Abstract
Oxytocin (Oxtr) and dopamine (Drd1, Drd2) receptors provide a canonical example for how differences in neuromodulatory receptors drive individual and species-level behavioral variation. These systems exhibit striking and functionally relevant differences in nucleus accumbens (NAc) expression across monogamous prairie voles (Microtus ochrogaster) and promiscuous meadow voles (Microtus pennsylvanicus). However, their cellular organization remains largely unknown. Using multiplex in situ hybridization, we mapped Oxtr, Drd1, and Drd2 expression in sexually naïve and mate-paired prairie and meadow voles. Prairie voles have more Oxtr+ cells than meadow voles, but Oxtr distribution across dopamine-receptor cell class was similar, indicating a general upregulation rather than cell class bias. Oxtr was enriched in cells that express both dopamine receptors (Drd1+/Drd2+) in prairie voles, suggesting these cells may be particularly sensitive to oxytocin. We found no species or pairing-induced differences in Drd1+ cell counts, suggesting prior reports of expression differences may reflect upregulation in cells already expressing these receptors. Finally, we used single-nucleus sequencing to provide the first comprehensive map of Oxtr and Drd1-5 across molecularly-defined NAc cell types in the prairie vole. These results provide a critical framework for understanding how nonapeptide and catecholamine systems may recruit distinct NAc cell types to shape social behavior.
Similar content being viewed by others
Introduction
Oxytocin and dopamine, acting via their receptors, play critical roles in regulating complex social behaviors, including pair bonding, parental care, and social recognition1,2,3. Comparisons of oxytocin (Oxtr - mRNA; OXTR - protein) and dopamine (Drd1 and Drd2 – mRNA; DRD1 and DRD2 - protein) receptor distributions within and across species provide classic examples of how differences in receptor distribution and/or levels within the brain contribute to species-appropriate and experience-dependent behaviors such as mating and aggression4,5. Socially monogamous prairie voles (Microtus ochrogaster) and promiscuous meadow voles (Microtus pennsylvanicus) exemplify this relationship, exhibiting strikingly different social behavior alongside distinct limbic oxytocin and dopamine receptor expression6,7,8,9.
Prairie voles form selective and long-lasting pair bonds, exhibit aggression toward non-partner individuals, and display robust biparental care, whereas meadow voles are primarily asocial, do not form mating-based bonds, and rely solely on dams for pup-rearing10. Compared to meadow voles, prairie voles have higher OXTR densities in the nucleus accumbens (NAc), which functionally contribute to species differences in social behavior6,11,12,13. Blockade of these receptors impairs bond formation, while overexpression accelerates bonding and facilitates alloparenting12,14,15. Additionally, histone deacetylase inhibitors facilitate partner preference formation, upregulating Oxtr transcript and OXTR protein, suggesting epigenetic modulation of social bonding that may be mediated via OXTR16. Of note, artificially increasing OXTR levels in adult meadow voles is not sufficient to convey the ability to form partner preference, suggesting it may be developmentally required for its effects on social behavior in prairie voles17.
Dopamine receptor expression in the NAc also differs across species and social experiences. DRD1-class (DRD1 + DRD5) and DRD2-class (DRD2 + DRD3 + DRD4) receptors play opposing roles in pair bonding in prairie voles, and their balance in the NAc is critical for modulating reward signaling and partner preference18,19. DRD2-class agonists promote partner preference formation, and DRD2-class antagonists block mating-induced partner preference formation20,21. Conversely, pharmacological manipulation of DRD1 receptors in prairie voles has opposite effects; agonism blocks pair bonding and leads to enhanced aggression18,22. DRD1 receptors are also upregulated following pair bond formation, potentially reinforcing social attachment by promoting aggressive rejection of non-partner voles23. In line with this, meadow voles exhibit higher DRD1 levels, which may contribute to their lack of bonding after mating18. Thus, the balance between DRD1 and DRD2—which differs across species and social experience, likely contributes to species and experience dependent differences in social bonding behaviors.
While previous studies have examined the distributions of oxytocin and dopamine receptors independently, their interaction is also critical. In prairie voles, pair bonding depends on the concurrent activation of OXTR and DRD2 in the NAc20. Studies to date have largely quantified receptors levels via autoradiography, or in a few cases, qRT-PCR or western blotting2,6,8,9,13,24. These and initial papers examining mRNA via radiolabeled in situ hybridization fall short of providing cell-type specific information regarding the expression of Oxtr, Drd1, and Drd2 mRNA expression. Recently, however, more sensitive techniques such as fluorescent in situ hybridization (FISH) has enabled higher-resolution analyses of receptor localization, revealing that Oxtr transcripts can be co-expressed with either Drd1 or Drd2 in sexually naïve prairie voles25. However, it remains unclear whether this co-expression is unique to prairie voles and/or if sociosexual experiences—such as pair bonding—alter its incidence or frequency. Addressing this question is critical for understanding the cellular basis of social bonding and how oxytocin-dopamine interactions support pair bonding. Furthermore, the role of these receptors in bonding is likely multifaceted, as DRD1 is not only implicated in non-partner aggression, but also in partner-directed motivation in bonded prairie voles26. Without a complete understanding of receptor distribution and co-expression, it remains unknown how DRD1 mediates both aggression and motivation within bonded relationships.
Recent transcriptomic studies in prairie voles further suggest that natural variation in Oxtr expression is associated with widespread changes in neural gene expression, across multiple brain regions and cell types, including those that do not express Oxtr directly27. These findings, based on single-nucleus RNA sequencing and systemic Oxtr knockout approaches, highlight the broad regulatory influence of OXTR signaling and suggest a potential role for immune-related genes (e.g., CTLR family members) in shaping neural circuits relevant to social behavior. Oxtr null mutant voles likewise display shifts in accumbal gene expression28. Together, these findings underscore the importance of mapping Oxtr expression at cellular resolution to better understand its contribution to neuromodulatory plasticity.
Therefore, to explore the cellular organization of these receptors across species and social experiences, we examined Oxtr, Drd1, and Drd2 mRNA expression using multiplex FISH. We compared sexually naïve prairie and meadow voles to their mated counterparts, a social experience that induces pair bonding in prairie but not meadow voles. Additionally, we leveraged well-powered tissue- and nuclear-level sequencing datasets to create a map that delineates the distribution of Oxtr and dopamine receptor transcripts (Drd1-5) in molecularly-defined NAc cell types in prairie voles29. Our analysis revealed species-specific and cell-type specific patterns of receptor expression, providing critical insights into how these neuromodulators may affect cellular and circuit function to drive differential social behaviors.
Results
All statistical results are reported in Supplementary Table S1. Only significant main effects or interactions (p < 0.05) and corresponding post-hoc tests are reported below.
Species and transcript identity interact to shape receptor expression patterns across the NAc
Based on prior evidence that prairie and meadow voles exhibit divergent social behaviors and neuromodulatory signaling6,8, we used multiplex FISH to compare the cellular distribution of Oxtr, Drd1, and Drd2 mRNA in the nucleus accumbens of both species (Fig. 1A, prairie voles; Fig. 1B, meadow voles). We outlined the nucleus of each cell and determined the presence or absence of each transcript (Oxtr, Drd1, Drd2, or none) within each nucleus (Fig. 1C). Binomial generalized linear mixed models (GLMMs) revealed significant main effects of transcript identity (Fig. 1D: core: χ²(3) = 2451.67; Fig. 1E: medial shell: χ²(3) = 2547.57; Fig. 1F: lateral shell: χ²(3) = 2344.20; all p < 0.0001) and significant species × transcript identity interactions (Fig. 1D: core: χ²(3) = 424.57; Fig. 1E: medial shell: χ²(3) = 338.74; Fig. 1F: lateral shell: χ²(3) = 782.79; all p < 0.0001). There were no main effects of species in any subregion (all p > 0.05) (Supplementary Table S1).
