeds14.
Herein, we assess the potential of fermented and hydrolysed seaweeds to inhibit selected methanogens responsible for CH4 production. Irish Ascophyllum nodosum and Himanthalia elongata were selected for use due to their abundance, affordability and reports of their polyphenol/ phlorotannin contents. The protein, ash, fibre, lipid, polyphenol and phlorotannin content of both seaweeds was determined. A. nodosum was fermented with a commercial lactic acid bacteria (LAB) preparation; H. elongata was hydrolysed using a commercially available xylanase enzyme, and the proximate composition of both were compared to raw seaweed biomass. Subsequently, the ability of molecular weight cut off (MWCO) fractions generated from the fermentate and hydrolysate to inhibit methanogenic bacteria was assessed in vitro using a high throughput 96-well microtiter plate assay using the methanogens Methanococcus maripaludis and Methanobrevibacter ruminantium. Methanogens are the dominant species in the rumen and include Methanobrevibacter ruminantium15. These Archaea are difficult to cultivate but once cultured reproducibly can be used to identify CH4 inhibitors. The seaweed hydrolysate and fermentate were further characterised using mass spectrometry and GC–MS to identify bioactive molecules within the fermentate and hydrolysate that could influence palatability and bioactivities of the treated seaweeds.
Materials and methods
Seaweeds used in the study
The brown seaweeds used in this study, Himanthalia elongata (Linnaeus) S.F. Gray (common name Sea Spaghetti) and Ascophyllum nodosum (Linnaeus) Le Jolis were harvested from the Northwest coast of Ireland in November 2020 and were supplied, dried and milled (size of 250-1 mm) by the Irish company SeaLac Limited (Sligo, Ireland). A low temperature drying method described previously16 where conditions do not exceed 28 °C was used to dry harvested biomass. Biomass was harvested once from the locations outlined.
Generation of Ascophyllum nodosum fermentate
Ascophyllum nodosum was fermented in triplicate (n = 3) using a commercially available LAB preparation (Ecosyl TM, Volac, UK) containing the bacterium Lactobacillus plantarum MDT/1. Fermentation was carried out by re-suspending dried, milled (250–1 mm size) A. nodosum supplied by SeaLac Ltd. at a ratio of substrate: ddH2O of 1:2 (w:v). To this mixture, the commercial bacterium mixture was added at a ratio of 24:1 (w:v, seaweed mixture to bacterium). This mixture was maintained at 25 °C for 5 days. The LAB strain used for fermentation was heat deactivated after fermentation at 95 °C for 15 min in a water bath and the fermentate was freeze-dried using an industrial scale freeze dryer, the FD 80 model (Cuddon Engineering, Marl-borough, New Zealand) following in-house drying protocols.
Generation of Himanthalia elongata hydrolysate
The Himanthalia elongata hydrolysate was generated using Xylanase powder (from Aspergillus oryzae > 2500 units/g, Merck, Dublin, Ireland) in triplicate (n = 3). 100 g of dried and milled H. elongata was placed in 1000 mL ddH2O in a bioreactor. The pH of the reaction was maintained at 3.8 and temperature was maintained at 70 °C using 1 M HCl and the temperature probe controller of the bioreactor. The hydrolysates were generated using a New Brunswick (Mason Technologies, Dublin, Ireland) 1.5 L bioreactor with temperature and pH control. Prior to initiating hydrolysis, the temperature of the mixture was adjusted to 70 °C. The reaction was maintained at these conditions for 3 h and subsequently the hydrolysate was heat deactivated by heating the mixture in a water bath to 95 °C for 15 min to deactivate the enzyme. The mixture was cooled to room temperature, frozen at -20 °C and subsequently freeze-dried as described earlier.
Nutritional and bioactive composition – analytical procedures
Elemental analysis was conducted using a LECO CNS928 Macro Analyzer (USA) to determine the carbon, nitrogen, and sulphur content of the seaweeds used in the study. Likewise, a TGM 800 Thermogravimetric Moisture Analyser (LECO Corporation, USA) was used to determine the moisture content following the AOAC Official Methods of Analysis 950.46. The ash content was measured by charring the sample using a Carbolite Muffle Furnace at 550 °C following the AOAC method 942.05.
The Oracle NMR Smart TRAC rapid Fat Analyser (CEM Corporation USA) measured fat content following the AOAC moisture and fat in meat and poultry products, Official Methods 985.14 and 985.26 as described previously17. Prior to the fat content analysis, the samples were pre-dried using a TGM 800 Thermogravimetric Moisture Analyser automated for oven drying.
Protein content was determined (n = 9) following the AOAC nitrogen-based combustion technique (Dumas method), Official method 992.15 (1990)18. Briefly, the protein concentration was measured using a LECO FP628 Protein analyser (LECO Corp., MI, USA) and a nitrogen factor of 5.13 was used to calculate the percentage protein of each sample19.
Total dietary fibre was determined using the Ankom (US) Dietary Fiber Automatic Analyser following the AOAC Total, Soluble, and Insoluble Dietary Fiber in Foods, Official methods 991.43 (1990)20.
Iodine content was measured using a spectrophotometric method (n = 3) and an Iodide colorimetric assay using a kit supplied by Biovision (Biovision, USA) as described previously17. A specific optical density at 330 nm was recorded during the study, wherein the principal relied on the iodide ion as a catalyst converting the yellow coloured substrate to a colourless solution.
Total phenolic content was determined using a spectrophotometric method with the Folin Ciocalteu’s reagent (purchased from Merck, Arklow, Ireland). The polyphenols were extracted following the method by Stankovic21 or Kupina et al.22. Briefly, phloroglucinol or Gallic acid (Merck, Ireland) was dissolved in methanol at different concentrations ~ 1–250 µg/mL to generate a standard curve. The test samples at a concentration of 1 mg/mL, 2.5 mL of 10% Folin-Ciocalteu’s reagent, and 2.5 mL of 7.5% Na2CO3 (Merck, UK) were used for the analysis. After incubation for 45 min at 45 °C, the absorbance was recorded at 765 nm and the results obtained were expressed as phloroglucinol equivalents (PGE eq. per mg of sample) or Gallic acid equivalents (mg GAE/100 g of sample), respectively.
The phlorotannin content of the hydrolysate and fermentate was determined by the method developed by Lopes and colleagues23. Briefly, the phlorotannins were extracted using 100 mL of acetone: water (70:30, v/v) added to 5 g of seaweed. The sample mixture was vortexed at 400 rpm for 1 h. After extraction, acetone remaining in the samples were separated using a rotary vacuum evaporator (Model no. 1000243991; Mason Technology, Buchi, Switzerland). Subsequently, the organic fractions were pooled and dried under nitrogen at 30 °C. A mixture of cellulose and methanol was used to purify phlorotannins. Pigments were removed using toluene. Phlorotannins were released from cellulose using acetone: water (70:30, v/v). The phlorotannin fractions obtained were dried under nitrogen and subsequently freeze-dried. Following hydrolysis and fermentation, different 10-kDa, 50-kDa, 100-kDa seaweed permeate fractions were generated using molecular weight cut-off (MWCO) filters (Merck Millipore, Carrigaline, Co. Cork, Ireland) using a centrifugation procedure described previously24. Permeates were frozen and subsequently freeze-dried as described previously.
