Abstract
Tej has inconsistent quality due to spontaneous fermentation. The aim of this study was therefore to develop mixed lactic acid bacteria (LAB) and yeast starter culture to improve the quality and safety of Tej. A total of 166 LAB and 193 yeasts, were characterized for their starter culture potential. Molecular identification was done for the LAB and yeast isolates. Reduced acid tolerances were recorded for the isolates. Better ethanol tolerances were observed for Lacticaseibacillus paracasei (L7) and Saccharomyces cerevisiae (Y4). A decrease in salt tolerance was recorded for the LAB and yeast isolates as the concentration of salt increased from 5.5 to 10%. The highest tolerance (p < 0.05) was recorded at 35ºC for Lacticaseibacillus paracasei (L8) and Saccharomyces cerevisiae (Y2). Four compatible yeast and LAB isolates were used to produce Tej under controlled conditions. Tej (T5) produced by L. paracasei (L6) and S. cerevisiae (Y6) had significantly higher overall acceptability (4.15 ± 0.47, p < 0.05) compared to Tej produced by other combinations of LAB and yeasts. Tej (T1) produced by L. pentosus and S. cerevisiae has shown equivalent acceptability to the control Tej. The lowest overall mean acceptability score was recorded for Tej (T2) produced by L. paracasei (L7) and S. cerevisiae (Y5). Most of the mixed lactic acid bacteria and yeast starter cultures improved the attributes of Tej.
Similar content being viewed by others
Introduction
Tej is an Ethiopian alcoholic beverage fermented commonly at the household level through traditional methods without inoculating defined starter cultures1,2. It is produced from honey, water and Gesho (Rhamnus prinoides) with some flavoring agents like Weira (Olea europaea) and Grawa (Vernonia amygdalina) for conditioning the fermentation vat3,4. Sugar is added in place of honey in some cases which necessitates the addition of coloring agents to make the final product look yellow5. The final product differs between different producers, but has effervescent nature2,6. The alcohol content of Tej is higher than other non-distilled Ethiopian fermented alcoholic beverages7. The quality of Tej varies depending on the raw materials used by the producers8. Reports show the presence of toxic metabolites such as high concentrations of methanol and fusel oils in the traditionally fermented Tej5. The microorganisms for Tej fermentation mainly come from the ingredients and equipment used for its preparation which varies between producers9. To produce products with consistent organoleptic quality and microbiological stability, starter culture development and optimization of the fermentation process were recommended10.
Starter cultures are microbial preparation of large numbers of cells of at least one type of microorganism to be added to a raw material to produce a fermented food11. They have been successfully applied to a wide verity of African fermented foods and drinks12. The selection of the right types of starter cultures is important to obtain products with the desired sensory attributes. Starter microorganisms should be resistant to low pH, high ethanol, high salt concentration, high temperature and be able to utilize various carbon sources10,11,12,13,14,15,16. Production of biotechnologically important products such as diacetyl and exopolysaccharides are also considered desirable17. Previous researches reported that LAB are mostly involved in the production of Tej18,19,20 and the yeasts of the genus Saccharomyces were responsible for the conversion of sugars to ethanol in Tej1.
Despite the huge economic values for venders which are usually females, and variability in the production processes of Tej from one locality to another, there is limited information about the development of starter culture for this valuable product. There are a few studies regarding the use of mixed starter cultures for production of Tej. A previous study2 used different types of lactic acid bacteria in combination with S. cerevisiae and found promising results for Tej produced by a combination of different lactic acid bacteria with S. cerevisiae. Similarly, our previous report21, for samples collected from different locality showed the potential of combined starter culture of Lactobacillus and Saccharomyces for production of Tej. The findings of the previous researches also indicated that, the potential starters for Tej production could differ depending on the Tej sources. The aim of this study was therefore to develop potential mixed lactic acid bacteria and yeast starter cultures for controlled production of Tej.
Materials and methods
Sampling site, sample collection and processing
Three cities (Debre Markos, DM; Finote Selam, FS and Bure, BU) in the Amhara region, Northern Ethiopia, were selected using lottery method from cities with potential for Tej and honey production. A total of 30 Tej samples, 10 samples from each city, were purposively collected from the best Tej producing and vending houses. Each sample was collected from the local venders with pre-sterilized containers, and the samples were transported to the Microbiology Laboratory, College of Natural and Computational Sciences, Addis Ababa University using Ice Box with a temperature of 4 °C. Then, isolation and enumerations of microorganisms in the Tej samples were done using the appropriate culture medium and culture conditions as described below.
