Abstract
Swallowing dysfunction, or dysphagia, is a critical concern among older residents in long-term care facilities because it contributes to malnutrition and increased frailty. Oral hypofunction, characterized by a decline in oral function, is recognized as a precursor to oral dysfunction. This study investigated the prevalence of oral hypofunction and assessed the effectiveness of oral health education and oral exercises in improving oral function. A total of 295 participants from seven long-term care facilities in Taiwan were recruited for this study, and they underwent comprehensive assessments. The key domains assessed were oral health, swallowing function, nutritional status, and frailty. The interventions included oral hygiene education and tongue–lip exercises, which were administered over a 6-month period. Outcomes such as swallowing function, oral health, tongue pressure, tongue–lip motor function, nutritional status, grip strength, and cheek bulging were measured at three time points. The prevalence of oral hypofunction in the participants was 58.3%. The intervention led to significant improvements in swallowing function 6 months later and in oral hygiene and tongue–lip motor function 3 months later in the participants with oral hypofunction. Additionally, in the oral hypofunction group, nutritional status and cheek bulging function were notably improved after 6 months. Oral hygiene education and oral exercises were demonstrated to effectively enhance swallowing function, oral hygiene, and tongue–lip motor function in older residents with oral hypofunction, thereby improving their nutritional status. Early identification and intervention are crucial for improving oral function in long-term care settings.
Similar content being viewed by others
Introduction
Swallowing dysfunction, commonly referred to as dysphagia, is characterized by difficulty or discomfort in swallowing. This condition impairs the movement of food, liquids, and saliva from the mouth, through the throat and esophagus, and into the stomach. Dysphagia can manifest at any stage of the swallowing process, including the oral, pharyngeal, or esophageal stage1. In older adults, the prevalence of swallowing dysfunction varies widely, ranging from 11 to 80%2. Dysphagia is associated with adverse outcomes, including poor nutritional status, compromised oral health, and increased frailty, all of which negatively affect daily functioning3. The concept of oral hypofunction, as defined by the Japanese Society of Gerodontology (JSG), refers to an early stage of oral dysfunction that can be evaluated and managed to prevent progression to more severe forms of oral dysfunction4. Understanding the impact of preventive interventions targeting oral hypofunction is crucial because early detection and management may mitigate further deterioration.
Oral hypofunction
Oral hypofunction refers to the decline of multiple aspects of an individual’s oral function while they remain otherwise relatively healthy. This condition is increasingly recognized as a precursor to systemic frailty5. Addressing oral hypofunction offers the opportunity to intervene before they progress to severe oral dysfunction, which can lead to severe frailty and disability. The JSG defines oral hypofunction based on seven diagnostic criteria, including poor oral hygiene, oral dryness, reduced occlusal force, decreased tongue–lip motor function, reduced tongue pressure, reduced masticatory function, and impaired swallowing function. Based on their previous study6, Minakuchi et al. established that impairment in three or more out of five specific criteria—oral hygiene, oral dryness, number of natural teeth, tongue pressure, and tongue–lip motor function—effectively identifies a preclinical stage of oral dysfunction, thereby facilitating the early detection of declining oral function4. The prevalence of oral hypofunction in older individuals within Japanese communities ranges from 43.6 to 60%7,8. In a study conducted in Spain among institutionalized older adults, the prevalence of oral hypofunction was found to be 71.5%, indicating a strong association between oral functional decline and physical frailty in this population9. In a South Korean study, the prevalence of oral hypofunction symptoms among older adults was reported to increase significantly with age10. The oral hypofunction is increasingly recognized as a global health issue, particularly in aging populations.
Oral hypofunction in long-term care facilities
The prevalence of oral dysfunction among older individuals in long-term care facilities is generally higher than that in community-dwelling older populations11,12,13. This higher risk is likely due to factors such as frailty, the presence of comorbidities, and limited access to comprehensive dental care. Old patient with oral dysfunction, such as dysphagia, could experience nutritional deficiencies, functional impairments, and social and emotional challenges in these setting14. Consequently, early detection of oral hypofunction is as crucial in long-term care facilities as it is in community settings to provide timely intervention. In a study considering Eating Assessment Tool-10 (EAT-10) scores, Minakuchi reported a higher prevalence of oral hypofunction in long-term care facilities (53.8%) compared with community settings (24.1%)4. Furthermore, Cruz-Moreira et al. reported that 71% of older residents in Japanese care homes had oral hypofunction, as defined by Minakuchi’s criteria9. These findings suggest that oral hypofunction is more prevalent in long-term care facilities in Japan than in the broader community.
