Abstract
Bronchopulmonary dysplasia (BPD) is a major complication in preterm infants, particularly those born before 33 weeks of gestation. Inhaled nitric oxide (iNO) is widely used to manage pulmonary hypertension (PH) and improve oxygenation, but its role in reducing BPD incidence in preterm infants with PH during the early postnatal period remains unclear. This study aimed to evaluate the impact of early iNO administration, both alone and in combination with pulmonary surfactant (PS), on the incidence of BPD in preterm infants diagnosed with PH within the first three days of life. A retrospective cohort study was conducted on 56 preterm infants (< 33 weeks gestation) with confirmed PH and hypoxemia (PaO₂ < 50 mmHg at FiO₂ ≥ 30%). Clinical outcomes, including BPD incidence, were compared between infants receiving iNO and/or PS and those who did not. Multivariate logistic regression was used to identify independent predictors of BPD. The incidence of BPD was significantly lower in the iNO group (15%) compared to the non-iNO group (63.9%) (P = 0.012). Infants receiving both iNO and PS demonstrated the best outcomes, with a marked reduction in BPD risk. Male gender and lack of PS therapy were associated with increased BPD risk. Multivariate analysis confirmed iNO (OR = 0.097, 95% CI: 0.014–0.682; P = 0.019) and PS (OR = 0.125, 95% CI: 0.021–0.728; P = 0.021) as independent protective factors against BPD. Early administration of iNO, particularly in combination with PS, significantly reduces the incidence of BPD in preterm infants with PH. These findings highlight the potential benefits of iNO and PS as preventive therapies in this high-risk population. Further prospective studies are needed to validate these results and guide clinical practice.
Similar content being viewed by others
Introduction
Bronchopulmonary dysplasia (BPD) is a chronic lung disease predominantly affecting preterm infants, characterized by impaired lung development and persistent respiratory symptoms. Epidemiological studies estimate that BPD affects approximately 10–15% of very low birth weight infants, with higher prevalence in those born before 32 weeks of gestation1. The development of BPD is multifactorial, involving a complex interplay between prenatal and postnatal factors. Prenatal contributors include maternal health conditions, intrauterine inflammation, and placental insufficiency, all of which can impair fetal lung development2,3. Postnatal risk factors such as oxygen toxicity, ventilator-induced lung injury, sepsis, and the presence of a patent ductus arteriosus (PDA) further exacerbate pulmonary damage in the premature infant. These combined insults disrupt normal alveolarization and pulmonary vascular development, ultimately leading to chronic respiratory disease and a spectrum of long-term complications.
Pulmonary Hypertension (PH), defined by elevated pulmonary vascular resistance and right ventricular strain, presents additional clinical challenges in preterm infants. The incidence of PH in this group is estimated to be 1–2%4 but can reach up to 10% in high-risk populations5. PH exacerbates oxygenation issues and heightens the risk of BPD. Conventional treatments, such as mechanical ventilation and supplemental oxygen, are essential but also increase BPD risk due to oxidative stress and inflammation.
Inhaled nitric oxide (iNO) is an established treatment for improving oxygenation and reducing the need for extracorporeal membrane oxygenation (ECMO) in term and near-term infants (> 34 weeks’ gestation) with hypoxic respiratory failure associated with PH6 .Although iNO has demonstrated potential in augmenting oxygen delivery and alleviating respiratory distress in preterm infants with severe respiratory failure who are less than 34 weeks of gestational age, the outcomes reported in various studies continue to exhibit inconsistencies. Some studies report significant benefits and improvements7,8,9,10, whereas others fail to demonstrate such outcomes. In fact, most studies have not demonstrated a significant benefit of iNO in this context1,49,10,11,12,13.
However, it should be noted that in all the aforementioned studies, the population under investigation for the use of iNO consisted of premature infants suffering from respiratory failure, or participants in randomized controlled trials, wherein PH was not explicitly designated as the primary indication for the administration of iNO. Given the high stakes involved in the management of BPD and PH, particularly in the vulnerable population of preterm infants, the potential of iNO as a therapeutic option remains an area of active research and investigation. Further research is necessary to establish a definitive conclusion regarding its efficacy and reliability.