Isolation and enumeration of the LAB and yeast isolates
LAB and yeast count of the Tej samples were analyzed according to the methods reported in Bahiru et al.22. Briefly, 25 mL of each sample was homogenized in 225 mL of sterile peptone water, followed by tenfold serial dilution (10−2 to 10−7) and plating 0.1 mL of the serially diluted sample on pre-sterilized agar media. The LAB was enumerated on pre-sterilized MRS (de Man, Rogosa, and Sharpe) agar after incubation at 32 °C for 24–48 h under anaerobic conditions using anaerobic jar. The yeasts were enumerated on yeast extract peptone dextrose (YPD) agar after incubation at 28 °C for 72 h.
Characterization of the LAB and yeasts
Cultural, morphological characteristics, catalase enzyme, gas and diacetyl production were done both for LAB and yeasts. However, Gram reaction (KOH test) was done only for LAB, and extracellular starch- like (amyloid) compound detection was done only for yeasts. Cultural (colony color, margin and elevation) and morphological (shape and arrangement of cells) were assessed according to the method described by23,24. Production of catalase was evaluated following the previously reported method25. Gas production was done according to the method of26. Production of diacetyl by the LAB and yeasts was done according to the method of27, exopolysaccharide production for the LAB was evaluated according to the method of28 and the production of extracellular starch-like (amyloid) compound was detected according to the method of29. The KOH test was done following the method of30.
Fermentation of carbohydrates
Four milliliters aliquot of the nitrogen base medium (4.5 g yeast extract, 7.5 g peptone, 0.5 g of bromothymol blue in 1000 mL distilled water) was dispensed in test tubes containing inverted Durham’s tube. After filtration, 1 mL aliquot of the sugar solutions was aseptically added to the test tubes containing the nitrogen base. A loop full of young cultures were inoculated into the tubes and incubated at 30 °C for 72 h. A blank consisting of inoculated basal medium devoid of any carbon source served as a control. The accumulation of gas in the Durham’s tubes and the color change of the indicator were recorded as positive results for carbohydrates fermentation31. The carbohydrates evaluated were glucose, fructose, galactose, lactose, sucrose, maltose, mannose, starch, trehalose and xylose.
Assimilation of carbon sources
Assimilation of carbon source was determined by the auxanographic carbohydrate assimilation method29 with slight modification, about 2 mL from 10% stock solution of carbon sources (mannose, lactose, trehalose, starch, maltose, fructose, sucrose, glucose, galactose, xylose and glycerol) were placed on the dried agar surface and inoculated with 10 µL of 48 h young yeast cultures. The Petri dishes were incubated at 30 °C for 10 days and examined for growth.
Physiological stress tests for LAB and yeast
The physiological stress tests were assessed for LAB and yeast isolates on MRS and YPD broths, respectively. Fresh overnight cell cultures were centrifuged (3000 rpm for 15 min at 24 °C) and washed twice with 0.1% w/v peptone water (pH 7). The cell cultures (OD600 of 0.2) in peptone water corresponding to ~ 107 cell/mL of yeast and LAB were centrifuged and re-suspended in every stress experiment and each experiment was conducted in triplicate.
pH tolerance
Tolerance of the yeasts and the LAB to low pH was tested using the method of32. One milliliter of the 24 h old yeast and LAB cultures centrifuged, washed and re-suspended in peptone water were separately inoculated into test tubes containing 5 mL of YPD and MRS broth adjusted to pH 2.5, 3.5 and 4.5 using 1N HCl and NaOH, respectively. The test tubes were incubated for 24 h at 32 °C, and optical density was measured at 600 nm using UV–visible spectrophotometer (Jenway, 6405, Felsted, Dunmow, UK).
Salt tolerance
Tolerance of the yeasts and the LAB to NaCl was tested using the method of33. One milliliter of the 24 h old yeast and LAB cultures centrifuged, washed and re-suspended in peptone water were separately inoculated into test tubes containing 5 mL of YPD and MRS broth supplemented with 5.5, 7.5 and 10% (w/v) NaCl, respectively. The test tubes were incubated for 24 h at 32 °C and optical density was measured at 600 nm using UV–visible spectrophotometer (Jenway, 6405, Felsted, Dunmow, UK).
Ethanol tolerance
Tolerance of the yeast and the LAB to ethanol was tested using the methods of33,34. One milliliter of the 24 h old yeast and LAB cultures centrifuged, washed and re-suspended in peptone water were separately inoculated into test tubes containing 5 mL of YPD and MRS adjusted to 10, 15 and 20% ethanol, respectively. The test tubes were incubated for 24 h at 32 °C and optical density was measured at 600 nm using UV–visible spectrophotometer (Jenway, 6405, Felsted, Dunmow, UK).
Temperature tolerance
Temperature tolerance of the yeasts and the LAB was tested using the method of35. One milliliter of the 24 h old yeast and LAB cultures that were centrifuged, washed and re-suspended in peptone water were separately inoculated into test tubes containing 5 mL of YPD and MRS, respectively. The tubes were then incubated at 35 °C, 40 °C and 45 °C for 24 h, and optical density was measured at 600 nm using UV–visible spectrophotometer (Jenway, 6405, Felsted, Dunmow, UK).
