Abstract
The association of the serum alanine aminotransferase to high-density lipoprotein cholesterol ratio(ALT/HDL-C) with NAFLD remains unclear. This study aimed to examine the association of the ALT/HDL-C ratio with the prevalence of NAFLD and liver fibrosis in the U.S. general population. 4764 participants from the 2017–2018 National Health and Nutrition Examination Survey were included in our cross-sectional study. The association of ALT/HDL-C with NAFLD was examined using a general additive model. Furthermore, we conducted subgroup analyses to evaluate the relationship between liver fibrosis, NAFLD risk, and the ALT/HDL-C ratio. Of the 4764 participants, 1513 (31.76%) were diagnosed with NAFLD. All three logistic regression models showed positive associations between NAFLD risk and ALT/HDL-C. Furthermore, in stratified analyses by body mass index (BMI), gender, and age, ALT/HDL-C was positively associated with NAFLD. Hepatic steatosis and fibrosis severity were strongly linked with the ALT/HDL-C. The ALT/HDL-C and the incidence of NAFLD exhibited a nonlinear distribution that was particularly noticeable in women with an inverted U distribution with an inflection point of 0.528. NAFLD was more accurately predicted by ALT/HDL-C than by ALT or HDL-C alone, according to receiver operating characteristic (ROC) analysis. A higher ALT/HDL-C ratio in the U.S. population is independently associated with a significantly higher risk of NAFLD and liver fibrosis. The ALT/HDL-C ratio is a useful noninvasive diagnostic tool to quickly and accurately identify those at high risk of developing NAFLD and liver fibrosis.
Similar content being viewed by others
.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is becoming increasingly prevalent. Therefore, it is crucial to identify those at risk of progression as early as possible. The gold standard for diagnosing steatohepatitis and determining the degree of fibrosis in NAFLD patients is a liver biopsy. However, there are several recognised drawbacks, including high expense, inaccurate sampling, variability between observers, and hazards related to the procedure itself, such as bleeding and infection. These factors may prevent its widespread use1,2,3. Serum liver enzyme levels are the most widely used and straightforward method of assessing liver function. In clinical practice, they are also the most commonly used enzymatic measure of the severity of liver damage. Patients with NAFLD may have mild to moderate elevations in serum liver enzymes. In addition, previous research has shown that liver enzymes can accurately indicate the degree of liver fat accumulation4,5. After excluding alcoholic liver disease, viral hepatitis, and other causes of liver disease, the most frequent reason for increased liver enzymes is NAFLD. Therefore, in epidemiological studies, serum liver enzymes can be used as a surrogate marker to examine the link between NAFLD, metabolic disorders, and other diseases. Several studies have identified low high-density lipoprotein cholesterol (HDL-C) as a sign of metabolic syndrome6,7,8,9. The risk of NAFLD rose dramatically as HDL-C levels fell. Evidence shows that people with NAFLD may have impaired antioxidant activity and poor HDL-C excretion10,11,12. HDL-C has antithrombotic, antioxidant, and nitric oxide-stimulating properties, and it can also prevent monocytes from adhering to endothelial cells. In addition, low levels of HDL-C may indicate a decrease in islet cell activity and insulin resistance. HDL-C has been shown to enhance pancreatic islet cell function and to motivate the body to absorb glucose13,14,15.
A recent study proposed a new indicator called the ALT/HDL-C ratio. Earlier studies have demonstrated a strong correlation between this ratio and the incidence of diabetes16,17. A cross-sectional survey of 7,599 individuals in the US from 2013 to 2020 suggested that elevated ALT/HDL-C was a significant indicator of insulin resistance (IR) in American adults18. These findings implied that the development and prevalence of NAFLD and liver fibrosis can be evaluated by combining ALT and HDL-C.
The present study therefore used data from the 2017–2018 National Health and Nutrition Examination Survey (NHANES) to examine the link between the ALT/HDL-C ratio and the progression of hepatic fibrosis and steatosis in the general US population. To further evaluate this relationship, subgroup analyses were performed according to age and gender categories.
.
Methods
Study design and population
The National Center for Health Statistics (NCHS), which funds and maintains the NHANES database, provided data for the cross-sectional secondary analysis used in this study. The data were supplied in two-year cycles, and the study used a stratified, multistage sampling methodology and individuals representative of the general non-institutional population of the U.S. The survey’s sophisticated design allowed for the collection of sufficient minority data by sampling Mexican Americans, Hispanics, non-Hispanic blacks, Asians, adolescents, seniors, and low-income individuals. The Centers for Disease Control (CDC) Research Ethics Review Board approved the study, and written informed consent was obtained from each participant. Researchers worldwide have unrestricted access to NHANES data. Data are available at https://www.cdc.gov/nchs/nhanes.htm.
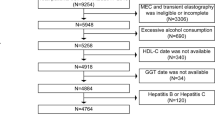
The transient elastography (T.E.) examination of the liver used in this analysis, for which data were gathered from NHANES 2017–2018, was primarily based on the diagnosis of NAFLD. A total of 9,254 people participated in the study, excluding 3,306 people with incomplete MEC examinations or no T.E. data. Next, 402 people with unavailable ALT levels and 6 people with incomplete HDL-C data were removed from the analysis. We then excluded 656 people who drank excessively, defined as more than three drinks per day for men and more than two drinks per day for women. We also excluded 70 people with hepatitis C and 50 people with hepatitis B. The final study included 4764 people (Fig. 1).
Definition of NAFLD
NAFLD was assessed using vibration-activated transient elastography (VCTE) and controlled attenuation parameter (CAP) data. CAP values range from 100 to 400 dB/m, with higher values indicating more significant liver steatosis. NAFLD is characterized by a CAP score of at least 288 dB/m, according to data from a previous population-based meta-analysis that examined CAP diagnostic cut-offs for the disease19.
