Abstract
This study explored the therapeutic potential of monocarboxylate transporter 4 (MCT4/SLC16A3) in KRAS-mutant colorectal cancer (CRC). By integrating immunohistochemistry with UCSC Xena database analysis, we identified a distinct MCT4 expression pattern in KRAS-mutant CRC. Cytoplasmic MCT4 expression was positively correlated with KRAS mutation status and mismatch repair (MMR) proficiency, but negatively associated with sex and tumor differentiation. Plasma membranous MCT4 expression was also positively correlated with KRAS mutations and negatively with differentiation grade. Notably, tumors harboring KRAS codon 13 mutations, particularly G13D, showed higher MCT4 expression than those with codon 12 mutations, such as G12D. Kaplan–Meier survival analysis revealed a significant association between high SLC16A3 expression and poor prognosis. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses indicated that MCT4 is mainly involved in immune regulation and metabolism-related pathways. Furthermore, CIBERSORT analysis combined with immunohistochemistry confirmed a relationship between KRAS mutations and immune cell infiltration. This is the first study to systematically characterize MCT4 expression across different KRAS-mutant CRC subtypes. Our findings suggest that MCT4 may modulate the tumor immune microenvironment in KRAS-mutant CRC and could serve as a potential target for precision therapy.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) is one of the most common malignancies worldwide, ranking third in incidence and second in cancer-related mortality. KRAS is one of the most frequently mutated oncogenes in CRC, with missense mutations detected in 30–50% of cases1. The majority of KRAS mutations occur at codons 12 and 13, with G12D, G12V, and G13D being the most prevalent subtypes2. Studies have shown that patients with KRAS-mutant CRC have a poorer prognosis compared to those with wild-type KRAS3,4. Clinical Practice Guidelines from the National Comprehensive Cancer Network (NCCN) also highlight the lack of effective targeted therapies for patients harboring KRAS mutations5. This underscores the urgent need to investigate the molecular mechanisms underlying the differences between KRAS-mutant and wild-type CRC.
Importantly, different KRAS mutations can activate downstream signaling pathways through distinct mechanisms, resulting in variable responses to targeted therapies6,7. This heterogeneity among KRAS mutation subtypes poses a major challenge in the clinical management of CRC. Therefore, it is essential to study the biological differences among KRAS mutation subtypes to inform the development of effective therapeutic strategies.
Lactate, traditionally considered a metabolic byproduct, has recently been implicated in modulating the tumor immune microenvironment8. It promotes immunosuppression and tumor progression. Monocarboxylate transporter 4 (MCT4, also known as SLC16A3) is typically expressed in hypoxic cells, where it facilitates the export of excess intracellular lactate to maintain cellular homeostasis9. Elevated expression of MCT4 has been reported in several malignancies, including breast cancer, lung cancer, and melanoma10,11,12. Accumulation of extracellular lactate creates an acidic tumor microenvironment, which enhances tumor invasiveness by promoting epithelial–mesenchymal transition (EMT) and facilitates immune escape13,14,15,16.
Moreover, the acidic microenvironment favors tumor immune evasion by impairing antigen presentation and promoting the polarization of immunosuppressive M2 macrophages17,18,19,20. Lactate also acts as a substrate for protein modification, altering the glycosylation of tumor-associated proteins and further contributing to immune escape21. Notably, overexpression of MCT4 has been shown to upregulate PD-L1 expression via the Wnt signaling pathway and to enhance its glycosylation, thereby increasing immune resistance22.
Despite these findings, the clinical significance of MCT4 in KRAS-mutant CRC and its role in immune suppression remain poorly understood. This study aims to investigate the expression of MCT4 in KRAS-mutant CRC and to explore the potential mechanisms by which MCT4 contributes to immunosuppression. Our findings may offer novel insights into overcoming the therapeutic challenges associated with KRAS-mutant CRC.
Methods
Gene expression analysis
SLC16A3 expression was analyzed using the GEPIA2 (http://gepia2.cancer-pku.cn) and CPTAC (https://proteomic.datacommons.cancer.gov/pdc/) databases. A p-value < 0.05 was considered statistically significant.