Molecular identification of the LAB and yeast isolates
Molecular identification of LAB
Extraction of the total DNA of the LAB was conducted following the method described in36. For identification of the LAB isolates, the full length 16S rRNA gene was amplified using the primer pair 27 F: 5’-AGA GTT TGA TCC TGG CTC AG-3′ and 1492 R: 5’ GGT TAC CTT GTT ACG ACT T-3. The PCR reaction parameters were: 94 °C for 10 min followed by 32 cycles at 94 °C for 30 s, 55 °C for 20 s, 72 °C for 55 s, and final extension at 72 °C for 5 min. The total reaction mixture per tube was 25 μL. The amplicons were sequenced using Sanger sequencing using the 27 F and 1492 R primers using the ABI PRISM® BigDye™ Terminator cycle sequencing kit. The ABI PRISM Sequencer platform 3730XL at the Beijing Sangon Biotechnology Company, China. The top match for the 16S rRNA sequence of the bacteria was searched against the GenBank database using the Basic Local Alignment Search Tool (BLAST). Sequence identity with a threshold of 97% or above was used to describe the bacterial species using the rRNA gene region37,38,39.
Molecular identification of the yeasts
The yeast isolates were grown overnight for 24 h at 30 °C on YPD medium and DNA extraction was performed following the method40. Primers NL1: 5’-GCA TAT CAA TAA GCG GAG GAA AAG-3’ and NL4: 5’-GGT CCG TGT TTC AAG ACG G-3’ were used to amplify the large sub unit (LSU) rDNA D1/D2 domain. The PCR conditions were: an initial denaturation at 98 °C for 2 min followed by 35 cycles at 98 °C for 10 s, annealing at 55 °C for 15 s, 72 °C for 15, and final extension at 72 °C for 5 min. The total volume of the reaction mixture per tube was 25 μL, and amplicon sequencing was performed methods described41, with minor modifications. Cycle sequencing was performed using the ABI PRISM® BigDye™ Terminator cycle sequencing kit. An ABI PRISM Sequencer platform 3730XL at the Beijing Sangon Biotechnology Company, China. The top match for the sequence of the yeasts in the current study was searched against the GenBank database using the Basic Local Alignment Search Tool (BLAST). Sequence identity with a threshold of 99% or above was used to describe the yeast species using the rRNA gene region37,38,39.
Starter culture development
Compatibility test
The candidate isolates’ inter-compatibility was evaluated by cross streaking each isolate against each other on YPD (for yeast) and MRS (for LAB) agar plates, and incubating at 32 °C for 48 h42. Isolates considered compatible were able to grow on the same substrate without an inhibition zone when cross streaked against each other.
Starter culture formulation
The combinations of the LAB and yeasts used for production of Tej were determined using Design Expert version 13.0.05, 2021 Statistical software. Accordingly, 5% viable cells of the pure isolate were mixed in a flask containing 25 mL of pasteurized honey (33.3%) dissolved in distilled water to determine the suitable combination to produce Tej43.
Propagation of the isolates
Pure cultures of LAB and yeasts were separately inoculated into MRS and YPD broth media, respectively. The LAB were incubated at 32 °C for 48 h and the yeasts were incubated at 30 °C for 72 h. Cells of the LAB and yeast were harvested from the media by centrifugation at 3000 rpm for 15 min. The viable counts of LAB (109 CFU/mL) and yeasts (107 CFU/mL) were determined using spread plate technique. The pellets (2 mL) were rinsed with peptone water and inoculated into 100 mL pasteurized honey in a 250 mL flask, and the mixture was incubated for 48 h at 32 °C44.
Laboratory preparation of Tej using starter cultures
For laboratory preparation of Tej using the starter cultures, 250 g of honey pasteurized at 62.5 °C for 15 min was mixed with 500 mL of sterile distilled water at a ratio of 1:2 in 1000 mL flasks. The mixture was inoculated with 5% of LAB and yeast suspension, and the flasks were tightly covered with aluminum sheet. After 3 days of fermentation, 10 g of sterile Rhamnus prenoides (Gesho) was added to the must and the flasks were covered again with sterile aluminum sheet and fermented continuously for 15 days at ambient temperature. After the fermentation period the prepared Tej was filtered through sterile muslin cloth to remove the sediments and the Rhamnus prenoides.
Sensory analysis
Panelists consisting of Tej producers and consumers (n = 10) were selected to determine the sensory acceptability of the product. Panelists ranked acceptability of various attributes of different Tej produced under laboratory conditions using 5-point hedonic scale.