Preparation of medium for cultivation of methanogens
The methanogens Methanococcus maripaludis (MM) and Methanobrevibacter ruminantium (MR) were purchased from The Leibniz Institute DSMZ—German culture collection of Microorganisms (Leibniz, Germany). Both the anaerobic Archaea cultures were grown in DSMZ 141 medium but lacked Wolin’s vitamin solution. Weimar and colleagues15 previously made the media for cultivation of methanogens. Briefly, the medium composition was as follows: KCl (0.34 g/L); MgCl2.6H2O (4 g/L); NH4Cl (0.25 g/L); MgSO4.7H2O (3.45 g/L); CaCl2.2H2O (0.14 g/L); K2HPO4 (0.14 g/L); NaCl (18 g/L); Fe(NH4)2(SO4)2.6H2O solution (0.1% w/v) (2 mL/L); CH3COONa (1 g/L); yeast extract (2 g/L); Trypticase peptone (2 g/L); Na Resazurin solution (0.1% w/v) (0.5 mL/L); NaHCO3 (5 g/L); L-Cysteine-HCl.H2O (0.5 g/L); Na2S.9H2O (0.5 g/L); and Modified Wolin’s mineral solution ~ 10 mL/L [Nitrilotriacetic acid (1.5 g/L); MgSO4.7H2O (3 g/L); MnSO4.7H2O (0.5 g/L); NaCl (1 g/L); FeSO4.7H2O (0.1 g/L); CoSO4.7H2O (0.18 g/L); CaCl2.2H2O (0.10 g/L); ZnSO4.7H2O (0.18 g/L); CuSO4.5H2O (0.01 g/L); KAl(SO4)2.12H2O (0.02 g/L); H3BO3 (0.01 g/L); Na2MoO4.2H2O (0.01 g/L); NiCl2.6H2O (0.03 g/L); Na2SeO3.5H2O (0.30 mg/L); Na2WO4.2H2O (0.04 mg/L)]. To prepare the Modified Wolin’s mineral solution, Nitrilotriacetic acid was dissolved first, and the pH was adjusted to 6.5 with KOH. Subsequently, the remaining minerals were added, and the final pH was adjusted to 7.0 with KOH. Once the Modified Wolin’s mineral solution was prepared, the mineral solution was added to the main medium, except for the bicarbonate, L-Cysteine-HCl and sodium sulphide. In order to make the medium anoxic, Nitrogen (N2) gas was used to sparge the mixture for 30–45 min. After the partial removal of dissolved oxygen from the medium, the bicarbonate was added, the final pH was adjusted to 7.0 and the medium were autoclaved. Following sterilization, L-Cysteine-HCl and sodium sulphide from sterile anoxic stock solution was added to the medium inside the anaerobic chamber (gas mix, 4% H2, 5% CO2, and 91% N2) with < 8 ppm O2. Note: L-Cysteine-HCl was filter sterilized using a syringe micro filter (0.2 mm).
For inoculum development both bacteria were transferred to the anoxic freshly prepared medium in plastic cell culture tubes and were incubated inside the anaerobic chamber (Whitley A35 anaerobic workstation, model number: NF237032833, PL ref: 799009 purchased from the UK) at 37 °C. Intermittently, the Optical Density (OD) was measured at 595 nm using a spectrophotometer purchased from Thermofisher scientific (Multiskan FC model number: NF980033586) provided with an inbuilt software (SKANIT software 4.1) package for determining the growth of bacterial cultures.
Microplate assay and seeding techniques (agar solid plate) for screening the methane inhibiting effect of seaweed hydrolysate and fermentate and MWCO fractions
A 96 well microplate assay was used to screen the effect of different seaweed treatments and fractions generated from the same on the ruminant specific methanogenic Archaea ~ Methanococcus maripaludis and Methanobrevibacter ruminantium following the method described by Weimar et al.15. 2-Bromoethanesulfonic acid (BES) was used as the positive control inhibitor and all the experiments were carried out inside an anaerobic chamber maintained at 37 °C. Prior to the experiment, the sample seaweed extracts or fractions were dissolved in distilled water (1 mg/mL). BES was used at a concentration of 1 mg/mL. The microplates and all other required equipment and materials were pre-incubated in the anaerobic chamber to remove traces of oxygen for at least 2–3 days prior to the experiment. Briefly, 100 µL of sample seaweed extracts or fraction was added to the 96 well plate. To this, 100 µL of bacterial cultures (in their exponential phase) were added. The final culture volume in the assay was 200 µL. Inoculum OD values were recorded at 595 nm. For the positive control, 100 µL of BES was added to 100 µL of bacterial cultures. Plates were incubated at 37 °C for 11 days. Similarly, for the negative control, 100 µL of only medium and 100 µL of each bacterial culture was added to individual wells on the plate. After addition of sample extracts and bacterial cultures, a thin plastic film was placed on top of the microplate to avoid spilling of the cultures during OD measurements. Subsequently, the OD values for each well were recorded at 405 nm using a spectrophotometer for time zero (T = 0, day 1) and on the 4, 6, 8 and 11th day post plate set up. The plate was always kept in anaerobic condition and an anoxic atmosphere was maintained using portable boxes preincubated with anaerobic gas packs (AGS AnaeroGen compact bag, Oxoid). The seaweed extracts and controls were tested in triplicate (n = 3).
Additionally, a spread plate technique was carried out (n = 3). Briefly, the bacterial cultures were seeded independently into the prepared medium and were examined for zones of inhibition in accordance with previously published antimicrobial screening assay procedures25. For seeding, 10 mL of bacterial cultures were added to 100 mL agar broth, and this was poured into petri-dishes. Following solidification, small holes were made in the agar plates using sterile borers, approximately 50 µL of each seaweed extracts including the hydrolysate, and fermentate fractions were added independently to the wells. Upon absorption of the extracts, the plates were inverted and incubated inside the same anaerobic chamber at 37 °C for 2–3 days. Following incubation, the appearance of zones of inhibition were observed. BES was used as a positive control.
Identification of secondary metabolites in the seaweed samples using HPLC-Q-TOF mass spectrometry
The Q-TOF Premier mass spectrometer connected to an Alliance 2695 HPLC system (Waters Corporation, Milford, MA, USA) was used for untargeted analysis of metabolites of the seaweed hydrolysate and fermentate following the method of Hossain et al.26 with slight modifications. Briefly, the separation of analytes was performed on an Atlantis T3 C18 column (100 × 2.1 mm, 3 µm) using a binary mobile phase of 0.1% aqueous formic acid (solvent A) and 0.1% formic acid in acetonitrile (solvent B). The temperature of the column was set at 40 °C. A stepwise gradient of solvent B was administered from 10 to 90% at a flow rate of 0.3 mL/min for 25 min. The capillary and cone voltages for the mass spectrometry were set at 3.0 kV and 50 V, respectively, whilst the source temperature and desolvation temperatures were set at 120 °C and 350 °C, respectively. Electrospray ionisation (ESI) mass spectra were recorded for mass scan range m/z 100 – 2,000 in negative ion mode. Waters untargeted (MSe) mode was applied with the low collision energy set at 5 eV, and the high collision energy ramped between 15–40 eV. Accurate masses of the recorded m/z values were achieved through the internal reference compound (i.e. leucine-enkephalin) delivered through lock spray.
Phlorotannin oligomer characterisation
Phlorotannin oligomers were detected by direct infusion of samples into the electrospray ionisation source of the quadrupole time of flight mass spectrometry (Q-ToF–MS) (Waters Corp. Milford, USA) as described previously by Kirke et al.27. This was a non-bias screening method. Mass spectral data were obtained in negative electrospray ion mode (ES −) with mass scan ranging from 300 to 3000 m/z. The capillary and cone voltages were set at 2.6 kV and 35 V, respectively. The desolvation gas was set at 800 L h−1 and the cone gas at 50 L h−1. The samples were infused at 1 μL min−1 for a total run time of 2 min.