Immunohistochemistry (IHC)
Seventy-five colorectal cancer samples that had undergone DNA sequencing were selected. The detected mutations included point mutations (SNV), small fragment insertions and deletions (INDEL), copy number variations (CNV), and fusions (FUSION). The genetic testing includes KRAS. Tissue sections from 37 KRAS-mutant and 38 KRAS wild-type CRC patients were collected from the Department of Pathology, First Affiliated Hospital of Chongqing Medical University. All patients provided informed consent, and the study was approved by the Ethics Committee (2024-402-01). All methods were performed in accordance with the relevant guidelines and regulations. Immunohistochemistry was performed using the UltraSensitive Kit (KIT-9710, Maixin) and anti-MCT4 antibody (1:2000, 22787-1-AP, Proteintech). Sections were scored independently by two pathologists in a double-blind manner, and mean values were used for analysis.
The staining intensity of MCT4 in the cytoplasm and plasma membrane was evaluated based on both staining intensity and the proportion of positive cells. Staining intensity was scored as follows: no staining = 0, weak = 1, moderate = 2, and strong = 3. The proportion of positive cells was scored as: <5% = 0; 5–25% = 1; 26–50% = 2; 51–75% = 3; and 76–100% = 4. The final score was obtained by multiplying the intensity and proportion scores, and categorized as follows: 0 (−), 1–4 (+), 5–8 (++), and 9–12 (+++).For stromal cells, MCT4 staining was assessed based on the percentage of positive cells: <10% (−), 10–30% (+), and > 30% (++).
Survival analysis
A total of 275 KRAS-mutant CRC patients were divided into high and low SLC16A3 expression groups based on the median mRNA expression level. Ten-year overall survival (OS), post-progression survival (PPS), and relapse-free survival (RFS) were assessed using the Kaplan–Meier Plotter database (http://kmplot.com/analysis/). A p-value < 0.05 was considered statistically significant.
Differentially expressed genes (DEGs), gene ontology (GO), and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis
Gene expression data and mutation information for the GDC TCGA colon cancer (COAD) cohort were downloaded from UCSC Xena database4 (https://xenabrowser.net/datapages/). 417 tumor tissues were obtained after excluding normal tissues. Subsequently, we merged the expression data and mutation information to obtain 164 KRAS-mutated CRC tissues. The limma package in R language was used to screen the differentially expressed genes between the low-expression and high-expression groups classified by the median value of SLC16A3 expression in 417 CRC tissue and 164 KRAS-mutant CRC tissues from the UCSC Xena database. The ClusterProfiler R language package was used for GO and KEGG function enrichment analysis. GO terms and KEGG pathway with p value<0.05 were considered statistically significant.
Tumor immune infiltration analysis
In CRC and KRAS mutant CRC, we assessed the proportion of 22 different types of immune cells in the SLC16A3 high and low expression groups using CIBERSORT. Subsequently, we plotted cumulative bar graphs and box plot using the ggplot2 R package to show comparisons of differences in immune cell infiltration.
Statistical analysis
T test was used to compare the expression of SLC16A3 in normal colorectal and colorectal cancer tissues. Immunohistochemical results were statistically analyzed by Mann-Whitney U test. P < 0.05 was statistically significant. Dunn’s multiple comparisons test was used for two-by-two comparisons between multiple groups, P < 0.05 was considered statistically significant. Spearman correlation coefficient was calculated for correlation analysis, and P < 0.05 was statistically significant. All statistical analysis were performed using GraphPad Prism software (10.1.2).
Results
SLC16A3 is overexpressed in CRC
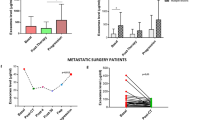
SLC16A3 mRNA expression in colon adenocarcinoma (COAD) and rectum adenocarcinoma (READ) was analyzed using the GEPIA2 database. Expression levels were significantly higher in both COAD and READ compared to normal tissues (Fig. 1A). Similarly, data from the CPTAC database showed elevated SLC16A3 protein levels in tumor tissues relative to adjacent normal tissues (Fig. 1B).