Data analysis
The data from the current study were analyzed using SPSS Software version 25.0.0, 2021. One way ANOVA was used to compare different means and mean comparison was done using Tukey’s HSD test. Statistical significance was determined at p < 0.05. The possible combinations of the isolates used for production of Tej under the laboratory conditions were identified using Design Expert software version 7.0.0 (2005).
Ethical approval
Ethical clearance was obtained from the Institutional Review Board of the College of Natural and Computational Sciences, Addis Ababa University with Ref No. SF/MCMB/345/14/2. After the approval of the proposal, informed consent was obtained from the panelists. The panelists were informed of the right to withdraw at any time if not comfortable and their participation was completely confidential. The ethical issue was conducted in accordance with the declaration of Helsinki and its amendments.
Results and discussion
Isolation, enumeration and characterization of the LAB and yeasts
LAB and yeasts were enumerated form Tej samples collected from the three study sites, and the mean counts (log CFU/mL) of both yeasts and LAB were significantly higher (p < 0.05) for the Tej samples collected from Finote Selam compared to the other two study sites (Supplementary Table 1). Other researchers also reported similar counts for LAB and yeasts22,45.
A total of 359 isolates of yeast (193) and LAB (166) were isolated from traditionally fermented Tej samples from the three sampling sites. The number of the isolates were reduced based on physiological stress tolerance tests, and four LAB and four yeasts which were compatible were used for production of Tej under the laboratory conditions. The details of their morphological, cultural and biochemical characteristics were summarized in Supplementary Tables 2 and 3, and identified using molecular techniques, respectively. The total number of each strain isolated was n = 1 for Lactiplantibacillus pentosus, n = 2 for Lacticaseibacillus paracasei and n = 1 for Lentilactobacillus hilgardii (Supplementary Fig. 1). All the yeast isolates used in the fermentation (n = 4) were Saccharomyces cerevisiae (Supplementary Fig. 2). The sequences were deposited in the NCBI database with the accession number provided in the bracket after the species name; Lactiplantibacillus pentosus (L2) (PV423076), Lacticaseibacillus paracasei (L6) (PV423077), Lacticaseibacillus paracasei (L7) (PV423078), Lentilactobacillus hilgardii (L8) (PV423079), and Saccharomyces cerevisiae (Y2) (PQ410281), Saccharomyces cerevisiae (Y4) (PQ410276), Saccharomyces cerevisiae (Y5) (PQ410274) and Saccharomyces cerevisiae (Y6) (PV416743). The predominance of Saccharomyces in the Tej samples of the current study was also in agreement with the report1. Various studies reported the presence of different types of LABs in Tej2,22.
Both the LAB and yeasts were able to produce diacetyl but only the LAB produced exopolysaccharides. Other researchers also reported the production of diacetyl by Lactobacillus species46. In yeasts, the concentration of diacetyl is linked to the degree of growth and the level of degradation of fermentable sugars47.
Carbon source utilization
All the yeast isolates were able to ferment glucose, and assimilate both glucose and fructose. Mannose, lactose and starch were neither assimilated nor fermented by the isolates. None of the yeast isolates utilized lactose, mannose and starch (Table 1), a similar study was also reported48. The differences in carbon source utilization depend on the presence of specific enzymes and sugar preferences by the yeasts.
Physiological stress tests for LAB and yeasts
pH tolerance
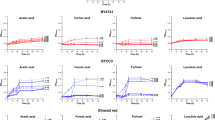
The LAB and yeasts were able to tolerate all the pH values tested. Significantly higher tolerances (4.6 ± 0.20) and (6.0 ± 0.14) were recorded, respectively at pH 3.5 and pH 4.5 for Lacticaseibacillus paracasei (L7) (Table 2). Other researchers also reported low pH tolerances of LAB and yeasts49,50,51,52,53. Microorganisms are reported to have acid tolerance mechanisms such as acid shock proteins, and the condition is strain specific54
Ethanol tolerance
Ethanol tolerances of the LAB and yeasts decreased as the concentrations increased. The highest tolerances of 0.86 ± 0.01 and 0.56 ± 0.03 were recorded respectively for Lacticaseibacillus paracasei (L7) and the yeast Saccharomyces cerevisiae (Y4) at 10% alcohol concentration (Table 3). High ethanol tolerances of the LAB55 and yeasts15,56 were also reported. Tolerance to ethanol depends on having specific tolerance mechanisms, and is conserved within species55. High ethanol tolerances in Lacticaseibacillus paracasei has been linked the SMN-LBK gene family57. In Saccharomyces cerevisiae, ethanol tolerance was associated with gene expression responses, pathway-based mechanisms and regulatory networks58.