Prairie voles have more Oxtr positive cells than meadow voles in the nucleus accumbens. (A, B) Representative in situ hybridization images of dopamine receptor 1 (Drd1), dopamine receptor 2 (Drd2), and oxytocin receptor (Oxtr) in the nucleus accumbens core of prairie voles (A) and meadow voles (B). Scale bar = 200 μm in low magnification images (left) and 50 μm in high magnification images (right). (C) Cell quantification using Fiji Image J: DAPI stained cells are computationally identified and thresholding is manually adjusted to remove background and detect nuclei. Nuclei are outlined, numbered and an outline mask is generated to overlay on other channels to then identify positive cells for each transcript channel. (D-F) Quantification of transcript presence or absence within a DAPI mask in the nucleus accumbens core (D), medial shell (E), and lateral shell (F) of prairie voles and meadow voles. There was a main effect of transcript identity and a significant interaction between species and transcript identity. Post hoc species tests revealed that prairie voles had significantly more Oxtr+ and no transcript(NT)+ cells than meadow voles in all subregions, while meadow voles had more Drd2+ cells. No consistent species differences were observed for Drd1+ cells. Post hoc transcript identity within species tests revealed that Drd1+ and Drd2+ cells were more abundant than Oxtr+ and NT+ cells in both species. In prairie voles, Drd1 predominated over Drd2 in the medial and lateral shell, while the two were comparable in the core. Oxtr+ cells were consistently more abundant than NT+ cells. In meadow voles, Drd2+ cells were most abundant across regions, and Oxtr+ cells more abundant than NT+ cells, except in the lateral shell. Species-level differences are annotated directly in the figure (Šidák-corrected post hoc comparisons). Due to the number of significant within-species differences between transcript identity groups, those comparisons are not shown visually but are fully reported in Supplementary Table S1. Error bars show SEM. n = 13 per group. ** p < 0.01,*** p < 0.001, **** p < 0.0001.
Sidak-corrected post hoc comparisons showed that prairie voles had significantly more Oxtr+ cells than meadow voles in all three subregions (all p < 0.0001, ORs > 2.0) and modestly more no transcript (NT)+ cells than meadow voles in all three subregions (all p < 0.0001, ORs ~ 1.2–1.5). Meadow voles had significantly more Drd2+ cells than prairie voles (all p < 0.0001, ORs > 1.4), and Drd1+ cell proportions did not differ significantly between species (all p > 0.05). Species-level comparisons are annotated in Fig. 1, with significance asterisks denoting pairwise differences.
Within-species post hoc comparisons of transcript identity revealed that most transcripts differed significantly from one another (z-values ranging from ± 4 to ± 41, all p < 0.0001), with a few nonsignificant comparisons in the core (e.g., prairie Drd1 vs. Drd2: z = -0.45, p = 0.651). Analyses showed consistent expression patterns within species across NAc subregions. Specifically, in prairie voles, Drd1+ cells were significantly more abundant than Drd2+, NT+, and Oxtr+ cells, and Drd2+ cells were also more abundant than NT+ and Oxtr+ cells. Drd1 and Drd2 expression were comparable in the core but diverged in the medial and lateral shell, where Drd1 predominated. NT+ cells were significantly less abundant than Oxtr+ cells in all regions.
In meadow voles, Drd2+ cells were the most abundant across regions, followed by Drd1+ cells, with both significantly exceeding NT+ and Oxtr+ cells. Oxtr+ cells were less abundant than NT+ cells in the lateral shell, but the reverse was true in the core and medial shell, though the difference in the core was not significant.
Full pairwise comparisons, including z statistics, p values, and odds ratios are reported in Supplementary Table S1. It is important to note that transcript categories Drd1, Drd2, and Oxtr are not mutually exclusive as individual cells may co-express multiple receptors, and therefore transcript level analyses should be interpreted with caution.
We also performed Oxtr SNP genotyping on our prairie vole samples post-mortem (Supplementary Table S2). Across all genotyped prairie voles (n = 13), genotype distribution was 69% heterozygous, 23% high-Oxtr, and 8% low-Oxtr. Due to the presence of only one low-Oxtr animal, we grouped it with the heterozygous animals. Comparison of high Oxtr animals to non-high Oxtr animals revealed no statistically significant differences in receptor distribution. Therefore, we pooled all genotypes for subsequent analyses.
Oxtr co-expression with dopamine receptor cell classes is greater in prairie voles than meadow voles in the lateral shell
To complement the transcript-level analyses described above, we next examined species differences in mutually exclusive cell classes defined by combinations of Oxtr, Drd1, and Drd2 expression. Unlike the GLMM analysis, which modeled each transcript independently and allowed for overlap between categories, this classification scheme groups cells into non-overlapping types based on their unique receptor expression profiles. This approach enables clearer comparisons of cell class prevalence between prairie and meadow voles, revealing how receptor co-expression is organized at the population level across species. Specifically, we classified cells based on receptor expression, yielding 8 transcript combinations: No transcript; Drd1+ only; Drd2+ only; Drd1 + Drd2; Oxtr+ only; Drd1 + Oxtr; Drd2 + Oxtr; and Drd1 + Drd2 + Oxtr. We replicated previous findings that Oxtr is expressed on both Drd1+ and Drd2+ cells in prairie voles (7–14% of cells, depending on the transcript combination and subregion examined)25 (Fig. 2A-C). Meadow voles also exhibited Oxtr co-expression but at significantly lower levels (2–8%, p < 0.05), consistent with lower overall Oxtr + cell counts. We also found that Oxtr is expressed on Drd1 + Drd2 cells, and this population is enriched in prairie voles in the lateral shell. Conversely, meadow voles exhibited higher proportions of Drd2+ only and Drd1 + Drd2 + cells, while no transcript and Drd1+ only transcript combinations did not differ significantly between species.
Oxtr co-expression with dopamine receptor cell classes is greater in prairie voles compared to meadow voles in the nucleus accumbens. (A–C) Quantification of cell proportions based on transcript combinations the nucleus accumbens core (A), medial shell (B), and lateral shell (C) in meadow voles and prairie voles (light green and darker green respectively in radar plot). Fold change of prairie:meadow vole of each transcript combination was quantified and compared between species for each subregion. Meadow voles had a significantly greater number of Drd2 only and Drd1 + Drd2 cells compared to prairie voles in all subregions. Prairie voles demonstrated significantly higher levels of Oxtr co-expressing transcripts as compared to meadow voles in the lateral shell. Co-expression of Oxtr does not appear to be biased towards a particular Drd receptor class as co-expression is found with Drd1, Drd2, and Drd1 + Drd2 cells in all subregions. Error bars show EP (error propagation). N = 12–13 per group. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
To analyze differences in relative proportions of transcript combinations between species, we calculated the mean prairie:meadow vole ratio and propagated measurement errors to estimate uncertainty. Using a one-sample t-test relative to null with FDR corrected p-values, we found that prairie voles have greater proportions of Oxtr co-expressing cells compared to meadow voles, particularly in the lateral shell (Fig. 2C: Drd1 + Oxtr Prairie vs. Drd1 + Oxtr Meadow: t = 4.4565, p < 0.0001; Drd2 + Oxtr Prairie vs. Drd2 + Oxtr Meadow: t = 2.4798, p = 0.0296; Drd1 + Drd2 + Oxtr Prairie vs. Drd1 + Drd2 + Oxtr Meadow: t = 2.3769, p = 0.0310). Meadow voles have greater levels of Drd2 only cells and Drd1 + Drd2 cells as compared to prairie voles across all subregions examined (Fig. 2A: Drd2 only Prairie vs. Drd2 only Meadow: t = -7.3465, p < 0.0001; Drd1 + Drd2 Prairie vs. Drd1 + Drd2 Meadow: t = -6.9505, p < 0.0001; Fig. 2B: Drd2 only Prairie vs. Drd2 only Meadow: t = -4.6578, p < 0.0001; Drd1 + Drd2 Prairie vs. Drd1 + Drd2 Meadow: t = -5.2969, p < 0.0001; Fig. 2C: Drd2 only Prairie vs. Drd2 only Meadow: t = -9.3429, p < 0.0001; Drd1 + Drd2 Prairie vs. Drd1 + Drd2 Meadow: t = -7.3221, p < 0.0001).