Intervention for oral hypofunction
Therapeutic interventions for oral dysfunction—such as tongue and pharyngeal muscle strengthening exercises, neuromuscular electrical stimulation (NMES), and compensatory swallowing techniques (e.g., the chin tuck and head turn)—are recommended to improve swallowing function15. To detect earlier stages of oral dysfunction, such as oral hypofunction, for timely intervention is recommended to enhance quality of life5,16.
Shirobe et al. demonstrated that preparatory oral exercises, mouth-opening training, tongue pressure training, prosodic training, and masticatory training improved the tongue–lip motor function and tongue pressure of 51 older individuals with oral frailty who lived in a community17. Furthermore, Onuki et al. demonstrated that oral health guidance and dental treatment improved oral hygiene, occlusal force, tongue–lip motor function (e.g., /pa/ repetition), mastication, and swallowing in 42 older outpatients with oral hypofunction18. These studies support the effectiveness of early intervention in improving swallowing function, which is essential for nutrition and muscle maintenance, particularly among older populations in Japan.
Correlates of oral hypofunction
Oral hypofunction has been associated with protein intake and malnutrition in community-dwelling individuals19,20 as well as with frailty9. Although the causal relationships between oral hypofunction, malnutrition, and frailty remain a subject of debate, their close association suggests that improving oral hypofunction could enhance oral intake, thereby positively influencing nutritional status and muscle strength.
Based on the literature review, we hypothesized that oral hypofunction is prevalent among older residents in long-term care facilities. As suggested by Minakuchi et al., interventions such as oral exercises and oral hygiene education can improve oral function. We also hypothesized these interventions can positively influence the correlates of oral hypofunction, such as nutritional status and frailty. Therefore, the objectives of this study were to investigate (1) the prevalence of oral hypofunction among residents of long-term care facilities in Taiwan and (2) the effect of oral health education and oral exercise on oral hypofunction and its associated outcomes.
Methods
This study, supported by the National Health Research Institutes of Taiwan, employed purposive sampling to recruit participants from seven long-term care facilities across Taiwan. The participating institutions were Baihe Veterans Home, Zhongzhang Veterans Home, Penghu Home for the Elderly, Jiali Veterans Home, Tianzhong Veterans Home, Taitung Canaan Nursing Home, and the Qijin Hospital Affiliate long-term Care Facility. Informed consent was obtained from all participants before their involvement in the study. All assessments and interventions in this study were performed in accordance with the Declaration of Helsinki. The study protocol was approved by the Institutional Review Board of Kaohsiung Medical University Hospital [KMUHIRB-SV(I)-20210041].
Participants
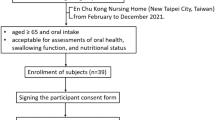
The study enrolled individuals with tongue mobility, full consciousness, the ability to express their thoughts and feelings in Mandarin or Taiwanese clearly, and the ability to follow various assessment instructions. The exclusion criteria were mental illness, unstable vital signs or medical conditions, or impaired consciousness. The required sample size was determined for a repeated measures design comparing two groups, assuming a two-tailed significance level of 0.05, a statistical power of 0.80, and a moderate effect size (Cohen’s d = 0.5), as demonstrated by previous literature of oral exercise21. After accounting for an anticipated 30% dropout rate, the total sample size necessary to maintain adequate statistical power was estimated to be at least 182 participants. A total of 373 participants were enrolled in the initial evaluation as considered the estimated conservative effect from oral exercise; 318 participants completed the second evaluation 3 months later, whereas 312 completed the third evaluation an additional 3 months later. The enrollment and dropout of the participants were detailed in Fig. 1. Of these, 295 participants completed all required assessments and received the interventions during the three evaluation periods, which were between December 2021 and September 2023.
