Abstract
Severe wounds (e.g., burns) often result in irreversible scarring, leading to cosmetic and functional impairments as well as secondary complications such as reduced skin strength and itching. In the case of chronic wounds and diabetic ulcers, impaired healing capacity is the primary clinical concern, not scarring. These wounds frequently fail to heal, presenting a therapeutic challenge. Despite ongoing research, the development of scarless, cost-effective, and clinically viable therapies for these complex wounds remains a significant challenge. Angiogenesis plays a crucial role in skin wound healing. Visfatin has been known to have angiogenic and wound healing effects. In our previous study, we derived two angiogenic peptides (Vis-1 and Vis-2) from the active site of visfatin. Therefore, this study is aimed to investigate the wound healing potential of these two peptides using a scratch assay system (in vitro) and full-thickness excision wound healing mice (in vivo). Only Vis-1 peptide had a significant wound healing effect in the in vitro assay by promoting proliferation and migration of keratinocytes and dermal fibroblasts, possibly through activation of Wnt/β-Catenin and MAPK signaling pathway. Vis-1 peptide also showed remarkable wound healing effects in the in vivo assay by accelerating wound healing, inducing angiogenesis, promoting neo-epithelium, decreasing granulation tissue, and increasing collagen fiber formation. These results suggest that the Vis-1 peptide has a potent wound healing activity and may contribute as a novel wound healing agent.
Similar content being viewed by others
Introduction
Human skin comprises of epidermis, dermis, and subcutaneous tissue and protects the body from extrinsic factors. A wound is a functional or structural damage to the skin and wound healing indicates the repair of the wound area. Wounds are generally classified as chronic wounds and acute wounds. Chronic wounds include vascular ulcers, diabetic ulcers, and pressure ulcers, while acute wounds involve abrasions, cut wounds, lacerations, and burns, etc1. Severe wounds (e.g., burns) often result in irreversible scarring, leading to cosmetic and functional impairments as well as secondary complications such as reduced skin strength and itching. In the case of chronic wounds and diabetic ulcers, impaired healing capacity is the primary clinical concern, not scarring. These wounds frequently fail to heal, presenting a therapeutic challenge and life-threatening risks, including progression to infection and potential limb amputation2,3. Despite ongoing research, the development of scarless, cost-effective, and clinically viable therapies for these complex wounds remains a significant challenge. Therefore, development of novel and effective methods for wound healing is necessary.
Wound healing is a sequential and highly complex cascade process, such as inflammation, proliferation, and remodeling phases4,5. Also it is associated with various factors such as resident skin cells, extracellular matrix, chemokines, cytokines, and growth factors6. Accordingly, several techniques including skin regenerative strategies have been explored to improve scarless wound healing outcomes7,8,9,10.
Angiogenesis plays a crucial role in skin regeneration-based wound healing as oxygen, nutrients, and bioactive substances are supplied through angiogenesis. In this respects, research on angiogenesis and wound healing has received extensive attention11,12. VEGF is a representative angiogenic factor and known to aid in skin regeneration by promoting keratinocyte proliferation13,14. Visfatin, a kind of adipokine, induces activation of VEGF and possess angiogenic potential15,16. It also promotes wound healing by enhancing proliferation and mobility of keratinocytes and human dermal fibroblasts17.
Computer-aided drug design (CADD), a computer simulation-based material screening technology, is fast, convenient, and efficient in deriving candidate substances. It also aids in screening protein-derived peptides that are identical or superior to existing proteins using the affinity of ligands and receptors18,19. In our previous study, we developed two peptides with superior angiogenic efficacy than the native visfatinbased on the active site of visfatin using computer simulation techniques20. Therefore, this study is aimed to investigate the skin regeneration effects of these peptides through in vitro and in vivo experiments.