To determine the effects of treatment with iNO on short-term outcomes in preterm newborn infants, we concentrate on observing the administration of iNO within three days post-birth in premature infants under 33 weeks of gestational age exhibiting clear signs of PH.
Methodology
Study design and participants
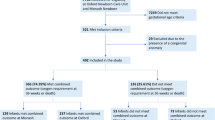
This retrospective cohort study analyzed premature infants admitted to the Neonatal Intensive Care Unit (NICU) at Xiangya Hospital, Central South University, from January 1, 2017, to July 1, 2023. The primary objective was to evaluate the incidence of BPD and associated factors in infants born before 33 weeks of gestation with confirmed PH. Ethical approval was obtained from Xiangya Hospital, and patient data were anonymized for privacy.
Inclusion and exclusion criteria
Inclusion criteria
Gestational ages between 24 + 1 weeks and 33 weeks infant manifested with hypoxemia (PaO₂ < 50 mmHg) on the condition of fraction of inspired oxygen (FiO₂) ≥ 30% within three days after birth, and cardiac ultrasound showed the following indicators: Right-to-left or bidirectional shunting through a patent ductus arteriosus (DA) with or without foramen ovale (PFO), and/or right ventricular systolic pressure (RVSP) > 30 mmHg.
Exclusion criteria
Infants were excluded from the study if they fulfilled any of the following criteria: Had been transferred from another hospital; PH resulting from congenital heart disease; or exhibited other severe congenital malformations.
Adverse short-term outcomes
Which encompass pneumothorax, BPD at any severity, retinopathy of prematurity (ROP) at any stage, intraventricular hemorrhage (IVH) of Grade II or higher, necrotizing enterocolitis (NEC) at stage IIB or above, hypothyroidism, and mortality at any time from birth to discharge. Infants were diagnosed with BPD was applied to infants who required any form of respiratory support for adequate oxygenation at 36 weeks postmenstrual age according to the 2018 diagnostic criteria8.The occurrence of systemic vasodilation side effects is signaled by the utilization of any vasoactive medications during the administration of iNO.
Main intervention
iNO was administered at a concentration of 20 parts per million (ppm) by the iNO inhaler (Guangdong Foshan Analyzer Co., Ltd.) via mechanical ventilation initially, as per the attending neonatologist’s clinical judgment. The therapy was initiated due to indications such as hypoxic respiratory failure and PH, which guided the decision-making process. If there is oxygenation response, inspired oxygen concentration is first weaned below 60% and then iNO is weaned only if PaO2 can be maintained ≥ 60 mmHg (or preductal SpO2 ≥ 90%) for 60 min, we wean iNO at a rate of 5 ppm every 4 h.Once the dose reached 5 ppm, further reductions were made in increments of 1 ppm every 30 min to 2 h, ensuring that the infant maintained adequate oxygenation with FiO₂ below 60% before discontinuation4,11,12,13 .This protocol is consistent with recommendations from the American Academy of Pediatrics (AAP) and the European Consensus Guidelines on the Management of Respiratory Distress Syndrome in Preterm Infants14.Pulmonary surfactant (PS) was administered at a dose of 200 mg/kg using Curosurf (Chiesi Pharmaceutical SpA), adhering to the following criteria: diagnosis of RDS via pulmonary ultrasound or X-ray, accompanied by PaO2 < 50 mmHg and FiO2 at least 30%, guided by criteria established in NICU protocols globally.
Statistical analysis
Data analysis was conducted using SPSS 29 (IBM Corporation, Armonk, NY, USA). Continuous variables were analyzed using the Kruskal-Wallis test (for comparisons among more than two groups) and the Mann-Whitney U test (for pairwise comparisons), as these variables did not follow a normal distribution. For categorical variables, the chi-square test was used, and Fisher’s exact test was employed when any expected cell count was less than 5.