Expression of SLC16A3 in colorectal cancer (CRC). (A) SLC16A3 mRNA expression levels in COAD and READ obtained from the GEPIA2 database. (B) SLC16A3 protein abundance levels in colon cancer obtained from the CPTAC database. (C-E) SLC16A3 mRNA expression levels in READ (C) and COAD (D) different gene subtypes and in CRC different stages (E).
To explore factors associated with SLC16A3 expression in colorectal cancer (CRC), its relationship with microsatellite instability (MSI) and clinical stage was examined using GEPIA2. No significant associations were found (Fig. 1C–E).
Expression of MCT4 in CRC and its relationship to clinical features
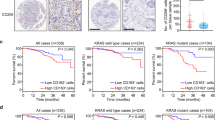
Immunohistochemical analysis showed that MCT4 was expressed in both the cytoplasm and plasma membrane of tumor cells (Fig. 2C). Cytoplasmic MCT4 expression was positively correlated with KRAS mutation (R = 0.50, P < 0.001) and MMR proficiency (R = 0.24, P = 0.04), and negatively correlated with sex (R = − 0.32, P = 0.01) and tumor differentiation (R = − 0.24, P = 0.04). No significant correlations were found with age (P = 0.23), vascular invasion (P = 0.99), lymph node metastasis (P = 0.92), or tumor location (P = 0.60) (Fig. 2A–B).
Correlation between MCT4 expression and clinicopathological features in CRC. (A) Spearman correlation heatmap showing correlation coefficients between MCT4 expression (cytoplasm, plasma membrane, stroma) and clinicopathological parameters. (B) Corresponding p-value heatmap indicating statistical significance. (C) Representative immunohistochemistry images of MCT4 in CRC tissues with different KRAS genotypes (WT, G13D, G12D, G12V) at 10×, 20×, and 40× magnifications.
At the plasma membrane, MCT4 expression was also positively associated with KRAS mutation (R = 0.56, P < 0.001) and negatively associated with tumor differentiation (R = − 0.27, P = 0.02), with no significant correlation with other clinical parameters.
Stromal MCT4 expression was positively correlated with KRAS mutations (R = 0.39, P < 0.01), but not with other clinical features. Notably, stromal MCT4 levels were significantly associated with MCT4 expression in tumor cells, both at the plasma membrane (R = 0.73, P < 0.001) and in the cytoplasm (R = 0.52, P < 0.001) (Fig. 2A–B).
MCT4 expression in the cytoplasm and on the cytomembrane between different KRAS codons mutation and KRAS wild type
MCT4 expression differs between KRAS-mutant and wild-type CRC in both the membrane and cytoplasm. However, its variation among KRAS mutation subtypes remains unclear. Our results showed elevated MCT4 expression in the membrane and cytoplasm of tumors harboring the three common KRAS mutations—G12D, G12V, and G13D—compared to KRAS wild-type CRC (Table 1).Further analysis revealed that membranous MCT4 expression was significantly higher in codon 13 mutants than in codon 12 mutants (P = 0.0116), while cytoplasmic expression did not differ significantly (P = 0.7836).
Among the three subtypes, KRAS(G13D) tumors showed higher membranous MCT4 expression than KRAS(G12D) (P = 0.0213), but not significantly different from KRAS(G12V) (P = 0.0692). Cytoplasmic MCT4 expression was similar across all three subtypes (P > 0.9999) (Table 1).
Expression of MCT4 in tumor stromal cells
Interestingly, while evaluating MCT4 expression in tumor cells, we also observed its expression in stromal cells (Fig. 3A). Although the exact stromal cell type could not be definitively identified, their morphology suggests they may be cancer-associated fibroblasts(CAF). Notably, MCT4 expression in stromal cells was higher in KRAS-mutant CRC compared to KRAS wild-type CRC. Further subtype analysis revealed that MCT4 expression was highest in KRAS(G13D) tumors (Table 1).