Intervention design
Prior to implementation of the intervention in the first evaluation, standardized predeparture training was conducted for research staffs. Data collection, including questionnaire administration and oral examinations, was conducted from December 2021 to September 2023. In the first evaluation, the intervention administered to all the participants addressed oral hygiene and oral exercise. The participants were then re-evaluated after 3 months and again after 6 months.
The following interventions were administered to residents of the long-term care facilities:
-
(A)
Teaching of Oral Cleaning Methods: The participants received instruction on proper oral cleaning techniques, and caregivers and nursing staff were trained to assist with daily oral hygiene checks. Then, the caregivers or nursing staff checked the participants’ oral hygiene every day.
-
(B)
Increasing Oral Cleaning Frequency: Participants who previously brushed their teeth only once in the morning had their frequency increased to include an additional cleaning session before bedtime every day.
-
(C)
Daily Oral Exercises or Bedside Speech Rehabilitation Exercises: Residents performed lip and tongue exercises thrice daily (Fig. 2). The lip exercises were the following: 1) firmly closing the lips and making a “pop” sound; 2) smiling in a horizontal line shape; 3) puckering the lips into a “woo"” shape; and 4) opening the mouth in an “ah” shape. The tongue exercises were the following: 1) extending the tongue as far forward as possible; 2) moving the tongue to the right (from the resident’s perspective); 3) moving the tongue to the left (from the resident’s perspective); and 4) sticking out the tongue to touch thee philtrum.
Measures in every evaluation
Demographic variables and body mass index (BMI) were recorded for all participants. Additionally, the participants were assessed for indicators of oral hypofunction and its associated factors, such as nutritional status and frailty. Key measurements such as calf circumference, height, and weight were recorded as relevant variables associated with oral hypofunction.
Indicators of oral hypofunction
This study evaluated five of seven criteria of oral hypofunction defined by Minakuchi et al.4 : swallowing function, oral hygiene, oral dryness, tongue–lip motor function, and tongue pressure. The exclusion of occlusal force and masticatory function was primarily due to practical limitations in the long-term care settings where our study was conducted. Specialized instruments required for these assessments, such as pressure-indicating films and masticatory testing systems, are not routinely available in most long-term care facilities in Taiwan. Given these resource constraints, we adopted a modified approach based on the core functional indicators that could be reliably and feasibly evaluated in institutional environments. Minakuchi et al. have suggested 3 or more in 5 criteria (oral hygiene, oral dryness, number of natural teeth, tongue pressure, and tongue–lip motor function) to identify oral hypofunction4,9. The participants exhibiting impairments in three or more of the five assessed domains were classified as having oral hypofunction, whereas those with fewer impairments were designated as controls in this study.
Eating assessment tool-10
The EAT-10 is a dysphagia screening instrument developed by Belafsky et al. in 200822. It comprises a 10-item self-assessment scale, and each item is scored in terms of five levels of difficulty, ranging from no problem to serious problem. The total score ranged from 0 to 40, with higher scores indicating greater levels of dysphagia.
Tongue coating index
The Tongue Coating Index (TCI) was developed by Shimizu et al.23 and comprises six items. Each item is rated on a scale from 0 to 2, where 0 indicates no coating, 1 indicates thin coating, and 2 indicates thick coating. The total score ranges from 0 to 12. Following the recommendations of Minakuchi et al., the participants with a TCI score of ≥ 9 (≥ 50%) were classified into the impaired group.
Oral health assessment tool
The Oral Health Assessment Tool (OHAT) was developed by Chalmers et al. in 200524. In this study, item 4—which assesses saliva through the question “Is the individual’s mouth dry?”—was used to assess oral dryness. A score of ≥ 1 suggests that the individual should seek consultation with an oral health care professional24.
Tongue pressure
The maximal tongue pressure is defined as the force exerted by the tongue bulb when the tongue is elevated and pressed against the hard palate. In this study, the IOPI Pro (IOPI Medical, WA, USA)25, was employed to assess tongue pressure. This test was conducted three times, with a 30-s rest period between each trial, and the highest recorded value was used to represent the participant’s maximal tongue pressure. A tongue pressure value of < 30 kPa, the median, indicated poor tongue strength25,26.