To identify independent predictors of BPD, we performed a multivariate logistic regression analysis using the stepwise regression method, which incorporated both forward selection (including variables with P < 0.05) and backward elimination (removing variables with P > 0.10). The final model was selected based on the Akaike Information Criterion (AIC) to achieve an optimal balance between model complexity and predictive accuracy. Odds ratios (ORs) with 95% confidence intervals (CI) were calculated for each predictor, and P-values were determined using the Wald chi-square test to assess statistical significance.
A p-value of < 0.05 was considered statistically significant for all tests.
Results
A comparison of perinatal conditions and short-term outcomes was performed between BPD and non-BPD preterm infants with PH (Table 1)
A total of 56 preterm infants, born before 33 weeks of gestation, developed PH within the initial three days of life. These infants were diagnosed with PH using cardiac ultrasound and displayed hypoxemia (PaO2 < 50 mmHg) when the fraction of inspired oxygen (FiO2) was at or above 30%.
Aside from the gestational age at birth(P = 0.011), no significant differences were noted in other perinatal factors, including gender, birth weight, mode of delivery (Cesarean), twin birth, small for gestational age (SGA), premature rupture of membranes (PROM), gestational diabetes mellitus (GDM), abortion history, pregnancy-induced hypertension (PIH), and the occurrence of asphyxia. There are no differences among the early comorbidities, such as respiratory distress syndrome (RDS), early-onset sepsis (EOS), patent ductus arteriosus (PDA), pneumothorax, and meconium aspiration syndrome (MAS).
There was no statistically significant difference in the rates of mechanical ventilation and in the Partial Pressure of Oxygen (PaO2)/ Fraction of Inspired Oxygen (FiO2) (P/F ratio) before and 24 h after treatment between with BPD and those without. It is important to note that the use of PS (P = 0.008) and iNO (P = 0.001) was significantly higher in the non-BPD group. ROP was significantly more common in the BPD group (P = 0.003). Vasoconstrictive drugs were used more frequently in the non-BPD group(P < 0.001). Other adverse outcomes, including IVH, NEC, and mortality, did not differ significantly between the two groups.
The characteristics of preterm infants who received iNO versus those who did not are detailed in Table 2
All cases treated with iNO exhibited no signs of methemoglobinemia, with methemoglobin levels consistently below 3%. An analysis of maternal and infant perinatal factors revealed no statistically significant differences between the iNO and N-iNO groups.
Among early comorbidities, only EOS showed a significant difference, with a higher incidence in the iNO group (P = 0.003). The P/F ratio outcomes before and 24 h after treatment showed no significant variation between the groups.
Regarding treatment strategies, milrinone use was significantly more common in the iNO group (P = 0.02), while vasoconstrictive drug use was not influenced by iNO therapy. Notably, the iNO group demonstrated significantly lower rates of BPD (P = 0.01).
The clinical characteristics of PH infants with and without PS administration are provided in Table 3
Given that the previous findings indicated a higher rate of PS usage in the group without BPD, we conducted a further analysis of the groups based on their PS usage. Upon examining the PS and non-PS groups, a notable disparity in gender distribution was observed, with the surfactant group consisting of 11 males and 19 females, in contrast to the non-surfactant group’s 19 males and 7 females (P = 0.006). Additionally, the incidence of RDS was markedly elevated in the PS group (P < 0.001). However, the PS group reported a lower incidence of BPD (P = 0.008). Other perinatal factors, early comorbidities, OI, and adverse outcomes did not exhibit significant variations between the groups.
The clinical characteristics of PH patients treated with different combinations of iNO and PS are summarized in Table 4
Gender distribution varied significantly among groups (p = 0.022), with more males in the iNO-only (5:1) and no iNO/PS (15:6) groups.The incidence of RDS was significantly higher in the groups that received iNO + PS (78.6%) and only PS (75%), respectively, when compared to the two control groups that did not undergo PS treatment (P = 0.004). Likewise, the occurrence of EOS was more prevalent in the iNO + PS (78.6%) and only iNO (83.3%) groups, in stark contrast to the two control groups that did not receive iNO administration (P = 0.024). When compared to the groups that received only PS (43.8%) and those that received neither iNO nor PS (80%), a notable reduction in BPD was observed in the groups that were treated with iNO + PS (14.3%) and iNO alone (16.7%) (P < 0.001).Other perinatal factors and adverse outcomes did not exhibit significant differences among the four groups.
