Abstract
To improve the solubility of atorvastatin and overcome the stability issues of liquid nanoemulsion, the current study aimed to synthesize solidified SNEDDS particles with aerodynamic diameter of ≤ 3 μm. The simple and chitosan-decorated liquid SNEDDS were dried by spray drying method and evaluated for their physicochemical properties, release characteristics and aerodynamic performance. A single dose pharmacokinetic study was performed in rabbits to establish the therapeutic performance of solidified nanoemulsion with respect to LIPITOR. The liquid SNEDDS were efficiently dried with pectin (1% w/w). The chitosan decorated solidified SNEDDS (SF10) have small particle size (2.02 μm), higher tapped density (0.733 g/cm3) and smooth surface as compared to uncoated solidified SNEDDS (SF8). The chitosan coated SNEDDS had higher drug content and significantly lower roughness than uncoated SNEDDS (student t-test; p ≤ 0.01). The uncoated SNEDDS exhibited significantly higher burst drug release as compared to the chitosan coated SNEDDS due to the porous structure, amorphous nature and small size of its associated nanoemulsion. The solidified nanoemulsion had relatively lower MMAD (1.0 to 1.5 μm) that supports higher FPF values of 45–54% for the uncoated SNEDDS and chitosan coated SNEDDS, respectively. The pharmacokinetic study revealed that the solidified SNEDDS are superior with respect to its bioavailability being 1.5 times higher than LIPITOR.
Similar content being viewed by others
Introduction
The pulmonary administration of microparticles having aerodynamic diameter of 0.5–5 μm is best suited for systemic drug delivery through the lung1. The pulmonary route of drug delivery is highly effective for the systemic administration of drugs due to high vascularization, the thin epithelial barrier, the large superficial alveolar area for absorption, and the low metabolic activity of the lungs2. It is a non-invasive route of drug administration with improved patient compliance3. The above advantages together with limited enzymatic activity further improve the bioavailability of drugs like atorvastatin that suffer from first-pass metabolism4. The capacity of the lung to absorb substances via the airways, i.e. the non-respiratory function of the lung, is now increasingly used for therapeutic purposes. The nano-carriers can maximize the benefits of pulmonary drug delivery due to their preferential deposition in the lung with high absorption capacity, reduction in drug dosages, reduced systemic toxicity and control of drug release5.
Atorvastatin is the drug of choice for atherosclerosis that belongs to BCS class-II drugs. It has limited oral bioavailability that supports its pulmonary administration in the form of microparticles for improved bioavailability6. Atorvastatin is a synthetic lipid-lowering agent that lowers plasma cholesterol levels by inhibiting the synthesis of HMG-CoA reductase, an enzyme responsible for cholesterol biosynthesis. It lowers the triglyceride levels in the blood while increasing the level of high-density lipoprotein7. The studies also signify its role as an anticancer agent8antifungal9anti-inflammatory and antioxidant10. The oral bioavailability of atorvastatin is around 14% due to lower aqueous solubility and first-pass metabolism. The currently available atorvastatin formulations are administered orally to the patients. However, the problems associated with daily oral administration of atorvastatin include lower oral bioavailability due to its lower aqueous solubility, dissolution rate, pre-systemic metabolism and vulnerability to efflux mechanisms. To address the above issues, the current pharmaceutical research has focused on developing novel drug delivery systems11.
Previous studies have shown that nanoparticles can improve bioavailability when administered orally12. Similarly, coating nanoparticles with chitosan has been found to exhibit better anti-hyperlipidemic effects as compared to non-coated nanoparticles and pure drug suspension13. The already synthesized carriers for pulmonary delivery of atorvastatin include micellar nanoparticles14microparticles8solid self-microemulsifying drug delivery systems15 for the management of lung cancer. The self micro- and nano-emulsifying drug delivery system serves as an optimum platform for the pulmonary delivery of atorvastatin. The pulmonary drug delivery improves the absorption of the drug from the lung parenchyma due to the large surface area, thin basement membrane and rich blood supply16. The dose and systemic toxicity of atorvastatin can be significantly reduced due to limited enzymatic activity in the lung for drug metabolism17. Liquid SNEDDS can achieve higher aerosolization and inhalation as compared to dry powder inhaler (DPI) formulations due to small particle size, uniformity of dosage, ease of inhalation and flexibility in dosage18. The liquid SNEDDS are also known to achieve rapid absorption with an improved bioavailability profile. However, the resultant nanoemulsion from liquid SNEDDS used for nebulization suffers from limitations of instability; prolonged time of administration as well as bacterial contamination that creates patient compliance problems19. The Solidified SNEDDS combines the advantages of liquid formulations (i.e., enhanced solubility and bioavailability) with those of solid dosage forms (e.g., low production cost, convenience of process control, high stability and reproducibility, and better patient compliance). The solid SNEDDS are relatively more robust formulations with improved stability in comparison to their liquid counterparts. They are more easily manufactured and delivered via dry powder inhalers (DPI’s). The liquid nanoemulsion was solidified using the spray drying method as mentioned earlier in our previous study19. The spray drying method was preferred to produce solidified SNEDDS as an inhalational powder for DPI to control over various particle attributes such as size distribution, surface morphology and energy by regulating parameters of the spray-drying process such as feed rate, inlet/outlet temperature, and aspiration20. Overall, the study intends to synthesize solidified SNEDSS for maximum deposition in the deep lung for systemic delivery of atorvastatin.
Materials and methods
Materials
Atorvastatin was provided as a gift sample by CCL Pharmaceuticals, Lahore, Pakistan. Oleic acid (Sigma Aldrich, USA) was used as an oil phase. The Tween 80 (Sigma Aldrich, USA) and Span 80 (Sigma Aldrich, USA) were used as surfactants in the fabrication of SNEDDS. The chitosan (Molecular weight of 50000, Sigma Aldrich, USA) was used as coating agent for nanoemulsion droplets. Pectin, lactose and mannitol (Sigma Aldrich, USA) were used as wall materials during the spray drying process. Phosphate buffer saline (Sigma Aldrich, USA) was utilized as a dissolution medium. All the other chemicals used in the study were of analytical grade.