The oligomer profile of phlorotannins for each extract was mapped using ultra performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) (Waters Corp. Mil-ford, USA) following the method of Tierney et al.28. The system consisted of a binary pump solvent manager coupled with an Acquity™ TQD-MS (Waters Corp., Milford, USA). It was operated in negative electrospray ion (ES−) mode with multiple reaction monitoring (MRM) method. The MRM method was developed and optimized with a phlorotannin-enriched sample using Intellistart™ software (Waters Corporation) according to molecular masses of phlorotannins containing 3–16 phloroglucinol units (maximum permitted by TQD). A Waters Acquity ™ UPLC HSS PFP column was used (100 Å, 1.8 μm particle size, 2.1 mm × 100 mm) at 40 °C. Mobile phases consisted of distilled water with 0.1% formic acid (A) and acetonitrile with 0.1% formic acid (B). The flow rate was 0.5 mL min−1 and the injection volume set at 1 μL. The elution gradient was set as follows: 0.5% B from zero to 10 min, 0.5–30% from 10 to 26 min, 30–90% B from 26 to 28 min, and 0.5% B from 28 to 30 min. The capillary voltage was set at 2.8 kV. The desolvation gas and cone gas were set at 1200 L h−1 and 50 L h−1, respectively. The source temperature and desolvation temperature were set at 450 °C and 150 °C, respectively, for maximum sensitivity. Methanol blanks were injected between samples to ensure that the peaks observed were in fact those of the sample and not resultant from the retention of compounds on the column and subsequent carry-over from previously analysed samples. Due to the lack of commercial phlorotannin standards, individual molecular weights detected (374–1987 Da) could not be quantified and only the relative abundance was expressed.
Peptide analysis
The hydrolysate and fermentate 10-kDa permeate fractions were processed for MS analysis using the Preomics Phoenix Clean-up Kit (96 ×), (Preomics, D-82152 Planegg/Martinsried, Germany) in accordance with the manufacturers’ instructions and as described previously29. Samples were acidified and hydrophobic and hydrophilic contaminants removed using a series of wash steps and peptides eluted from the cartridge and prepared in loading buffer for LC–MS analysis. Peptides were identified using a mass spectrometer nano ESI-QqTOF (6600 plus TripleTOF, AB SCIEX, Framingham, MA, USA) using liquid chromatography and tandem mass spectrometry (LC–MS/MS). A total of 1 μL of the seaweed permeates were loaded independently onto a trap column (3 µ C18-CL 120 Ᾰ, 350 μM × 0.5 mm; Eksigent, Redwood City, CA, USA) and desalted with 0.1% TFA (trifluoroacetic acid) at 5 µL/min during 5 min. The peptides were then loaded onto an analytical column (3 µ C18-CL 120 Ᾰ, 0.075 × 150 mm) equilibrated in 5% acetonitrile 0.1% FA (formic acid). Elution was carried out with a linear gradient from 7 to 45% B in A for 20 min, where solvent A was 0.1% FA and solvent B was ACN (acetonitrile) with 0.1% FA) at a flow rate of 300 nL/min. The sample was ionized in an electrospray source Optiflow < 1 μL Nano applying 3.0 kV to the spray emitter at 200 °C. Analysis was carried out in a data dependent mode. Survey MS1 scans were acquired from 350 to 1400 m/z for 250 ms. The quadrupole resolution was set to ‘LOW’ for MS2 experiments, which were acquired from 100 to 1500 m/z for 25 ms in ‘high sensitivity’ mode. The following switch criteria were used: charge: 1+ to 4+ ; minimum intensity; 100 counts per second (cps). Up to 50 ions were selected for fragmentation after each survey scan. Dynamic exclusion was set to 15 s. The system sensitivity was controlled by analysing 500 ng of K562 protein extract digest (SCIEX); in these conditions, 2260 proteins were identified (FDR < 1%) in a 45 min gradient. Protein Pilot v 5.0. (SCIEX) default parameters were used to generate peak list directly from 6600 plus Triple TOF wiff files. The Paragon algorithm of ProteinPilot v 5.0 was used to search different databases. Peptides were identified with a confidence of ≥ 95%.
Volatile analysis
SPME analysis - sample preparation
0.5 g of the fermentate was added to a 20 mL screw capped SPME vial and equilibrated to 40 °C for 10 min with pulsed agitation of 5 s at 500 rpm. Sample introduction was accomplished using a Gerstel MPS auto-sampler.
GC–MS analysis for volatiles
GC–MS analysis was carried out as described previously30 in triplicate (n = 3). Briefly, a single 50/30 µm CarboxenTM/divinylbenzene/polydimethylsiloxane (23 Ga DVB/CAR/PDMS, Agilent Technologies Ireland Limited) fibre was used. The SPME fibre was exposed to the headspace above the samples for 20 min at depth of 1 cm at 40 °C. The fibre was retracted and injected into the GC inlet and desorbed for 2 min at 250 °C. Injections were made on a Shimadzu 2010 Plus GC with an Agilent DB-624 UI (60 m × 0.32 mm × 1.8 μm) column using a split/splitless injector in a splitless mode. A merlin microseal was used as the septum. The temperature of the column oven was set at 40 °C, held for 5 min, increased at 5 °C/min to 230 °C, held for 15 min, then increased at 15 °C/min to 260 °C, held for 5 min yielding at total GC run time of 65 min. The carrier gas was helium held at a constant flow of 1.2 ml/min. The detector was a Shimadzu TQ8030 mass spectrometer detector, ran in single quad mode. The ion source temperature was 220 °C and the interface temperature was set at 260 °C. The MS mode was electronic ionization (70v) with the mass range scanned between 35 and 250 amu. Compounds were identified using mass spectra comparisons to the NIST 2014 mass spectral library, a commercial flavour and fragrance library (FFNSC 2, Shimadzu Corporation, Japan) and an in-house library created using authentic compounds with target and qualifier ions and linear retention indices for each compound using Kovats index. Retention indices were matched against peer reviewed publications where possible to confirm compound identification. Spectral deconvolution was also performed to confirm identification of compounds using AMDIS. Batch processing of samples was carried out using MetaMS31. MetaMS is an open-source pipeline for GC–MS-based untargeted metabolomics. An auto tune of the GCMS was carried out prior to the analysis to ensure optimal GCMS performance. A set of external standards: 1-butanol, dimethyl disulphide, butyl acetate and cyclohexanone (Sigma Aldrich Ltd, Arklow, Ireland) at 10 ppm concentration were run at the start and end of the sample set and abundances were compared to known amounts to ensure that both the SPME extraction and MS detection was performing within specification.
FAME analysis
Lipids from the fermentate and hydrolysate were extracted using an ethanol: ethyl acetate mixture (1:1, w/w), as described by Lin and colleagues32. Extracted lipids were then directly converted to fatty acid methyl esters (FAMEs) using boron trichloride in methanol (14%, w/v) without previous derivatization, with slight modifications of the method previously described by Araujo et al.33. Separation and analysis of the FAMEs was done using an Agilent 7890A/5975C GC-MSD system (Agilent Technologies, Santa Clara, CA, USA) equipped with Agilent J&W DB-Fast FAME column (30 m × 0.25 mm, 0.25 µm), (n = 3). A modified method from Agilent Application note 5991-8706EN was used for analysis34. Hydrogen was used as carrier gas in constant pressure mode at 8 PSI and sample injection volume was 1 µL in inlet split mode (25:1). The temperature program of the oven was as follows: 50 °C (0.5 min), then 15 °C/min to 194 °C (4 min) and finally 4 °C/min to 240 °C (1 min). Mass spectra were acquired in scan mode in the 40–550 AMU mass range.
Chromatographic peaks (total ion chromatogram, TIC) were identified by comparison of retention times (RT) and mass spectra with peaks of analysed FAMEs in the standard mixture. Identification was confirmed by searching the generated MS spectra within available spectral database (NIST11) using Agilent ChemStation software. Supelco Fame 37 mix (Sigma Aldrich, Darmstadt, Germany) was utilized for external calibration by making series of appropriate dilutions with hexane, with individual compound peaks used to construct a five-point calibration curve. Each individual FAME was quantified by measuring response (peak area after integration) of a selected quantifier ion from the compound spectrum using ChemStation software. 1 mL of glyceryl-tri-heptadecanoate (Sigma Aldrich, Darmstadt, Germany) in hexane (1 mg/mL, w/v) was added to lipid samples prior to derivatization, to correct for transesterification efficiency and procedural losses. All samples were analysed in triplicates and results expressed as concentration (mg/g) of each fatty acid in extracted fat.