Tongue–lip motor function
The diadochokinetic (DDK) test was conducted to evaluate tongue–lip function. This test requires participants to rhythmically repeat a consonant–vowel sequence (e.g., PA-TA-KA) that engages different places of articulation: bilabial, alveolar, and velar. This assessment typically comprises three items, each corresponding to one syllable: “pa,” “ta,” and “ka.” The total scores range from 0 to a practical maximum of approximately 30 repetitions in 5 s.
The factors associated with oral hypofunction—including nutritional status, frailty, grip strength, and daily function—were assessed to evaluate the effects of the intervention.
Mini nutritional asessment—short form
The Mini Nutritional Assessment–Short Form (MNA-SF) is a validated tool commonly used in clinical and research settings to rapidly assess the nutritional status of older patients and identify those at risk of malnutrition27.
The study of osteoporotic fracture method for frailty assessment
The Study of Osteoporotic Fractures (SOF) method is a tool widely used for assessing frailty in older adults and helps identify individuals at risk of adverse health outcomes. Participants who exhibit none of three components are classified as robust, whereas those demonstrating one component are considered to be in an intermediate stage of frailty28.
Grip strength
The grip strength of each participant’s dominant hand was measured using an electronic dynamometer. Their maximal grip strength was measured, and the highest value recorded from three trials, each separated by a 1-min interval, was used in the analysis.
Cheek bulging
The strength, symmetry, and control of the participants’ buccinator muscles were assessed by instructing the participants to puff out their cheeks. A score of 3 was assigned if the patient could puff out both cheeks normally, 2 if they could do so only partially (such as weakly or asymmetrically), and 1 if they were unable.
Statistical analysis
Data were analyzed using SPSS v26. The percentage of participants with each score for oral hypofunction was calculated. An independent t test was performed to assess differences in age, body mass index, nutritional status, frailty, grip strength, calf circumference, cheek bulging, and activities of daily living between the participants with and without oral dysfunction. The generalized estimating equation (GEE) model was then employed to examine the effect of the intervention (the secondary or third evaluation versus first evaluation) and the effect of oral hypofunction on the scores of various variables. The dependent variables were indicators of oral hypofunction, such as swallowing dysfunction (assessed using the EAT-10), tongue pressure, tongue–lip motor function (evaluated through the DDK test with the syllables “pa,” “ta,” and “ka”), and oral hygiene (measured using the TCI). Additionally, associative factors of oral hypofunction—such as nutritional status (assessed using the MNIA-SF), frailty (assessed by grip strength), and cheek bulging—were included in the analysis. The effect of the interaction between oral hypofunction and intervention effect was calculated first. If it was nonsignificant, the interaction term was removed from the model. P < 0.05 was considered significant in all analyses.
Results
Participants characteristics
A total of 295 long-term care facility residents (mean age: 83.44 ± 10.59 years; range: 51–102 years) were enrolled in the study. Among them, 75.6% were male. The prevalence of oral hypofunction, defined as having three or more impaired oral functions, was 58.3% (Table 1). The most commonly impaired functions were tongue-lip motor function (97.3%), tongue pressure (65.4%), and oral hygiene (53.9%), while oral dryness was the least prevalent (24.1%). Distribution of the number of impaired oral functions was as follows: 11.2% had one impairment, 30.5% had two, 35.6% had three, 17.3% had four, and 5.4% had five.
Oral hypofunction
Table 2 compares residents with (n = 155) and without (n = 140) oral hypofunction. The results of an independent t-test revealed a significant difference in BMI, ability to puff out their cheeks, grip strength, calf circumference, and frailty between the oral hypofunction group and controls. These results support that participants with oral hypofunction have lower nutrition status, impaired oral function, and vulnerability to frailty. Furthermore, the participants with oral hypofunction exhibited significant differences in swallowing dysfunction, oral hygiene, tongue–lip function, and tongue pressure compared to controls. As predicted, those with oral hypofunction have lower performance in these areas, which indicates oral hypofunction.