MCT4 expression in stromal cells and prognostic value of SLC16A3 in KRAS-mutant CRC. (A) Immunohistochemistry staining of MCT4 in stromal cells from KRAS-mutant and wild-type CRC tissues (40×). (B-D) Kaplan–Meier curves for relapse-free survival (RFS), overall survival (OS), and post-progression survival (PPS) based on SLC16A3 expression using four probes (202855_s_at, 202856_s_at, 213522_s_at, 217691_x_at). Hazard ratios (HR), 95% confidence intervals, and log-rank p-values are shown.
Prognosis curves analysis based on SLC16A3 mRNA expression in KRAS-mutant CRC
Using the Kaplan–Meier Plotter database, we identified four probe IDs corresponding to SLC16A3 (202855_s_at, 202856_s_at, 213522_s_at, and 217691_x_at) and analyzed their prognostic significance in KRAS-mutant CRC based on mRNA expression levels. KRAS-mutant CRC patients with lower SLC16A3 expression exhibited poorer RFS[HR = 2.19(1.28–3.77), logrank p = 0.0036 for 202855_s_at; HR = 1.82(1.16–2.84), logrank p = 0.0079 for 202856_s_at; HR = 1.51 (0.99–2.28), logrank p = 0.052 for 213522_s_at; HR = 1.97(1.3-3), logrank p = 0.0012 for 217691_x_at] (Fig. 3B).
For overall survival (OS), only 217691_x_at showed a statistically significant association, with higher SLC16A3 expression linked to poorer outcomes [HR = 1.83 (1.1–3.04), p = 0.018]. The other probes showed no significant correlation with OS [HR = 0.68(0.43–1.08), logrank p = 0.1 for 202855_s_at; HR = 0.7(0.4–1.22), logrank p = 0.2 for 202856_s_at; HR = 1.45 (0.91–2.3), logrank p = 0.11 for 213522_s_at] (Fig. 3C).
Regarding post-progression survival (PPS), 213522_s_at was the only probe showing a significant association between higher SLC16A3 expression and worse prognosis [HR = 2.44 (1.42–4.19), p = 0.0009]. The remaining probes did not show significant correlations [HR = 0.59(0.31–1.13), logrank p = 0.11 for 202855_s_at; HR = 1.61(0.9–2.87), logrank p = 0.11 for 202856_s_at; HR = 1.4(0.83–2.39), logrank p = 0.21 for 217691_x_at] (Fig. 3D).
DEGs analysis and GO/KEGG enrichment in KRAS mutant CRC
To investigate the effects of SLC16A3 expression in KRAS-mutant CRC, we integrated mutation and expression data, identifying 164 KRAS-mutant and 307 non-mutant samples. The KRAS-mutant group was divided into high and low SLC16A3 expression groups based on the median expression level. Compared to the low-expression group, the high-expression group showed 66 upregulated and 98 downregulated genes (criterions: p value < 0.05 and |Fold Change|>1; Fig. 4A). Then, GO enrichment analysis revealed that these DEGs were significantly associated with immune responses and metabolic processes, including: humoral immune response (GO:0006959), hormone metabolic process (GO:0042445), protein O-linked glycosylation (GO:0006493), antibacterial humoral response (GO:0019731) and CD4-positive, CD25-positive, alpha-beta regulatory T cell differentiation (GO:0002361) (Fig. 4B&C). Meanwhile, DEGs were mainly enriched to KEGG pathways such as Retinol metabolism(hsa00830), Drug metabolism - cytochrome P450(hsa00982), Metabolism of xenobiotics by cytochrome P450(hsa00980), Bile secretion(hsa04976), Salivary secretion(hsa04970), Staphylococcus aureus infection(hsa05150), Mucin type O-glycan biosynthesis(hsa00512), TGF-beta signaling pathway(hsa04350) and Chemical carcinogenesis - DNA adducts(hsa05204) (Fig. 4D).