Oxtr expression in dopamine receptor cell classes is largely unaffected by sociosexual experience
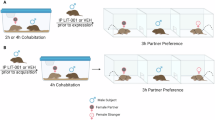
We next investigated whether mating influenced receptor distribution in either species. The experimental timeline for behavioral evaluation and tissue collection is shown in Fig. 3A. Partner preference testing confirmed species-specific differences social behaviors. Prairie voles spent more time huddling with their partner than with a novel conspecific (Fig. 3B: 2-way ANOVA: main effect of conspecific: F(1,13) = 10.24, p = 0.007; main effect of sex: F(1, 13) = 0.3043, p = 0.8560; interaction [conspecific x sex]: F(1,13) = 0.2546, p = 0.6223), while meadow voles did not display a partner preference (Fig. 3C). Overall differences in sociality between species were also evident in the amount of time spent in social chambers, with the unexpected finding that male meadow voles spent significantly more time in a social chamber compared to female meadow voles (Fig. 3B: unpaired t-test: t = 3.034, p = 0.0126). Animals indicated by solid gray triangles or circles are those used in in situ experiments (Fig. 3B and C).
Oxtr distribution in dopamine receptor cell classes does not vary based on sociosexual experience. (A) Experimental timeline for pairing and behavioral test for paired prairie and meadow vole cohorts. (B, C) A partner preference test confirmed pair bond formation in prairie voles, but not meadow voles. Male and female prairie voles, and male meadow voles more time spent in social chambers compared to female meadow voles. Solid gray triangles and circles indicate animals used in in situ experiments. (D, E) Representative images demonstrating co-labeling of Oxtr with Drd1, or Drd2, or both Drd1 and Drd2 in the nucleus accumbens core occurs in all cohorts: sexually naïve prairie voles (D: top row), pairbonded prairie voles (D: bottom row), sexually naïve meadow voles (E: top row) and paired meadow voles (E: bottom row). Image inset represents 40× image with 4× zoom. Scale bar = 50 μm. (F, G) Prairie and meadow voles showed a main effect of cell class but no main effect of bond status on the percentage of cells with Oxtr across dopamine receptor cell class, indicating that this metric is not influenced by sociosexual experience but does scale as a function of cell dopamine receptor identity in both species. (H, I) The number of Oxtr puncta was counted, and the frequency of puncta number was calculated in each dopamine receptor cell class. Naïve prairie voles had a greater puncta density in Drd1 + Drd2 cells compared to Drd1 cells (H: left). Pairbonded prairie voles had a greater puncta density in Drd1 + Drd2 cells compared to all other dopamine receptor cell classes (H: right). Naïve meadow voles (I: left) and paired meadow voles (I: right) had no statistically significant differences in Oxtr puncta frequency across dopamine receptor cell classes. Error bars show SEM. n = 4–8 per group. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Oxtr co-labeling with dopamine receptor cell classes occurred in both species and both sexually naïve and paired animals (Fig. 3D-E). Mating had no effect on Oxtr distribution across dopamine receptor cell classes in either species, a pattern consistent across all subregions examined (Fig. 3F-G; Supplementary Fig. S1A-B, E-F). Classification of the same 8 transcript combinations as in Fig. 2 revealed that cell ratios do not differ in prairie voles before and after bonding, except for two differences: naïve prairie voles had greater no transcript expression in the core (Fig. 4A: t = 3.4716, p = 0.0061) and greater Drd1 + Drd2 expression in the medial shell (Fig. 4B: t = -4.0282, p = 0.0009) (all non-significant results in Table S1). Somewhat surprisingly, meadow voles showed more pronounced mating-induced differences, especially in the lateral shell. Naïve meadow voles exhibited greater Drd2 only expression in the core and lateral shell (Fig. 4A: t = -3.8622, p = 0.0016; Fig. 4C: t = -3.0634, p = 0.0056, respectively), while Drd1 + Drd2 expression was higher in naïve animals in the medial and lateral shell (t = -3.6401, p = 0.0035; t = -3.0945, p = 0.0056). Interestingly, naïve meadow voles had greater Drd2 + Oxtr and Drd1 + Drd2 + Oxtr cells compared to paired counterparts, though absolute levels in both groups remained low (< 3% and < 4%, respectively; Fig. 4C: t = -2.8353, p = 0.0089; t = -5.0947, p < 0.0001). Additionally, paired meadow voles had significantly more Drd1-only cells than naïve meadow voles (Fig. 4C: t = 3.0992, p = 0.0056).
Sociosexual experience does not affect overall cell class receptor distribution in prairie voles. (A–C) Quantification of Oxtr, Drd1, and Drd2 positive cells and their various co-expression combinations in the nucleus accumbens core (A), medial shell (B), and lateral shell (C) in prairie voles (Light green = sexually naïve. Dark green = pairbonded) and in meadow voles (Light purple = sexually naïve. Dark purple = paired). Fold change of paired to naive vole of each transcript category was quantified and compared between sociosexual experience for each subregion. Pair bonded and sexually naïve prairie voles exhibit similar receptor expression across all cell classes and subregions, except for two differences: naïve prairie voles had greater no transcript expression in the core and greater Drd1 + Drd2 expression in the medial shell. In meadow voles, sexually naïve and paired individuals showed more pronounced differences, particularly in the lateral shell. Naïve meadow voles exhibited greater Drd2 only expression in both the core and lateral shell, while Drd1 + Drd2 expression was higher in naïve animals in the medial and lateral shell. Naïve meadow voles had fewer Drd2 + Oxtr and Drd1 + Drd2 + Oxtr cells compared to paired counterparts, though absolute levels in both groups remained low (< 3% and < 4%, respectively). Additionally, paired meadow voles had significantly more Drd1 only cells than naïve meadow voles. Error bars show EP (error propagation). N = 6–8 per group. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
To gain a more nuanced assessment of relative transcript abundance in different cell classes, we counted fluorescent puncta per cell, which was feasible for Oxtr due to its low abundance. Across sexually naïve and mated prairie voles, more Oxtr puncta were observed in Drd1 + Drd2 + positive cells. (Fig. 3H: Naïve- Generalized Fisher’s Exact Test: p = 0.0003; Post hoc Fisher’s Exact Test with Bonferroni correction: Drd1 vs. Drd1 + Drd2: p = 0.0009; Pairbonded - Chi Squared Test of Independence: χ2 = 28.9829, p < 0.0001; Post hoc Chi Squared Test for Trend with Bonferroni correction: No Drd vs. Drd1 + Drd2: χ2 = 20.1709, p < 0.0001; Drd1 vs. Drd1 + Drd2: χ2 = 30.3492, p < 0.0001; Drd2 vs. Drd1 + Drd2: χ2 = 18.2546, p < 0.0001). Similar trends were observed in the medial and lateral shell of naïve and pairbonded prairie voles (Supplementary Fig. S1C & S1G; see Supplementary Table S1).
In contrast, meadow voles had lower Oxtr puncta counts, with no significant differences in puncta frequency across Drd receptor cell classes in either naïve or paired conditions (Fig. 3I: naïve meadow: Generalized Fisher’s Exact Test: p = 0.0951; paired meadow: Generalized Fisher’s Exact Test: p = 0.1938). Similar patterns were observed in the medial and lateral shell of meadow voles (Supplementary Fig. S1D & S1H; see Supplementary Table S1).
Mapping of oxytocin and dopamine receptor expression across cell types in the prairie vole NAc
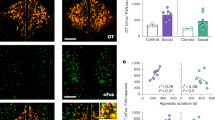
Finally, we used existing RNA sequencing datasets from the prairie vole NAc to comprehensively map Oxtr and Drd transcripts in the prairie vole NAc30. Tissue level sequencing revealed that Drd1 and Drd2 are expressed 10-20x higher levels than Drd3, Drd4, Drd5, and Oxtr. This pattern aligns with our FISH findings, which also indicate a higher relative abundance of Drd1 and Drd2 compared to Oxtr. (Fig. 5A). In addition, we observed that Drd3 and Drd5 transcripts are expressed in the NAc at levels commensurate with that of Oxtr, a gene with a well-established role in bonding.