TGA analysis
The A. nodosum fermentate was analysed thermogravimetrically to determine the cellulosic material present in the samples using a Mettler-Toledo GmbH TGA analyser instrument no. B848840113; serial no. 1630/1 (Mason Technology, Switzerland). Approximately 10–15 mg of the sample was placed in a special aluminium crucible and subsequently in the sample holder. The temperature range selected for the assay was 25 to 500 °C with a constant heat rate of 10 °C min−1 and a Nitrogen gas flow rate of 30 mL min−1. Cellulose (Merck, Dublin, Ireland) was used as a standard. Mathematical derivations were calculated using the Star e software (version: V16.00). Analysis was performed in triplicate (n = 3).
Confocal microscopy
A small spatula (~ 10 mg) of the A. nodosum fermentate was placed onto a slide. 100 µL of a stain mixture comprising Nile Red (0.10 g/L in polyethylene glycol 200), Fast green (0.01 g/L in water) and Fluorescent Brightener 28 (0.125 g/L in water) mixed together in a ratio of 3:1:3 respectively, was added to the surface and a coverslip placed on top. The sample was examined with a Leica SP5 confocal scanning laser microscope (Leica Microsystems, Mannheim, Germany) using blue diode (405 nm), argon (488 nm) and HeNe (633 nm) lasers in three channels simultaneously. Cellulosic material stained by the fluorescent brightener is shown in blue. Material within the cell wall is indicated by the red colour (stained by Fast green) and the Nile Red labels trace amounts of lipid a green colour. Sequential images were acquired using triple channel imaging. Emission signals for FB28, Nile Red and Fast Green were sequentially collected using band pass filters 450 – 490 nm; 510 – 550 nm and 650–700 nm, respectively. Digital 8-bit images (512 × 512 pixels) were obtained for each separate excitation wavelength using 10 × air and 63 × oil immersion lenses and combined images were collected.
Statistical analysis
All the experiments were performed in triplicates and the results of each study were expressed as the mean ± standard deviation (SD) of the three independent analysis. Additionally, two way ANOVA and p- values were determined and excel ANOVA packages were used for the analysis. Tukey–Kramer test was also performed using the excel packages.
Results and discussion
Nutritional composition analysis
The A. nodosum fermentate had a moisture, protein, lipid, ash and dietary fibre content of 3.205 ± 0.33, 6.79 ± 0.21, 3.20 ± 0.32, and 27.66 ± 0.56 and 49.16 ± 7.97%, respectively. Amongst other elemental analysis, the carbon, nitrogen, and sulphur percentage (%) values for the fermentate were 32.3 ± 0.14, 1.04 ± 0.035, and 0.59 ± 0.05, respectively. The phlorotannin content in the phenolic extracts (acetone: water, 70:30, v/v) of the fermentate and unprocessed seaweed was determined by UV–vis spectroscopy using the Folin-Ciocalteu (FC) assay. The total phenolic and phlorotannin contents expressed in phloroglucinol (PGE) equivalents per gram of the fermentate and unprocessed seaweed biomass were 4.97 mg PGE/g (raw seaweed) and 0.513 mg PGE/g (fermentate). The phlorotannin content was calculated as 0.6 mg PGE/g of fermentate. The iodine content of the fermentate and raw seaweed were determined as 7.37 ± 0.035 and 6.5 ± 0.08 µg/L. H. elongata hydrolysate had a protein, ash and dietary fibre content of 7.77 ± 0.16, 24.63 ± 0.04 and 45.15 ± 4.30 respectively. The total polyphenol content of the hydrolysate was determined using the method of Kupina et al.22 and expressed as Gallic Acid Equivalents (mg GAE/100 g sample). The total content of polyphenols were 109.5 mg GAE/100 g sample (~ 1.09 mg GAE/ g hydrolysate).
Previously, Biancarosa et al. (2017) studied the protein content of several Norwegian seaweeds and found that the protein content varied widely and in particular the brown seaweeds showed the lowest protein content (Ascophyllum nodosum ~ 4.5% crude protein & 3% true protein; Fucus serratus ~ 4.8% crude protein & 3.9% true protein)35. A. nodosum used in this study had a protein content of 4.85%, however, a slight increase in protein content was observed for the fermentate. The protein content of H. elongata whole seaweed was 4.73% and the xylanase hydrolysate was 7.77%. In this study, the level of iodine observed for A. nodosum and fermented A. nodosum are similar to those reported previously for brown seaweeds such as Fucus vesiculosus, Undaria pinnatifida and Bifurcaria bifurcata36,37,38. These studies recommend that seaweeds could serve as a beneficial source of iodine whilst providing other micronutrients required by the animals and humans. In 2013, the FEEDAP Panel suggested reducing the iodine content in animal diets in order to limit exposure to consumers. Upper tolerable limits (UL) for toddlers were set at 200 µg/day and for adults 600 µg/day39. According to FEEDAP Panel, the reduced recommended iodine contents in complete animal feed are as follows: dairy cows and minor dairy ruminants ~ 2 mg I/kg; laying hens ~ 3 mg I/kg; horses ~ 3 mg I/kg; dogs ~ 4 mg I/kg; cats ~ 5 mg I/kg39. Following the FEEDAP recommendations, the fermented A. nodosum in this study with an iodine content of 0.00737 ± 0.035 mg/Kg ~ 7.37 ± 0.035 µg/L and untreated A. nodosum with an iodine content of 0.00651 ± 0.08 mg/Kg ~ 6.51 ± 0.08 µg/L fall within the recommended iodine limits for ruminants and are suitable for use as animal feed additives. Furthermore, the fibre, proteins and fat content of whole and fermented A. nodosum also indicate their suitability for use as feed additives.
In a recent study Martelli et al. (2020), reported the total phenolic content of H. elongata as 2.94 mg GAEs/g Dry weight40. The total phenolic content of Palmaria palmata and Undaria pinnatifida was found to be 2.45–4.46 and 0.14–0.16 mg GAE/g respectively40. Marteilli also observed that Undaria pinnatifida showed 10 times less phenolic content than the other seaweeds assessed in this study40. In another study, Zhang and colleagues reported total phenolic content of the brown seaweeds Carpophyllum flexuosum, Carpophyllum plumosum and Ecklonia radiata in PGEs % dry weight following the FC method and found that the total phenolic content varied between 15, 10 & 4%41. Additionally, Abdelhamid reported a phlorotannin content of 873.14-µg PGE/g dry matter for the seaweed Cystoseira sedoides collected from Mediterranean (Tunisia) coast42. The geographical location and season of harvest and extraction method have an impact on phlorotannin content of seaweeds.