Differential expression and functional enrichment analysis in KRAS-mutant CRC with high vs. low SLC16A3 expression. (A) Volcano plot of differentially expressed genes (DEGs) between high and low SLC16A3 expression groups (|log2FC| > 1, p < 0.05). (B) Circular plot showing GO enrichment of DEGs across biological process, cellular component, and molecular function categories. (C) Bar plot of top GO terms enriched in DEGs. (D) KEGG pathway enrichment bubble plot showing significantly enriched pathways. Dot size indicates gene count; color represents p-value.
Correlation between SLC16A3 and immune infiltration
To investigate the association between MCT4 expression and the immune microenvironment, we analyzed immune cell infiltration using the CIBERSORT algorithm in both CRC and KRAS-mutant CRC samples (Fig. 5A–D). In the overall CRC cohort, eight immune cell types differed significantly between the high and low SLC16A3 expression groups (Fig. 5A). The high-expression group showed increased proportions of CD8⁺ T cells, follicular helper T cells, M0 macrophages, and M1 macrophages, and decreased proportions of resting CD4⁺ memory T cells, M2 macrophages, and resting dendritic cells. In the KRAS-mutant subgroup, only two immune cell types showed significant differences (Fig. 5C): M0 macrophages were enriched, while resting CD4⁺ memory T cells were reduced in the high-expression group.
Immune infiltration analyses. Box plots show the differences in immune cell types between the high- SLC16A3 expression and low-expression groups in CRC (A) and KRAS mutant CRC (C). Distribution of 22 immune cells in CRC tissue (B) and KRAS mutant CRC tissue (D), with different colors representing different immune cells. (E-F) Relationship between CD4 and MCT4 expression in KRAS MUT and WT. (CP: cytoplasm, CM: Plasma membrane, MC: Stromal cells) (G) Immunohistochemistry showed the relationship between CD4 and MCT4(40x).
To further examine the relationship between MCT4 and T cells, we performed immunohistochemical analysis in KRAS-mutant CRC tissues. Tumor CD4⁺ T cell density was negatively correlated with MCT4 expression in both the cytoplasm (R = − 0.47, P = 0.029) and the stroma (R = − 0.52, P = 0.013) (Fig. 5E–G). No significant correlation was observed between CD4⁺ T cell density at the invasive front and MCT4 expression in either KRAS-mutant or wild-type CRC.
Discussion
CRC is a malignancy with high incidence and mortality, posing a significant threat to human health1,23. Approximately 40% or more of CRC patients harbor KRAS mutations, which are associated with poorer prognosis compared to wild-type KRAS cases24. Moreover, KRAS mutations are recognized as negative predictors for the efficacy of anti-EGFR therapies such as cetuximab24,25. However, emerging evidence suggests heterogeneity in treatment responses even among KRAS-mutant patients, indicating that differences in the tumor immune microenvironment may influence drug sensitivity26. These findings highlight the need for deeper molecular stratification of KRAS-mutant CRC to identify predictive markers and therapeutic targets.
In this study, we focused on MCT4, a lactate transporter highly expressed in glycolytic and hypoxic tumor cells27. Analysis of public databases and our immunohistochemical results demonstrated that MCT4 is overexpressed in CRC tissues, particularly in KRAS-mutant subtypes. Notably, MCT4 expression was highest in the KRAS G13D subtype, suggesting subtype-specific regulation and possible links to differential treatment responses. These findings identify MCT4 as a potentially valuable biomarker in the molecular stratification of KRAS-mutant CRC.
CIBERSORT analysis indicated that high SLC16A3 expression is associated with altered immune cell infiltration, particularly CD4 + T cells and M0 macrophages. These observations were supported by immunohistochemical data, which showed a negative correlation between MCT4 expression and CD4⁺ T cell density in both the tumor and stromal compartments. Existing studies have shown that lactic acid modulates the tumor immune microenvironment (TME) through acidification and immune suppression28,29,30. These results suggest that MCT4 may contribute to immune evasion mechanisms in KRAS-mutant CRC.