Preparation and characterization of liquid SNEDDS
The SNEDDS were prepared by spontaneous emulsification method as mentioned previously21. Briefly, 0.621% w/w of atorvastatin was added to 24.84% w/w of oleic acid and stirred continuously to obtain a clear homogeneous oily solution. The oil phase was added dropwise to the mixture of Tween 80 (14.91% w/w) and Span 80 (59.63% w/w) being utilized in the ratio of 1:4. The stirring was continued for an additional 3 h until homogeneous SNEDDS were synthesized. The chitosan-coated SNEDDS was prepared by adding 1% w/w chitosan to the fabricated SNEDDS for improved permeation through the lung parenchyma22. The concentration of chitosan was kept at 1% w/w due to optimization with respect to Zeta potential. There was a significant increase in the particle size and viscosity of formulation when the chitosan was used beyond 1% w/w that creates hurdles in aerosolization of SNEDDS from the nebulizer23. The SNEDDS were characterized for their size, homogeneity, Zeta potential, viscosity, density, surface tension, pH, drug content and drug release behavior23 as mentioned in Table 1.
Preparation and characterization of solidified SNEDDS
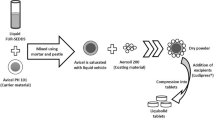
The liquid nanoemulsion for nebulization suffers from limitations of physicochemical instability, risk of bacterial contamination, poor reproducibility with respect to aerodynamic performance and degradation of the drug due to shear force during nebulization24. To overcome the above limitations of liquid SNEDDS current study undertakes the conversion of liquid SNEDDS to its solidified counterparts using the spray drying technique as mentioned previously19. Briefly, the solution of wall materials (lactose, mannitol and pectin) was prepared by dissolving the wall material in 500 ml of distilled water using magnetic stirring at 1000 rpm for 2 h. 5 ml of liquid SNEDDS formulation (F1) was added dropwise to the solution of wall material with continuous magnetic stirring at 1000 rpm to get feed solution for spray drying (Table 2). The spray drying feed solution was further stirred for 2 h before spray drying using Pilotech spray dryer (YC-015, Shanghai, China). The spray drying conditions used in the study include an inlet air temperature of 120 ºC, feed rate of 20 ml/minute, atomizing air pressure of 6 bar and outlet air temperature of 75 ºC. The solidified SNEDDS powder was collected in air-tight glass vials and stored in desiccator till further use. The chitosan-coated SNEDDS (F2) was similarly solidified as mentioned above.
Particle size analysis
The particle size and size distribution of the solidified SNEDDS were determined using Horiba particle size analyzer (Horiba Ltd., LA-300, Kyoto, Japan). Briefly, 5 mg of the sample was added to HPLC-grade cyclohexane, mixed thoroughly and vortexed for 2 min to homogeneously disperse the samples in dispersion medium. The liquid dispersion unit of the analyzer was supplemented with a homogeneous dispersion. The determination of the particle size distribution involved the measurement of light intensity scattered when a laser beam passed through the dispersed particulate sample25. The volume median diameter and span value were used to express the particle size distribution26.
Morphological features
The morphology of the solidified SNEDDS was assessed utilizing a scanning electron microscope (SEM). A quantity of 2 mg of the solidified SNEDDS was evenly distributed across the surface of a double-sided carbon adhesive tape that was affixed to an aluminum stub. The extra particles were eliminated by a delicate tapping technique, thereafter applying a platinum coating with a current intensity of 20 mA using an automated fine coater (JFC1600, JEOL Tokyo, Japan). The desired sections were photographed at different magnifications and the photograph of the desired magnification was shown to elaborate the morphology of solidified SNEDDS27.
Roughness and circularity
The roughness and the circularity of solidified SNEDDS were evaluated using ImageJ software (version 1.54i, NIH, Bethesda, MD, USA, https://imagej.net/ij/index.html)28. In this study, the scanning electron microscope (SEM) micrographs of optimized formulation were carefully chosen at an appropriate magnification level of 5000×. These images were then subjected to quantitative analysis using a specialized plugin to calculate the roughness and circularity values. The values were presented as the mean of five measurements.
Flow characteristics
The bulk and tap density of the powder-solidified SNEDDS were determined gravimetrically as mentioned previously29. Briefly, the weight of the solidified powder sample was determined. The powder sample was poured into 5 ml graduated cylinder to determine the bulk volume. The sample was tapped 500 times or till the tapped volume remained constant. The data of bulk and tapped density was used to calculate Carr’s index and Hausner’s ratio using Eqs. (1) and (2), respectively.
Drug content and entrapment efficiency
The drug content and entrapment efficiency of solidified SNEDDS were determined spectrophotometrically as mentioned previously30. Briefly, solidified SNEDDS (equivalent to 2 mg of atorvastatin) was added to 10 ml phosphate buffer having pH 7.4. The sample was stirred at 1000 rpm for 10 min until complete solubilization of pectin. The sample was then centrifuged at 4000 for 15 min using a benchtop centrifuge (Atlas Medical Italiano, Berlin, Germany). The drug content in the supernatant layer was determined spectrophotometrically (UV-1601, Shimadzu, Japan) at λmax of 242 nm, using similarly processed blank samples as control31.
To extract the entrapped medication from the solid SNEDDS, the sediment was combined with 1 ml of methanol and vortexed for 5 min. Then it was diluted with buffer having pH 7.4 and processed for determination of atorvastatin content as mentioned previously. The drug content was calculated by combining the drug present in both the supernatant and the sediment layer32. The drug entrapment efficiency was calculated as mentioned in Eq. (3).
X-ray diffraction analysis
The crystalline/amorphous nature of the solidified SNEDDS together with the nature of atorvastatin, chitosan and pectin was evaluated in intact form as well as in formulation. Briefly, the samples were placed in aluminum sample holder of X-ray diffractometer (Ultima IV, Rigaku Corporation, Tokyo, Japan). The samples were scanned within the range of 4 to 80 degrees and position 2 Theta (θ), with increments of 0.008 degrees, at a rate of 3 degrees per minute. This scanning process was conducted at an ambient temperature, utilizing a radiation source of Cu-Kα with a wavelength of 1.5418 A°, operating at a voltage of 40 kV and a current of 30 mA. The raw data was transformed into graphical format using Origin Pro 2021 (OriginLab Corporation in Northampton, MA, USA).
FTIR analysis
The ATR-FTIR analysis was performed to evaluate the possible interaction of the drug with formulation components in the solidified SNEDDS. The FTIR Spectra of atorvastatin, Tween 80, span 80, oleic acid, chitosan and the formulations (SF8-SF10) were obtained using ATR-FTIR (Perkin Elmer, Waltham, MA, USA) by scanning the components between 400 and 4000 cm− 133.