Effects of the intervention on indicators of oral hypofunction
The GEE model revealed no significant effect of the interaction between oral hypofunction status and the intervention on the scores for swallowing dysfunction, oral hygiene, tongue pressure, or tongue–lip motor function. Therefore, the main effects of the intervention and oral hypofunction were included in the model, whereas the interaction term was excluded. The results in Table 3 revealed a significant difference in swallowing dysfunction (reduced EAT-10 scores) at the third evaluation compared with the first evaluation after controlling for gender, age, and oral hypofunction (Table 3). When stratifying the analysis by oral hypofunction status, the GEE model demonstrated that the oral hypofunction group and the control group experienced an alteration in swallowing dysfunction after 6 months of the intervention (Tables 4 and 5; Fig. 3A). These results indicated that both participants had oral hypofunction or did not have improved swallowing function during the oral exercise intervention course.
The GEE analysis also revealed a significant alteration in oral hygiene, as measured using the TCI. Oral hygiene scores were more favorable at the second and third evaluations than at the first (Table 3). A further analysis stratified by oral hypofunction status revealed that oral hygiene was altered 3 months after the intervention in the oral hypofunction group (Table 4; Fig. 3B) and 6 months after the intervention in the control group (Table 5; Fig. 3B). These results indicated that oral hygiene improved during the early intervention stage in the oral hypofunction group and during the later stage in controls.
Tongue pressure was significantly altered at the third evaluation, 6 months later, compared with the first evaluation (Table 3). A separate analysis revealed that tongue pressure was altered 6 months after the intervention only in the controls (Table 5; Fig. 3C). These results indicated that tongue function deteriorated during the intervention course among controls.
Lastly, the GEE analysis indicated no significant change in tongue–lip motor function. However, a separate analysis demonstrated that tongue–lip motor function had altered three months after the intervention in the oral hypofunction group (Table 4; Fig. 3D). This indicates that the integrated function of tongue-lip improved during the early stage of the intervention course among participants with oral hypofunction.
Effects of intervention on correlates of oral hypofunction
The GEE model revealed no significant effect of the interaction between oral hypofunction and intervention (Visit 3 vs. 1 or Visit 2 vs. 1) on the scores for nutritional status (Fig. 3E), grip strength (Fig. 3F), or cheek bulging (Fig. 3G). Therefore, we included the main effects of intervention and oral hypofunction in the model, but not their interaction term. Nutritional status, as assessed using the MNA, was significantly altered at the second and third evaluations compared with the first evaluation, after adjustments were made for gender, age, and oral hypofunction(Table 3). A separate analysis demonstrated that both the oral hypofunction group and controls exhibited alteration in nutritional status at 3 and 6 months after the intervention (Tables 4 and 5, and Fig. 3E). These results suggested that the nutritional status improved during the intervention course among participants with or without oral hypofunction.
The analysis revealed a significant alteration in cheek bulging at the third evaluation compared with the first evaluation, when controlling for gender, age, and oral hypofunction (Table 3). A separate analysis demonstrated that the oral hypofunction group exhibited alterations in cheek bulging (Table 4; Fig. 3G) 6 months after the intervention. Still, the control group did not show any significant change. These results indicated that the cheek bulging function increased later in the intervention course among participants with oral hypofunction.
Discussion
Oral hypofunction
Studies have demonstrated that the prevalence of oral hypofunction among the older population in community settings in Japan ranges from 43.6 to 60% based on varied definitions7,8. In the present study, 58.3% of the enrolled residents of long-term care facilities had oral hypofunction. This prevalence is consistent with Minakuchi’s prediction of approximately 53.8% in long-term care facilities, derived from earlier data using the EAT-104. By contrast, Cruz-Moreira et al. reported a high prevalence of oral hypofunction, at 71%, among older individuals residing in care homes based on three or more of the 5 criteria for oral hypofunction9. Our assessment did not address occlusion force or masticatory function, whereas Cruz-Moreira et al.’s study excluded tongue pressure and tongue–lip function from their evaluation. This difference in assessment criteria may contribute to the observed differences in the prevalence of oral hypofunction. Modifications to the assessment criteria should be considered to facilitate more convenient assessments, potentially incorporating physical examinations or simple assessment tools. However, both studies underscore the elevated prevalence of oral hypofunction in long-term care facilities. Establishing a simple method for routine evaluation of oral hypofunction could enable timely intervention and improve the oral health outcomes of facility residents.