VCTE
The VCTE is replacing liver biopsy in clinical practice to determine the prevalence and stage of NAFLD20. The FibroScan model 502 V2 Touch (Echosens, North America) and medium (M) or extra large (XL) probes were used in the NHANES MEC in the 2017–2018 cycle. A large body of clinical data shows that liver steatosis and fibrosis increase with the degree of CAP and liver stiffness measurement (LSM) values, respectively21. Decibels per meter (dB/m) and kilopascals (kPa) were used to report CAP and LSM values, respectively. In this review, tests were considered credible if at least 10 LSM values with an interquartile range/median (IQR) of less than 30% were obtained after an overnight fast of at least 8 h21. Studies have shown that significant liver fibrosis (F2), advanced liver fibrosis (F3), and cirrhosis (F4) are indicated by LSM values of 8.0, 9.7, and 13.7 kPa, respectively22,23.
Variables
The independent variable was ALT/HDL-C, while the dependent variables were the grade of steatosis in the liver, the severity of liver fibrosis, and the prevalence of NAFLD. This study used confounders demonstrated in earlier research and clinical practice. Type 2 diabetes (T2DM), sex, race, physical activity level, smoking status, and hypertension status were covariates in our study. We then used the following continuous variables as covariates Gamma-glutamyl transpeptidase (GGT), systolic/diastolic blood pressure (S/DBP), alkaline phosphatase (ALP), body mass index (BMI), aspartate aminotransferase (AST), total bilirubin (TBIL), serum uric acid (SUA), C-reactive protein (CRP), platelets (PLT), fast glucose, serum lipids, glycated hemoglobin (HbA1c) and fast insulin.
T2DM was diagnosed using the following American Diabetes Association criteria: First, use of diabetes medications; second, detection of a random plasma glucose reading of at least 200 mg/dl (11.1 mmol/L); third, self-reported diabetes; fourth, fasting plasma glucose (FPG) result of 126 mg/dl (7 mmol/L) or higher; in addition, HbA1c levels above 6.5%24. Hypertension was defined as (1) current use of antihypertensive medication; (2) blood pressure measured with a diastolic reading more significant than 90 mmHg or a systolic reading greater than 140 mmHg; (3) self-reported hypertension22. Hepatitis B surface antigen testing is used to identify HBV, while the presence of HCV RNA or a positive HCV antibody test verifies HCV infection. In addition, physical activity recommendations were followed to classify the activity level as active, moderate, or inactive25.
Statistical analysis
This study used the R package version 3.4.3 and EmpowerStats software for data analysis. The data were analyzed using the NCHS recommended weighting guidelines to ensure that the outcomes represented the U.S. general population fairly. The two-tailed P value must be less than 0.05 to be statistically significant. Where continuous variables had a normal distribution, they were presented as the weighted mean and weighted standard deviation; otherwise, they were presented as the median and interquartile range (IQR). Categorical variables were described using frequencies and weighted proportions. After quarterly categorization of ALT/HDL-C, three types of logistic regression models were constructed to explore the association between ALT/HDL-C ratio, NAFLD, and liver fibrosis: (1) no covariate adjustment was made; (2) age, sex, and race adjustments were made; (3) all relevant factors including age, sex, race, physical activity level, hypertension, BMI, ALP, GGT, AST, waist circumference (W.C.), T2DM, smoking, LSM, DBP, SBP, fast insulin, HBA1C, SUA, CRP, PLT, TBIL, total cholesterol (T.C.), triglycerides (T.G.) and fast glucose were adjusted.
To identify relevant groups, we also performed subgrouping by sex, age, and BMI using categorized multivariate logistic regression. In addition, threshold effect analysis and smooth curve fitting were used to investigate the nonlinear relationship between the level of ALT/HDL-C and the incidence of NAFLD. Binary linear regression models were fitted on either side of the threshold after identifying non-linearities. The effectiveness of ALT/HDL-C and other diagnostic markers in diagnosing NAFLD was evaluated using receiver operator characteristic (ROC) curve analysis. The primary objective was to find the ideal ALT/HDL-C ratio to assess NAFLD’s occurrence and development risk in this cohort. The ideal cut-offs were found by maximizing the Yoden index. A p-value of less than 0.05 indicates that the result is statistically significant.
Results
Study population demographic and clinical features
4764 people took part in the survey. The average age of the participants was 43.35 ± 19.44 years; 45.85% were men, and 54.15% were women. Table 1 presents the weighted distribution based on the categorization of NAFLD status. Individuals with NAFLD had elevated rates of smoking, hypertension, and T2DM and tended to be older, male, and Mexican-American. People with NAFLD had significantly higher BMI, LSM, CAP, T.G., fast glucose, hip circumference (H.C.), ALT, AST, T.C., GGT, fast insulin, low-density lipoprotein cholesterol (LDL-C), W.C., S/DBP, HbA1c, PLT, CRP, and SUA, but significantly lower levels of ALP, TBIL, and HDL-C (P < 0.001). Notably, ALT/HDL-C was considerably lower in the non-NAFLD group than in the NAFLD group (0.37 ± 0.31 vs. 0.64 ± 0.51, P < 0.001). On the other hand, there were no significant differences in physical activity levels.
Relationship between ALT/HDL-C and NAFLD
Multiple regression analysis was used to assess the association between ALT/HDL-C and NAFLD prevalence in Table 2. The risk of NAFLD and ALT/HDL-C showed positive associations in each of the three models using multivariable logistic regression: model 1 (OR = 7.595, 95% CI: 6.132, 9.407), model 2 (OR = 8.151, 95% CI: 6.455, 10.294) and model 3 (OR = 2.915, 95% CI: 2.290, 3.709). After converting the continuous ALT/HDL-C ratio into a categorical variable (quartiles), individuals in quartiles 2 (ALT/HDL-C 0.216–0.317), 3 (ALT/HDL-C 0.318–0.508) and 4 (ALT/HDL-C 0.509–12.500) had higher odds of having NAFLD than those in quartile 1 (ALT/HDL-C 0.032–0.215), which were 41.9%, 125.3% and 357.1%, respectively. The results showed that people with a higher ratio of ALT to HDL-C were more likely to develop NAFLD than those with a lower ratio.