Determination of methanogen inhibition study using the seaweed extracts
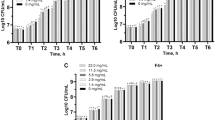
Use of the growth medium containing formate, L-Cysteine-HCl, and sodium sulphide (0.2% w/v) as the reducing agent as developed previously by Weimar et al.15 resulted in fast growth of the selected methanogens in the 96-well microtiter plate assay. Resazurin was used to detect the presence of oxygen and helped to maintain anoxic condition throughout the experiments (Colour change indication is provided in (Supplementary Fig. 1). Batch experiments were carried out in an anaerobic chamber at 37 °C for 11 days and while taking the OD measurements the microtiter plates were carried in portable boxes preincubated with anaerobic Oxoid gas packs. Equal volumes of bacterial cultures, the control BES and seaweed extracts were added to the media and growth conditions described for the methanogens that yielded reproducible growth. M. maripaludis and M. ruminantium inoculated in the 96-well microtiter plate, with just the growth media, had average OD405 values of 0.35 ± 0.034 (M. maripaludis) and 0.28 ± 0.015 (M. ruminantium) on the 8th day post incubation, reaching the stationary phase of growth (Fig. 1a,b). However, in the presence of BES, there was a steady decline in the OD405 values observed with figures less than OD405 of 0.05 (Fig. 1a,b) observed on day 4 post incubation. This indicates inhibition by BES (an analogue of methyl-coenzyme M and a potent inhibitor of methanogens) of the methanogens. These findings correlate with previous work carried out by MacMillan and Weimar43,44. Significant inhibition activity by the H. elongata xylanase hydrolysate and A. nodosum fermentate (supernatant) on M. maripaludis and M. ruminantium was also observed when included in the growth media at concentrations of 1 mg/mL (Fig. 1a). Significant difference between the different types of seaweed extracts on growth inhibition study were observed following a two-way ANOVA, where p < 0.05; p value: 6.88634E-25 (F > F crict ~ 88.59 > 2.003) for M. maripaludis and 1.41748E-26 (F > F crict ~ 108.46 > 2.0034) for M. ruminantum. Additionally, Tukey–Kramer comparison test further confirmed the significant differences between the inhibition activity of the seaweed extracts (Ascophyllum sp. polyphenol, xylanase, tannin, and Himanthalia xylanse) on both the methanogens, wherein the absolute difference value was greater than their critical range (M. maripaludis ~ 0.19 > 0.083, 0.196 > 0.083, 0.192 > 0.083, 0.1924 > 0.083; M. ruminantum ~ 0.169 > 0.064, 2.05 > 0.064, 0.163 > 0.064, 0.17 > 0.064). Furthermore, when these extracts were tested against agar plates seeded with M. maripaludis and M. ruminantium, zones of inhibition were observed. The treated seaweeds that demonstrated positive inhibition activity were passed through MWCO filters to generate different 10-kDa, 50-kDa, and 100-kDa seaweed permeate fractions. These fractions were tested against the methanogens M. maripaludis and M. ruminantium, which were inoculated in 96-well microtiter plates as described previously. Figure 2a,b demonstrate inhibition activity against the methanogens especially for MWCO fractions of A. nodosum polyphenol extract (100 and 50-kDa); A. nodosum tannin 100-kDa; and H. elongata tannin-50-kDa over an 11-day period. From Fig. 2, significant difference between the MWCO fractions on growth inhibition were observed following a two-way ANOVA, where p < 0.05; p value: 0.00234 (F > F crict ~ 4.041 > 2.266) for M. maripaludis and 6.02128E-13 (F > F crict ~ 19.246 > 2.003) for M. ruminantum. Additionally, Tukey–Kramer comparison test further confirmed the significant differences between the inhibition activity of the MWCO fractions on both the methanogens, wherein the absolute difference value was greater than their critical range (M. maripaludis ~ 0.250 > 0.039, 0.063 > 0.039, 0.314 > 0.039, 0.29 > 0.039; M. ruminantum ~ 0.33 > 0.32, 0.4 > 0.32, 0.35 > 0.32, 0.37 > 0.32). A. nodosum fermentate and H. elongata xylanase hydrolysate have potential for use as CH4 inhibitors as an alternative to compounds like bromoform, a known carcinogen. Figure 3a and b demonstrate the inhibitory activity of the seaweed hydrolysate and fermentate enriched fractions using the well diffusion assay method.
Growth of M. maripaludis and M. ruminantum in the presence and absence of the methanogen inhibitor BES or the seaweed-derived fractions added at a concentration of 1 mg/mL (100 µL) observed over a period of 11 days at an absorbance OD405 using a 96 well plate reader. (a) Growth of M. maripaludis reached an OD405 of 0.35 (day 8). In the presence of the tests and inhibitors, growth reached a maximum of 0.05 (day 8). (b) Growth of M. ruminantium reached an OD405 of 0.26 (day 8). In the presence of the tests and inhibitors, growth reached a maximum of 0.05 (day 1). Two-way ANOVA confirmed significant differences, where p < 0.05; p value: 6.88634E-25 (F > F crict ~ 88.59 > 2.003) for M. maripaludis and 1.41748E-26 (F > F crict ~ 108.46 > 2.0034) for M. ruminantum.
Growth of M. maripaludis and M. ruminantum in the presence and absence of the methanogen inhibitor BES or the seaweed derived MWCO fractions added at a concentration of 1 mg/mL (100 µL) observed over a period of 11 days at an absorbance OD405 using a 96 well plate reader. (a) Growth of M. maripaludis reached an OD405 of 0.35 (day 8). In the presence of the tests and inhibitors, growth reached a maximum of 0.03 (day 8). (b) Growth of M. ruminantium reached an OD405 of 0.52 (day 8). In the presence of the tests and inhibitors, growth reached a maximum of 0.02 (day 8). Two-way ANOVA confirmed significant differences, where p < 0.05; p value: 0.00234 (F > F crict ~ 4.041 > 2.266) for M. maripaludis and 6.02128E-13 (F > F crict ~ 19.246 > 2.003) for M. ruminantum.
Peptide analysis
One hundred and forty-seven (147) peptides were identified in the A. nodosum fermentate permeate fraction using mass spectrometry (MS). Peptides with > 99% confidence were subjected to in silico analysis using methods described previously by Hayes et al.29,45. Eighty-two (82) peptides were identified in the H. elongata xylanase hydrolysate permeate fraction. Again, peptides with > 99% confidence were put through in silico analysis procedures. Peptide Ranker (http://distilldeep.ucd.ie/PeptideRanker/, accessed on 11th of December 2023) is an open-source software resource, used to predict the potential bioactivity of peptides based on a novel N-to-1 neural network. It ranks the probability that a peptide sequence will be bioactive. The peptides with amino acid sequences, PMGIWGSCIGLFF (Peptide ranker score 0.96), GSPGGGGGGGGGGGAG (Peptide Ranker score 0.91) and PAFNFPAP (Peptide Ranker score 0.90) derived from H. elongata hydrolysate had the highest probability of being antimicrobial. The peptides MGPPMGPM (Peptide ranker score 0.931315), GAGLGNGLPF (Peptide Ranker score 0.83), GSSFYYGK (Peptide ranker score 0.82) and FGDVLNF (Peptide Ranker score 0.80) were identified in the A. nodosum fermentate. PeptideRanker scores indicate that these peptides are likely to have bioactivity, specifically antimicrobial activity. Acceptable probability values for bioactivity are between 0.5 and 1.0. The potential anti-inflammatory activity of these peptides was also assessed using PreAIP and results are reported in (Table 1).
The novelty of peptides was confirmed by searching the database BIO-PEP-UWM, https://biochemia.uwm.edu.pl/biopep-uwm/, accessed on 10 May 2024, for similar peptides. The peptide sequences found in Table 1 are novel and were not identified in BIOPEP-UWM. Peptide MGPPMGPM derived from the A. nodosum fermentate had the highest Peptide Ranker value (0.9313). Peptide PMGIWGSCIGLFF from the H. elongata hydrolysate has the highest Peptide Ranker value of peptides derived from H. elongata. Both peptide warrant further investigation regarding their antimicrobial activities against these methanogens. Additionally, both peptides are predicted to have anti-inflammatory activities using PrepAIP.