Multivariate analysis of the impact of PH treatment on BPD
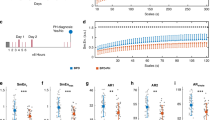
The results of an exhaustive multifactorial analysis, which omitted variables pertaining to perinatal, demographic, and early-life comorbidities—including EOS, PDA, and RDS—bolster the evidence that PS and iNO can independently and effectively diminish the occurrence of BPD. In this analysis, the employment of iNO was linked to a marked reduction in BPD incidence, with an odds ratio (OR) of 0.09 (95% CI: 0.002, 0.45; P = 0.004). Likewise, the administration of PS also exhibited a protective effect, with an OR of 0.19 (95% CI: 0.05, 0.77; P = 0.02). Other medications that lower pulmonary artery pressure do not significantly differ in their incidence of BPD. (Table 5, Fig. 1)
Discussion
This study demonstrated that the administration of iNO within the first three days of life serves as an effective standalone therapy to reduce the risk of BPD in preterm infants born before 33 weeks of gestation who have PH. Furthermore, its effectiveness is augmented when combined with PS. Infants who received iNO exhibited better respiratory outcomes and required shorter durations of mechanical ventilation, likely due to iNO’s role in enhancing oxygenation, reducing pulmonary vascular resistance, and minimizing lung injury through its anti-inflammatory effects1,9,15 .
Notably, the primary causes of ROP and IVH in preterm neonates include early exposure to high oxygen concentrations and circulatory instability16.The rationale behind using PS and iNO early in treatment is to rapidly lower the inhaled oxygen requirement and shorten the duration of mechanical ventilation, potentially reducing the risk of oxygen toxicity-related complications. However, our data revealed no significant difference in the P/F ratio at 24 h post-treatment, nor in the overall duration of mechanical ventilation between groups. This suggests that PS and iNO may reduce BPD through mechanisms beyond mere oxygen reduction, likely involving direct anti-inflammatory and endothelial-protective pathways17.
The development of BPD is closely associated with inflammation in the lungs of preterm infants, and iNO may assist in alleviating this inflammation by influencing pulmonary vascular remodeling. Research on animals indicates that iNO can diminish pulmonary inflammation and promote vascular remodeling, processes that are critical for lowering the risk of BPD. This is consistent with the increase in EOS proportion among iNO administration in our research. These outcomes are consistent with the findings of Zheng et al.18 who reported a reduction in BPD incidence with early administration of iNO in premature infants. Other studies, including one by Praveen Kumar et al.19 indicate that iNO’s efficacy in BPD prevention is not consistently supported, possibly due to differences in study designs, patient populations, and iNO administration protocols.
To date, randomized controlled trials have not demonstrated a definitive benefit of prophylactic iNO administration in preventing BPD in preterm infants. As a result, major professional organizations including the National Institutes of Health20 the American Academy of Pediatrics21, and the American Heart Association in conjunction with the American Thoracic Society recommend against routine use of iNO, except in select rescue scenarios. These include cases such as preterm infants with a history of prolonged rupture of membranes leading to pulmonary hypoplasia22, or in those with documented pulmonary hypertension20. Nevertheless, off-label iNO use remains prevalent, with the most significant increase observed among neonates born at < 26 weeks gestational age21,23.
Currently, the U.S. Food and Drug Administration (FDA), approves iNO only for term and near-term infants (≥ 34 weeks gestation) with hypoxic respiratory failure associated with PH, where its primary objective is to reduce the need for extracorporeal membrane oxygenation (ECMO)24. There is no approved indication for its use in preterm infants < 34 weeks, making this study a valuable contribution to the growing evidence base supporting its off-label application in this vulnerable group.
This study stands out by focusing on a high-risk group of infants diagnosed with PH and respiratory failure; conditions that substantially increasethe risk of developing BPD. By confirming PH through cardiac ultrasound and identifying infants with hypoxemia (PaO₂ < 50 mmHg at FiO₂ ≥ 30%), the study establishes a specific context for assessing the therapeutic roles of iNO and surfactant replacement therapy.