Reconstituted SNEDDS
The reconstituted solidified SNEDDS were assessed with respect to their particle size distribution, Zeta potential and morphological features as mentioned previously34. Briefly, 1 mg of the solidified SNEDDS was reconstituted with the same amount of solvent that was initially added before spray-drying. The reconstituted nanoemulsion was assessed for particle size and Zeta potential in triplicates using Zetasizer while morphological features of reconstituted nanoemulsion droplets was visualized using scanning electron microscope35.
In-vitro release behavior
The in-vitro release behavior of atorvastatin from the solidified SNEDDS was evaluated using the dialysis bag diffusion method36. Briefly, the specified amount of solidified SNEDDS (equivalent to 10 mg of atorvastatin) was introduced into the activated dialysis membrane (with a molecular weight cut-off diameter of 1.2 kDa, ZelluTrans/ROTH T4). The dialysis bag was introduced into 300 ml of dissolution medium mimicking the human lung environment. The dissolution medium, containing a sealed dialysis bag, was subjected to agitation at a speed of 50 rpm using a mechanical shaker (Biobase, Jinan, China) at a temperature of 37 ºC for a duration of 24 h. At specified time points 0.5, 1, 2, 4, 8, 16, and 24 h, a 5 ml sample of the medium was withdrawn and filtered using a syringe filter with a pore size of 0.45 μm (Durapore, Millipore Corporation, Cork, Ireland). The samples were analyzed using a UV-visible spectrophotometer at a λmax of 242 nm. The cumulative drug release (%) was plotted against the time intervals25. The data was further processed using DD-solver to determine the mechanism of drug release from the fabricated SNEDDS37.
Assessment of aerodynamic performance
The in-vitro aerodynamic performance of spray dried SNEDDS was evaluated using Anderson Cascade impactor (ACI, Copley Scientific, Nottingham, UK), that fractionates the aerosol based on aerodynamic diameter. The ACI was assembled from stage ‘0’ to filter. After stage 7, a glass fiber filter was inserted to collect the small particles that escape from the stage 7. The stages were sealed together with FDA approved silicone rubber O-rings (Copley Scientific Ltd, Nottingham, UK) to prevent inter-stage leak. The stage 0 was connected to pre-separator that joins induction port. The induction port was further attached to the mouthpiece adopter to provide an airtight seal between the induction port and the HANDIHALER device (Boehringer Ingelheim, Germany). The vacuum source was attached on the other side of ACI through a PVC tubing to create a pressure drop of 4 kPa through aerosol device. The ACI was assembled and operated in accordance with USP General Chapter < 601 > to assess the aerodynamic properties of solidified SNEDDS38.
The solidified SNEDDS (25 mg) was added to size ‘2’ hard gelatin capsule (Gelcap LTD, Karachi, Pakistan). The filled capsules were placed in the sample chamber of HANDIHALER. The capsules were pierced to activate the solidified SNEDDS powder into the ACI. The aspiration flow rate of 60 L/min was applied for 4 s to create a pressure drop of 4 kPa over the inhaler device, to aerosolize the capsule content. After aerosolization, the ACI was dismantled, and the powder deposited on different stages of ACI together with the induction port and pre-separator were carefully collected using phosphate buffer pH 7.4 into separate glass vials. The respective atorvastatin content was quantified in aerosol mass deposited on different stages of ACI spectrophotometrically at a λmax of 242 nm, as mentioned under drug content analysis section “Drug content and entrapment efficiency”. The data was processed to determine different aerodynamic parameters including percent dispersed (PD), percent inhaled (PI), mass median aerodynamic diameter (MMAD), geometric standard deviation (GSD), fine particle fraction (FPF) and respirable fraction (RF). Each value was expressed as the mean ± standard deviation.
In-vivo Pharmacokinetic analysis
A single dose pharmacokinetic study of pure atorvastatin, LIPITOR tablet and solidified SNEDDS (SF10) containing 10 mg of the drug was conducted in white albino male rabbits, purchased from National Institute of Health (NIH), Islamabad, Pakistan. This animal study was performed in accordance with ARRIVE guidelines. The protocols for the animal study were approved by the ethical review board (ERB) of Gomal University, Pakistan via letter number 118/ERB/GU and all experiments were performed in accordance with relevant guidelines and regulations. Healthy white albino male rabbits, weighing 2.0 to 2.5 kg were divided into 3 groups and each group comprised of 5 rabbits as recommended in previous studies39. All the rabbits were housed in animal house maintained on 12 h light/dark at room temperature 25 ºC with free access to water and food pellets. The rabbits were anesthesized using xylazine HCl at a dose rate of 5 mg/kg, intramuscularly (i.m), followed by Ketamine at a dose rate of 35 mg/kg, i.m. Pure atorvastatin was administered to Group I animals in the form of suspension in distilled water using microsprayer aerosolizer (Model IA-1B, Penn-century, USA). Group-II was administered with LIPITOR tablet orally while optimized solidified SNEDDS (F2) was given to Group-III using microsprayer aerosolizer. The blood sample (1 ml) was collected from the marginal ear vein of each rabbit and processed for determination of drug content using high performance liquid chromatographic (HPLC) analysis as mentioned earlier40. Briefly, the blood samples were subjected to centrifugation at 10,000 rpm for 10 min using high-speed mini-centrifuge (Microspin 12, Boeco, Germany). The supernatant plasma layer was collected in micro-centrifuge tubes and was kept in freezer at -20ºC until further use. The concentration of drug in the isolated plasma was determined by thawing the samples to ambient temperature followed by addition of methanol in equal volume ratio (1:1) for the proteins precipitation. The resulting mixture was vortexed for 10 min and then subjected to centrifugation at 10,000 rpm for further 10 min. The supernatant layer was isolated and dried using block heater and re-dissolved in mobile phase consisting of acetonitrile, stabilizer free tetrahydorfurane and buffer in a volume ratio of 27:20:53. The samples were vortexed for 5 min followed by centrifugation at 10,000 rpm for 5 min to get a clear supernatant layer that is filtered and analyzed for drug content using HPLC (1260 Infinity II, Agilent technologies, US).
Statistical analysis
The findings were presented as mean ± standard deviation. The data was analyzed using GraphPad Prism version 8 (San Diego, CA, USA). A statistically significant difference was indicated by a p-value of ≤ 0.05. The statistical tests employed in this study included the student’s t-test, ANOVA and Pearson correlation, when applicable.