Volatile compound analysis
In total, 50 volatile compounds were identified in the A. nodosum fermentate consisting of acidic oxides (1), amines (1), alkanes (7), alcohols (10), ketones (12), sulphides (1), furans (2), esters (1), chloroalkanes (1), hydrocarbons (2), carboxylic acids (1), disulphides (1), aldehydes (2), alkenes (1), formamides (1), pyrazines (5) and acetamides (1). Ketones dominated the A. nodosum fermentate sample, which contained 11 of the 12 ketone compounds identified. In addition, the compounds 2-pentanone, acetoin, 2,2,6-trimethyl-cyclohexanone, 2-undecanone and dihydro-beta-ionone were present only in the A. nodosum fermentate sample. Acetone and 2-butanone were also found with significant concentrations of acetone present. The A. nodosum fermentate did not contain ethanol and 1-methoxy-2-propanol. Additionally, a number of alcohols including 2-methyl-1-propanol, 3-pentanol, 3-methyl-1-butanol, 3-octanol and 2-cyclobutyl-2-propen-1-ol, were found in this sample. Benezene and 1,3-ditertiarybutylbenzene were also present in the A. nodosum fermentate sample. 2-methyl-furan was also found in the A. nodosum fermentate. It was previously reported that almost no methane was produced in the presence of 5 g/L of the aldehyde hexanal, e-2-hexenal, and nonanal. These were not found in the fermentate. Ester compounds such as methyl butanoate, ethyl butanoate, ethyl hexanoate, and hexyl acetate start to inhibit the digestion process at concentrations of 10 g/L46,47. Again, these were not identified in the samples analyzed.
Fatty acid methyl ester (FAME) analysis
The H. elongata hydrolysate and A. nodosum fermentate contained significant levels of Palmitic acid—1536.37 µg fatty acid/g lipid extract (DW) found in the H. elongata hydrolysate and 1139.37 µg fatty acid/g lipid extract (DW) was found in the A. nodosum fermentate, respectively (Table 2). The content of alpha- Linolenic acid in each sample was 736.28 µg fatty acid/g lipid extract (DW) in the H. elongata extract and 355.05 µg fatty acid/g lipid extract (DW) was found in the A. nodosum fermentate, respectively. Dohme showed that supply of certain fatty acids to ruminant diets has potential to reduce methane (CH4) release48. Fatty acids C12:0, Lauric acid, and C18:3, alpha-Linolenic acid (ALA), were previously shown to have an inhibitory effect on methane production compared with other fatty acids in diets49. CH4 emissions are not affected by total concentration of saturated fatty acid but CH4 emissions are depressed by total concentrations of mono- and poly-unsaturated fatty acids (PUFAs) in diets48. Both products also contained significant quantities of EPA—137.4 µg fatty acid/g lipid extract (DW) for the H. elongata hydrolysate and 112.82 µg fatty acid/g lipid extract (DW) for the A. nodosum fermentate respectively.
HPLC Q-TOF analysis
The molecular weight distribution of phlorotannins found in the different MWCO fractions generated from the H. elongata hydrolysate varied from 3–15 phloroglucinol units (PGUs) equating to between 498–1867.5 Da (Table 3). This composition is in agreement with other studies analysing the phlorotannin profiles of H. elongata50. The most abundant phlorotannins were consistently detected at 3–7 PGUs. Mannitol and glucuronic acid were detected in all MWCO fractions along with unknown compounds corresponding to m/z 819 and 423 (Table 3).
Thermogravimetric (TGA) analysis
The percentage of hemicellulose (percentage by weight) found in the fermented A. nodosum was 4.65 ± 0.35% and cellulose was 18.60 ± 0.40% compared to 4.75 and 41.64% in the dried, milled A. nodosum which was untreated (Fig. 4). No lignin was found in this seaweed which was in agreement with the previously reported study by Olsson et al.51. Additionally, Olsson suggested that the absence of lignin in the cell wall of the seaweeds makes them a favourable feedstock for fermentation in comparison to other land based lignocellulosic biomasses. Thus, the absence of lignin may have supported the fermentation process undertaken in this study that enhanced the methanogen inhibitory activity of the bioactives generated.
Confocal microscopy
From the Fig. 5, it can be noted that the fermented A. nodosum contains large amount of cellulosic material and comparatively lesser fat/lipid & proteins. Due to the auto-fluorescence of the cells, which was quite strong, protein content could not be identified from the microscopic view alone. It was also observed from the proximate analysis that fat and protein content of the fermentate was low, and total fibre was significantly higher which was evident from the microscopic analysis.
Discussion
The phlorotannin content in the phenolic extract (acetone: water, 70:30, v/v) of fermented A. nodosum and dried, milled A. nodosum was determined by UV-vis spectroscopy using the Folin-Ciocalteu (FC) assay. The concentration of phenolics was found to be 4.97 mg PGE/g for whole seaweed. The fermented biomass contained 0.513 mg PEG/g and the phlorotannin content of fermented A. nodosum was determined as 0.6 mg PEG/g of sample. It was reported previously that phenolic content of seaweeds varies depending on geographical location and time of harvest as well as the extraction procedure applied to the biomass. Biomass collected previously during the period January to July for example, was reported to have variable phenolic content (between 1 to 30 PEG/g for Ascophyllum nodosum). The highest phenolic content was reported as 30 PEG/g for A. nodosum harvested in the period February-May. In this study, the seaweed used was harvested in the month of November. Overall, it can be observed that irrespective of these factors A. nodosum does contain phlorotannins in a high proportion with respect to the dry biomass weight.
Peptide Ranker determines the potential of peptides to be antimicrobial by ranking peptides from 0–1. Peptides with ranker scores closer to one are predicted to be antimicrobial. The sequences of peptides with Peptide ranker values close to one and predicted to be antimicrobial are shown in (Table 1). Peptides like LL32, Lpep 19–2.5, and NK2 derivatives of porcine NK lysin have demonstrated activity against methanogenic archaeal strains previously52. Three tested methanoarchaea were found to be sensitive to human cathelicidin of porcine lysin, and a synthetic antilipopolysaccharide peptide (Lpep) with antimicrobial peptide concentrations affecting growth observed at a concentration of 10 μM52. Cathelicidins are linear peptides of between 23–37 amino acid residues in length, similar to the peptides derived from A. nodosum and H. elongata in this study. More recently, a study by Shi and colleagues identified that antimicrobial peptides can improve the growth performance of animals while resisting viruses and harmful bacteria53. The study identified that antimicrobial peptides act on the rumen microbiome and metabolome affecting the performance of castrated bulls positively. However, CH4 production is phenotypically and genetically positively associated with traits important to the profitability of the beef industry such as dry matter intake and live weight. Varnana collated details of the effects of antimicrobial peptides on methanogens, including Archaea recently54.Volatile compounds and aromatics in feed additives and feeds may have an impact on feed intake by sheep, cattle and dairy cows. In a previous study, the palatability of 14 concentrates was assessed in lambs and mature dry ewes. Ewes demonstrated a clear dislike for sulphur compounds, which was thought to negatively influence the palatability of the tested feeds, especially in ewes55. Feed behaviour and performance of lambs is influenced by flavour diversity and lambs are known to like umami55. The predicted umami of peptides identified in Table 1 shows that several peptides might impart umami flavours, which could influence uptake of the fermentate or hydrolysate by sheep. Dairy cows are thought to prefer vanilla or fenugreek flavours when offered these choices. Components influencing flavour include volatiles like monoterpenes, sesquiterpenes, ethers, arenes, phenolics, and lactones. Ketones dominated the A. nodosum fermentate sample, which contained 11 of the 12 ketone compounds identified. In addition, the compounds 2-pentanone, acetoin, 2,2,6-trimethyl-cyclohexanone, 2-undecanone and dihydro-beta-ionone were present only in the A. nodosum fermentate sample.
Fermentation and hydrolysis were used in this study to treat the brown seaweeds Ascophyllum nodosum and Himanthalia elongata, respectively and treated seaweeds were subsequently characterised for their potential to reduce CH4 in vitro. The ability of molecular weight cut off (MWCO) fractions generated from the fermentate and hydrolysate to inhibit methanogenic Archaea was determined using antimicrobial assays incorporating the rumen specific methanogens Methanococcus maripaludis and Methanobrevibacter ruminantium. Inhibition of both methanogens was observed in the presence of polyphenol rich MWCO fractions generated from both treated seaweeds.