Mapping of oxytocin and dopamine receptor expression across cell types in the prairie vole nucleus accumbens. (A) Oxtr and Drd transcript expression levels from prairie vole nucleus accumbens. Drd1 and Drd2 transcripts are expressed at high levels, whereas Drd3, Drd4, Drd5, and Oxtr transcript levels are significantly less abundant. (B) Single-nucleus RNA sequencing data from prairie voles identifying 15 molecularly-defined cell types in the nucleus accumbens. (C, D) Visualization of Oxtr and Drd transcript expression across all nucleus accumbens cell types (UMAP and violin plots). Single-nucleus RNA sequencing results support our FISH results in that Oxtr is expressed in both Drd1 and Drd2 cells, as well as in some astrocytes, mature oligodendrocytes, and all interneurons populations identified (somatostatin/neuropeptide Y, parvalbumin and GABAergic interneurons). Drd1 and Drd2 transcripts are expressed in medium spiny neuron populations (MSN’s) as well as astrocytes, oligodendrocytes, microglia, and various interneuron populations. Drd5 transcripts appear selectively enriched in GABAergic and cholinergic interneurons, whereas Drd3 transcripts are expressed across several cell types including MSN’s and sparsely across any other cell types.
To further investigate receptor expression in molecularly defined cell types in the NAc, we examined transcript expression in a single-nucleus RNA sequencing dataset from 39 prairie voles. This dataset was used to identify 15 primary cell types in the NAc, characterized by the expression of key marker genes, including Drd1 and Drd2 (Fig. 5B)29. However, low-abundance transcripts such as Oxtr, Drd3, Drd5, suffer from high-levels of dropout (e.g. a failure to detect the transcript or false negative)31,32. This was evident when comparing Oxtr detection rates:1% via snRNA-seq vs. 34–38% (depending on the NAc subregion) via FISH. Thus, while mapping low-abundance transcripts onto UMAP projections provides insights into which cell types express a given transcript, it does not accurately estimate the proportion of expressing cells.
To better visualize Oxtr and Drd transcript distribution across cell types, we plotted transcript-positive cells on a UMAP. This approach overlays transcript-expressing cells on top of the entire cell cluster. To provide a more accurate representation of expression levels for each cell type, we also plotted transcript counts per cell cluster (Fig. 5C). Using this approach, we found that Drd1 and Drd2 were enriched in subsets of medium spiny neurons (MSN’s), but also detectable across all identified cell types (Fig. 5C). Due to high dropout rates associated with their low abundance, quantitative estimates of Drd3, Drd5, and Oxtr expression remain inconclusive. Despite dropout effects, UMAP projections suggest that Oxtr is expressed in MSNs and Sst-Npy interneurons, Drd3 is localized to various MSN subtypes, and Drd5 is potentially expressed in GABAergic and cholinergic interneurons (Fig. 5D). These distributions suggest potential cell-type specific roles for Oxtr, Drd3 and Drd5 signaling in modulating cell functions and behavior.
Discussion
This study presents the first detailed comparison of accumbal Oxtr, Drd1, and Drd2 expression at the cellular level in sexually naïve and mated prairie and meadow voles. We demonstrate that the previously reported difference in Oxtr and OXTR expression between these two species (prairie > meadow)6,7,8,9 is due, at least in part, to differences in the total number of cells expressing Oxtr transcripts in the NAc. Further, Oxtr expression occurs across Drd cell classes in both species, and this distribution does not differ by sex or as a function of mating/bonding. Oxtr puncta are enriched in Drd1 + Drd2 co-expressing cells, especially in prairie voles, suggesting preferential recruitment of these cells by oxytocin. Finally, we provide the first expression atlas for Oxtr and all known Drd receptors across molecularly defined cell types in the prairie vole NAc.
We note that while prairie voles exhibit higher Oxtr levels overall, the distribution of Oxtr across Drd-expressing cell classes appears to be similar across species. This suggests that species differences in OXTR signaling occurs via a greater proportion of cells overall rather than preferentially engaging a specific DRD cell class in prairie voles. Given that oxytocin is released upon mating in prairie voles33, this widespread activation may function as a filtering mechanism that enhances the saliency of mating-related sensory and reward signals, thereby reinforcing both the mating experience and the identity of the partner. Similar mechanisms have been proposed in other brains regions where OXTR-mediated modulation of local signal-to-noise ratios can selectively enhance relevant stimuli34. While this remains a working model, it provides a plausible cellular-level framework for how species differences in OXTR prevalence may contribute to the formation of enduring social bonds.
Notably, we observed that prairie voles had a higher number of Oxtr-expressing and co-expressing cells, particularly in the lateral shell of the NAc. This is especially intriguing, as most research has focused on the medial shell and core, leaving the lateral shell largely unexplored in the context of social behavior and pair bonding. Our findings highlight significant species differences in Oxtr expression and its co-localization with Drd receptors in the lateral shell, contributing to the growing literature on NAc heterogeneity35,36,37. These results suggest that the lateral shell may play a previously underappreciated role in social bonding, particularly in monogamous species by integrating oxytocin and dopamine signaling in a unique way.
While prior studies have reported increased Drd1 and DRD1 levels following pair bonding in prairie voles18, these studies have not provided cellular-level resolution. Our findings suggest that increased levels may result from upregulation of Drd1 within existing populations rather than recruitment of new Drd1+ cells. This distinction is important, as it implies that dopaminergic plasticity in bonding likely occurs through receptor upregulation in cells already expressing the receptor rather than new expression in cells without a history of Drd1 expression. This suggests an enhancement of Drd1 signaling in a specific circuit rather than engagement of new circuits mediating bonding-dependent effects on behavior, although this needs to be experimentally tested in future work.
Our results provide particular relevance for understanding potential oxytocin:dopamine interactions at a cellular level. Oxtr expression within Drd2+ cell classes creates an opportunity for heterodimer formation. In vitro studies using bioluminescence resonance energy transfer (BRET) demonstrated formation of DRD2-OXTR heterodimers, suggesting a direct molecular interaction between these receptors38. In these heterodimer-specific experiments, oxytocin activation enhanced DRD2 signaling and increased inhibition of the cAMP-PKA-CREB pathway39. Separately, radioligand binding experiments using 3H-raclopride showed that oxytocin increased DRD2-class binding, suggesting an additional mechanism by which oxytocin modulates DRD2 function40. Together, these findings suggest that oxytocin may enhance DRD2-class receptor function via two mechanisms: (1) direct heteromeric interactions that increase DRD2 affinity for dopamine, thereby amplifying inhibitory Gi/o signaling and reducing cAMP levels, and (2) increased receptor availability, potentially via upregulation of receptor density or altered receptor conformation. Both mechanisms would converge on enhanced DRD2-mediated inhibition of the cAMP-PKA-CREB pathway, ultimately suppressing CREB-dependent transcription of genes involved in synaptic plasticity and reward processing such as c-Fos, TH, BDNF, and ∆FosB, all of which are known to contribute to pair bonding41,42,43,44,45,46. Of course, an important question remains: do these receptors merely colocalize within the same cells or do they form true heterodimers in vivo? Future research should explore the dynamic interactions between these receptor systems using super-resolution imaging or functional assays to address this question.
Additionally, our data revealed that Oxtr is expressed on a subset of cells positive for both Drd1 and Drd2, and we observed increased Oxtr puncta density in these Drd1 + Drd2 cells, suggesting that these cells may exhibit enhanced oxytocinergic sensitivity. Overall, our data are consistent with prior work showing that 10–30% of NAc MSNs in other species exhibit dopamine receptor co-expression47,48,49,50. Notably, coordinated activation of multiple receptors (Oxtr, Drd1, Drd2) in the same cell may engage distinct intracellular signaling pathways that regulate bond-relevant transcriptional programs and/or neuronal activity. Oxtr, Drd1, and Drd2 each activate distinct intracellular signaling cascades, yet they may also integrate signals to shape social behavior. OXTR is typically Gq-coupled, leading to phospholipase C (PLC) activation and intracellular calcium release, which modulates excitability and synaptic plasticity. In contrast, D2-like receptors (including DRD2) are Gi-coupled, inhibiting adenylate cyclase and reducing cAMP levels dampening excitability, while D1-like receptors (including DRD1) are Gs-coupled, stimulating adenylate cyclase and increasing cAMP production, promoting excitatory signaling51,52,53. Beyond their independent signaling roles, DRD1-DRD2 heteromers in rat NAc neurons and have been found to induce a unique pathway where the heteromer is Gq-coupled, stimulates the PLC pathway to release intracellular calcium from IP3 receptor-sensitive stores, leading to CaMKII activation and BDNF production. This mechanism may underlie synaptic plasticity and reinforce socially motivated behaviors48. Given the role of dopaminergic plasticity in social bonding, the co-expression of Oxtr, Drd1, and Drd2 within the same cells–and the potential formation of heteromers—may enable complex intracellular cross-talk between these pathways, fine-tuning cellular responses to social stimuli. These interactions could shape neural circuits involved in pair bonding, and reinforce behaviors critical for maintaining social attachment52,53.