Salt tolerance
Salt tolerances of the LAB and yeasts decreased with increase in the salt concentration from 5.5 to 10%. The highest salt tolerances were recorded for Lacticaseibacillus paracasei (L6) and Saccharomyces cerevisiae (Y6) at 5.5% salt concentration (Table 4). This is not in line with the report by48. However, halophilic LAB were reported to be widely used as starter cultures and were the dominant microorganisms in many fermented foods59,60. The presence of highly conserved pathways that mediate salt tolerance was reported for Saccharomyces cerevisiae61. The differences in salt tolerance could be attributed to having mechanisms such as Na+ and K+ fluxes, modulation of cell wall properties and synthesis of compatible solutes33.
Temperature tolerance
A general decreasing trend in tolerance of the LAB and yeasts was observed. Best tolerance was recorded at 35 °C for both LAB (L8) and yeast (Y2) (Table 5). Similar pattern of tolerance was recorded for LAB62. Strain dependence of temperature tolerance in LAB was reported by63. Significant growth of Saccharomyces cerevisiae at 42 °C was reported by64. But, a report by48 showed the complete absence of yeast growth at 40 °C. The differences in temperature tolerances of yeasts, and LAB could be attributed to differences in heat shock responses65, and mutations linked to high temperature growth was associated with high temperature tolerances in Saccharomyces cerevisiae53.
Starter culture development
Compatibility of the isolates
The isolates that were able to tolerate the stresses were assessed for compatibility in cross streaking method. Four isolates from the yeast and four from the LAB were found to be compatible (Fig. 1).
Starter culture formulation
There were eight possible combinations generated by the Design expert software (F1 to F8). But, two of the combinations contained either the LAB or the yeast and weren’t included in the laboratory production of Tej. The LAB and yeast isolates used in the formulation were: F1 (L2 and Y4); F2 (L7 and Y5); F3 (All combinations); F4 (L8 and Y2); F5 (L6 and Y6) and the control was fermented without spontaneously without the addition of starter culture (Table 6).
Propagation of inoculants and production of Tej
Inoculants were prepared by mixing 2 mL of washed pellets of LAB and yeast isolates into 25 g of honey diluted with 50 mL of distilled water (Fig. 2a). The Tej produced using the starter cultures before filtration (Fig. 2b) and after filtration (Fig. 2c). The produced Tej samples were yellow in color and had pleasant honey odor.
Sensory analysis
Panelists consisting of Tej producers and consumers (n = 10) were selected to determine the sensory acceptability of the products. Panelists ranked acceptability of various attributes of different Tej samples using a 5-point hedonic scale. The sensory attributes of the Tej such as color, flavor, odor, and overall sensory acceptability were assessed. The overall mean sensory acceptability of the Tej types ranged from 1.72 to 4.15 (Table 7). The difference between the best rated Tej samples (T4 and T5) and the control was not statistically significant. The second Tej sample (T2) was the least rated by the panelists.
Conclusion
Yeasts and LAB were the dominant microorganisms in the Tej samples collected from the Northern part of Ethiopia. Among the total of 359 isolates, four potential isolates of LAB and four yeasts were selected for the purpose of testing their starter culture potential in Tej production based on their stress tolerance capabilities. Two of the LAB isolates were identified to be Lacticaseibacillus paracasei, one each of Lactiplantibacillus pentosus and Lentilactobacillus hilgardii. All the yeasts belonged to Saccharomyces cerevisiae. The isolates were able to pass all the hurdles tested and were able to produce Tej under controlled conditions. From the sensory analysis, Tej (T5) produced by the combination of Lacticaseibacillus paracasei (L6) and Saccharomyces cerevisiae (Y6) was the most rated compared to all other combinations of Tej produced under laboratory conditions. Thus, we are able to produce acceptable Tej using defined mixed starter cultures under the laboratory conditions which has strong implications for consumers and vendors with regard to the quality and safety of the product. Further testing of the isolates at larger scales is important to scale up Tej production.
Data availability
Data is provided within the manuscript or supplementary information.
References
Vogel, S. & Abeba, G. Ethiopian ‘Tej’. In Handbook of Indigenous Fermented Foods (ed. Steinkraus, K. H.) 363–365 (Marcel Dekker Inc, 1983).
Fentie, E. G. et al. Physicochemical properties, antioxidant activities and microbial communities of Ethiopian honey wine, ‘Tej’. Food Res. https://doi.org/10.1016/j.foodres.2021.110765 (2022).
Bahiru, B., Meharie, T., Tetemke, M. & Ashenafi, M. Chemical and nutritional properties of ‘Tej’’, an indigenous Ethiopian honey wine: Variations within and between production units. J. Food Technol. Afr. 6, 104–108 (2001).
Berhanu, M., Desalegn, A., Birri, D., Ashenafi, M. & Tigu, F. Microbial, physicochemical and proximate analysis of Tej collected from Amhara regional state of Ethiopia. Heliyon 9, e16911. https://doi.org/10.1016/j.heliyon.2023.e16911 (2023).