Gender-stratified subgroup analyses for all three models showed that NAFLD risk in men and ALT/HDL-C were positively associated in model 1 (OR = 5.877, 95% CI: 4.502, 7.671), model 2 (OR = 6.810, 95% CI: 5.138, 9.027) and model 3 (OR = 2.345, 95% CI: 1.747, 3.148). Women showed similar outcomes, with each of the three models displaying a positive association: model 1 (OR = 13.243, 95% CI: 8.753, 20.037), model 2 (OR = 12.083, 95% CI: 7.951, 18.360), and model 3 (OR = 3.665, 95% CI: 2.369, 5.670). According to the subgroup analysis stratified by age, those over 60 years with higher ALT/HDL-C levels were also more likely to develop NAFLD compared to those in other age groups: model 1 (OR = 9.469, 95% CI: 5.668, 15.818), model 2 (OR = 8.462, 95% CI: 4.969, 14.411) and model 3 (OR = 4.737, 95% CI: 2.663, 8.424). For all BMI groups, there was a positive association with the incidence of NAFLD. People with a BMI between 25 and 30 kg/m2 were more likely to develop NAFLD than people in other categories: model 1 (OR = 4.216, 95% CI: 2.942, 6.043), model 2 (OR = 4.269, 95% CI: 2.882, 6.322) and model 3 (OR = 3.906, 95% CI: 2.561, 5.957).
In the comorbid population we found that the risk of NAFLD was higher with increasing ALT/HDL-C ratio in the population with type 2 diabetes: model 1 (OR = 6.247, 95% CI: 3.615, 10.796), model 2 (OR = 5.545, 95% CI: 3.081, 9.980) and model 3 (OR = 4.340, 95% CI: 2.308, 8.160). Meanwhile, we found that the risk of NAFLD was higher with increasing ALT/HDL-C ratio in the hypertensive population: model 1 (OR = 7.042, 95% CI: 5.003, 9.912), model 2 (OR = 6.271, 95% CI: 4.330, 9.081) and model 3 (OR = 3.693, 95% CI:2.534, 5.380).
Association between severity of liver steatosis and ALT/HDL-C
Supplementary Table 1 illustrates the relationship between ALT/HDL-C and hepatic steatosis depending on CAP levels. The level of liver steatosis was shown to be strongly and positively associated with ALT/HDL-C in models 1 (β = 44.000, 95% CI: 40.157, 47.843), 2 (β = 42.699, 95% CI: 38.884, 46.514) and 3 (β = 18.884, 95% CI: 15.441, 22.327), with a trend P less than 0.001. Moreover, in comparison to the lower quartile, the higher ALT/HDL-C quartile had a notably higher level of hepatic steatosis (P for trend < 0.001). Following the adjustment of all pertinent factors, the adjusted mean difference (β) in CAP of ALT/HDL-C participants in Q2, Q3 and Q4 compared to Q1 was 6.040 dB/m (95% CI: 2.013, 10.067), 14.813 dB/m (95% CI: 10.579, 19.047) and 33.629 dB/m (95% CI: 28.961, 38.297), respectively. After accounting for all pertinent variables, there was a significant correlation found between the level of liver steatosis and ALT/HDL-C in both men (β = 2.345, 95% CI: 1.747, 3.148, P < 0.001) and women (β = 3.665, 95% CI: 2.369, 5.670, P < 0.001). Simultaneously, examination of subgroups by age and BMI showed a robust correlation between ALT/HDL-C and the severity of hepatic steatosis, especially in those over 60 years of age (β = 4.737, 95% CI: 2.663, 8.424, P < 0.001) and those weighing between 25 and 30 kg/m2 (β = 3.906, 95% CI: 2.561, 5.957, P < 0.001).
Correlation between severity of liver fibrosis and ALT/HDL-C
Table 3 illustrates the relationship between the three stages of liver fibrosis and ALT/HDL-C. Our findings demonstrated that in all three models, ALT/HDL-C was associated with significant liver fibrosis, advanced liver fibrosis, and cirrhosis (P < 0.001). After adjustment for all relevant factors, ALT/HDL-C was explicitly implicated in liver fibrosis model 3 (OR = 2.274, 95% CI: 1.848, 2.797), advanced hepatic fibrosis model 3 (OR = 2.200, 95% CI: 1.763, 2.746) and cirrhosis model 3 (OR = 1.870, 95% CI: 1.447, 2.418).
According to three multivariable logistic regression models, subgroup analysis stratified by gender, ALT/HDL-C was strongly correlated with significant fibrosis, advanced fibrosis, and cirrhosis in both males and females (P < 0.001). The research found that in men, after adjustment for each of the relevant variables, there was a robust association between ALT/HDL-C and the risk of significant hepatic fibrosis (OR = 1.915, 95% CI: 1.485, 2.468), advanced hepatic fibrosis (OR = 1.983, 95% CI: 1.493, 2.633) and cirrhosis (OR = 1.604, 95% CI: 1.220, 2.108). Similar outcomes were observed for women: after controlling for all pertinent covariates, the correlation of ALT/HDL-C and the development of severe liver fibrosis was (OR = 3.785, 95% CI: 2.478, 5.782), advanced liver fibrosis was (OR = 2.919, 95% CI: 1.952, 4.364) and cirrhosis was (OR = 2.832, 95% CI: 1.891, 4.241) after adjusting for every relevant covariates (Table 3).