The role of DRD1-DRD2 heteromers in reward processing extends beyond social bonding. Studies have demonstrated that repeated amphetamine (AMPH) administration leads to functional super sensitivity of the DRD1-DRD2 heteromer, as evidenced by enhanced GTPγS binding, and also increases heteromer density in the NAc, as assessed by FRET techniques54. These findings are particularly relevant given that pair bonding in prairie voles protects against AMPH’s rewarding effects, with this buffering mediated via a DRD1-class-specific mechanism in males55. This suggests that pair bonding may modulate dopaminergic plasticity in a way that counteracts drug-induced neuroadaptations, highlighting a potential role for Oxtr-Drd1-Drd2 co-expression in reshaping neural circuits involved in both natural and drug-related reward processing.
Additionally, we provide a comprehensive map of Drd expression across molecularly-defined cell types in the prairie vole NAc, including for less-commonly studied dopamine receptors. Although Drd3 and Drd5 are expressed at lower levels in the NAc, evidence suggests they may play important roles in social behavior. DRD3 agonists disrupt social behavior and social recognition in rats56,57, while dopamine DRD5 receptors modulate sexual behavior in mice58, although these functions may be localized outside of the NAc. Furthermore, preliminary data, although underpowered, also suggests that DRD3, rather than DRD2 receptors, may be more important for bond formation in prairie voles59. Accordingly, our expression map offers a valuable reference for studying dopaminergic contributions to social behavior and underscores the need for receptor-specific investigations to clarify the role of DRD3 and DRD5 receptors in social bonding.
Finally, while we provide insight into accumbal Oxtr, Drd1, and Drd2 expression and distribution at the cellular level in prairie and meadow voles, our study is not without its limitations. Our behavioral cohort sample sizes were relatively small, (6–9 animals/group). Also, because our sample includes prairie voles with different Oxtr genotypes, future studies should stratify animals by genotype to assess whether genetic variation contributes to species differences. Additionally, our workflow relied on a nuclear (DAPI) mask to identify transcript-positive cells, meaning transcripts outside the nucleus (e.g., in axon terminals or dendrites) were not captured. This is particularly relevant for Drd1, which has been shown to change with social experience at the mRNA level60, but may not always translate to nuclear-proximal expression. Further, determining the relationship between the numbers of cells expressing a transcript and what this means for protein abundance and localization could provide further insight into their regulation25. More broadly, our findings highlight Oxtr-Drd co-expression as a potential mechanism for integrating oxytocin and dopamine signaling in the NAc, but the functional relevance of our observations has yet to be established.
In sum, our findings may have implications for understanding social deficits in neuropsychiatric conditions characterized by oxytocin-dopamine dysregulation, such as autism spectrum disorder (ASD) and schizophrenia. Given that disruptions in Oxtr-Drd interactions could impair social reward processing, future research should explore whether similar neuromodulatory mechanisms contribute to altered social motivation in these conditions. Investigating whether pharmacological modulation of Oxtr or Drd signaling in the NAc can rescue social deficits may provide a more targeted framework for therapeutic interventions. Beyond clinical applications, investigating the circuit-specific mechanisms through which oxytocin modulates social reward will be essential for bridging the gap between animal and human studies. A deeper understanding of these neuromodulatory interactions could refine our models of dopaminergic plasticity in social bonding and inform the development of target approaches for addressing social behavior impairments.
Methods
Animals
Prairie voles were bred in-house from colonies originating from the University of California Davis and Emory University, with all animals originally descended from wild animals collected in Illinois. Meadow voles were also bred in house from colonies originating from Smith College and University of California San Francisco. All voles were weaned at postnatal day 21. Animals were then housed in standard static rodent cages (17.5 L x 9.0w. x 6.0 h. in.) in groups of 2–4 with either same sex siblings or same sex voles from similar weaning time frames. Animals were given ad libitum access to water and rabbit chow (5326-3 by PMI Lab Diet). Rabbit chow was supplemented with sunflower seeds, dehydrated fruit bits, and alfalfa cubes. Cages were enriched with cotton nestlets, a plastic igloo, and a PVC pipe. Animals were kept in a temperature (23–26 °C) and humidity-controlled room with a 14:10 h light-dark cycle.
All voles used in the studies ranged in ages of 62–104 days at the start of experiments and weighed between 28.4 and 48.4 g. All procedures were performed in the light phase, approved under the University of Colorado’s Institute of Animal Care and Use Committee (IACUC) and conducted in accordance with relevant guidelines and regulations. All authors complied with the ARRIVE guidelines.
Sample collection timeline
All sexually naïve animals used in this study were housed in groups of 2–4 with same sex siblings or same sex voles from similar weaning time frames.
All paired animals used in this study consisted of one female and one male from different genetic backgrounds. Females were reproductively intact and were primed to induce estrus via subcutaneous injection of 20 ug/mL of estradiol benzoate (Cayman Chemical) every 24 h for 3 days. On the third day of priming, females were paired with their designated partner and placed in a smaller standard rodent cage (11.0 L x 6.5w. x 5.0 h. in) with fresh bedding, food, water, and cage enrichment.
Sixteen days after pairing, we measured partner preference in female animals, and 18 days after pairing, we measured partner preference in male animals. Nineteen days after pairing, we extracted brains for analysis in 16 prairie vole pairs (8 pairs for a female focal animal and 8 pairs for a male focal animal) and 7 meadow vole pairs. Tissue from a subset of 4 animals per sex was used in in situ hybridization studies. These animals were selected based on partner preference score and total test huddle time. Specifically, all paired prairie voles used in in situ studies had a partner preference (> 66% time spent huddling with partner) and spent at > 2% of total test time (216 s) huddling). The subset of prairie voles selected for the in situ studies were those with highest partner preference scores. All paired meadow voles used in in situ studies had no partner preference ((< 66% time spent huddling with partner) and/or < 2% of total test time huddling).
Partner preference test
Partner preference tests (PPT) were performed as described in Scribner et al. 202061. Briefly, partner and novel animals were tethered to opposite end walls of three-chamber plexiglass arenas (76.0 cm long, 20.0 cm wide, and 30.0 cm tall). Tethers consisted of an eye bolt attached to a chain of fishing swivels that slid into the arena wall. Animals were briefly anesthetized with isoflurane and attached to the tether using a zip tie around the animal’s neck. Two pellets of rabbit chow were given to each tethered animal and water bottles were secured to the wall within their access while tethered. After tethering the partner and novel animals, the experimental animal was placed in the center chamber of the arena. After a 10-minute acclimation period, opaque dividers between the chambers were removed, allowing the subject to move freely about the arena for three hours. Overhead cameras (Panasonic WVCP304) were used to video record tests.
The movement of all three animals in each test was scored using TopScan High-Throughput software v3.0 (Cleversys Inc) using the parameters from Ahern et al.62 Behavior was analyzed using a Python script developed in-house (https://github.com/donaldsonlab/Cleversys_scripts) to calculate the following metrics: time spent in partner, novel, or center chamber, the time spent huddling with the partner/novel, and total locomotion.