Fite, A., Tadesse, A., Urga, K. & Seyoum, E. Methanol, fusel oil and ethanol contents of some Ethiopian traditional alcoholic beverages. SINET Ethiop. J. Sci. 14, 19–27 (1991).
Steinkraus, K. Handbook of Indigenous Fermented Foods (Marcel Dekker Inc, 1983).
Bacha, K. & Nemo, R. Microbial, physicochemical and proximate analysis of selected Ethiopian traditional fermented beverages. LWT 131(9), 109713. https://doi.org/10.1016/j.lwt.2020.109713 (2020).
Yohannes, T., Melak, F. & Siraj, K. Preparation and physicochemical analysis of some Ethiopian traditional alcoholic beverages. Afr. J. Food Sci. 7, 399–403. https://doi.org/10.5897/AJFS2013.1066 (2013).
Hotessa, N. & Robe, J. Ethiopian indigenous traditional fermented beverage: The role of the microorganisms toward nutritional and safety value of fermented beverage. Int. J. Microbiol. 2020, 1–11 (2020).
Kimaryo, V. M., Massawe, G. A., Olasupo, N. A. & Holzapfel, W. H. The use of a starter culture in the fermentation of cassava for the production of kivunde, a traditional Tanzanian food product. Int. J. Food Microbiol. 56, 179–190 (2000).
Leroy, F. & De Vuyst, L. Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci. Technol. 15(2), 67–78 (2004).
Wesley, B., Azuwike, C. O. & Adeleye, S. A. The role of microorganisms in the production of some indigenous fermented foods in Nigeria. Int. J. Adv. Res. Biol. Sci. 5, 86–94 (2018).
Lin, C. C. & Fung, D. Y. Conventional and rapid methods for yeast identification. Crit. Rev. Microbiol. 14(4), 273–289 (1987).
Balakumar, S. & Arasaratnam, V. Osmo- thermo and ethanol- tolerances of Saccharomyces cerevisiae. Braz. J. Microbiol. 43(1), 157–166 (2012).
Walker, G. & Stewart, G. Saccharomyces cerevisiae in the production of fermented beverages. Beverages 2(4), 30. https://doi.org/10.3390/beverages2040030 (2016).
Bintsis, T. Lactic acid bacteria as starter cultures: An update in their metabolism and genetics. AIMS Microbiol. 4(4), 665–684 (2018).
Reale, A., Zotta, T., Ianniello, R. G., Mamone, G. & Di Renzo, T. Selection criteria of lactic acid bacteria to be used as starter for sweet and salty leavened baked products. LWT 133(14), 23–38. https://doi.org/10.1016/j.lwt.2020.110092 (2020).
Mehari, T. & Ashenafi, M. Microbiology of siljo, a traditional Ethiopian fermented legume product. World J. Microbiol. Biotechnol. 11(3), 338–342. https://doi.org/10.1007/BF00367113 (1995).
Abegaz, K. Isolation, characterization and identification of lactic acid bacteria involved in traditional fermentation of borde, an Ethiopian cereal beverage. Afr. J. Biotechnol. 6(12), 14691478 (2007).
Bacha, K., Mehari, T. & Ashenafi, M. Microbiology of the fermentation of ‘Shamita’, a traditional Ethiopian fermented beverage. SINET Ethiop. J. Sci. 22, 113–126 (2012).
Girma, B., Desalegn, A. & Tigu, F. Development of a defined mixed starter culture for the improvement of Tej, Ethiopian honey wine. Food Sci. Technol. Int. https://doi.org/10.1177/10820132241251866 (2024).
Bahiru, B., Meharie, T. & Ashenafi, M. Yeast and lactic acid flora of ‘Tej’, an indigenous Ethiopian honey wine: Variations within and between production units. Food Microbiol. 23, 277–282 (2006).
Singleton, P. Bacteria in Biology, Biotechnology and Medicine 3rd edn. (John Willey and Sons, London, 1995).
Lodder, J. The Yeasts. A Taxollomic Study (North Holland Publishing Company, 1971).
Roberts, D., Hooper, W. & Greenwood, M. Practical Food Microbiology (Public Health Laboratory Service, 1995).
Schillinger, U. & Lücke, F. K. Identification of lactobacilli from meat and meat products. Food Microbiol. 4, 199–208 (1987).
Barritt, M. The intensification of the Voges–Proskauer reaction by the addition of α-naphthol. J. Pathol. Bacteriol. 42, 441–454 (1936).
Ruas-Madiedo, P. & de los Reyes-Gavilán, G. Invited Review: Methods for 466 the screening, isolation, and characterization of exopolysaccharides produced by 467 lactic acid bacteria. JDS 88, 843–856 (2005).
Barnette, A., Payne, R. & Yarrow, D. A Guide to Identifying All Classifying Yeasts (Cambridge University Press, 1979).