Effect of the intervention on the index of oral hypofunction: promoting oral hygiene and lip and tongue exercise
The intervention implemented in this study included educational initiatives aimed at enhancing oral hygiene practices and encouraging lip and tongue exercises. The GEE analysis revealed positive effects of the intervention on swallowing function, oral hygiene, and tongue pressure among the participants. Notably, further analysis indicated significant improvements in swallowing function at 6 months after the intervention in the oral hypofunction group. Additionally, significant short-term effects on oral hygiene and tongue–lip function were found as early as 3 months after the intervention. A study demonstrated that oral health education and dental treatment led to improvements in oral hygiene, occlusal force, tongue–lip motor function, mastication, and swallowing within a 6-month timeframe in 86 patients with oral hypofunction within an outpatient setting18. This study suggested that interventions targeting oral health and dental function not only improve oral hygiene but also oral muscle function. Our intervention, which combined oral health education with exercises for the tongue and lips, was found to positively affect both oral hygiene and swallowing function. These findings are consistent with those of Matsuo et al., who reported that a 12-week regimen of oral exercise and a textured diet reduced the prevalence of oral hypofunction in a community from 56 to 26%29.
The principles of experience-dependent neural plasticity indicate that repetition, intensity, and specificity of practice are crucial for leveraging neuroplasticity to facilitate functional recovery30. Thus, the present study instructed the participants to perform the lip and tongue exercises three times a day. This approach capitalizes on repeated activation of neuron synapses, which can strengthen the connections between neurons—an essential process for learning and developing efficient circuits, as suggested by Hebbian plasticity theory31. Thus, the neurons involved in swallowing could be activated and interconnected effectively through repeated practice of the lip and tongue exercises. Our findings regarding the improvement in swallowing function are in line with the concept of task-specific training, which induces plastic changes in the motor cortex to support recovery32.
Our study did not reveal a significant intervention effect on tongue pressure in the oral hypofunction group. Additionally, the improvements in tongue–lip function were only short-term improvements. By contrast, the study conducted by Matsuo demonstrated significant effects of a similar exercise on tongue pressure and tongue–lip motor function. One potential explanation for our finding is the higher mean age (84.00 years) of the participants in our study compared with those of Matsuo’s study (76.7 years). These results suggest that interventions should be initiated as early as possible. Furthermore, Matsuo’s study incorporated a textured lunch box, which may have facilitated direct training regarding the swallowing process. This approach aligns with the principle of task-specific training, which indicates that the exercises most similar to the swallowing process are most effective in enhancing swallowing function.
Conversely, the intervention significantly improved cheek bulging function 6 months after the intervention. The tongue is primarily controlled by the hypoglossal nerve (cranial nerve XII), whereas the facial nerve controls the cheek and lip muscles. The four lip exercises employed in this study simultaneously engage the cheek muscles. The movement involved in cheek bulging is relatively simple and similar to the exercise of “popping”. This targeted training effect may account for the observed improvement in cheek bulging following 6 months of exercise training. Furthermore, the tongue plays critical roles in shaping sounds during speech and in coordinating swallowing. These functions require precise control and fine motor movements, characterized by high complexity and flexibility33. Effective coordination of the tongue with other upper airway muscles is essential for functional speech and swallowing. Fine motor control of the tongue is heavily reliant on cortical regulation34. Given the intricate neural circuitry involved, achieving a sustained training effect for tongue function may require a longer-duration targeted intervention. Therefore, future studies should consider developing simple and easily executable oral exercises that closely mimic the swallowing process, coupled with an adequate duration of practice, to enhance an intervention’s efficacy.
Effects of the intervention on correlates of oral hypofunction
Oral hypofunction has been associated with inadequate protein intake and malnutrition in community-dwelling participants19,20. Our study demonstrated a significantly lower BMI in the oral hypofunction group. Furthermore, this group also had higher levels of frailty, lower grip strength, and smaller calf circumference. These findings indicate a strong association between oral hypofunction, malnutrition, and frailty.
Our study demonstrated that interventions aimed at enhancing oral hygiene and exercise interventions significantly improved the nutritional status of both the oral hypofunction and control groups. Another study demonstrated that, within 12-week trials, oral exercise can improve appetite and reduce the risk of malnutrition29. These findings collectively support the notion that interventions for oral hypofunction can lead to improvements in nutritional status within 3–6 months of starting the oral exercise training. This suggests that addressing oral hypofunction is particularly important for individuals at risk of malnutrition. Future studies should investigate the mechanisms through which these interventions improve nutritional status.