Data availability
The datasets form part of a patent application submitted by Teagasc but are available from the corresponding and first authors (M.H. and G. De B.) on reasonable request. Please contact the corresponding author for queries.
References
Niderkorn, V. & Jayanegara, A. Opportunities offered by plant bioactive compounds to improve silage quality, animal health and product quality for sustainable ruminant production: A review. Agronomy 11, 86. https://doi.org/10.3390/agronomy11010086 (2021).
Martin, C. et al. The use of plant bioactive compounds to reduce greenhouse gas emissions from farmed ruminants. In Reducing Greenhouse Gas Emissions from Livestock Production (ed. Baines, R.) (Burleigh Dodds Science Publishing, 2020).
Rizzo, G., Baroni, L. & Lombardo, M. Promising sources of plant-derived polyunsaturated fatty acids: A narrative review. Int. J. Environ. Res. Public Health 20 (3), 1683. https://doi.org/10.3390/ijerph20031683 (2023).
Vaou, N., Stavropoulou, E., Voidarou, C., Tsigalou, C. & Bezirtzoglou, E. Towards advances in medicinal plant antimicrobial activity: A review study on challenges and future perspectives. Microorganisms 10, 2041. https://doi.org/10.3390/microorganisms9102041 (2021).
Lorenz, A. J., Beissinger, T. M., Silva, R. R. & de Leon, R. Selection for silage yield and composition did not affect genomic diversity within the wisconsin quality synthetic maize population. Genes Genomes Genet. 5 (4), 541–549. https://doi.org/10.1534/g3.114.015263 (2015).
Mala, A. et al. Review: Potential of using lactic acid bacteria as inoculant for seaweed silage towards sustainable aquaculture. Aquac. Rep. 28, 101440. https://doi.org/10.1016/j.aqrep.2022.101440 (2023).
Catarino, M. D., Silva, A. M. S. & Cardoso, S. M. Phycochemical constituents and biological activities of Fucus spp.. Mar. Drugs 16, 249. https://doi.org/10.3390/md16080249 (2018).
Aboagye, I. A., Oba, M., Koenig, K. M., Zhao, G. Y. & Beauchemin, K. A. Use of gallic acid and hydrolyzable tannins to reduce methane emission and nitrogen excretion in beef cattle fed a diet containing alfalfa silage. J. Anim. Sci. 97 (5), 2230–2244. https://doi.org/10.1093/jas/skz101 (2019).
Deaville, E. R., Givens, D. I. & Mueller-Harvey, I. Chestnut and Mimosa tannin silages: Effects in sheep differ for apparent digestibility, nitrogen utilization and losses. Anim. Feed Sci. Technol. 157 (3–4), 129–138. https://doi.org/10.1016/j.anifeedsci.2010.02.007 (2010).
Min, B. R. et al. The role of seaweed as a potential dietary supplementation for enteric methane mitigation in ruminants: Challenges and opportunities. Anim. Nutr. 7 (4), 1371–1387. https://doi.org/10.1016/j.aninu.2021.10.003 (2021).
Bruhn, A. et al. Fermentation of sugar kelp (Saccharina latissima)—Effects on sensory properties, and content of minerals and metals. J. Appl. Phycol. 31, 3175–3187. https://doi.org/10.1007/s10811-019-01827-4 (2019).
Caplice, E. & Fitzgerald, G. F. Food fermentation: Role of microorganisms in food production and preservation. Int. J. Food Microbiol. 50 (1–2), 131–149. https://doi.org/10.1016/S0168-1605(99)00082-3 (1999).
Kohajdová, Z. & Karovičová, J. Sensory and chemical evaluation of lactic acid-fermented cabbage-onion juices. Chem. Pap. 59 (1), 55–61 (2005).
Harris, A. D. & Ramalingan, C. Xylanases and its application in food industry: A review. J. Exp. Sci. 1, 7 (2010).
Weimar, M. R. et al. Development of multiwell-plate methods using pure cultures of methanogens to identify new inhibitors for suppressing ruminant methane emissions. Appl. Environ. Microbiol. 83 (15), e00396-e417. https://doi.org/10.1128/AEM.00396-17 (2017).
Krizsan, S. J. et al. Characterization and in vitro assessment of seaweed bioactives with potential to reduce methane production. Front. Anim. sci. 3, 1062324. https://doi.org/10.3389/fanim.2022.1062324 (2022).
Association of Official Analytical (AOAC) Official methods of analysis of AOAC international. (Maryland: AOAC International). (1998).
Association of Official Analytical (AOAC). Official methods of analysis. The association of official analytical chemists. 17th Edn (2000).
Angell, A. R., Leonardo, M., de Nys, R. & Paul, N. A. The protein content of seaweeds: a universal nitrogen to protein conversion factor of five. J. Appl. Phycol. 28, 511–514. https://doi.org/10.1007/s10811-015-0650-1 (2016).
AOAC Method 991.43–1994; Total Dietary Fiber in Foods—Enzymatic-Gravimetric Method MES-TRIS Buffer. Association of Official Analytical Chemists: Rockville, MD, USA. http://www.eoma.aoac.org/methods/info.asp?ID=26991 (1994).
Stankovic, M. Total phenolic content, flavonoid concentration and antioxidant activity of leaves and bark extracts of Celtis australis L.. Int. J. Pharm. Sci. Nanotechnol. 9, 3188–3192. https://doi.org/10.37285/ijpsn.2016.9.2.5 (2011).
Kupina, S., Fields, C., Roman, M. C. & Brunelle, S. L. Determination of total phenolic content using the folin-C assay: Single-laboratory validation, first action 2017. J. AOAC Int. 101 (5), 1466–1472. https://doi.org/10.5740/jaoacint.18-0031 (2018).
Lopes, G. et al. Can phlorotannins purified extracts constitute a novel pharmacological alternative for microbial infections with associated inflammatory conditions?. PLoS ONE 7 (2), e31145. https://doi.org/10.1371/journal.pone.0031145 (2012).
Shannon, E., Conlon, M. & Hayes, M. Seaweed components as potential modulators of the gut microbiota. Mar. Drugs 23 (7), 358. https://doi.org/10.3390/md19070358 (2021).
Morgan, S. M., Hickey, R., Ross, R. P. & Hill, C. Efficient method for the detection of microbially-produced antibacterial substances from food systems. J. Appl. Microbiol. 89 (1), 56–62. https://doi.org/10.1046/j.1365-2672.2000.01081.x (2000).
Hossain, M., Rai, D. K. & Brunton, N. Characterization of phenolic composition in Lamiaceae spices by LC-ESI-MS/ MS. J. Agric. Food Chem. 58 (19), 10576–10581. https://doi.org/10.1021/jf102042g (2010).
Kirke, D. A., Rai, D. K., Smyth, T. J. & Stengel, D. B. An assessment of temporal variation in the low molecular weight phlorotannin profiles in four intertidal brown macroalgae. Algal Res. 41, 101550. https://doi.org/10.1016/j.algal.2019.101550 (2019).
Tierney, M. S. et al. UPLC-MS profiling of low molecular Fucus spiralis. Metabolomics 10, 524–535. https://doi.org/10.1007/s11306-013-0584-z (2014).
Hayes, M., Aluko, R. E., Aurino, E. & Mora, L. Generation of bioactive peptides from Porphyridium sp. and assessment of their potential for use in the prevention of hypertension. Inflamm. Pain. Mar. Drugs 21, 422. https://doi.org/10.3390/md21080422 (2023).