Results and discussion
Optimization of spray dried SNEDDS
The spray dried SNEDDS were optimized with respect to particle size distribution and yield as mentioned in Table 3. The matrix former used in the solidification of optimized SNEDDS (F4) formulation includes lactose (SF1-SF3), mannitol (SF4-SF6) and pectin (SF7-SF9) in three different concentrations of 0.5%, 1% and 1.5% w/w. The powders were evaluated with respect to particle size distribution to get spray dried powder of ≤ 3 μm being suitable for systemic drug delivery via inhalation. The synthesis of lactose-based solidified SNEDDS was possible only when lactose concentration was above 0.5%. At lower lactose concentrations, the SNEDDS droplets were impossible to be dried efficiently (SF1, Table 3). The higher lactose concentration resulted in the synthesis of coarse particles with the diameter (D50) above 20 μm being not suitable for pulmonary drug delivery. To get particles of optimum diameter, the solidification of SNEDDS was attempted with mannitol. The solidified SNEDDS was in the particle size range of 10–23 μm (D50), well above the diameter needed for deep lung inhalation. In the next phase, pectin was used as a wall material for the solidification of SNEDDS using spray drying process. It was observed that SF8 was the optimum formulation with respect to particle size (≤ 3 μm) and percent yield (≥ 50%). Generally, the spray drying yield increased with increasing concentration of wall material (Table 3). The coating of SNEDDS droplets with chitosan (F2) when spray dried at already optimized spray drying conditions and wall material concentration of 1% pectin also resulted in the fabrication of optimum size particles. The smaller particle size of SF10 compared to SF8 was attributed to surfactant properties of chitosan at lower concentrations, which led to the atomization of smaller droplets. The smaller droplets were effectively dried due to higher inlet temperature thereby, resulting in the fabrication of compact particles with higher yield (Table 3).
Physicochemical characterization
Particle size distribution
The particle size and size distribution data of the solidified SNEDDS are shown in Table 4. The chitosan coated solidified SNEDDS (SF10) was found to be significantly smaller than simple solidified SNEDDS (SF8) (student t-test; p ≤ 0.01). The smaller particle size of SF10 was attributed to its compact nature being introduced due to the surface coating of chitosan during spray drying process41. This could be partly due to ionic interaction between the negatively charged oleic acid and positively charged chitosan, while surfactant properties of chitosan also augment the stabilization effect of added surfactants (Tween-80 and Span-80). The higher span value of SF8 depicts the heterogeneous nature of particles as compared to SF10. The chitosan reduced the tackiness of nanoemulsion thereby, resulting in the formation of relatively homogenous particles42.
Morphology
The morphological features of simple (SF8) and chitosan coated solidified SNEDDS (SF10) are shown in Fig. 1. The SEM analyses depict the porous nature of SF8, while SF10 has compact structure. The higher inlet air temperature of 120 ºC during spray drying of the feed solution (liquid SNEDDS and pectin) results in rapid evaporation of the aqueous phase leaving behind pores in the solidified SNEDDS (SF8). Previous studies have shown that the pores in the solidified SNEDDS are introduced when the inlet temperature of the spray dryer rises above the boiling point of the aqueous phase43. In SF10, no pores were observed in the solidified SNEDDS due to the presence of chitosan that stabilized the SNEDDS droplets during the spray drying process resulting in the formation of compact particles. The majority of the particles exist as separate entities with negligible agglomeration tendency (Fig. 1). However, some of the smaller particles accommodate themselves in the grooves of the larger particles. The majority of the solidified SNEDDS have dimples on their surface while some particles have cup-shaped morphology. The crust formed on the surface of the solidified SNEDDS was due to rapid evaporation of the aqueous phase at higher inlet temperature44. The particles of SF10 get doughnut shape for large particles while the compactness and homogeneity of other particles also increased (Fig. 1). When the hydrodynamic diameter of spray dried SNEDDS was compared, it was found that particle size data was in close resemblance to that of scanning electron microscopy results (Table 4Vs Fig. 1). Overall, the simple solidified SNEDDS (SF8) had irregular morphology when compared to its chitosan-coated counterpart due to higher concentration of surfactant that imparts plasticity to the particles during the drying process (Fig. 1). Furthermore, the presence of chitosan coating on the oil droplet surface forms a thicker shell around the oil droplets and thus prevents the oil leakage and coalescence during the spray drying process45.
Roughness and circularity
The surface properties that include surface area, morphology and roughness play a significant role in determining the inter particulate interactions, stability, ease of dispersion from DPI, and the de-agglomeration tendency of aerosol particles46. The surface roughness of solidified SNEDDS produced from reconstituted nanoemulsion with pectin (1% w/w) as wall material is shown in Table 4. The roughness of SF8 was significantly higher (student t-test; p ≤ 0.01) than SF10. The higher roughness together with the porous nature of SF8 was due to higher lipid content47. The particles have fissures and grooves that can accommodate smaller particles to help in the dispersion of these particles during aerosolization from HANDIHALER. Previous studies have shown that the higher roughness of particles was attributed to the higher association of nanoemulsion in the spray dried SNEDDS particles48.
The circularity of spray dried particles affects not only the aerosolization potential of the carrier, but also the inhaled fraction of DPI powder49. The higher circularity of DPI powders decreases the aggregation tendency of inhaled particles, thereby promoting improved aerodynamic performance50. The circularity of the chitosan coated solidified SNEDDS (SF10) was higher than its non-coated counterpart (Table 4). However, there was no significant difference between the circularity of the two formulations (SF8 Vs SF10, p ≥ 0.05).
Drug content and entrapment efficiency
The drug content and entrapment efficiency of solidified SNEDDS are shown in Table 4. The higher drug content and entrapment efficiency of SF10 was attributed to the presence of chitosan on nanoemulsion droplets being dried at higher inlet air temperature (120ºC). The additional shell of chitosan layer not only supports efficient drying of SNEDDS droplets encapsulating atorvastatin but also gives additional protection against the degradation of the drug during spray drying process. The drug content was in the range of 2.30–2.35 mg/g which indicates that the solidified SNEDDS components effectively preserved the drug during spray drying.
Flow properties
The aerodynamic performance of aerosol depends not only on the size and shape but also on the density of particles51. The large porous particles can be preferentially inhaled when compared to large dense particles52,53. The powder flow characteristics are important parameters to ensure the reproducible dosage emission from DPI’s. The theoretical aerodynamic diameter was found in the range of 1–2 μm depicts that the fabricated SNEDDS powder is suitable for deep lung inhalation (Table 5)54.