Tissue extraction & brain sectioning
Animals were sedated with isoflurane and euthanized via rapid decapitation. Brains were immediately extracted, rinsed in sterile saline, and then frozen on powdered dry ice. Samples were stored at -80° C for a minimum of 24 h. 90–120 min before cryostat slicing, brains were moved to a -20° C freezer to acclimate to the temperature of the cryostat. Brain tissue was sliced coronally at 12 μm and nucleus accumbens tissue was collected between bregma 1.54 mm to 0.74 mm as per The Mouse Brain Atlas in Stereotaxic Coordinates63 and mounted on Superfrost Plus slides (Fisherbrand). During slicing, slides were kept inside the cryostat to maintain a stable temperature and minimize RNA degradation. After slicing, tissues slices were stored at -20° C for 2 h to support better adhesion of tissue to the slide and then moved to -80° C for subsequent storage.
Assessment of pregnancy status
After rapid decapitation, females were examined for embryos by dissection. The uterus was identified, and number of embryos was based on the number of independent protrusions.
Fluorescent RNAscope in situ hybridization
Fluorescent in situ hybridization triple labeling of oxytocin receptor (Oxtr), dopamine receptor 1 (Drd1), and dopamine receptor 2 (Drd2) mRNA expression in the nucleus accumbens was performed using RNAscope Multiplex Fluorescent v2 Kit (ACD Bio). Species specific (Microtus orchrogaster) probes were used for dopamine receptor 1 (Mo-Drd1, Catalog #: 588161-C3); dopamine receptor 2 (Mo-Drd2, Catalog #: 534471-C2); and oxytocin receptor (Mo-Oxtr, Catalog #: 500721); 3-plex positive control probes Mo- Pol2ra (polypeptide A 220 kDa, Catalog # 563291), Ppib (peptidylprolyl isomerase B, Catalog # 533491), and Ubb (ubiquitin B, Catalog #300040); 3 plex negative control probe DapB (4-hydroxy-tetrahydrodipicolinate reductase from Bacillus subtilis, Catalog # 320871). Sections were fixed using 4% PFA in 1X PBS and then dehydrated in a series of ethanol washes. Endogenous peroxidases were blocked via RNAscope H2O2 and tissues were permeabilized using RNAscope protease III. Slides were hybridized with the probes in a HybEZ oven (ACD), at 40 °C for 2 h and then slides were subjected to signal amplification according to manufacturer’s instructions. Hybridization signals were detected using Perkin Elmer Opal Dyes 520 (Catalog #: fp1487001kt), 570 (Catalog #: fp1488001kt), and 650 (Catalog #: fp1496001kt) at a 1:50 dilution. Slides were counterstained with RNAscope DAPI for nuclear visualization and coverslipped with ProLong Diamond Antifade Mountant (Invitrogen, Catalog # P36970). Slides were stored covered at 4 °C and imaged within 2 weeks.
Microscopy
Slices were imaged using a Nikon A1 Laser Scanning confocal Microscope at the University of Colorado Boulder MCDB Light Microscopy Core Facility (RRID: SCR_018993). Images were taken in one sitting of the anterior portion of the nucleus accumbens at Bregma 1.54 mm as defined according to The Mouse Brain Atlas in Stereotaxic Coordinates63 on both left and right hemispheres. Confocal images were taken with a 40x lens in four channels (blue, green, red, and infared) for each hemisphere of the core, and medial and lateral shell of the nucleus accumbens. Confocal stacks were projected as single images using maximum fluorescence and used for analysis.
Image analysis
Images were analyzed using Fiji Image J software (version 2.14.0/1.54f)64. Images were split into 4 channels: channel 0 / DAPI, channel 1 / Drd1, channel 2 / Drd2, and channel 3 / Oxtr. Regions of interest (ROI’s) outlining DAPI nuclei labeling were automatically generated in Fiji Image J. Thresholds for DAPI nuclear staining were established by eye to eliminate background and provide an accurate overlay of nuclei in each image. Accuracy of DAPI masks was quantified for a subset of images (10%, 1 image per animal) comparing experimenter number of identified nuclei per image vs. automatically identified number of nuclei per image. Number of nuclei identified by person vs. machine did not differ by more than 5% and thus, automatically generated ROI’s for DAPI were used for all further analysis. This DAPI mask overlay was then applied to Drd1, Drd2, and Oxtr images (Fig. 1C). Images underwent a white top-hat transformation to enhance contrast and improve detection of localized bright features facilitating identification and quantification of puncta. Signal data for Drd1, Drd2, and Oxtr images was then collected including the region of interest number / nuclei number, minimum, mean and maximum intensity values, and % area for both 16- and 8-bit depth data. This final cellular distribution data was analyzed via custom-generated Python code (https://github.com/donaldsonlab/RNAscope paper) to identify the number of positively labeled nuclei for each channel, as well as co-expression in the form of double and triple labeled cells. Oxtr puncta were quantified using Morphological Filters Plugin (https://github.com/ijpb/MorphoLibJ) and Object Inspector (2D/3D) Plugin65 in Fiji Image J.
Methods for bulk RNAsequencing
Animals were sacrificed by rapid decapitation and the NAc was dissected out of the brain prior to processing for RNA-Seq. Sequence mapping and counting was performed as described in Sadino et al.30 (GEO: GSE192661).
Methods for single nucleus RNAsequencing
Methods were as described in Brusman et al.29. Briefly, single nuclei were isolated, and samples were sequenced using Chromium next GEM Single Cell 5’ kit v2 from 10X Genomics. Single nuclei RNA-seq (snRNA-seq) libraries were prepared according to the manufacturer’s instructions. Library quality was assessed using the Agilent High Sensitivity D5000 ScreenTape System and were subsequently sequenced using paired-end sequencing on an Illumina NovaSeq6000. Raw reads were aligned and counted using the Cellranger v3.1.0 analysis pipeline, and Seurat v4.3.0 in R v4.2.2 was used to analyze single nuclei data. GEO (GSE255620).
Statistical analysis
Data are shown as means ± standard error of the mean. Statistical significance α was set at 0.05, unless otherwise specified. All n values represent number of animals. Depending on the statistical test, statistical analyses were carried out using GraphPad PRISM (version 10.4.1, Graphpad, San Diego, CA); R (v 4.2.3, (2023-03-15 ucrt)), or Python (v 3.12.4) in a reproducible computing environment. RStudio (v 2024.12.0.467, Boston, MA) was used as the integrated development environment (IDE) for R analyses, while Python scripts were executed in Juypter Notebook (v 7.0.8) via Anaconda Navigator (v 2.6.4). In R, the following packages were used: ggplot2 (v 3.4.2); ggpubr (v.0.6.0); TMB (v 1.9.11); lme4 (v1.1-35.1); emmeans (v1.10.1); dplyr (v1.1.4); car (v3 3.1.2).and openslsx (v4.2.5). In Python, the following packages were used: NumPy: 1.26.4; SciPy: 1.13.1;pandas: 2.2.2; matplotlib: 3.8.4; and seaborn: 0.13.2.
Normality of the data was assessed using Shapiro-Wilk tests in GraphPad PRISM, and parametric tests were chosen accordingly. Outliers were tested for using the Robust Regression and Outlier Removal (ROUT) method in GraphPad Prism (10.4.1), with a False Discovery Rate (Q) set at 1%.
Additional statistical tests included 2-way repeated measures ANOVA’s; post hoc Šidák’s multiple comparisons test; one sample t-tests with error propagation and false discovery rate (FDR) correction; generalized Fisher’s Exact Test with post hoc Fisher’s Exact Test with Bonferroni correction; Chi Square Test of Independence with post hoc Chi Squared Test for Trend with Bonferroni correction; and binomial generalized linear mixed models (GLMM’s; used to model proportions while accounting for repeated sampling and non-mutually exclusive transcript categories). For GLMM analyses, the number of transcript-positive cells (Oxtr, Drd1, Drd2, or NT) was modeled as a proportion of total DAPI + cells per animal per region. Species and transcript identity were treated as fixed effects, and animal ID was included as a random effect. The significance of main effects and interactions was evaluated using Type III Wald χ² tests. Post hoc pairwise comparisons of estimated marginal means were performed using Šidák correction, and FDR-adjusted p-values are reported. In cases where differences were not found between males and females, sexes were combined.
Oxtr SNP genotyping
All prairie voles were genotyped post-mortem for nucleotide 213,739 (NT213739), as this robustly predicts Oxtr expression in the nucleus accumbens of prairie voles, where CC > CT > TT66.