Results
Effects of visfatin-derived peptides on wound healing in vitro
Two in vitro assays were conducted to evaluate the wound-healing effects of visfatin-derived peptides (Vis-1 and Vis-2) on HaCaT cells and HDFs. The first test assessed the proliferation effect using an MTT assay, and the other measured the degree of wound closure using a cell scratch assay in HaCaT cells and HDFs. In HaCaT cells, the groups treated with 0.1, 1.0, and 10 µM Vis-1 peptide showed 106.49%, 113.78%, and 109.25% cell proliferation, respectively, a significant increase compared to the 100% of the control group reference. In HDFs, 106.44% and 106.01% cell proliferation were noted in the groups treated with 0.1 and 10 µM Vis-1 peptide, respectively, which was higher than the control group, but the change was not significant (Supplementary Fig. 1). These results suggest that Vis-1 peptide has a proliferation effect on these two cells. The degree of wound closure was measured by scratching the cultured cells and calculating the changes in the wound area before and after treatment with the peptides at various concentrations (0.1, 1, and 10 µM).
Wound closure after Vis-2 peptide treatment was not significantly different compared to that of the control group in both HaCaT (Fig. 1A, B) and HDF (Fig. 1C, D), whereas wound closure after Vis-1 peptide treatment was significantly different at a rate of 64.8% at 0.1 µM and 63.7% at 1 µM compared to the control group (47.1%) and positive control group (hEGF, 49.1%) in HaCaT cells (Fig. 1A, B). Vis-1 peptide also significantly increased wound closure rates in HDFs by 71.7% at 0.1 µM and 67.0% at 1 µM compared to control (54.8%) and positive control (hFGF, 57.1%)(Fig. 1C, D). These results indicate that Vis-1 peptide exerts a wound healing effect by promoting cell proliferation and migration of keratinocytes and skin fibroblasts.
Effects of Vis-1 peptide on cell proliferation and migration on HaCaT cells and HDFs. Representative image and quantification of visfatin-derived peptides (Vis-1 and Vis-2) on HaCaT (A,B) and HDF cells (C,D). Data represent the average of five independent experiments and are expressed as a mean ± SEM. * p < 0.05 and ** p < 0.01 (vs. control). Con control, hEGF human epidermal growth factor, hFGF human fibroblast growth factor, Vis-1 visfatin-derived peptide-1, Vis-2 visfatin-derived peptide-2.
Effects of the Vis-1 peptide on Wnt/β-Catenin and MAPK signaling pathway
Thereafter, western blot analysis was performed to further investigate whether Vis-1 peptide could affect the Wnt/β-Catenin and MAPK signaling pathway in HaCaT cells and HDFs in vitro. In HaCaT cells, the expressions of β-Catenin and p-p38 were increased at 0.1 and 1 µM, and the expressions of p-JNK was increased at 1 µM concentration (Fig. 2A, B), respectively. In HDFs, only β-Catenin expression was significantly increased at 1 µM concentration (Fig. 2C, D). These results indicate that treatment with Vis-1 peptide increases the expression of β-Catenin and p-p38 at low concentrations.
Effects of Vis-1 peptide on Wnt/β-Catenin and MAPK signaling pathway. Expression of the β-Catenin and MAPK-related protein (p-ERK, p-JNK, and p-p38)on HaCaT (A,B) and HDF cells (C,D) by western blot analysis. Data represent the average of three independent experiments and are expressed as a mean ± SEM. * p < 0.05 and **p < 0.01 (vs. control). Con control, hEGF human epidermal growth factor, hFGF human fibroblast growth factor, Vis-1 visfatin-derived peptide-1.