Nonlinear relationship analysis
The present study examined the possibility of a nonlinear association between ALT/HDL-C and NAFLD using smooth curve fits. In addition, there was a nonlinear relationship between the prevalence of NAFLD and ALT/HDL-C in subgroup analyses adjusted for age, race, sex, and BMI categories (Table 4; Fig. 2, and Fig. 3). The population inflection point was 1.054 for all individuals, 1.042 for the subgroup with a BMI greater than 30 kg/m2, and 1.029 for the cohort aged 40–59. The nonlinear trend is most pronounced in women with an inverted U-shaped curve with an inflection point of 0.528. In the female population, the risk of NAFLD increased with increasing ALT/HDL-C ratio at ALT/HDL-C ratios less than 0.528 and decreased with increasing ALT/HDL-C ratio at ALT/HDL-C ratios greater than 0.528.
Associations between alanine aminotransferase to high-density lipoprotein cholesterol ratio and CAP values or prevalence of NAFLD. (A) and (B): Associations between ALT/HDL-C ratio and CAP values. (C) and (D): Associations between ALT/HDL-C ratio and prevalence of NAFLD. Each black point represents a sample. The solid red line represents the smooth curve fit between variables. Blue bands represent the 95% confidence interval from the fit. They were adjusted for age, gender, race, hypertension, BMI, T2DM, smoke, W.C., LSM, DBP, SBP, CRP, fast glucose, fast insulin, HbA1c, TBIL, ALP, GGT, AST, T.G., and SUA.
Associations between alanine aminotransferase to high-density lipoprotein cholesterol ratio and the prevalence of NAFLD by gender (A), age (B), BMI (C), and race (D). They were adjusted for age, gender, race, hypertension, BMI, T2DM, smoke, W.C., LSM, DBP, SBP, CRP, fast glucose, fast insulin, HbA1c, TBIL, ALP, GGT, AST, T.G., and SUA. In subgroup analyses, the model was not adjusted for the classified variables.
A ROC analysis of ALT/HDL-C as a NAFLD predictor
Prior studies have found ALT, AST, GGT, HDL-C, and TG to be significant predictors of NAFLD based on ROC curve analysis. The ROC for ALT/HDL-C is compared to these predictors (Fig. 4, Supplementary Table 2, Supplementary Fig. 1, and Supplementary Fig. 2). In the ROC study, ALT/HDL-C had a substantially higher area under the curve (AUC) of 0.7357 than ALT, AST, GGT, HDL-C, and T.G. (95% CI: 0.7205–0.7509) (P < 0.001) (Supplementary Table 2). In addition, we conducted a gender subgroup investigation and found that in the ROC analysis, the AUC of ALT/HDL-C was more significant than the other markers (Supplementary Fig. 2 and Supplementary Table 2). The AUC for ALT/HDL-C was 0.7317 (95% CI: 0.7098–0.7535) in men and 0.7359 (95% CI: 0.7143–0.7576) in women. Meanwhile, we performed an age-based subgroup analysis and found that ALT/HDL-C had a higher AUC in the ROC analysis than the other markers. The AUC for ALT/HDL-C was 0.8261 (95% CI: 0.7850–0.8671) in individuals under 20 years of age, 0.7857 (95% CI: 0.7558–0.8156) in individuals between 20 and 39 years of age, 0.7165 (95% CI: 0. 6874-0.7456) for those aged 40–59 years and 0.6735 (95% CI: 0.6450–0.7021) for those aged 60 years and over (Fig. 4 and Supplementary Table 2).
Discussion
Patients with NAFLD often lack specific clinical symptoms, and elevated liver enzymes are the most common abnormality in the biochemical indices of patients with NAFLD. There are different degrees of hepatocyte inflammation and necrosis in fatty liver disease. The more severe the fatty liver, the more pronounced the hepatocyte damage, which is often manifested by increased serum transaminases, especially ALT26. When NAFLD manifests with elevated liver enzymes, it indicates the progression of liver inflammation27,28. Meanwhile, a growing body of recent clinical data suggests that dyslipidemia and lipoproteins are strongly associated with the development and progression of NAFLD and that patients with dyslipidemia and NAFLD have a significantly increased risk of cardiovascular and cerebrovascular disease29. This suggests that the ratio of liver enzyme markers to blood lipids or lipoproteins could be an effective diagnostic indicator of NAFLD.
Our investigation showed a significant correlation between a high ALT/HDL-C ratio and the incidence of NAFLD, the severity of liver steatosis, and the elevated risk of hepatic fibrosis in the general population. After controlling for all relevant confounding variables, there was a 1.915-fold increase in the risk of NAFLD for every unit elevation in ALT/HDL-C. Additional subgroup analyses were conducted depending on the population’s age, gender, and BMI. The findings indicated that the incidence of NAFLD in each group remained positively correlated with the ALT/HDL-C ratio rise. However, it is important to note that these subgroups exhibit a threshold saturation effect and a non-linear relationship between an increase in the ALT/HDL-C ratio and the incidence of NAFLD. Subgroup analysis by gender revealed a non-linear connection between NAFLD incidence, particularly in women. This was represented by an inverted U-shaped curve with an inflection point of 0.528. Therefore, when assessing NAFLD risk, the role of the ALT/HDL-C ratio should be considered alongside gender, age, and BMI. This is the most extensive research to evaluate the relationship between NAFLD and the ALT/HDL-C ratio in the US population. This study provides evidence-based evidence for the clinical use of the ALT/HDL-C ratio in NAFLD screening. Because ALT and HDL-C are routine clinical tests, obtaining and calculating the ALT/HDL-C ratio is straightforward. In clinical practice, physicians can incorporate the ratio and its threshold values into their routine screening processes. Further investigations (e.g., liver ultrasound, elastography, etc.) in patients with ratios higher than the threshold can help in the early detection of NAFLD and hepatic fibrosis, leading to early diagnosis and treatment of the disease. The ALT/HDL-C ratio is particularly well suited for large-scale population-based screening and early intervention in high-risk individuals. In the future, prospective interventional trials are needed to validate its value as a dynamic surveillance marker and to explore strategies for its integration with genetic risk scores and imaging parameters to construct a more accurate stratified management pathway for NAFLD.