Limitations
The study has several limitations that should be considered when interpreting the results. First, the reliance on subjective assessments, such as the EAT-10, may have led to variability in the findings. Second, the study’s focus on older residents in long-term care facilities may limit the generalizability of the findings to other, more diverse populations. Third, we only assess five of the 7 domains defined by Minakuchi et al.4. While the omission of occlusal force and masticatory function assessments may limit the comprehensiveness of the diagnostic evaluation, it may result in an underestimation of prevalence. However, this pragmatic model may serve as a foundation for broader implementation of oral health assessments in geriatric care settings where access to advanced diagnostic tools is limited. Furthermore, future studies should incorporate more extended observation periods and a more diverse participant pool to better assess the interventions’ efficacy.
Conclusion
This study revealed that 58.3% of residents in long-term care facilities meet the criteria for oral hypofunction. The participants with oral hypofunction had a lower BMI, lower grip strength, a smaller calf circumference, and a higher level of frailty, underscoring the need for targeted interventions to address their oral function. Implementation of oral health hygiene and lip and tongue exercises make change in swallowing function, oral hygiene, and tongue–lip motor function. These findings support the notion that early intervention for individuals with oral hypofunction can enhance their oral function. Therefore, oral hypofunction should be identified as early as possible to facilitate timely interventions to save adequate oral function.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to the restriction from IRB but are available from the corresponding author upon reasonable request.
References
Jones, E. et al. Health-Related quality of life and oropharyngeal dysphagia: A systematic review. Dysphagia 33, 141–172 (2018).
Abu-Ghanem, S., Chen, S. & Amin, M. R. Oropharyngeal dysphagia in the elderly: evaluation and prevalence. Curr. Otorhinolaryngol. 8, 34–42 (2020).
Xue, W., He, X., Su, J., Li, S. & Zhang, H. Association between dysphagia and activities of daily living in older adults: a systematic review and meta-analysis. Eur Geriatr. Med. 14, 1812 (2024).
Minakuchi, S. et al. Oral hypofunction in the older population: position paper of the Japanese society of gerodontology in 2016. Gerodontology 35, 317–324 (2018).
Minakuchi, S. Philosophy of oral hypofunction. Gerodontology 39, 1–2 (2022).
Matsuo, K. T. H. et al. Relationships between deterioration of oral functions and nutritional status in elderly pa-tients in an acute hospital. Ronen Shika Igaku. 31, 11 (2016).
Kugimiya, Y. et al. Rate of oral frailty and oral hypofunction in rural community-dwelling older Japanese individuals. Gerodontology 37, 342–352 (2020).
Shimazaki, Y. et al. Oral hypofunction and its association with frailty in community-dwelling older people. Geriatr. Gerontol. Int. 20, 917–926 (2020).
Cruz-Moreira, K. et al. Prevalence of frailty and its association with oral hypofunction in older adults: a gender perspective. BMC Oral Health. 23, 140 (2023).
Park, H. J. et al. Assessment of oral hypofunction and its association with age among Korean community-dwelling older adults. BMC Oral Health. 24, 441 (2024).
Montero, L. et al. A systematic evaluation for oropharyngeal dysphagia in Non-institutionalized elderly patients with home Care-based in the community. Dysphagia 40, 607-613 (2025).
Sarabia-Cobo, C. M. et al. The incidence and prognostic implications of dysphagia in elderly patients institutionalized: A multicenter study in Spain. Appl. Nurs. Res. 30, e6–e9 (2016).
Tanaka, T. et al. Oral frailty as a risk factor for physical frailty and mortality in Community-Dwelling elderly. J. Gerontol. A-biol. 73, 1661–1667 (2017).
Leira, J. et al. Dysphagia and its association with other health-related risk factors in institutionalized older people: A systematic review. Arch. Gerontol. Geriatr. 110, 104991 (2023).
Yang, S. et al. Clinical practice guidelines for oropharyngeal dysphagia. Ann. Rehabil Med. 47, S1–s26 (2023).