FTIR analysis
The FTIR spectra of pure atorvastatin, pectin, components of formulations, as well as the solidified SNEDDS (SF8 and SF10), are shown in Fig. 2 (A-H). The characteristic peaks of atorvastatin at 3364 cm− 1, 2968 cm− 1, 1650 cm− 1 and 1435 cm− 1 were attributed to amine group stretching, C-H stretching, carbonyl group C = O and hydroxyl groups respectively. The pectin gives spectral peaks at 3363 cm− 1, 1747 cm− 1 and 1442 cm− 1 corresponding to the OH, CO, and COC stretching respectively. The amino group of cationic polymer (chitosan) depicts spectral peaks at 1635 cm− 1 representing amide I, the backbone of chitosan. The other peaks in the FTIR spectra of chitosan at 3427 cm− 1 and 1531 cm− 1 correspond to the OH and amide II respectively. When the FTIR spectra of solidified SNEDDS (SF8 and SF10) were compared with the formulation components, the peak at 1639 cm− 1 in SF10 dictates the presence of chitosan in the spray dried particles. The appearance of spectral peaks at 2930 cm− 1 and 2940 cm− 1 in SF8 and SF10 corresponds to the matrix former material (pectin). The characteristic groups (NH, C = O) of atorvastatin intermingle with the spectral bands of chitosan and pectin showing successful encapsulation of the drug within the matrix former thereby resulting in an amorphous micro-particulate carrier that provides efficient absorption of the drug from the lung. The spectral peak of atorvastatin at 3364 cm− 1 disappears in SF8 while the same peak gets broader in SF10. The broadening of the peak in SF10 depicts that the amino group peaks of the drug intermingle with the spectral band of the OH group in the chitosan. The characteristic peak of the drug at 2968 cm− 1 was shifted to 2930 cm− 1 and 2940 cm− 1 in the SF8 and SF10 respectively, representing the preservation of the drug within the solidified SNEDDS55.
XRD analysis
The XRD pattern of atorvastatin, pectin, chitosan and solidified SNEDDS (SF8 and SF10) is shown in Fig. 3. The XRD diffractogram of atorvastatin shows numerous sharp peaks at diffraction angle of 9.41º, 10.18º, 16.21º and 19.12º depicting the crystalline nature of drug56. The broader peak at 14.61º of pectin depicts the amorphous nature of the matrix former57. The XRD analysis of chitosan depicts the broader peak at 2Ɵ value of 20.5º that is well matched with the literature58. The broader peak of chitosan illustrates its amorphous nature. There were no sharp peaks in the XRD diffractogram of SF8 and SF10 that illustrate the amorphous nature of solidified SNEDDS. The XRD results of the prepared formulations was in accordance with the results previously reported by Wong et al.59.
Reconstituted SNEDDS
The data of reconstituted SNEDDS is shown in Table 6. The particle size of the SNEDDS droplets increased significantly, however the nanostructure was retained. The increase in droplet size of F1 was higher than F2, due to higher steric stabilization of nanoemulsion due to an effective coating of chitosan60. The structural analysis using SEM have shown that the reconstituted nanoemulsion droplets were spherical. The morphological feature of the SNEDDS after reconstitution depicts that the hydrodynamic particle size was higher than its actual particle size being visualized using photon correlation spectroscopy. The larger particle size of the reconstituted nanoemulsion during zeta size analysis was due to multi-scattering phenomenon (Table 6)61. The PDI values lower than 0.5 that confirm the preserved nature of nanoemulsion in the solidified SNEDDS (Table 6). When the reconstituted nanoemulsion was compared to its primary nanoemulsion (F1 & F2; Table 6), the droplet size was found to be higher in reconstituted nanoemulsion (S1). This phenomenon was due to the agglomeration of oil droplets during spray drying process (Table 6). The decrease in Zeta potential values of SF8 and increase in case of SF10 based nanoemulsion was due to the masking of charge during spray drying process (S2)62. Overall, the anionic polysaccharides pectin acts efficiently as wall material to preserve the SNEDDS droplets during spray drying process.
In-vitro drug release
The release pattern of solidified SNEDDS in simulated lung fluid (phosphate buffer, pH 7.4) is shown in Fig. 4. The drug dissolution from both formulations (SF8 and SF10) follows biphasic release pattern whereby, the first 20 to 30% of the drug released within first 1 h, followed by sustained drug release. The burst drug release was significantly higher in case of SF8 (p ≤ 0.05) due to its porous structure, amorphous nature and small size of its associated nanoemulsion (F4). In case of SF10, the chitosan coated solidified nanoemulsion droplets significantly reduced the burst drug release (20%) due to compact structure, relatively crystalline nature as well as large droplets of nanoemulsion (F5). Additionally, the chitosan coated droplets further retards the drug release from the SNEDDS. The sustained drug release pattern of the solidified SNEDDS was attributed to the nature of the drug (BCS-II), dosage form (SNEDDS) as well as the physicochemical characteristics of solidified SNEDDS63. The results were in close resemblance to previously reported studies whereby nanoemulsion droplets were encapsulated within the matrix former19.
The mechanism of drug release from solidified SNEDDS was evaluated using different kinetic models including zero order, first order, Higuchi model and korsmayer-peppas model. The parameters of above kinetic models are shown in Table 7. The different values of correlation coefficient (R2) indicated the best fit to kinetic model. The in-vitro release data followed the korsmeyer-peppas model with R2 of approximately 1. The n-value of less than 0.5 indicates Fickian diffusion as mentioned in Table 7. The Fickian diffusion of drug from the solidified SNEDDS depicts sustained drug release profile due to the presence of pectin in the spray dried SNEDDS particles64.
Aerodynamic behavior
The aerosol dispersion and aerodynamic performance of solidified SNEDDS (SF8 and SF10) from HANDIHALER using ACI are shown in Table 8. Both the formulations were well dispersed without the addition of large carriers with emitted dose of 0.19 to 0.20 mg for SF10 and SF8 respectively. The dispersed fraction of SF8 was higher than SF10 due to its large particle size, higher roughness at the nanoscale and lower tapped density (Table 4Vs Tables 5 and 5Vs Table 8). The emitted dose of our fabricated solidified SNEDDS was higher than 60%, that is considered adequate for inhalation products50. The aerodynamic diameter of aerosol particles ≤ 5 μm is suggested for localized treatment within the lung while ≤ 3 μm has been recommended for systemic drug delivery through the lung65. An MMAD of 1.0 to 1.5 μm for fabricated SNEDDS particles elaborates their suitability for systemic delivery of atorvastatin via DPI65. The relatively lower MMAD was attributed to the higher inhaled fraction of aerosol from the solidified SNEDDS (70%, Table 8). There was no significant difference in the aerodynamic particle size distribution with GSD value of around 3.0. The fine particle and respirable fractions were evaluated at two different levels i.e. ≤3 μm and ≤ 5 μm. It was observed that the FPF≤ 3 μm of SF10 was significantly higher than SF8 due to small particle size and relatively spherical structure (p ≤ 0.05; Table 8Vs Table 3). When the aerodynamic performance of our solidified nanoemulsion was compared to encapsulated liposomes, it was observed that higher FPF was achieved without the addition of large carrier35.