Effects of Vis-1 peptide on wound healing and angiogenesis in vivo
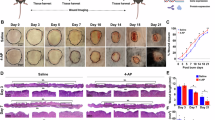
Since Vis-1 peptide showed potential in promoting cell proliferation on HDFs and HaCaT cells in vitro, the effect of Vis-1 peptide on the healing of full-thickness skin wounds in vivo was investigated by using an excision wound healing test in mice. In the preliminary experiments using 0.1, 1, 10, and 20 µM of Vis-1 peptide, we observed that 1 and 20 µM were more effective concentrations in promoting wound closure than other concentrations (Supplementary Fig. 2). Therefore, the following experiments were conducted with concentrations of 1 and 20 µM of Vis-1. On post-injury day 8, wounds were almost healed at a low concentration (1µM) of Vis-1 peptide, but the wounds in the control group were not closed. On post-injury day 10, the wound closure rates were at 76.9% in the control group, 87.5% in the positive control group (20 µM hEGF), 90.8% using 1µM of Vis-1 peptide, and 86.9% using 20 µM of Vis-1 peptide. In wound tissues at post-injury, the wound area of mice treated with Vis-1 peptide and hEGF was smaller than that of the control group, suggesting that 1µM of Vis-1 peptide accelerated wound healing (p < 0.05) (Fig. 3). Furthermore, there were no adverse effects on body weight, overall health status, or behavior of the mice during the treatment with Vis-1 peptide.
Wound healing effects of Vis-1 in full-thickness wound models. Representative images of wounds healing on day 0, 2, 4, 6, 8, and 10 after injury (A). Quantification of the wound closure rate in different groups (Control, 20µM hEGF, 1µM Vis-1, and 20µM Vis-1) at various time points (Day 2, 4, 6, 8 and 10) (B). Data represent the average of three independent experiments and are expressed as a mean ± SEM.*p < 0.05 (vs. control). hEGF human epidermal growth factor, Vis-1 visfatin-derived peptide-1.
Considering that Vis-1 peptide accelerated wound healing in vivo, we hypothesized that this peptide may also contribute to the promotion of angiogenesis during wound healing. The analysis of subcutaneous wound tissues collected on days 10 post-injury revealed that the wounds in subcutaneous tissues showed a marked increase in new vessel formation, as evidenced by increased vessel size and number of vessels in the Vis-1 peptide-treated groups compared to the control group. These results suggest that the Vis-1 peptide appeared to effectively promote wound healing (Fig. 4).
Angiogenic activity of Vis-1 on subcutaneous wound tissues. Representative images of the newly formed blood vessels at the wound area (A) on day 10 post-injury in the mice treated with control (DPBS), positive control (hEGF), 1 µM and 20 µM of Vis-1 peptide. Quantification of vessel area (B), number of junctions (C), and number of endpoints (D) from the images using AngioTool. Data are expressed as a mean ± SEM. *p < 0.05, **p < 0.01 and ***p < 0.001 (vs. control). hEGF human epidermal growth factor, Vis-1 visfatin-derived peptide-1.
Effects of Vis-1 peptide on re-epithelialization, granulation tissue formation, and collagen formation
We performed hematoxylin &eosin (H&E) and Masson’s trichrome staining to observe the wound area on days 4, 8, and 10 after the operation. H&E staining showed that the Vis-1 peptides thickened the neo-epithelium (Fig. 5A, B) and decreased granulation tissues on day 10 compared with the control group (Fig. 5C). In particular, when treated with a high concentration (20 µM) of the Vis-1 peptide, neo-epithelium was thicker than that in the positive control group on days 4, 8, and 10. On post-injury day 8, the largest amount of granulosa tissue was formed in all test groups. The Masson’s staining results showed that more collagen was deposited in the wound tissues in the Vis-1 peptide group compared to the control group. These results indicate that Vis-1 peptide has skin regenerative effects by increasing the thickness of neo-epithelium in wound tissue, reducing the formation of granulation tissues, and increasing collagen formation.
Effects of Vis-1 peptide on re-epithelialization, granulation tissue formation, and collagen formationin full-thickness wound healing mouse model. H&E staining results of the wound tissues (A). Quantification of the neo-epithelium thickness (B) and the granulation tissues (C). Masson’s trichrome staining results of the wound sites (D). Data represent the average of three independent experiments and are expressed as a mean ± SEM. *p < 0.05 and **p < 0.01 (vs. control). hEGF human epidermal growth factor, Vis-1 visfatin-derived peptide-1, ES eschar, GT granulation tissue, K keratin, NE neo-epithelium, yellow dotted line and a block arrow: neo-epithelium, and block triangle: collagen fiber.