Gregersen, T. Rapid methods for distinction of gram-negative from gram-positive bacteria. Eur. J. Appl. Bacteriol. 5, 123–127 (1978).
Van der Walt, J. P. Criteria and methods used in classification. In The Yeasts Taxonomic Study (ed. Lodder, J.) 34–113 (North Holland Publishing Co., 1971).
Subashini, D., Ejilane, J., Radha, A., Jayasri, M. A. & Suthindhiran, K. Ethanol production from sago waste using Saccharomyces cerevisiae Vits-M1. Curr. Res. J. Biol. Sci. 3, 42–51 (2011).
Ogunremi, O., Oboh, L. & Ogbe, F. Characterization and stress tolerance of yeasts isolated from palm wine. SAU Sci. Tech. J. 2(1), 36–68 (2017).
Iticha, T. N. Isolation and screening of ethanol tolerant yeast for bio-ethanol production in Ethiopia. Global J. Life Sci. Biol. Res. 2(2), 1–7 (2016).
Pons, M., Rajab, A. & Engasser, J. Influence of acetate on growth kinetics and production control of Saccharomyces cerevisiae on glucose and ethanol. Appl. Microbiol. Biotechnol. 24, 193–198 (1986).
Han, P. J. et al. Species-level understanding of the bacterial community in Daqu based on full-length 16S rRNA gene sequences. Food Microbiol. 123, 104566. https://doi.org/10.1016/j.fm.2024.104566 (2024).
Vu, D. et al. DNA barcoding analysis of more than 9000 yeast isolates contributes to quantitative thresholds for yeast species and genera delimitation. Stud. Mycol. 85, 91–105 (2016).
Fell, J. W., Boekhout, T., Fonseca, A., Scorzetti, G. & Statzell-Tallman, A. Biodiversity and systematics of basidiomycetous yeasts as determined by large-subunit rDNA D1/D2 domain sequence analysis. Int. J. Syst. Evol. Microbiol. 50, 1351–1371 (2000).
Kurtzman, C. P. & Robnett, C. J. Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Antonie Van Leeuwenhoek 73, 331–371 (1998).
Makimura, K., Murayama, Y. S. & Yamaguchi, H. Detection of a wide range of medically important fungi by the polymerase chain reaction. J. Med. Microbiol. 40, 358–364 (1994).
Bai, F. Y. et al. Reclassification of the Sporobolomyces roseus and Sporidiobolus pararoseus complexes, with the description of Sporobolomyces phaffii sp. nov. Int. J. Syst. Evol. Microbiol. 52, 2309–2314 (2002).
Gupta, A. Natural preservation of orange juice to enhance its shelf stability and microbial safety using purified bacteriocin of Brevibacillus borstelensis AG1. AJPTR 5, 168–179 (2015).
Luangkhlaypho, A., Pattaragulwanit, K., Leepipatpiboon, N. & Yompakdee, C. Development of a defined starter culture mixture for the fermentation of sato, a Thai rice-based alcoholic beverage. Sci. Asia 40, 125. https://doi.org/10.2306/scienceasia1513-1874.2014.40.125 (2014).
Asmahan, A. & Muna, M. Use of starter cultures of lactic acid bacteria and yeast in the preparation of Kisra, a Sudanese fermented food. Pak. J. Nutr. 8, 49–53 (2009).
Abebe, B. et al. Isolation and screening of antibacterial producing lactic acid bacteria from traditionally fermented drinks (“Ergo” and “Tej”) in Gondar town, Northwest Ethiopia. Glob. Res. J. Public Health Epidemiol. 1(3), 18–22 (2014).
Jyoti, B. D., Suresh, A. K. & Kareenhalli, V. Diacetyl production and growth of Lactobacillus rhamnosus on multiple substrates. WJMB 19(5), 509–514 (2003).
Mansouri, F., Chouchene, K., Roche, N. & Ksibi, M. Removal of pharmaceuticals from water by adsorption and advanced oxidation processes: State of the art and trends. Appl. Sci. 2021, 6659. https://doi.org/10.3390/app11146659 (2021).
Esayas, A. Development of starter cultures for the production of ‘Tej’: A traditional home made honey wine in Ethiopia, M.Sc. thesis, Faculty of Chemical and Food Engineering, Bahir Dar University. Thesis: 35 pages (2017).
Syal, P. & Vohra, A. Probiotic potential of yeasts isolated from traditional Indian fermented foods. Int. J. Microbiol. Res. 5(2), 390–398 (2013).
Jose, N., Bunt, C. & Hussain, M. Comparison of microbiological and probiotic characteristics of Lactobacilli isolates from dairy food products and animal rumen contents. Microorganisms 3(2), 198–212. https://doi.org/10.3390/microorganisms020198 (2015).