DNA was extracted from toes using The Jackson Laboratory Quick DNA purification protocol. The Oxtr SNP genotyping assay was designed using IDT’s rhAmp SNP Genotyping technology (Design ID: CD.GT.GFDG0397.1, IDT ) and performed on an Applied Biosystems QuantStudio 3 qPCR machines. Samples were genotyped in duplicate in MicroAmp Fast Optical 96 well reaction plate (Applied BioSystems) in a reaction containing 2 µL of DNA, 0.25 µL of probe rhAmp SNP Assay, 0.1 µL nuclease free H2O, 0.13 µL rhAmp Reporter Mix, and 2.52 µL rhAmp Genotyping Master Mix for a total volume of 5 µL per well. Genotyping was performed using the following primers: 5’GAATCATCCCACCGTGC; 5’ GGAATCATCCCACCGTGT; and GCGTCAGTCCCTTATCGACCT. The cycling conditions were those as determined by the manufacturer, specifically: 95 °C for 10 min, 40 × (95 °C for 10 s, 60 °C for 30 s, and 68 °C for 20s) and 99 °C for 15 min.
Data availability
The RNA sequencing datasets generated and/or analyzed during the current study are available in the GEO repository: (Bulk RNA sequencing data: GSE192661 and single nuclei RNA sequencing data: GSE255620). All lab specific code is available on Donaldson Lab GitHub (https://github.com/donaldsonlab/RNAscope-paper) and multiplex FISH data will be publicly available on Dryad (https://doi.org/10.5061/dryad.s1rn8pkk3) as of the date of publication. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
Lieberwirth, C. & Wang, Z. The neurobiology of pair bond formation, bond disruption, and social buffering. Curr. Opin. Neurobiol. 40, 8–13 (2016).
Gobrogge, K. & Wang, Z. The ties that bond: neurochemistry of attachment in voles. Curr. Opin. Neurobiol. 38, 80–88 (2016).
Young, K. A., Gobrogge, K. L., Liu, Y. & Wang, Z. The neurobiology of pair bonding: insights from a socially monogamous rodent. Front. Neuroendocrinol. 32, 53–69 (2011).
Donaldson, Z. R., Young, L. J. & Oxytocin Vasopressin, and the neurogenetics of sociality. Science 322, 900–904 (2008).
Resendez, S. L., Kuhnmuench, M., Krzywosinski, T. & Aragona, B. J. κ-Opioid receptors within the nucleus accumbens shell mediate pair bond maintenance. J. Neurosci. 32, 6771–6784 (2012).
Insel, T. R. & Shapiro, L. E. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc. Natl. Acad. Sci. 89, 5981–5985 (1992).
Lim, M. M., Murphy, A. Z. & Young, L. J. Ventral striatopallidal Oxytocin and vasopressin V1a receptors in the monogamous prairie vole (Microtus ochrogaster). J. Comp. Neurol. 468, 555–570 (2004).
Young, L. J., Young, M., Hammock, E. A. & A. Z. & D. Anatomy and neurochemistry of the pair bond. J. Comp. Neurol. 493, 51–57 (2005).
Hammock, E. A. D. & Young, L. J. Oxytocin, vasopressin and pair bonding: implications for autism. Philos. Trans. R Soc. Lond. B Biol. Sci. 361, 2187–2198 (2006).
Dewsbury, D. A. The comparative psychology of monogamy. Neb. Symp. Motiv. Neb. Symp. Motiv. 35, 1–50 (1987).
Johnson, Z. V., Walum, H., Xiao, Y., Riefkohl, P. C. & Young, L. J. Oxytocin receptors modulate a social salience neural network in male prairie voles. Horm. Behav. 87, 16–24 (2017).
Ross, H. E. et al. Variation in Oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. J. Neurosci. 29, 1312–1318 (2009).
Smeltzer, M. D., Curtis, J. T., Aragona, B. J. & Wang, Z. Dopamine, oxytocin, and vasopressin receptor binding in the medial prefrontal cortex of monogamous and promiscuous voles. Neurosci. Lett. 394, 146–151 (2006).
Keebaugh, A. C., Barrett, C. E., Laprairie, J. L., Jenkins, J. J. & Young, L. J. RNAi knockdown of Oxytocin receptor in the nucleus accumbens inhibits social attachment and parental care in monogamous female prairie voles. Soc. Neurosci. 10, 561–570 (2015).
Keebaugh, A. C. & Young, L. J. Increasing Oxytocin receptor expression in the nucleus accumbens of pre-pubertal female prairie voles enhances alloparental responsiveness and partner preference formation as adults. Horm. Behav. 60, 498–504 (2011).
Wang, H., Duclot, F., Liu, Y., Wang, Z. & Kabbaj, M. Histone deacetylase inhibitors facilitate partner preference formation in female prairie voles. Nat. Neurosci. 16, 919–924 (2013).
Ross, H. E. et al. Variation in Oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. J. Neurosci. 29, 1312 (2009).
Aragona, B. J. et al. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat. Neurosci. 9, 133–139 (2006).
Aragona, B. J. Dopamine regulation of social choice in a monogamous rodent species. Front Behav. Neurosci 3, (2009).
Liu, Y. & Wang, Z. X. Nucleus accumbens Oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience 121, 537–544 (2003).
Gingrich, B., Liu, Y., Cascio, C., Wang, Z. & Insel, T. R. Dopamine D2 receptors in the nucleus accumbens are important for social attachment in female prairie voles (Microtus ochrogaster). Behav. Neurosci. 114, 173–183 (2000).
Hostetler, C. M., Harkey, S. L., Krzywosinski, T. B., Aragona, B. J. & Bales, K. L. Neonatal exposure to the D1 agonist SKF38393 inhibits pair bonding in the adult prairie vole. Behav. Pharmacol. 22, 703–710 (2011).
Aragona, B. J., Liu, Y., Curtis, J. T., Stephan, F. K. & Wang, Z. A critical role for nucleus accumbens dopamine in Partner-Preference formation in male prairie voles. J. Neurosci. 23, 3483–3490 (2003).
Liu, Y. et al. Nucleus accumbens dopamine mediates amphetamine-induced impairment of social bonding in a monogamous rodent species. Proc. Natl. Acad. Sci. 107, 1217–1222 (2010).
Inoue, K., Ford, C. L., Horie, K. & Young, L. J. Oxytocin receptors are widely distributed in the prairie vole (Microtus ochrogaster) brain: relation to social behavior, genetic polymorphisms, and the dopamine system. J. Comp. Neurol. 530, 2881–2900 (2022).
Pierce, A. F. et al. Nucleus accumbens dopamine release reflects the selective nature of pair bonds. Curr. Biol. 34, 519–530e5 (2024).
Boender, A. et al. (ed, J.) Natural variation in Oxytocin receptor signaling causes widespread changes in brain transcription: a link to the natural killer gene complex. BioRxiv 20231026564214 https://doi.org/10.1101/2023.10.26.564214 (2023).
Sharma, R. et al. Oxytocin receptor controls promiscuity and development in prairie voles. eLife 14, (2025).
Brusman, L. E. et al. Single nucleus RNA-sequencing reveals transcriptional synchrony across different relationships. 03.27.587112 Preprint at (2024). https://doi.org/10.1101/2024.03.27.587112 (2024).
Sadino, J. M. et al. Prolonged partner separation erodes nucleus accumbens transcriptional signatures of pair bonding in male prairie voles. eLife 12, NA-NA (2023).
Li, W. V. & Li, J. J. An accurate and robust imputation method scimpute for single-cell RNA-seq data. Nat. Commun. 9, 997 (2018).
Miao, Z. et al. ScRecover: discriminating true and false Zeros in Single-Cell RNA-Seq data for imputation. Stat. Med. 44, e10334 (2025).
Ross, H. E. et al. Characterization of the Oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience 162, 892–903 (2009).
Froemke, R. C., Young, L. J. & Oxytocin Neural plasticity, and social behavior. Annu. Rev. Neurosci. 44, 359–381 (2021).
Resendez, S. L. et al. µ-Opioid receptors within subregions of the striatum mediate pair bond formation through parallel yet distinct reward mechanisms. J. Neurosci. 33, 9140–9149 (2013).