Discussion
The present study shows that only Vis-1 peptide had a significant wound healing effect in in vitro assayusing human keratinocytes and dermal fibroblasts by promoting proliferation and migration of these cells, possibly through activation of Wnt/β-Catenin and MAPK signaling pathway. Furthermore, Vis-1 peptide also showed remarkable wound healing effects in the in vivo assay using full-thickness excision wound healing mouse model by accelerating wound healing, inducing angiogenesis, promoting neo-epithelium, decreasing granulation tissue, and increasing collagen fiber formation. In addition, Vis-1 peptide administration increased the rate of healing, formation of new vessels, and re-epithelialization during the early stage (Figs. 3, 4 and 5). These results suggest that Vis-1 peptide may contribute as a novel wound healing agent.
The most valuable finding of this study is the identification of peptide (LEYKLHDFGY), with more effective angiogenic activity than original visfatin. It had superior skin regeneration and wound healing abilities than the positive control, hEGF. To the best of our knowledge, this is the first study to show that the Vis-1 peptide had these effects.
Visfatin has angiogenic activity and promotes VEGF expression. Hence, it is a very potential angiogenic activator and might be a therapeutic agent for wound healing. However, as visfatin has high molecular weight, its development as a therapeutic drugis challenging. The limitation includes increased immunogenicity, unstable activity and loss of bioactivity21. Therefore, there has been a growing interest toward therapeutic peptides in recent years22,23,24. Various computer simulation techniques including molecular docking simulation have been very useful in screening potential therapeutic peptides based on the affinity for ligands and receptors18,25. We derived peptides from the active site of visfatin, with similar or superior angiogenic activity using computer simulation techniques. Indeed, many efforts have been pursued to develop peptides with wound healing properties in recent years26,27,28,29.
Several studies have shown that the proliferation and migration of keratinocytes and dermal fibroblasts are important steps in the wound healing process, which promotes re-epithelialization and wound repair30,31. Many peptides have been reported to be involved in the proliferation phase of wound healing by promoting the proliferation and migration of keratinocytes and fibroblast28,29,32. In the present study, Vis-1 peptide significantly stimulated cell proliferation and migration in immortalized HaCaT cells and HDFs (Supplementary Fig. 1). Indeed, culturing primary keratinocytes can be demanding, but their use would be an important addition to the research. Therefore, this study attempted to isolate primary keratinocytes from donor skin according to the methods reported by Henro et al. and Supp et al.33,34. Unfortunately, the cells did not grow well and could not be used in the experiments. In fact, primary human keratinocytes have limitations such as difficulty in isolation and culture from donors and limited passage number35. Some studies have reported that HaCaT cells are similar to primary human keratinocytes and are also suitable as an inflammation/repair response model for skin diseases36. Indeed, many studies have used HaCaT cells instead of primary human keratinocytes37,38,39,40. These results are thought to support that the use of HaCaT cells can partially compensate for the shortcomings of our inability to use primary human keratinocytes. These facts suggest that Vis-1 peptide exerts a wound healing effect although this study was performed on HaCaT cells and HDFs.
Wound healing is a very complex process regulated by a variety of different signaling pathways41,42. Among them, the Wnt/β-catenin pathway plays an important role in cell proliferation during the wound healing process43,44. MAPK signaling is also critically involved in cell proliferation and migration in skin keratinocytes and fibroblasts45,46. Some peptides exert significant effects on wound healing through the MAPK signaling pathway29,47,48. Lu et al. showed that dracorhodin perchlorate promoted the wound healing activity through the β‑catenin, ERK, p38 MAPK and AKT signaling pathways by western blot experiment49. In the present study, the results of western blotting showed that the treatment of Vis-1 peptide increased the expression of β-Catenin and p-p38 at low concentrations (1 µM).