Prior research on the ALT/HDL-C ratio has mainly focused on the relationship between the ratio and the risk of developing diabetes16,17. It has been widely recognized that there are common pathophysiological pathways between T2DM and NAFLD, such as inflammation and IR30,31. Most studies suggested a negative link between HDL-C and NAFLD and a positive relationship between ALT and NAFLD27,32. This relationship considered the impact of metabolic disorders, serum lipids, and liver function. Therefore, we hypothesized that integrating these two characteristics could improve the assessment and prediction of NAFLD incidence. There has been relatively little study on the connection between NAFLD and the ALT/HDL-C ratio. Using cross-sectional data from the NAGALA project in Japan from 1994 to 2016, Zou et al. performed a retrospective analysis of data from 14,251 participants in the health screening project. The results show that there is a strong positive correlation between the prevalence of NAFLD and the ALT/HDL-C ratio in this Japanese population, and it is suggested that the adjusted odds ratio (OR) of NAFLD increases by 3.05 with each standard deviation of the ALT/HDL-C ratio33. Another prospective cohort study of 11,975 non-obese participants found a positive and nonlinear association between the NAFLD risk in lean Chinese individuals and the ALT/HDL-C ratio34. The results of our current investigation support previous findings showing a substantial association between the ALT/HDL-C ratio and NAFLD. With an AUC of 0.736, we found that ALT/HDL-C was much more accurate for NAFLD than other diagnostic indications in the study population. We found that the AUC of ALT/HDL-C for NAFLD was significantly higher than that of other diagnostic markers, with an AUC of 0.736, in the entire study population.
Previous studies have mostly focused on individuals from East Asia. Our research initially examined the relationship between a high ALT/HDL-C ratio and the risk of NAFLD in a large sample of the American population. Based on these studies, the ALT/HDL-C ratio could be a valuable indicator of liver inflammation and metabolism, as well as a means of identifying NAFLD risk in populations in Asia and the USA. Currently, there is no precise mechanism that explains the link between NAFLD and a higher ALT/HDL-C ratio. Based on a substantial body of previous research, the following factors are considered the most likely causes: Firstly, major pathological changes in the development and progression of NAFLD include steatosis, lipotoxicity, and chronic inflammation. High levels of ALT are associated with hepatic steatosis and chronic inflammation35,36. Additionally, a condition characterised by elevated ALT and decreased HDL-C frequently leads to elevated serum fatty acids. These fatty acids are converted into excess T.G. and cause further organ damage, including lipotoxicity, when they exceed the processing capacity of adipose tissue35. Low-grade systemic inflammation is one of the significant factors in the pathophysiology of NAFLD. During an inflammatory response, HDL particles have anti-inflammatory properties by reducing the expression of adhesion molecules and preventing the synthesis of specific pro-inflammatory cytokines and chemokines37. HDL-C is an anti-atherosclerotic lipoprotein. It transports cholesterol from extrahepatic tissues to the liver for metabolism and promotes reverse cholesterol transport. It also has a protective effect in hepatic steatosis38. Secondly, insulin resistance is a leading factor in NAFLD, and numerous studies have also shown an association between increased ALT and decreased HDL with insulin resistance (I.R.)39,40. ALT may serve as a marker both for an early symptom of an insulin signaling problem and for liver damage caused by fatty liver disease. Therefore, some researchers believe that ALT may indicate the future development of metabolic diseases39.
Notably, the study’s age-stratified subgroup analysis showed that, compared with other age groups, people over 40 had a significantly higher risk of developing NAFLD if their ALT/HDL-C ratio was elevated. The mechanism behind this association is not well understood. We speculate that it may be due to the following reasons: Age is an essential factor in dyslipidemia, which may be related to growth hormone (GH) levels. Studies have shown that G.H. can increase intermediate-density lipoprotein (IDL) catabolism and decrease T.C. synthesis41. As people get older, their body produces less G.H. and more T.C., T.G., and LDL-C, while HDL-C goes down. There is a link between age and HDL-C levels. Levels of HDL-C decrease significantly, particularly among individuals aged 40–49.
Moreover, in the stratified analysis according to sex, we also found that the risk of NAFLD was higher in women with an elevated ALT/HDL-C ratio than in men. We speculate that there may be some correlation with the age of the female population in this study. The mean age of the women in this study was 44.88 ± 19.47 years, and a significant proportion were menopausal or perimenopausal. The underlying biological mechanism of increased risk of NAFLD in postmenopausal women is mainly related to multiple metabolic disorders caused by decreased estrogen levels. Estrogen regulates liver lipid metabolism by activating the estrogen receptor α (ERα), promotes the expression of fatty acid β-oxidation-related enzymes, and inhibits new fat production, thereby reducing liver triglyceride deposition42. Postmenopausal estrogen deficiency leads to impaired insulin signaling pathways, which are manifested as increased phosphorylation of IRS-1 serine, inhibiting the function of insulin receptor substrates, and aggravating IR43. In addition, low estrogen levels cause a decrease in sex hormone-binding globulin (SHBG) and a relative increase in androgen, further promoting visceral fat accumulation and lipotoxicity44. In terms of metabolism, estrogen deficiency leads to a decrease in the oxidative capacity of fatty acids in the liver, while the expression of fatty acid synthetases is upregulated, and the secretion of extremely low-density lipoprotein (VLDL) is reduced, which together aggravates the accumulation of lipids in the liver (45). In terms of inflammation pathways, estrogen reduces the release of proinflammatory factors by inhibiting the JNK and NF-κB signaling pathways, thereby blocking the inflammatory-fibrosis cascade45.