Tigu, F., Assefa, F., Mehari, T. & Ashenafi, M. Probiotic property of lactic acid bacteria from traditional fermented condiments: Datta and Awaze. Int. Food Res. J. 23(2), 770–776 (2016).
Okoth, R. A., Matofari, J. W. & Nduko, J. M. Effectiveness of Levilactobacillus brevis fermentation on antinutrients and protein quality of leaves of selected cassava verities. Appl. Food Res. 2(2), 100134. https://doi.org/10.1016/j.afres.2022.100134 (2022).
Huang, C. J., Lu, M. Y., Chang, Y. W. & Li, W. H. Experimental evolution of yeast for high-temperature tolerance. Mol. Biol. Evol. 35(8), 1823–1839 (2018).
Fang, F., Xu, J., Li, Q., Xia, X. & Du, G. Characterization of a Lactobacillus brevis strain with potential oral probiotic properties. BMC Microbiol. 18, 221. https://doi.org/10.1186/s12866-018-1369-3 (2018).
Pittet, V., Morrow, K. & Ziola, B. Ethanol tolerance of lactic acid bacteria, including relevance of the exopolysaccharide gene Gtf. Am. Soc. Brew. Chem. 69(1), 57–61 (2011).
Khaing, T., Weine, N. & Mya, M. Isolation, characterization and screening of thermo tolerant, ethanol tolerant indigenous yeasts and study on the effectiveness of immobilized cell for ethanol production. Asian J. Sci. Technol. 1, 12–14 (2008).
Guo, J. et al. Transcriptome analysis of Lactobacillus paracasei SMN-LBK under ethanol stress. J. Dairy Sci. 103, 7813–7825 (2019).
Ma, M. & Liu, Z. L. Mechanisms of ethanol tolerance in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 87, 829–845. https://doi.org/10.1007/s00253-010-2594-3 (2010).
Ogasawara, M., Yamada, Y. & Egi, M. Taste enhancer from the long-term ripening of miso (soybean paste). Food Chem. 99, 736–741 (2006).
Udomsil, N., Rodtong, S., Tanasupawat, S. & Yongsawatdigul, J. Proteinase-producing halophilic lactic acid bacteria isolated from fish sauce fermentation and their ability to produce volatile compounds. Int. J. Food Microbiol. 141, 186–194 (2010).
Dhar, D., Sagesser, R., Weikert, C., Yuan, J. & Wanger, A. Adaptation of Saccharomyces cervisiae to saline stress through laboratory evaluation. J. Evol. Biol. 24, 1136–1153 (2011).
Serna, L., Oscar, J. S. & Pedro, J. B. Review of Lactobacillus in the food industry and their culture media. Rev. Colomb. Biotecnol. 21(2), 63–76 (2019).
Pelinescu, D. R. et al. Isolation and identification of some Lactobacillus and Enterococcus strains by a polyphasic taxonomical approach. Rom. Biotechnol. Lett. 14(2), 4225–4233 (2009).
Arachchige, M. S. A., Yoshida, S. & Toyama, H. Thermo-and salt-tolerant Saccharomyces cerevisiae strains isolated from fermenting coconut toddy from Sri Lanka. Biotechnol. Biotechnol. Equip. 33(1), 937–944. https://doi.org/10.1080/13102818.2019.1631213 (2019).
Verghese, J., Abrams, J., Wang, Y. & Morano, K. A. Biology of the heat shock response and protein chaperones: Budding yeast (Saccharomyces cerevisiae) as a model system. Microbiol. Mol. Biol. Rev. MMBR 76(2), 115–158. https://doi.org/10.1128/MMBR.05018-11 (2012).
Acknowledgements
This work was supported by the Addis Ababa University 7th Round Thematic Research Fund (RD/LT076, 2019). The authors would like to thank the Department of Microbial, Cellular and Molecular Biology, Addis Ababa University for facilitating the study. We would also like to thank the Chinese Academy of Science President’s International Fellowship Initiative (CAS-PIFI-2024) award for supporting us in molecular identification of the microorganisms.
Author information
Authors and Affiliations
Contributions
K.D.: Methodology, Validation and Statistical analysis, Investigation, Data curation, Writing – original draft. A.D.: Conceptualization, Methodology, Supervision, Writing—original draft, Review & editing, Funding acquisition and Project administration. F.T.: Conceptualization, Methodology, Supervision, Writing – review & editing, funding acquisition. M.A.: Conceptualization, Methodology, Supervision, Review & editing, Funding acquisition. DJB: Conceptualization, Methodology, Supervision, Review & editing, Funding acquisition. F.Y.B.: Molecular identification of the yeasts and bacteria, review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Denekew, K., Tigu, F., Birri, D.J. et al. Production of improved Ethiopian Tej using mixed lactic acid bacteria and yeast starter cultures. Sci Rep 15, 33460 (2025). https://doi.org/10.1038/s41598-025-05552-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-05552-6