Enrichment analyses revealed that high MCT4 expression in KRAS-mutant CRC is linked to immune-related and metabolic pathways. Enrichment of the mucin-type O-glycan biosynthesis pathway suggests that lactate exported via MCT4 may influence glycosylation processes, potentially contributing to immune evasion and metastasis31. Retinol metabolism pathway enrichment implies interference with vitamin A signaling, known to regulate immune differentiation and epithelial integrity32. Associations with cytochrome P450 drug metabolism pathways raise the possibility of MCT4 involvement in modulating drug efficacy.
A particularly novel finding of our study is the high expression of MCT4 in stromal cells, especially those with morphology consistent with cancer-associated fibroblasts (CAFs). These MCT4-positive CAFs were more prevalent in KRAS-mutant tumors, indicating a possible role in modulating the TME and promoting therapeutic resistance. Previous studies have shown that CAFs rely on MCT4-mediated lactate export to support tumor progression and immune modulation33. Our KEGG pathway analysis also showed enrichment of the TGF-β signaling pathway, a key regulator of CAF activation34,35. It was found in fibroblasts that hypoxia can accelerate the TGF-β1 signaling pathway and the transformation of normal fibroblasts into CAFs. At the same time, the autophagy induced by the combination of hypoxia and TGF-β1 can promote aerobic glycolysis in cancer stromal cells and the expression of MCT4. There is a positive correlation between glucose consumption in CAFs and the expression of MCT436. Therefore, MCT4 may participate in the transformation of CAFs through regulating the glycolysis of fibroblasts under the regulation of TGF-β1.
In this study, high MCT4 expression was consistently associated with shorter RFS across multiple probes. However, results for OS and PPS were less consistent. These differences may be due to probe-specific detection of transcript variants, as different probes target distinct regions of the gene33. In addition, RFS reflects early recurrence, while OS and PPS are influenced by post-recurrence treatments and other clinical factors, which may dilute the impact of MCT437,38. Despite these variations, the consistent association with RFS supports a potential role for MCT4 in early disease progression.
Clinically, these results suggest that MCT4 is not only a potential biomarker for KRAS-mutant CRC subtypes but also a candidate therapeutic target, particularly in the context of overcoming resistance to EGFR-targeted therapies. Existing MCT4 inhibitors used in other malignancies may warrant evaluation in CRC, especially in combination with immunotherapy or agents targeting the TME.
This study has several limitations. First, the sample size for immunohistochemical validation was limited, which may affect the generalizability of our findings. Second, the mechanistic link between MCT4 expression and TGF-β signaling in CAFs remains speculative and requires further functional validation. Third, although we observed differences in immune infiltration patterns, functional assays confirming causality were not conducted. Future studies incorporating larger patient cohorts and in vivo experiments are needed to validate these findings and explore therapeutic implications.
Data availability
Expression data supporting the results of this study are available in the Gene Expression Profiling Interactive Analysis 2 (GEPIA2) database (http://gepia2.cancer-pku.cn/#index), the National Cancer Institute Clinical Tumor Proteomics Analysis Consortium (CPTAC) database (https://proteomic. datacommons.cancer.gov/pdc/) and the UCLA Xena database4 (https://xenabrowser.net/datapages/). Survival analysis data are available in the Kaplan-Meier plotter database (http://kmplot.com/analysis/).
References
Sung, H. et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Dienstmann, R., Connor, K., Byrne, A. T. & Consortium, C. Precision therapy in RAS mutant colorectal Cancer. Gastroenterology 158, 806–811 (2020).
Rui, Y., Wang, C., Zhou, Z., Zhong, X. & Yu, Y. K-Ras mutation and prognosis of colorectal cancer: A meta-analysis. Hepatogastroenterology 62, 19–24 (2015).
Zhu, G., Pei, L., Xia, H., Tang, Q. & Bi, F. Role of oncogenic KRAS in the prognosis, diagnosis and treatment of colorectal cancer. Mol. Cancer. 20, 143 (2021).