Another interesting finding of our research was that a higher ALT/HDL-C ratio was associated with the development of liver fibrosis in the population, especially in women. These results may be explained by the influence of HDL-C on liver fibrosis. Our study’s mean HDL-C level is 53.66 ± 14.670 mg/dL, higher than the average. The mean value in women was 58.179 ± 14.814 mg/dL, significantly higher than in men (48.331 ± 12.557 mg/dL). High HDL-C levels were found to be an independent predictor of advanced liver fibrosis in previous research by Klisic et al., and the degree of fibrosis was correlated with increases in serum HDL-C levels46,47,48. A recent study using gerbils as an animal model of NAFLD cirrhosis found a steady-state positive association between HDL-C and hepatic stellate cells (HSCs)49. Similar literature has documented the increasing trend of HDL-C in the course of fibrosis and its association with a rising abnormal prevalence of ALT and AST levels50,51. This is consistent with our research findings.
Recent studies have proposed lean metabolism-associated fatty liver disease (lean MAFLD), which refers to patients with fatty liver who have normal body mass index (BMI < 25Kg/m2) but metabolic abnormalities, and whose pathomechanisms differ from obese MAFLD: lean patients are more likely to exhibit visceral fat accumulation, mitochondrial dysfunction, and intestinal dysbiosis, and the risk of hepatic fibrosis progression is comparable to that of the obese type52,53,54,55. Despite the low proportion of lean-type NAFLD patients in this study (5.12%), subgroup analysis showed that their ALT/HDL-C was still significantly correlated with the risk of NAFLD (OR = 2.345), suggesting that this ratio may serve as a BMI-independent metabolic marker. As lean MAFLD is easily missed and lacks specific diagnostic indicators, the threshold effect of ALT/HDL-C in this population and its synergistic effect with visceral fat and insulin resistance need to be further explored in the future to optimize the early screening strategy for metabolic abnormality-associated fatty liver.
These are the main strengths of our study. First, this study is the first to examine the U.S. population with a large sample size; second, liver steatosis and fibrosis were accurately assessed using VCTE technology; and third, a subgroup analysis of the study participants was performed. However, our investigation has several limitations. First, since the NHANES database is an observational cross-sectional survey, it is practically difficult to determine the cause-and-effect relationship between NAFLD, liver fibrosis, and the ALT/HDL-C ratio. Interventional and longitudinal studies are needed to overcome this limitation. In the future, it is necessary to clarify the time series relationship between exposure and outcome through a prospective cohort study, and analyze the direct/indirect effects of estrogen level on gender differences by mediation analysis. At the same time, the causal relationship between genetic proxy variables and NAFLD can be verified by combining causal inference methods such as Mendelian randomization, or the intervention effect of hormone replacement therapy on risk ratio can be explored by using a natural experimental design to break through the bottleneck of cross-sectional study in causal chain analysis. Secondly, the use of CAP ≥ 288 dB/m instead of liver biopsy to diagnose NAFLD may lead to false positive or false negative results, and numerous factors such as ascites and obesity may affect the detection value of CAP. Thirdly, people who did not complete or only partially complete the examination were not included in the study, which may have led to selection bias. Fourthly, unmeasured dietary patterns, drug use, and environmental exposures may introduce residual confounding, and although sensitivity analyses suggest that the main findings are robust (E-value > 1.5), further validation is needed in a causal inference framework. Finally, as the study population was all American. To improve the global applicability of the ALT/HDL-C ratio, future studies should aim to: establish race-specific risk stratification models; integrate multiomics data to resolve the influence of genetic and environmental factors on the dynamics of the ratio; and promote international multi-center studies to validate its cross-population robustness.
Conclusion
In the U.S. population, a higher ALT/HDL-C ratio is independently associated with a significantly higher risk of NAFLD and liver fibrosis. This independent association was robust in women and adults over 40. The ALT/HDL-C ratio is a useful noninvasive diagnostic tool to quickly and accurately identify those at high risk of developing NAFLD and liver fibrosis.
Data availability
The datasets collected and analyzed during the current study are available on the NHANES website (http://www.cdc.gov/nchs/nhanes.htm).
Abbreviations
- NAFLD:
-
Nonalcoholic fatty liver disease
- NASH:
-
Nonalcoholic steatohepatitis
- T2DM:
-
Type 2 diabetes
- BMI:
-
Body mass index
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- PLT:
-
Platelet
- CRP :
-
C-Reactive Protein
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- GGT:
-
Gamma-glutamyl transpeptidase
- AKP:
-
Alkaline phosphatase
- TBIL:
-
Total bilirubin
- HbA1c :
-
Glycosylated hemoglobin A1c
- TG:
-
Triglycerides
- T.C.:
-
Total cholesterol
- HDL-C:
-
High-density lipoprotein cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
- SUA:
-
Serum uric acid
- VCTE:
-
Vibration-controlled transient elastography
- LSM :
-
Liver stiffness measurement
- CAP:
-
Controlled attenuation parameter
- OR :
-
Odds ratios
- CI :
-
Confidence interval
- β :
-
Standardization regression coefficient
- ROC:
-
Receiver operating characteristic
References
Sheka, A. C. et al. Nonalcoholic steatohepatitis: A review. Jama 323 (12), 1175–1183 (2020).
Niu, L. et al. Noninvasive proteomic biomarkers for alcohol-related liver disease. Nat. Med. 28 (6), 1277–1287 (2022).
Lin, H. et al. Vibration-Controlled transient elastography scores to predict liver-Related events in steatotic liver disease. Jama 331 (15), 1287–1297 (2024).
Wu, C. Z. et al. First-phase insulin secretion is positively correlated with Alanine aminotransferase in young adults. Advances in clinical and experimental medicine: official organ. Wroclaw Med. Univ. 30 (1), 35–40 (2021).
Chen, Q. C. et al. Longitudinal changes in liver aminotransferases predict metabolic syndrome in Chinese patients with nonviral hepatitis. Biomed. Environ. Sci.: BES. 29 (4), 254–266 (2016).
Nosrati, M., Safari, M., Alizadeh, A., Ahmadi, M. & Mahrooz, A. The atherogenic index log (Triglyceride/HDL-Cholesterol) as a biomarker to identify type 2 diabetes patients with poor glycemic control. Int. J. Prev. Med. 12, 160 (2021).