Coughlan, R., Kilcawley, K., Skibinska, I., Moane, S. & Larkin, T. Analysis of volatile organic compounds in Irish rapeseed oils. Curr. Res. Food Sci. 6, 100417. https://doi.org/10.1016/j.crfs.2022.100417 (2023).
van Den Dool, H. & Dec Kratz, P. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. 11, 463–471 (1963).
Wehrens, R., Weingart, G. & Mattivi, F. MetaMS: An open-source pipeline for GC-MS-based untargeted metabolomics. J. Chromatogr. B 966, 109–116. https://doi.org/10.1016/j.jchromb.2014.02.051 (2014).
Lin, H. Z., Guo, Z., Yang, Y., Zheng, W. & Li, Z. J. Effect of dietary probiotics on apparent digestibility coefficients of nutrients of white shrimp Litopenaeus vannamei boone. Aquac. Res. 35 (15), 1441–1447. https://doi.org/10.1111/j.1365-2109.2004.01169.x (2004).
Araujo, P., Nguyen, T. T., Frøyland, L., Wang, J. & Kang, J. X. Evaluation of a rapid method for the quantitative analysis of fatty acids in various matrices. J. Chromatogr. A 1212 (1–2), 106–113. https://doi.org/10.1016/j.chroma.2008.10.006 (2008).
De Bhowmick, G. & Hayes, M. In vitro protein digestibility of selected seaweeds. Foods 11 (3), 289. https://doi.org/10.3390/foods11030289 (2022).
Biancarosa, I. et al. Amino acid composition, protein content, and nitrogen-to-protein conversion factors of 21 seaweed species from Norwegian waters. J. Appl. Phycol. 29, 1001–1009. https://doi.org/10.1007/s10811-016-0984-3 (2017).
Antaya, N. T., Ghelichkhan, M., Pereira, A. B. D., Soder, K. J. & Brito, A. F. Production, milk iodine, and nutrient utilization in Jersey cows supplemented with the brown seaweed Ascophyllum nodosum (kelp meal) during the grazing season. J. Dairy Sci. 102 (9), 8040–8058. https://doi.org/10.3168/jds.2019-16478 (2019).
Soares, C. et al. Mineral composition of subcritical water extracts of Saccorhiza Polyschides, a brown seaweed used as fertilizer in the North of Portugal. J. Mar. Sci. Eng. 8, 244. https://doi.org/10.3390/jmse8040244 (2020).
Milinovic, J., Rodrigues, C., Diniz, M. & Noronha, J. P. Determination of total iodine content in edible seaweeds: Application of inductively coupled plasma-atomic emission spectroscopy. Algal Res. 53, 102149. https://doi.org/10.1016/j.algal.2020.102149 (2021).
European Food Safety Authority (EFSA). Scientific opinion on the safety and efficacy of iodine compounds (E2) as feed additives for all animal species: calcium iodate anhydrous, based on a dossier submitted by Calibre Europe SPRL/BVBA. EFSA J. 11 (2), 3100 (2013).
Martelli, F., Cirlini, M., Lazzi, C., Neviani, E. & Bernini, V. Edible Seaweeds and spirulina extracts for food application: In vitro and in situ evaluation of antimicrobial activity towards foodborne pathogenic bacteria. Foods 9, 1442 (2020).
Rajauria, G., Foley, B. & Abu-Ghannam, N. Characterization of dietary fucoxanthin from Himanthalia elongata brown seaweed. Food Res. Int. 99 (3), 995–1001. https://doi.org/10.1016/j.foodres.2016.09.023 (2017).
Zhang, R. et al. A comparative assessment of the activity and structure of phlorotannins from the brown seaweed Carpophyllum flexuosum. Algal Res. 29, 130–141. https://doi.org/10.1016/j.algal.2017.11.027 (2018).
Abdelhamid, A. et al. Phytochemical analysis and evaluation of the antioxidant, anti-inflammatory, and antinociceptive potential of phlorotannin-rich fractions from three mediterranean brown seaweeds. Mar. Biotechnol. 20, 60–74. https://doi.org/10.1007/s10126-017-9787-z (2018).
McMillan, D. G. et al. A1Ao-ATP synthase of Methanobrevibacter ruminantium couples sodium ions for ATP synthesis under physiological conditions. J. Biol. Chem. 286 (46), 39882–39892. https://doi.org/10.1074/jbc.M111.281675 (2011).
Purcell, D., Packer, M. A. & Hayes, M. Identification of bioactive peptides from a Laminaria digitata protein hydrolysate using in silico and in vitro methods to identify angiotensin-1-converting enzyme (ACE-1) inhibitory peptides. Mar. Drugs 21, 90. https://doi.org/10.3390/md21020090 (2023).
Yanti, H., Wikandari, R., Millati, R., Niklasson, C. & Taherzadeh, M. J. Effect of ester compounds on biogas production: beneficial o detrimental?. Energy. Sci. Eng. 2 (1), 22–30 (2014).
Dohme, F., Machmuller, A., Wasserfallen, A. & Kreuzer, M. Ruminal methanogenesis as influenced by individual fatty acids supplemented to complete ruminant diets. Lett. Appl. Microbiol. 32 (1), 47–51 (2008).
Rasmussen, J. & Harrison, A. The benefits of supplementary fat in feed rations for ruminants with particular focus on reducing levels of methane production. ISRN Vet Sci. 2011, 613172. https://doi.org/10.5402/2011/613172 (2011).
Kirke, A.D. Impacts of natural and induced abiotic factors on phlorotannins in brown algae. University of Galway, PhD Thesis https://aran.library.nuigalway.ie/handle/10379/7154 (2017).
Olsson, J., Toth, G. B. & Albers, E. Biochemical composition of red, green and brown seaweeds on the Swedish west coast. J. Appl. Phycol. 32, 3305–3317. https://doi.org/10.1007/s10811-020-02145-w (2020).
Bang, C. et al. Effects of antimicrobial peptides on methanogenic archaea. Antimicrob. Agents Chemother. 56 (8), 4123–4130 (2012).
Shi, J. et al. Antimicrobial peptides act on the rumen microbiome and metabolome affecting the performance of castrated bulls. J. Anim. Sci. Biotechnol. 14, 31. https://doi.org/10.1186/s40104-023-00832-5 (2023).
Varnava, K. G., Ronimus, R. S. & Sarojini, V. A review on comparative mechanistic studies of antimicrobial peptides against archaea. Biotech. Bioeng. 114 (11), 2457–2473 (2017).
Villalba, J. J., Bach, A. & Ipharraguerre, I. R. Feeding behavior and performance of lambs are influenced by flavor diversity. J. Anim. Sci. 89 (8), 2571–2581. https://doi.org/10.2527/jas.2010-3435 (2011).
Acknowledgements
This research was carried out as part of the SeaSolutions Project funded by the European Research Area on Sustainable Animal Production (ERA-NET) SUSAN and ICT-Agri-2, 2018 Joint Call on Novel Technologies, solutions, and systems to reduce greenhouse gas emissions in animal production systems.
Funding
The project received funding from the European Union’s Horizon 2020 Research and Innovation Programme under grant agreement no. 696231. This research was made possible by funding from the 2018 Joint Call.
Author information
Authors and Affiliations
Contributions
Conceptualization, M.H.; methodology, G. De B., E. S., K. K., D. R. O. O., M.H.; software, G. De B.; validation, M.H., G. De B. and D. K. R.; formal analysis, G. De B.; investigation, M.H.; resources, M.H; data curation, G. De B.; writing—original draft preparation; M. H. and G. De B; writing—review and editing, M.H.; visualization, D.W supervision, M.H.; project administration. M.H.; funding acquisition, M.H. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
De Bhowmick, G., Rai, D.K., Olatunji, O. et al. Development and characterisation of brown seaweed hydrolysates and fermentates with potential to reduce enteric methane. Sci Rep 15, 21445 (2025). https://doi.org/10.1038/s41598-025-05387-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-05387-1