Benson, A. B. et al. Colon cancer, version 2.2021, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Canc Netw. 19, 329–359 (2021).
Yuan, T. L. et al. Differential effector engagement by oncogenic KRAS. Cell. Rep. 22, 1889–1902 (2018).
Chong, W. et al. Integrated multi-omics characterization of KRAS mutant colorectal cancer. Theranostics 12, 5138–5154 (2022).
Reuss, A. M., Groos, D., Buchfelder, M. & Savaskan, N. The acidic brain-glycolytic switch in the microenvironment of malignant glioma. Int. J. Mol. Sci 22. (2021).
Liu, T., Han, S., Yao, Y. & Zhang, G. Role of human monocarboxylate transporter 1 (hMCT1) and 4 (hMCT4) in tumor cells and the tumor microenvironment. Cancer Manag Res. 15, 957–975 (2023).
Roche, M. E. et al. TP53 induced Glycolysis and apoptosis regulator and monocarboxylate transporter 4 drive metabolic reprogramming with c-MYC and NFkB activation in breast cancer. Int. J. Cancer. 153, 1671–1683 (2023).
Qian, Y. et al. MCT4-dependent lactate secretion suppresses antitumor immunity in LKB1-deficient lung adenocarcinoma. Cancer Cell. 41, 1363–1380 (2023). e7.
Kanekura, T. CD147/Basigin is involved in the development of malignant tumors and T-Cell-Mediated immunological disorders via regulation of Glycolysis. Int J. Mol. Sci. 24, 17344 (2023).
Zhang, S. et al. Pirfenidone inhibits TGF-beta1-induced metabolic reprogramming during epithelial-mesenchymal transition in non-small cell lung cancer. J. Cell. Mol. Med. 28, e18059 (2024).
Jena, B. C. et al. TGF-beta1 induced autophagy in cancer associated fibroblasts during hypoxia contributes EMT and Glycolysis via MCT4 upregulation. Exp. Cell. Res. 417, 113195 (2022).
Feng, Q. et al. Severely polarized extracellular acidity around tumour cells. Nat. Biomed. Eng. 8, 787–799 (2024).
Babl, N. et al. MCT4 Blockade increases the efficacy of immune checkpoint Blockade. J. Immunother. Cancer 11, e007349 (2023).
Li, X., Du, G., Li, L. & Peng, K. Cellular specificity of lactate metabolism and a novel lactate-related gene pair index for frontline treatment in clear cell renal cell carcinoma. Front. Oncol. 13, 1253783 (2023).
Gao, Y. et al. Enhanced osteosarcoma immunotherapy via CaCO(3) nanoparticles: remodelling tumour acidic and immune microenvironment for photodynamic therapy. Adv. Healthc. Mater. 13, e2400538 (2024).
Lai, S. W., Lin, H. J., Liu, Y. S., Yang, L. Y. & Lu, D. Y. Monocarboxylate transporter 4 regulates glioblastoma motility and monocyte binding ability. Cancers (Basel) 12, 380 (2020).
Reinfeld, B. I. et al. Cell-programmed nutrient partitioning in the tumour microenvironment. Nature 593, 282–288 (2021).
Chakraborty, M., Kaur, J., Gunjan, Kathpalia, M. & Kaur, N. Clinical relevance of glycosylation in triple negative breast cancer: a review. Glycoconj. J. 41, 79–91 (2024).
Duan, X. et al. MCT4/Lactate promotes PD-L1 glycosylation in Triple-Negative breast Cancer cells. J. Oncol. 2022, 3659714 (2022).
Saoudi González, N. et al. Cetuximab as a key partner in personalized targeted therapy for metastatic colorectal Cancer. Cancers (Basel) 16, 412 (2024).
Pellatt, A. J., Bhamidipati, D., Subbiah, V. & Ready Set, Go: Setting Off on the Mission to Target KRAS in Colorectal Cancer. JCO Oncol Pract : Op2400295. (2024).