Zvintzou, E. et al. High-Density Lipoprotein in Metabolic Disorders and Beyond: An Exciting New World Full of Challenges and Opportunities. Pharmaceuticals (Basel, Switzerland). ;16(6). (2023).
Kosmas, C. E. et al. The Triglyceride/High-Density lipoprotein cholesterol (TG/HDL-C) ratio as a risk marker for metabolic syndrome and cardiovascular disease. Diagnostics (Basel Switzerland) ;13(5). (2023).
Cho, J., Hong, H., Park, S., Kim, S. & Kang, H. Insulin resistance and its association with metabolic syndrome in Korean children. Biomed. Res. Int. 2017, 8728017 (2017).
Rhainds, D. & Tardif, J. C. From HDL-cholesterol to HDL-function: cholesterol efflux capacity determinants. Curr. Opin. Lipidol. 30 (2), 101–107 (2019).
Hoekstra, M. & Van Eck, M. High-density lipoproteins and non-alcoholic fatty liver disease. Atherosclerosis Plus. 53, 33–41 (2023).
Karami, S. et al. Association of anti-oxidative capacity of HDL with subclinical atherosclerosis in subjects with and without non-alcoholic fatty liver disease. Diabetol. Metab. Syndr. 13 (1), 121 (2021).
von Eckardstein, A., Nordestgaard, B. G., Remaley, A. T. & Catapano, A. L. High-density lipoprotein revisited: biological functions and clinical relevance. Eur. Heart J. 44 (16), 1394–1407 (2023).
Mackey, R. H. et al. Lipoprotein particles and incident type 2 diabetes in the multi-ethnic study of atherosclerosis. Diabetes Care. 38 (4), 628–636 (2015).
Lam, D. W. & LeRoith, D. Metabolic syndrome. In: (eds Feingold, K. R., Anawalt, B., Blackman, M. R., Boyce, A., Chrousos, G., Corpas, E. et al.) Endotext. South Dartmouth (MA): MDText.com, Inc. Copyright © 2000–2024, MDText.com, Inc.; (2000).
Cao, C. et al. The nonlinear correlation between Alanine aminotransferase to high-density lipoprotein cholesterol ratio and the risk of diabetes: a historical Japanese cohort study. BMC Endocr. Disorders. 23 (1), 124 (2023).
He, S. et al. Alanine aminotransferase to high- density lipoprotein cholesterol ratio is positively correlated with the occurrence of diabetes in the Chinese population: a population-based cohort study. Front. Endocrinol. 14, 1266692 (2023).
Zhou, X., Xu, J. & Dai, H. The ratio of Alanine aminotransferase to high-density lipoprotein cholesterol is positively correlated with the insulin resistance in American adults: a population-based cohort study. Front. Med. 11, 1418364 (2024).
Rinella, M. E. et al. AASLD practice guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatol. (Baltimore Md). 77 (5), 1797–1835 (2023).
Ferraioli, G. et al. Liver ultrasound elastography: an update to the world federation for ultrasound in medicine and biology guidelines and recommendations. Ultrasound. Med. Biol. 44 (12), 2419–2440 (2018).
Ciardullo, S. & Perseghin, G. Statin use is associated with lower prevalence of advanced liver fibrosis in patients with type 2 diabetes. Metab. Clin. Exp. 121, 154752 (2021).
Kim, D., Konyn, P., Cholankeril, G. & Ahmed, A. Physical activity is associated with nonalcoholic fatty liver disease and significant fibrosis measured by fibroscan. Clin. Gastroenterol. Hepatology: Official Clin. Pract. J. Am. Gastroenterological Association. 20 (6), e1438–e55 (2022).
Roulot, D. et al. Liver stiffness values in apparently healthy subjects: influence of gender and metabolic syndrome. J. Hepatol. 48 (4), 606–613 (2008).
Wang, L. et al. Trends in prevalence of diabetes and control of risk factors in diabetes among US adults, 1999–2018. Jama 326 (8), 1–13 (2021).
Seebacher, F., Zeigerer, A., Kory, N. & Krahmer, N. Hepatic lipid droplet homeostasis and fatty liver disease. Semin. Cell Dev. Biol. 108, 72–81 (2020).
Man, S. et al. Prevalence of liver steatosis and fibrosis in the general population and various High-Risk populations: A nationwide study with 5.7 million adults in China. Gastroenterology 165 (4), 1025–1040 (2023).
Torres, D. M. & Harrison, S. A. NAFLD: predictive value of ALT levels for NASH and advanced fibrosis. Nat. Reviews Gastroenterol. Hepatol. 10 (9), 510–511 (2013).
Sugimoto, K. et al. Multiparametric US for identifying patients with High-Risk NASH: A derivation and validation study. Radiology 301 (3), 625–634 (2021).
Musso, G., Saba, F., Cassader, M. & Gambino, R. Lipidomics in pathogenesis, progression and treatment of nonalcoholic steatohepatitis (NASH): recent advances. Prog. Lipid Res. 91, 101238 (2023).
En Li Cho, E. et al. Global prevalence of non-alcoholic fatty liver disease in type 2 diabetes mellitus: an updated systematic review and meta-analysis. Gut 72 (11), 2138–2148 (2023).
Fuchs, A. et al. Associations among adipose tissue immunology, inflammation, exosomes and insulin sensitivity in people with obesity and nonalcoholic fatty liver disease. Gastroenterology 161 (3), 968–81e12 (2021).
Di Costanzo, A. et al. HDL-Mediated cholesterol efflux and plasma loading capacities are altered in subjects with Metabolically- but not genetically driven Non-Alcoholic fatty liver disease (NAFLD). Biomedicines ;8(12). (2020).
Cao, C. et al. Association between Alanine aminotransferase to high-density lipoprotein cholesterol ratio and nonalcoholic fatty liver disease: a retrospective cohort study in lean Chinese individuals. Sci. Rep. 14 (1), 6056 (2024).