When the aerosol deposition on different stages of ACI was evaluated, maximum deposition of SNEDDS particles was achieved on stage 5, 6 and 7 corresponding to an aerodynamic cut-off diameter of ≤ 1.0 μm (Fig. 5). The drug retention in the pre-separator (PS) and throat (IP) may be considered as extrathoracic loss of atorvastatin that was found to be significantly lower than formulations developed with large carriers66. This trend of aerosol deposition in the lung elaborates the success of designed solidified SNEDDS with respect to systemic drug delivery through the lung.
In-vivo pharmacokinetic analysis
The single dose pharmacokinetic profile of atorvastatin, LIPITOR and Solidified SNEDDS had been shown in Table 9. The atorvastatin achieved significantly higher AUC0 − t for solidified SNEDDS formulation as compared to pure atorvastatin and LIPITOR. The spray dried solidified SNEDDS get dissolved in the lung fluid resulting in the liberation of SNEDDS droplets. The liberated oily droplets stabilized by surfactants with the particle size of 272.7 nm, were efficiently absorbed resulting in significantly higher AUC values (305.98 ± 26.05 ng/ml*h) when compared to pure atorvastatin (136.13 ± 16.75 ng/ml*h) and LIPITOR (220.06 ± 15.60 ng/ml*h). The higher AUC could be further attributed to lower first-pass metabolism in the lung when compared to orally administered formulation (LIPITOR). The pure atorvastatin achieved its maximum plasma concentration within 4 h in comparison to LIPITOR and inhaled SNEDDS that reached its Cmax within 2 h. The atorvastatin being class II drug dissolved slowly in the lung fluid allowing sufficiently longer time for absorption as well as elimination (Ke) from the body. The slow elimination of inhaled atorvastatin results in higher MRT values as compared to its counterparts (LIPITOR and solidified SNEDDS). However, the atorvastatin being a foreign material was engulfed by the alveolar macrophages making it therapeutically less effective from marketed as well as inhaled solidified SNEDDS formulation (SF10).
The data in Fig. 6 depicts that the atorvastatin achieved its therapeutic concentration within 2 h of administration of atorvastatin through oral or intra-tracheal instillation. The drug maintains its therapeutic level within the plasma for its hypolipidemic effect due to its higher MRT values. Overall, the solidified SNEDDS was found to be superior with respect to its bioavailability as shown by the AUC values being 1.5 times higher than the marketed tablets (LIPITOR).
Conclusion
The simple and chitosan decorated SNEDDS were solidified by spray drying method using pectin as matrix former to produce nanoemulsion loaded microspheres. The chitosan decorated solidified SNEDDS (SF10) was superior to SF8 based on physicochemical properties, morphological features and aerodynamic performance for systemic delivery of atorvastatin. The chitosan decorated solidified SNEDDS achieved dispersed fraction of 85% with MMAD of 1.24 μm that supports higher FPF of 54% when delivered via HANDIHALER. The deposition pattern of aerosol on different stages of ACI elaborates maximum aerosol deposition on stages 5, 6 and 7 with an aerodynamic cut-off diameter of ≤ 1 μm that highlights the importance of solidified SNEDDS for systemic delivery of atorvastatin. The single dose pharmacokinetic study depicted that the chitosan decorated solidified SNEDDS achieved higher bioavailability than the marketed tablets (LIPITOR). Though, the fabricated SNEDDS served as an alternative platform for the systemic delivery of atorvastatin through the lungs. However, the toxicological evaluation of the designed carriers and the effect of DPI on the performance of drug loaded carriers first needs to be investigated before further studies.
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.
References
d’Água, R. B. & Pereira, J. in Journal of Aerosol Medicine and Pulmonary Drug A16-A16 (Mary Ann Liebert, Inc 140 Huguenot Street, 3rd FL, New Rochelle, NY 10801 USA).
Chassot, J. M. et al. Beclomethasone dipropionate-loaded polymeric nanocapsules: development, in vitro cytotoxicity, and in vivo evaluation of acute lung injury. J. Nanosci. Nanotechnol. 15, 855–864 (2015).
Bajracharya, R., Song, J. G., Back, S. Y. & Han, H. K. Recent advancements in non-invasive formulations for protein drug delivery. Comput. Struct. Biotechnol. J. 17, 1290–1308 (2019).
Ghadiri, M., Young, P. M. & Traini, D. Strategies to enhance drug absorption via nasal and pulmonary routes. Pharmaceutics 11, 113 (2019).
Paranjpe, M. & Müller-Goymann, C. C. Nanoparticle-mediated pulmonary drug delivery: a review. Int. J. Mol. Sci. 15, 5852–5873 (2014).
Jain, H., Bairagi, A., Srivastava, S. & Singh, S. B. Mehra, N. K. Recent advances in the development of microparticles for pulmonary administration. Drug Discovery Today. 25, 1865–1872 (2020).
Dong, W. et al. Preparation, characterization, and in vitro/vivo evaluation of polymer-assisting formulation of Atorvastatin calcium based on solid dispersion technique. Asian J. Pharm. Sci. 13, 546–554 (2018).
Tulbah, A. S. & Gamal, A. Design and characterization of Atorvastatin dry powder formulation as a potential lung cancer treatment. Saudi Pharm. J. 29, 1449–1457 (2021).
Eita, A. S., Makky, M. A., Anter, A., Khalil, I. A. & A. & Repurposing of Atorvastatin emulsomes as a topical antifungal agent. Drug Deliv. 29, 3414–3431 (2022).
Bradbury, P., Traini, D., Ammit, A. J., Young, P. M. & Ong, H. X. Repurposing of Statins via inhalation to treat lung inflammatory conditions. Adv. Drug Deliv. Rev. 133, 93–106 (2018).
Tiwari, R. & Pathak, K. Statins therapy: a review on conventional and novel formulation approaches. J. Pharm. Pharmacol. 63, 983–998 (2011).
Shaker, M. A., Elbadawy, H. M., Thagfan, A., Shaker, M. A. & S. S. & Enhancement of Atorvastatin oral bioavailability via encapsulation in polymeric nanoparticles. Int. J. Pharm. 592, 120077 (2021).