Tanaka, T. et al. Oral frailty as a risk factor for physical frailty and mortality in Community-Dwelling elderly. J. Gerontol. Biol. Sci. Med. Sci. 73, 1661–1667 (2018).
Shirobe, M. et al. Effect of an oral frailty measures program on Community-Dwelling elderly people: A Cluster-Randomized controlled trial. Gerontology 68, 377–386 (2022).
Onuki, W. et al. Evaluating the effect of management on patients with oral hypofunction: A longitudinal study. Gerodontology 40, 308–316 (2023).
Iwasaki, M. et al. Oral hypofunction and malnutrition among community-dwelling older adults: evidence from the Otassha study. Gerodontology 39, 17–25 (2022).
Nishi, K. et al. Relationship between oral hypofunction, and protein intake: A Cross-Sectional study in local Community-Dwelling adults. Nutrients 13, 4377 (2021).
Kim, H. J. et al. I. Improvements in oral functions of elderly after simple oral exercise. Clin. Interv Aging. 14, 915–924 (2019).
Belafsky, P. C. et al. Validity and reliability of the eating assessment tool (EAT-10). Ann. Otol Rhinol Laryngol. 117, 919–924 (2008).
Shimizu, T., Ueda, T. & Sakurai, K. New method for evaluation of tongue-coating status. J. Oral Rehabilitation. 34, 442–447 (2007).
Chalmers, J. M., King, P. L., Spencer, A. J., Wright, F. A. C. & Carter, K. D. The oral health assessment tool—validity and reliability. Aust Dent. J. 50, 191–199 (2005).
Adams, V., Mathisen, B., Baines, S., Lazarus, C. & Callister, R. A systematic review and meta-analysis of measurements of tongue and hand strength and endurance using the Iowa oral performance instrument (IOPI). Dysphagia 28, 350–369 (2013).
Adams, V., Mathisen, B., Baines, S., Lazarus, C. & Callister, R. Reliability of measurements of tongue and hand strength and endurance using the Iowa oral performance instrument with elderly adults. Disabil. Rehabil. 37, 389–395 (2015).
Rubenstein, L. Z., Harker, J. O., Salvà, A., Guigoz, Y. & Vellas, B. Screening for undernutrition in geriatric practice: developing the short-form mini-nutritional assessment (MNA-SF). J. Gerontol. Biol. Sci. Med. Sci. 56, M366–M372 (2001).
Ensrud, K. E. et al. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J. Am. Geriatr. Soc. 57, 492–498 (2009).
Matsuo, K. et al. Improvement of oral hypofunction by a comprehensive oral and physical exercise programme including textured lunch gatherings. J. Oral Rehabil. 48, 411–421 (2021).
Kleim, J. A. & Jones, T. A. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J. Speech Lang. Hear. Res. 51, S225–239 (2008).
Markram, H., Lübke, J., Frotscher, M. & Sakmann, B. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science 275, 213–215 (1997).
Nudo, R. J., Wise, B. M., SiFuentes, F. & Milliken, G. W. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science 272, 1791–1794 (1996).
Mu, L. & Sanders, I. Human tongue neuroanatomy: nerve supply and motor endplates. Clin. Anat. 23, 777–791 (2010).
Ross, C. F., Laurence-Chasen, J. D., Li, P., Orsbon, C. & Hatsopoulos, N. G. Biomechanical and cortical control of tongue movements during chewing and swallowing. Dysphagia 39, 1–32 (2024).
Acknowledgements
The study received support from the National Health Research Institutes, Taiwan (B1-11202) and Kaohsiung Municipal Siaogang Hospital (Proj-111-15).
Author information
Authors and Affiliations
Contributions
Chih-Hung Ko: conducted statistical analyses and drafted the initial version of the manuscript; Chia-Ling Chao, Meng-Ling Hou, Ming-Feng Wu, and Sheng-Hsiu Wu: implemented the study project and participant recruitment; Chih-Hsing Hung: supervised the project; Ming-Chu Feng: designed the methodology and reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ko, CH., Feng, MC., Chao, CL. et al. Effects of oral hygiene and oral exercise on oral hypofunction in residents of long-term care facilities. Sci Rep 15, 20612 (2025). https://doi.org/10.1038/s41598-025-05403-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-05403-4