Notably, the high rate of surfactant use in the non-BPD group highlights the potential protective effects of PS in reducing the incidence of BPD. While RDS is highly prevalent and often associated with PH, excluding perinatal conditions, demographic factors, and early comorbidities such as EOS, PDA, and RDS, PS therapy emerges as a potential intervention to lower the risk of BPD in preterm infants (< 33 weeks gestation) with PH during the critical first three days of life. These findings align with previous studies advocating for the use of PS in neonates with PH caused by conditions like meconium aspiration syndrome, which demonstrated efficacy in improving oxygenation and reducing PH severity25. However, long-term studies indicate that preterm infants treated with PS may still exhibit pulmonary function abnormalities in adolescence, including reduced lung capacity and increased airway reactivity26.Further, the combination of early PS administration and iNO has shown significant improvement in oxygenation and reduction in the progression to severe hypoxemic respiratory failure in neonates with persistent PH27,28 .
Current recommendations limit iNO dosing to 20 ppm, as higher doses have been associated with the formation of methemoglobin and nitric dioxide (NO₂). The Neonatal Inhaled Nitric Oxide Research Group (NiNOS) observed the peak level of methaemoglobin to be 2.4% +/- 1.85% in the iNO-treated group compared to controls29,30,31. Once initiated, daily methaemoglobin and nitrogen dioxide levels require close vigilance. In our study, all cases treated with iNO showed no signs of methemoglobinemia. In the CINRGI study, hypotension was the sole observed adverse effect between the control and iNO-treated groups4. While short-term benefits include improved oxygenation, long-term studies suggest potential neurodevelopmental effects, including altered vascular remodeling and increased risk of cognitive impairments32.However, our study did not observe any differences in the utilization of vasoconstrictive drugs.
Other pulmonary vasodilators, including sildenafil, have the potential to reduce pulmonary pressure and enhance outcomes for patients with BPD. Nonetheless, additional research is required to assess their effectiveness in preventing BPD and in treating the associated pulmonary hypertension in infants33.Milrinone has shown the capacity to mitigate pulmonary hypertension and bronchoconstriction, which are pivotal factors in the progression of BPD34. While milrinone may indirectly enhance respiratory function by sustaining cardiovascular stability, the existing evidence does not definitively demonstrate a direct effect on the reduction of BPD. The varied outcomes from these studies underscore the imperative for additional research to elucidate the precise roles of these agents in preventing BPD in preterm infants with PH.
We acknowledge that this study is a retrospective observational analysis, which inherently carries limitations such as selection bias, potential confounding factors, and variability in clinical management. These limitations may influence the interpretation and generalizability of our findings. In light of this, we plan to conduct a prospective, randomized, double-blind controlled trial in the future to further validate these conclusions. Such a study will allow for a more robust assessment of the efficacy and safety of early iNO combined with PS therapy in preventing BPD among preterm infants with PH.
Study limitations
The retrospective approach and single-center structure of this research restrict the applicability of its results to a broader context. Furthermore, the relatively small size of the sample may diminish the statistical power to identify minor differences, which could compromise the strength of some conclusions. Subsequent studies should contemplate multi-center, prospective frameworks involving larger cohorts to corroborate these outcomes and assist in establishing guidelines for the administration of iNO and PS in averting BPD in preterm infants at high risk.