Zahm, D. S. An integrative neuroanatomical perspective on some subcortical substrates of adaptive responding with emphasis on the nucleus accumbens. Neurosci. Biobehav Rev. 24, 85–105 (2000).
Reynolds, S. M. & Berridge, K. C. Glutamate motivational ensembles in nucleus accumbens: rostrocaudal shell gradients of fear and feeding. Eur. J. Neurosci. 17, 2187–2200 (2003).
Romero-Fernandez, W., Borroto-Escuela, D. O., Agnati, L. F. & Fuxe, K. Evidence for the existence of dopamine d2-oxytocin receptor heteromers in the ventral and dorsal striatum with facilitatory receptor–receptor interactions. Mol. Psychiatry. 18, 849–850 (2013).
Borroto-Escuela, D. O. et al. Moonlighting characteristics of G protein-coupled receptors: focus on receptor heteromers and relevance for neurodegeneration. IUBMB Life. 63, 463–472 (2011).
Fuxe, K. et al. On the role of volume transmission and receptor–receptor interactions in social behaviour: focus on central catecholamine and Oxytocin neurons. Brain Res. 1476, 119–131 (2012).
Tregub, P. P. et al. Brain plasticity and cell competition: immediate early genes are the focus. Cells 14, 143 (2025).
Manning, C. E., Williams, E. S. & Robison, A. J. Reward network immediate early gene expression in mood disorders. Front Behav. Neurosci 11, (2017).
McClung, C. A. & Nestler, E. J. Neuroplasticity mediated by altered gene expression. Neuropsychopharmacology 33, 3–17 (2008).
Sanna, F. et al. c-Fos, ∆fosb, BDNF, TrkB and Arc expression in the limbic system of male Roman High- and Low-Avoidance rats that show differences in sexual behavior: effect of sexual activity. Neuroscience 396, 1–23 (2019).
Pitchers, K. K. et al. ∆FosB in the nucleus accumbens is critical for reinforcing effects of sexual reward. Genes Brain Behav. 9, 831–840 (2010).
Gandolfi, D. et al. Activation of the CREB/c-Fos pathway during Long-Term synaptic plasticity in the cerebellum granular layer. Front Cell. Neurosci 11, (2017).
Bertran-Gonzalez, J. et al. Opposing patterns of signaling activation in dopamine D1 and D2 Receptor-Expressing striatal neurons in response to cocaine and haloperidol. J. Neurosci. 28, 5671–5685 (2008).
Hasbi, A., O’Dowd, B. F. & George, S. R. Dopamine D1-D2 receptor heteromer signaling pathway in the brain: emerging physiological relevance. Mol. Brain. 4, 26 (2011).
Perreault, M. L. et al. The dopamine D1-D2 receptor heteromer localizes in dynorphin/enkephalin neurons: INCREASED HIGH AFFINITY STATE FOLLOWING AMPHETAMINE AND IN SCHIZOPHRENIA*. J. Biol. Chem. 285, 36625–36634 (2010).
Hasbi, A. et al. Calcium signaling cascade links dopamine D1–D2 receptor heteromer to striatal BDNF production and neuronal growth. Proc. Natl. Acad. Sci. 106, 21377–21382 (2009).
Bergson, C. et al. Regional, cellular, and subcellular variations in the distribution of D1 and D5 dopamine receptors in primate brain. J. Neurosci. Off J. Soc. Neurosci. 15, 7821–7836 (1995).
Beaulieu, J. M., Espinoza, S. & Gainetdinov, R. R. Dopamine receptors – IUPHAR review 13. Br. J. Pharmacol. 172, 1–23 (2015).
Loth, M. K., Donaldson, Z. R. & Oxytocin Dopamine, and opioid interactions underlying pair bonding: highlighting a potential role for microglia. Endocrinology 162, bqaa223 (2021).
Perreault, M. L. et al. The dopamine D1-D2 receptor heteromer localizes in dynorphin/enkephalin neurons: increased high affinity state following amphetamine and in schizophrenia. J. Biol. Chem. 285, 36625–36634 (2010).
Liu, Y., Young, K. A., Curtis, J. T., Aragona, B. J. & Wang, Z. Social bonding decreases the rewarding properties of amphetamine through a dopamine D1 Receptor-Mediated mechanism. J. Neurosci. 31, 7960–7966 (2011).
Watson, D. J. et al. Selective Blockade of dopamine D3 receptors enhances while D2 receptor antagonism impairs social novelty discrimination and novel object recognition in rats: A key role for the prefrontal cortex. Neuropsychopharmacology 37, 770–786 (2012).
Watson, D. J. G., Marsden, C. A., Millan, M. J. & Fone, K. C. F. Blockade of dopamine D3 but not D2 receptors reverses the novel object discrimination impairment produced by post-weaning social isolation: implications for schizophrenia and its treatment. Int. J. Neuropsychopharmacol. 15, 471–484 (2012).
Kudwa, A. E., Dominguez-Salazar, E., Cabrera, D. M., Sibley, D. R. & Rissman, E. F. Dopamine D5 receptor modulates male and female sexual behavior in mice. Psychopharmacol. (Berl). 180, 206–214 (2005).
Nevarez, N. Dopaminergic Regulation of Pair Bonding in the Socially Monogamous Prairie Vole. at (2017). http://deepblue.lib.umich.edu/handle/2027.42/140824
Resendez, S. L. et al. Dopamine and opioid systems interact within the nucleus accumbens to maintain monogamous pair bonds. eLife 5, e15325 (2016).
Scribner, J. L. et al. A neuronal signature for monogamous reunion. Proc. Natl. Acad. Sci. 117, 11076–11084 (2020).
Ahern, T. H., Modi, M. E., Burkett, J. P. & Young, L. J. Evaluation of two automated metrics for analyzing partner preference tests. J. Neurosci. Methods. 182, 180–188 (2009).
Paxinos, G. & Watson, C. Paxinos and Watson’s the rat brain in stereotaxic coordinates.
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 9, 676–682 (2012).
Jan Brocher. biovoxxel/bv3dbox: BioVoxxel 3D Box - v1.22.3. (2024). https://doi.org/10.5281/ZENODO.5987322
King, L. B., Walum, H., Inoue, K., Eyrich, N. W. & Young, L. J. Variation in the Oxytocin receptor gene predicts brain Region–Specific expression and social attachment. Biol. Psychiatry. 80, 160–169 (2016).
Acknowledgements
We thank the voles for their sacrifice and contribution to research. We thank Jessica Abazaris and the rest of the animal care staff at the University of Colorado Boulder for their excellent care of the voles. Kelly Winther, Katie Gallagher, and Kresil Gordon managed the animal colony and provided experimental support. We acknowledge the Light Microscopy Core Facility, Porter B047, B049, B051 and B059 at the University of Colorado Boulder (RRID: SCR_018993) for help and advice with microscopy and thank Dr. James D. Orth for his assistance. We thank the Donaldson lab for their feedback and support. This work was supported by awards from the Dana Foundation, the Whitehall Foundation, National Science Foundation (NSF) IOS-1827790, and National Institute of Health (NIH) DP2MH119421, UF1NS122124, R01MH125423, IOS-2045348, U01NS131406 to Z.R.D.; National Institute of Health (NIH) 1F31MH132278-01A1, 5T32GM008759-20, 1T32GM142607-01 to L.E.B, and T32 DA 17637 support to M.K.L.
Author information
Authors and Affiliations
Contributions
Conceptualization: MKL, ZRD. Formal analysis: MKL, JCS, CAG, LEB, JMS, KEW, DSWP. Funding acquisition: MKL, LEB, ZRD. Investigation: MKL, JCS, CAG, LEB, JMS. Project management: ZRD. Resources: ZRD. Visualization: MKL, LEB, JMS; Writing – original draft: MKL, ZRD. Writing – review & editing: MKL, LEB, JMS, ZRD.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Loth, M.K., Schmidt, J.C., Gonzalez, C.A. et al. Mapping the cellular basis of species differences in oxytocin and dopamine receptor expression in the vole nucleus accumbens. Sci Rep 15, 26908 (2025). https://doi.org/10.1038/s41598-025-06367-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-06367-1