Fayed, N. D., Goda, A. E., Essa, E. A. & El Maghraby, G. M. Chitosan-encapsulated niosomes for enhanced oral delivery of Atorvastatin. J. Drug Deliv. Sci. Technol. 66, 102866 (2021).
Tulbah, A. S. Inhaled Atorvastatin nanoparticles for lung cancer. Curr. Drug Deliv. 19, 1073–1082 (2022).
Ishak, R. A. & Osman, R. Lecithin/TPGS-based spray-dried self-microemulsifying drug delivery systems: in vitro pulmonary deposition and cytotoxicity. Int. J. Pharm. 485, 249–260 (2015).
Jung, S. & Fraser, R. Development and functional anatomy of the respiratory system. In Cotes’ Lung Function, (eds Maynard, R.L., Pearce, S.J., Nemery, B., Wagner, P.D., and Cooper, B.G.). https://doi.org/10.1002/9781118597309.ch3 33–43 (2020).
Kole, E. et al. Nanotherapeutics for pulmonary drug delivery: an emerging approach to overcome respiratory diseases. J. Drug Deliv. Sci. Technol. 81, 104261 (2023).
Akhtar, N. et al. Self-Generating nano-emulsification techniques for alternatively-routed, bioavailability enhanced delivery, especially for anti-cancers, anti-diabetics, and miscellaneous drugs of natural, and synthetic origins. J. Drug Deliv. Sci. Technol. 58, 101808. https://doi.org/10.1016/j.jddst.2020.101808 (2020).
Shah, K., Chan, L. W. & Wong, T. W. Conversion of liquid chitosan-based nanoemulsions into inhalable solid microparticles: process challenges with polysaccharide. Int. J. Biol. Macromol. 253, 126991 (2023).
Mehta, P. Imagine the superiority of dry powder inhalers from carrier engineering. J. Drug. Deliv. 2018, 5635010 (2018).
Merchant, J., Müllertz, A., Rades, T. & Bannow, J. Functionalized calcium carbonate (FCC) as a novel carrier to solidify supersaturated self-nanoemulsifying drug delivery systems (super-SNEDDS). Eur. J. Pharm. Biopharm. 193, 198–207 (2023).
Harugade, A., Sherje, A. P., Pethe, A. & Chitosan A review on properties, biological activities and recent progress in biomedical applications. Reactive Funct. Polymers, 191, 105634 https://doi.org/10.1016/j.reactfunctpolym.2023.105634 (2023).
Saeed, M. D. et al. Self-nanoemulsifying drug delivery system for nebulization: fabrication and evaluation for systemic delivery of Atorvastatin. Naunyn. Schmiedebergs Arch. Pharmacol. https://doi.org/10.1007/s00210-024-03494-w (2024).
Adams, S. P., Tsang, M. & Wright, J. M. Atorvastatin for lowering lipids. Cochrane Database Syst. Reviews https://doi.org/10.1002/14651858.CD008226.pub3(2015).
Naz, F. F. et al. Polymeric microparticles: synthesis, characterization and in vitro evaluation for pulmonary delivery of rifampicin. Polymers 14, 2491 (2022).
Aghdam, M. H., Ghanbarzadeh, S., Javadzadeh, Y. & Hamishehkar, H. Aggregated nanotransfersomal dry powder inhalation of Itraconazole for pulmonary drug delivery. Adv. Pharm. Bull. 6, 57 (2016).
Khalid, W. et al. 5-Fluorouracil-Loaded hyaluronic Acid-Coated Niosomal vesicles: fabrication and ex vivo evaluation for skin drug delivery. ACS Omega 8(48), 45405–45413 https://doi.org/10.1021/acsomega.3c04457 (2023).
Alhajj, N. et al. Critical physicochemical attributes of Chitosan nanoparticles admixed lactose-PEG 3000 microparticles in pulmonary inhalation. Asian J. Pharm. Sci. 15, 374–384 (2020).
Ghosh, D. et al. Impact of solidification on micromeritic properties and dissolution rate of self-nanoemulsifying delivery system loaded with docosahexaenoic acid. Drug Dev. Ind. Pharm. 46, 597–605 (2020).
Mohanty, D. et al. Preparation and evaluation of transdermal Naproxen niosomes: formulation optimization to preclinical anti-inflammatory assessment on murine model. J. Liposome Res. 30, 377–387 (2020).
Burki, F. A. et al. Optimization of chitosan decorated solid lipid nanoparticles for improved flurbiprofen transdermal delivery. JDDST-D-22-00271 8(22), 19302–19310 (2023).
Nawaz, A. & Wong, T. W. Microwave as skin permeation enhancer for transdermal drug delivery of chitosan-5-fluorouracil nanoparticles. Carbohydr. Polym. 157, 906–919 (2017).
Singh, H. et al. Development and characterization of solid-SNEDDS formulation of DHA using hydrophilic carrier with improved shelf life, oxidative stability and therapeutic activity. J. Drug Deliv. Sci. Technol. 54, 101326 (2019).
Irfan, M. M. et al. Impact of formulation design and lyophilisation on the physicochemical characteristics of finasteride nanosystems. J. Microencapsul. 40, 106–123 (2023).
Honmane, S., Hajare, A., More, H., Osmani, R. A. M. & Salunkhe, S. Lung delivery of nanoliposomal salbutamol sulfate dry powder inhalation for facilitated asthma therapy. J. Liposome Res. 29, 332–342. https://doi.org/10.1080/08982104.2018.1531022 (2019).
Li, L., Zhou, C. H. & Xu, Z. P. in Nanocarriers for drug delivery 421–449 Elsevier, (2019).
Jadach, B., Misek, M. & Ferlak, J. Comparison of hydroxypropyl Methylcellulose and alginate gel films with meloxicam as fast orodispersible drug delivery. Gels 9, 687 (2023).
Kang, J. H., Yang, M. S., Kwon, T. K., Kim, D. W. & Park, C. W. Inhaled deep eutectic solvent based-nanoemulsion of Pirfenidone in idiopathic pulmonary fibrosis. J. Controlled Release. 352, 570–585 (2022).
Biswal, P. K., Pani, N. R. & Dixit, P. K. Effects of carbohydrate polymers in self-microemulsified tablets on the bioavailability of atorvastatin: in vitro–in vivo study. Life Sci. 135, 92–100 (2015).
Shulyak, N. et al. Development of a novel, fast, simple HPLC method for determination of Atorvastatin and its impurities in tablets. Sci. Pharm. 89, 16 (2021).
Pourashouri, P. et al. Impact of wall materials on physicochemical properties of microencapsulated fish oil by spray drying. Food Bioprocess Technol. 7, 2354–2365 (2014).