Conclusion
Our research indicates that administering iNO and PS during the initial three days of life markedly decreases the occurrence of BPD among preterm infants with PH. Early intervention using iNO and PS appears to be a hopeful approach for enhancing respiratory outcomes in this vulnerable group of infants. Nonetheless, additional research, particularly randomized controlled trials, is imperative to confirm these results and to inform the creation of evidence-based guidelines for the use of iNO and PS in preterm infants who are susceptible to BPD.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- BPD:
-
Bronchopulmonary dysplasia
- iNO:
-
Inhaled nitric oxide
- PH:
-
Pulmonary hypertension
- PS:
-
Pulmonary surfactant
- NICU:
-
Neonatal intensive care unit
- RDS:
-
Respiratory distress syndrome
- P/F ratio:
-
Partial pressure of oxygen (PaO₂)/fraction of inspired oxygen (FiO₂)
- IVH:
-
Intraventricular hemorrhage
- NEC:
-
Necrotizing enterocolitis
- PDA:
-
Patent ductus arteriosus
- EOS:
-
Early-onset sepsis
- ROP:
-
Retinopathy of prematurity
- PROM:
-
Premature rupture of membranes
- SGA:
-
Small for gestational age
References
Barrington, K. J., Finer, N. & Pennaforte, T. Inhaled nitric oxide for respiratory failure in preterm infants. Cochrane Neonatal Group, editor. Cochrane Database of Syst. Rev. https://doi.org/10.1002/14651858.CD000509.pub5 (2017).
Thébaud, B. et al. Bronchopulmonary dysplasia. Nat. Rev. Dis. Primers. 5(1), 78 (2019).
Mourani, P. M. & Abman, S. H. Pulmonary vascular disease in bronchopulmonary dysplasia: pulmonary hypertension and beyond. Curr. Opin. Pediatr. 25(3), 329–337 (2013).
Inhaled nitric oxide. In full-term and nearly full-term infants with hypoxic respiratory failure. N Engl. J. Med. 336(9), 597–604 (1997).
Zamanian, R. T. et al. Outpatient inhaled nitric oxide in a patient with vasoreactive idiopathic pulmonary arterial hypertension and COVID-19 infection. Am. J. Respir Crit. Care Med. 202(1), 130–132 (2020).
Sokol, G. M., Konduri, G. G. & Van Meurs, K. P. Inhaled nitric oxide therapy for pulmonary disorders of the term and preterm infant. Semin Perinatol. 40(6), 356–369 (2016).
Hasan, S. U. et al. Effect of inhaled nitric oxide on survival without bronchopulmonary dysplasia in preterm infants: A randomized clinical trial. JAMA Pediatr. 171(11), 1081 (2017).
Higgins, R. D. et al. Bronchopulmonary dysplasia: executive summary of a workshop. J. Pediatr. 197, 300 (2018).
Sahni, M. & Bhandari, V. Patho-mechanisms of the origins of bronchopulmonary dysplasia. Mol. Cell. Pediatr. 8(1), 21 (2021).
Huang, X. et al. Influence of inhaled nitric oxide on bronchopulmonary dysplasia in preterm infants with PPHN or HRF at birth: a propensity score matched study. Front. Pharmacol. https://doi.org/10.3389/fphar.2024.1515030/full (2024).
Banks, B. A. et al. Changes in oxygenation with inhaled nitric oxide in severe bronchopulmonary dysplasia. Pediatrics 103(3), 610–618 (1999).
Peliowski, A. Canadian paediatric society, fetus and newborn committee. Inhaled nitric oxide use in newborns. Paediatr. Child. Health. 17(2), 95–100 (2012).
Barrington, K. J., Finer, N., Pennaforte, T. & Altit, G. Nitric oxide for respiratory failure in infants born at or near term. Cochrane Database Syst. Rev. 1(1), CD000399 (2017).
Sweet, D. G. et al. European consensus guidelines on the management of respiratory distress syndrome: 2022 update. Neonatology 120(1), 3–23 (2023).
Grisham, M. B., Jourd’Heuil, D. & Wink, D. A. I. Physiological chemistry of nitric oxide and its metabolites: implications in inflammation. Am. J. Physiology-Gastrointestinal Liver Physiol. 276(2), G315–G321 (1999).
Fierson, W. M. et al. Screening examination of premature infants for retinopathy of prematurity. Pediatrics 142(6), e20183061 (2018).
Van Meurs, K. P. et al. Inhaled nitric oxide for premature infants with severe respiratory failure. N Engl. J. Med. 353(1), 13–22 (2005).
Zheng, Y., Wu, Q. & Han, S. Inhaled nitric oxide in premature infants for preventing bronchopulmonary dysplasia: a meta-analysis. BMC Pediatr. 23, 139 (2023).