Qiu, J. et al. The newly proposed Alanine aminotransferase to high-density lipoprotein cholesterol ratio has shown effectiveness in identifying non-alcoholic fatty liver disease. Front. Endocrinol. 14, 1239398 (2023).
Jou, J., Choi, S. S. & Diehl, A. M. Mechanisms of disease progression in nonalcoholic fatty liver disease. Semin. Liver Dis. 28 (4), 370–379 (2008).
Omagari, K. et al. Serum Alanine aminotransferase concentration as a predictive factor for the development or regression of fatty liver. J. Clin. Biochem. Nutr. 49 (3), 200–206 (2011).
Cruz, M. A. et al. Association of the nonalcoholic hepatic steatosis and its degrees with the values of liver enzymes and homeostasis model Assessment-Insulin resistance index. Gastroenterol. Res. 8 (5), 260–264 (2015).
Deprince, A., Haas, J. T. & Staels, B. Dysregulated lipid metabolism links NAFLD to cardiovascular disease. Mol. Metabolism. 42, 101092 (2020).
Hanley, A. J., Wagenknecht, L. E., Festa, A., D’Agostino, R. B. Jr. & Haffner, S. M. Alanine aminotransferase and directly measured insulin sensitivity in a multiethnic cohort: the insulin resistance atherosclerosis study. Diabetes Care. 30 (7), 1819–1827 (2007).
Feng, G., Feng, L. & Zhao, Y. Association between ratio of γ-glutamyl transpeptidase to high-density lipoprotein cholesterol and prevalence of nonalcoholic fatty liver disease and metabolic syndrome: a cross-sectional study. Annals Translational Med. 8 (10), 634 (2020).
Cuneo, R. C., Salomon, F., Watts, G. F., Hesp, R. & Sönksen, P. H. Growth hormone treatment improves serum lipids and lipoproteins in adults with growth hormone deficiency. Metab. Clin. Exp. 42 (12), 1519–1523 (1993).
Palmisano, B. T., Zhu, L. & Stafford, J. M. Role of estrogens in the regulation of liver lipid metabolism. Adv. Exp. Med. Biol. 1043, 227–256 (2017).
Della Torre, S. Beyond the X factor: relevance of sex hormones in NAFLD pathophysiology. Cells ;10(9). (2021).
Pafili, K. et al. Non-alcoholic fatty liver disease through the female lifespan: the role of sex hormones. J. Endocrinol. Investig. 45 (9), 1609–1623 (2022).
Della Torre, S. Non-alcoholic fatty liver disease as a canonical example of metabolic Inflammatory-Based liver disease showing a Sex-Specific prevalence: relevance of Estrogen signaling. Front. Endocrinol. 11, 572490 (2020).
Klisic, A. et al. Older age and HDL-cholesterol as independent predictors of liver fibrosis assessed by BARD score. Minerva Med. 110 (3), 191–198 (2019).
Klisic, A. et al. Relationship between oxidative stress, inflammation and dyslipidemia with fatty liver index in patients with type 2 diabetes mellitus. Experimental and clinical endocrinology & diabetes: official journal. German Soc. Endocrinol. [and] German Diabetes Association. 126 (6), 371–378 (2018).
Benedict, M. & Zhang, X. Non-alcoholic fatty liver disease: an expanded review. World J. Hepatol. 9 (16), 715–732 (2017).
Li, W. et al. A nonalcoholic fatty liver disease cirrhosis model in gerbil: the dynamic relationship between hepatic lipid metabolism and cirrhosis. Int. J. Clin. Exp. Pathol. 11 (1), 146–157 (2018).
Jiang, Z. G., Mukamal, K., Tapper, E., Robson, S. C. & Tsugawa, Y. Low LDL-C and high HDL-C levels are associated with elevated serum transaminases amongst adults in the united states: a cross-sectional study. PloS One. 9 (1), e85366 (2014).
Christakoudi, S., Riboli, E., Evangelou, E. & Tsilidis, K. K. Associations of body shape index (ABSI) and hip index with liver, metabolic, and inflammatory biomarkers in the UK biobank cohort. Sci. Rep. 12 (1), 8812 (2022).
Alarabi, M., Pan, Z., Romero-Gómez, M., George, J. & Eslam, M. Telomere length and mortality in lean MAFLD: the other face of metabolic adaptation. Hep. Intl. 18 (5), 1448–1458 (2024).
Pan, Z. et al. MAFLD: an ideal framework for Understanding disease phenotype in individuals of normal weight. Therapeutic Adv. Endocrinol. Metabolism. 15, 20420188241252543 (2024).
Alharthi, J. et al. Loss of metabolic adaptation in lean MAFLD is driven by endotoxemia leading to epigenetic reprogramming. Metab. Clin. Exp. 144, 155583 (2023).
Eslam, M. et al. Metabolic (dysfunction)-associated fatty liver disease in individuals of normal weight. Nat. Reviews Gastroenterol. Hepatol. 19 (10), 638–651 (2022).
Acknowledgements
The authors thank the staff and the participants of the NHANES study for their valuable contributions.
Funding
This study was supported by the Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (Grant No. 2025KY1377).
Author information
Authors and Affiliations
Contributions
YX, Y.Z., and J.Y. contributed to the conception and study design. Y.J., D.W., and X.Y. were responsible for execution, data acquisition, analysis, and interpretation. Y.X., Y.Z., and D.W. drafted, revised, and critically reviewed the article. All authors provided final approval of the version to be published, agreed on the journal for article submission, and accepted responsibility for all aspects of the work.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The National Center for Health Statistics ethics review board approved all NHANES protocols. The participants provided written informed consent to participate in this study. This study uses publicly available secondary data.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xuan, Y., Wu, D., Jin, Y. et al. Utility of the serum alanine aminotransferase to high density lipoprotein cholesterol ratio in evaluating nonalcoholic fatty liver disease and liver fibrosis. Sci Rep 15, 21292 (2025). https://doi.org/10.1038/s41598-025-06171-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-06171-x