Chaudhary, S., Kumar, S., Kumar, V. & Sharma, R. Chitosan nanoemulsions as advanced edible coatings for fruits and vegetables: composition, fabrication and developments in last decade. Int. J. Biol. Macromol. 152, 154–170 (2020).
Botrel, D. A. et al. Application of cashew tree gum on the production and stability of spray-dried fish oil. Food Chem. 221, 1522–1529 (2017).
Dukovski, B. J. et al. Spray-dried nanoparticle-loaded pectin microspheres for dexamethasone nasal delivery. Drying Technology 37(15), (2019).
Sodalee, K. et al. Preparation of redispersible dry nanoemulsion using chitosan-octenyl succinic anhydride starch polyelectrolyte complex as stabilizer. J. Drug Deliv. Sci. Technol. 73, 103433 (2022).
Peng, T. et al. Influence of physical properties of carrier on the performance of dry powder inhalers. Acta Pharm. Sinica B. 6, 308–318 (2016).
Kwamman, Y. & Klinkesorn, U. Influence of oil load and maltodextrin concentration on properties of tuna oil microcapsules encapsulated in two-layer membrane. Drying Technol. 33, 854–864 (2015).
Noello, C., Carvalho, A., Silva, V. M. & Hubinger, M. D. Spray dried microparticles of Chia oil using emulsion stabilized by Whey protein concentrate and pectin by electrostatic deposition. Food Res. Int. 89, 549–557 (2016).
Hamishehkar, H. et al. Effect of carrier morphology and surface characteristics on the development of respirable PLGA microcapsules for sustained-release pulmonary delivery of insulin. Int. J. Pharm. 389, 74–85 (2010).
Healy, A. M., Amaro, M. I., Paluch, K. J. & Tajber, L. Dry powders for oral inhalation free of lactose carrier particles. Adv. Drug Deliv. Rev. 75, 32–52 (2014).
Littringer, E. M. et al. Spray dried mannitol carrier particles with tailored surface properties–The influence of carrier surface roughness and shape. Eur. J. Pharm. Biopharm. 82, 194–204 (2012).
Patel, B., Gupta, V. & Ahsan, F. PEG–PLGA based large porous particles for pulmonary delivery of a highly soluble drug, low molecular weight heparin. J. Controlled Release. 162, 310–320 (2012).
Ullah, F. et al. Synthesis, characterization and in vitro evaluation of Chitosan nanoparticles physically admixed with lactose microspheres for pulmonary delivery of Montelukast. Polymers 14, 3564 (2022).
Agnihotri, V., Agrawal, Y., Goyal, S., Sharma, C. & Ojha, S. An update on advancements and challenges in inhalational drug delivery for pulmonary arterial hypertension. Molecules 27, 3490 (2022).
Ravindran, A. & C. H, A. & BSA nanoparticle loaded Atorvastatin calcium-a new facet for an old drug. PloS One. 9, e86317 (2014).
Choudhary, A., Rana, A. C., Aggarwal, G., Kumar, V. & Zakir, F. Development and characterization of an Atorvastatin solid dispersion formulation using skimmed milk for improved oral bioavailability. Acta Pharm. Sinica B. 2, 421–428 (2012).
Wang, W. et al. Characterization of pectin from grapefruit peel: A comparison of ultrasound-assisted and conventional heating extractions. Food Hydrocoll. 61, 730–739 (2016).
Govindan, S., Nivethaa, E., Saravanan, R., Narayanan, V. & Stephen, A. Synthesis and characterization of chitosan–silver nanocomposite. Appl. Nanosci. 2, 299–303 (2012).
Wong, P. C. H., Heng, P. W. S. & Chan, L. W. Determination of solid state characteristics of spray-congealed ibuprofen solid lipid microparticles and their impact on sustaining drug release. Mol. Pharm. 12, 1592–1604 (2015).
Franconetti, A., Carnerero, J. M., Prado-Gotor, R., Cabrera-Escribano, F. & Jaime, C. Chitosan as a capping agent: insights on the stabilization of gold nanoparticles. Carbohydr. Polym. 207, 806–814 (2019).
Teo, A., Lam, Y., Lee, S. J. & Goh, K. K. Spray drying of Whey protein stabilized nanoemulsions containing different wall materials–maltodextrin or Trehalose. LWT 136, 110344 (2021).
Masum, A., Chandrapala, J., Adhikari, B., Huppertz, T. & Zisu, B. Effect of lactose-to-maltodextrin ratio on emulsion stability and physicochemical properties of spray-dried infant milk formula powders. J. Food Eng. 254, 34–41 (2019).
Shakeel, F., Haq, N., Alanazi, F. K. & Alsarra, I. A. Polymeric solid self-nanoemulsifying drug delivery system of Glibenclamide using coffee husk as a low cost biosorbent. Powder Technol. 256, 352–360 (2014).
Hanmantrao, M. et al. Development of Guar Gum-Pectin-Based Colon targeted solid Self-Nanoemulsifying drug delivery system of Xanthohumol. Pharmaceutics 14, 2384 (2022).
Ceschan, N. E., Bucala, V., Mateos, M. V. & Smyth, H. D. C. Ramírez-Rigo, M. V. Carrier free indomethacin microparticles for dry powder inhalation. Int. J. Pharm. 549, 169–178 (2018).
Deb, P. K. et al. Elsevier,. in Drug Delivery Systems 521–577 (2020).
Acknowledgements
The authors would like to extend their sincere appreciation to the Ongoing Research Funding Program (ORF-2025-1118), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
Conceptualization, original draft writing, reviewing, and editing: Muhammad Danish Saeed, Kifayat Ullah Shah. Formal analysis, investigations, funding acquisition, reviewing, and editing: Khalid S. Almaary, Musaab Dauelbait. Resources, data validation, data curation, and supervision: Faiqa Falak Naz, Asif Nawaz, Mohammed Bourhia.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Statement of human and animal rights
This study was performed in accordance with ARRIVE guidelines. The protocols for the animal study were approved by the ethical review board (ERB) of Gomal University, Pakistan via letter number 118/ERB/GU and all experiments were performed in accordance with relevant guidelines and regulations.
This article does not contain any studies with human subjects.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Saeed, M.D., Shah, K.U., Nawaz, A. et al. Fabrication and evaluation of solidified nanoemulsion designs for systemic delivery of atorvastatin through the lung. Sci Rep 15, 20884 (2025). https://doi.org/10.1038/s41598-025-05646-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-05646-1