Kumar, P. et al. Use of inhaled nitric oxide in preterm infants. Pediatrics 133(1), 164–170 (2014).
Cole, F. S. et al. NIH Consensus Development Conference statement: inhaled nitric-oxide therapy for premature infants. Pediatrics. 127(2), 363–9 (2011).
Kumar, P. & Committee on Fetus and Newborn, American Academy of Pediatrics. Use of inhaled nitric oxide in preterm infants. Pediatrics 133(1), 164–170 (2014).
Abman, S. H. et al. Pediatric pulmonary hypertension: guidelines from the American heart association and American thoracic society. Circulation 132(21), 2037–2099 (2015).
Ellsworth, M. A., Harris, M. N., Carey, W. A., Spitzer, A. R. & Clark, R. H. Off-label use of inhaled nitric oxide after release of NIH consensus statement. Pediatrics 135(4), 643–648 (2015).
American Academy of Pediatrics. Committee on fetus and newborn. Use of inhaled nitric oxide. Pediatrics 106(2 Pt 1), 344–345 (2000).
Rosati, E., Butera, G., Bossone, E., De Felice, C. & Latini, G. Inhaled nitric oxide and oral Nifedipine in a preterm infant with bronchopulmonary dysplasia and pulmonary hypertension. Eur. J. Pediatr. 166(7), 737–738 (2007).
van Dokkum, N. H., de Kroon, M. L. A., Reijneveld, S. A. & Bos, A. F. Neonatal stress, health, and development in preterms: A systematic review. Pediatrics 148(4), e2021050414 (2021).
Enderby, C. Y. & Burger, C. Medical treatment update on pulmonary arterial hypertension. Therapeutic Adv. Chronic Disease. 6(5), 264–272 (2015).
Bhogal, S. et al. Sildenafil for pulmonary arterial hypertension. Am. J. Ther. 26(4), e520–e526 (2019).
Neonatal Inhaled Nitric Oxide Study Group. Inhaled nitric oxide in full-term and nearly full-term infants with hypoxic respiratory failure. N Engl. J. Med. 336(9), 597–604 (1997).
Kinsella, J. P. Inhaled nitric oxide in the term newborn. Early Hum. Dev. 84(11), 709–716 (2008).
Witek, J. & Lakhkar, A. D. Nitric oxide. In StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK554485/ (Accessed 6 Dec 2024) (StatPearls Publishing, 2023).
Pravia, C. I. & Benny, M. Long-term consequences of prematurity. CCJM 87(12), 759–767 (2020).
Ling, R. & Greenough, A. Advances in emerging treatment options to prevent bronchopulmonary dysplasia. Expert Opin. Orphan Drugs. 1–11. (2017).
Harris, M. N., Daborn, A. K. & O’Dwyer, J. P. Milrinone and the pulmonary vascular system. Eur. J. Anaesthesiol. Suppl. 5, 27–30 (1992).
Acknowledgements
We wish to convey our profound gratitude to the NICU teams at Xiangya Hospital, Central South University, for their invaluable support and unwavering dedication to patient care, which were pivotal to the successful completion of this study.
Funding
This work was supported by the National Natural Science Foundation of China (81873852). The funding body had no role in the design, data collection, analysis, or interpretation of the study.
Author information
Authors and Affiliations
Contributions
MW contributed to the study design; TA conducted data collection and study selection, extracted the data, and assessed the methodological quality. TA drafted and revised the manuscript. LL, SW, SY, XY, ZL, CC, YD, ML, TL, MC, and MW reviewed and edited the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was reviewed and approved by the Institutional Review Board (IRB) of Xiangya Hospital, Central South University (Approval Number: 202406249). The research complies with the ethical standards of the institution and international ethical guidelines for human subject research. Given the retrospective nature of the study, the requirement for informed consent was waived by the IRB.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Azad, T., Li, L., Wang, S. et al. Inhaled nitric oxide as an independent intervention to lower the risk of bronchopulmonary dysplasia in preterm infants (< 33 weeks) with pulmonary hypertension within the initial 3 days of life. Sci Rep 15, 22517 (2025). https://doi.org/10.1038/s41598-025-06055-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-06055-0