Zhou, Y. et al. The relationship of KRAS expression with KRAS status, prognosis, and tumor-infiltrated T lymphocytes in colorectal cancer. Th. Adv. Gastroenterol. 17, 17562848241249387 (2024).
McFall, T., Schomburg, N. K., Rossman, K. L. & Stites, E. C. Discernment between candidate mechanisms for KRAS G13D colorectal cancer sensitivity to EGFR inhibitors. Cell. Commun. Signal. 18, 179 (2020).
Zhang, Y. et al. Macrophage MCT4 Inhibition activates reparative genes and protects from atherosclerosis by histone H3 lysine 18 lactylation. Cell. Rep. 43, 114180 (2024).
Espelage, L., Wagner, N., Placke, J. M., Ugurel, S. & Tasdogan, A. The interplay between metabolic adaptations and diet in cancer immunotherapy. Clin. Cancer Res. 30, 3117–3127 (2024).
Liu, S., Jia, M. & Dai, R. Deciphering the tumour immune microenvironment of hepatocellular carcinoma. Scand. J. Immunol. 98, e13327 (2023).
Chen, Y. et al. Progress in the regulation of immune cells in the tumor microenvironment by bioactive compounds of traditional Chinese medicine. Molecules 29, 2374 (2024).
Xu, Y. et al. PD-L2 glycosylation promotes immune evasion and predicts anti-EGFR efficacy. J. Immunother. Cancer 9, e002699 (2021).
Esposito, M., Amory, J. K. & Kang, Y. The pathogenic role of retinoid nuclear receptor signaling in cancer and metabolic syndromes. J. Exp. Med. 221, e20240519 (2024).
Affinito, A. et al. MCT4-driven CAF-mediated metabolic reprogramming in breast cancer microenvironment is a vulnerability targetable by miR-425-5p. Cell. Death Discov. 10, 140 (2024).
Wang, Y. et al. Targeting Src SH3 domain-mediated glycolysis of HSC suppresses transcriptome, myofibroblastic activation, and colorectal liver metastasis. Hepatology (2024).
Calon, A., Tauriello, D. V. & Batlle, E. TGF-beta in CAF-mediated tumor growth and metastasis. Semin Cancer Biol. 25, 15–22 (2014).
Jena, B. C. et al. TGF-β1 induced autophagy in cancer associated fibroblasts during hypoxia contributes EMT and Glycolysis via MCT4 upregulation. Exp. Cell. Res. 417, 113195 (2022).
Zhao, Y. et al. Integrative analysis identified MCT4 as an independent prognostic factor for bladder Cancer. Front. Oncol. 11, 704857 (2021).
Tong, Y. H., Hu, X. P., Xiang, X. P. & Fang, L. High expression of monocarboxylate transporter 4 (MCT 4), but not MCT 1, predicts poor prognosis in patients with non-small cell lung cancer. Transl Cancer Res. 10, 1336–1345 (2021).
Funding
This research was supported by Future Medical Youth Innovation team support program of Chongqing Medical University (W0096), funded by Open Research Projects of the Key Laboratory of Tumor Immunopathology, Ministry of Education (2021jsz707), and the Natural Science Foundation of Chongqing (CSTB2022NSCQ-BHX0678). Additionally, it was funded by Smart medicine project of Chongqing Medical University (ZHYX2019014).
Author information
Authors and Affiliations
Contributions
Qian Gong was responsible for downloading, collating and analyzing data, and was responsible for manuscript writing. Jing Huang processed the immunohistochemical staining of tissue sections. Qingshu Li and Shuxian Zhang participated in the collection of clinical information of CRC patients. Ming Xiao and Ming Li were responsible for scoring the degree of staining of the pathological sections. Tang Yi assisted in the experimental design and participated in manuscript modification. Yalan Wang and Yaying Yang were responsible for funding acquisition.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gong, Q., Huang, J., Li, Q. et al. Exploring the role of monocarboxylate transporter 4 in diverse KRAS mutation subtypes of colorectal Cancer. Sci Rep 15, 23257 (2025). https://doi.org/10.1038/s41598-025-06254-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-06254-9