In the study of wound healing, the selection of appropriate in vitro and in vivo models is necessary for two reasons: first, the process of wound healing is very complex, and second, each models must reflect a specific stage of wound healing well50,51. In vitro models are required to be rapid, simple, cost effective, and have few ethical considerations52. In this respect, the most frequently used technique is a scratch test assay in a single-cell system29,32,37,38,52. The present study also adopted this system using HDFs and HaCaT cells, and was able to confirm that Vis-1 peptide promotes the cell proliferation and migration of both cells. One thing to note in the scratch assay is that cell proliferation may affect scratch closure. Accordingly, some studies have chosen to block cell proliferation with mitomycin C53 while others have used culture medium with lower serum concentration (1%) to minimize cell proliferation17,29,54,55. The present study also performed the scratch assay using culture medium containing 1% serum, which is lower than 10%.
Various animal models, including diabetic mice and full-thickness wound healing mice, have been proposed as models for the healing of impaired wounds51. Effective skin wound healing requires full re-epithelialization in the wound, and angiogenesis56. Therefore, animal models should be readily available for visual inspection to measure changes in wound size, and for assessing the rate of wound healing by measuring epithelialization, vascularisation, and ECM deposition, and histological studies32,37,38. In this regard, full-thickness wound healing mice model, as in our study, is also widely used for wound healing through skin regeneration29,32,37,57,58. In the present study, we adopted full-thickness wound healing mice model considering laboratory conditions, cost, and convenience. These assessments showed that Vis-1 peptide significantly decreased wound area and promoted angiogenesis around the wound site. In addition, the H&E staining and Masson’s trichrome staining confirmed that Vis-1 peptide induced an increase in the thickness of neo-epithelium, a decrease in granulation tissue, and an increase in collagen deposition in wound tissues.
Determining treatment dose in in vitro and in vivo studies to investigate the functional activity of a new substance is challenging. Therefore, we employed a four-step process to determine the appropriate treatment concentration of the Vis-1 and Vis-2 peptides. First, we reviewed the literature on peptide treatment concentrations. In many previous studies, the treatment concentrations of peptides in in vitro and in vivo experiments ranged from 10 nM to 100 µM32,38,59,60 and from 1µM to 100 µM, or up to 200 µg/ml32,37,38,58, respectively. Second, we performed the MTT cell cytotoxicity assay for various concentrations of Vis-1 and Vis-2, ranging from 0.1,1 to 10.0 µM (Supplementary Fig. 1). Thereby, we observed that these concentrations had no cytotoxicity. Third, based on these results, we carried out angiogenic activity assay for two concentrations: minimum (0.5 µM) and maximum (2.0 µM)as in our previous study20. Finally, we performed preliminary experiments using concentrations of 0.1, 1, 10, and 20 µM to determine the in vivo treatment concentration for Vis-1 peptide, which shows wound healing effect in vitro assay using HaCaT and HDF cells. Based on these results, we chose 1µM and 20 µM as the final concentrations for the following in vivo experiments.
When studying the functional activity of a substance, the choice of a positive control substance is important. The aim of this study was to investigate the wound healing effect of the peptides, not its angiogenic activity. Many studies on skin regeneration have used hEGF as a positive control17,27,37,38. For this reason, our study choseh EGF as a more reliable positive control than VEGF or visfatin.
The peptides used in this study were derived from the active site of visfatin by computer simulation. Visfatin has been reported to influence glucose metabolism and inflammation61,62. However, visfatin also induces the proliferation and migration of HaCaT and HDF cells by regulating the ERK1/2 and JNK1/2 signaling pathways, and promotes wound healing by increasing the expression of VEGF63. In this respect, the present study focused on the wound healing efficacy of visfatin-derived peptides rather than the effects on glucose metabolism or inflammation promotion. In addition, this study did not investigate whether the peptide caused an increase in obesity and/or insulin resistance for two reasons. First, this study only applied the peptide to the skin, not administered orally or injection. It was thought that such application would have little effect on systemic obesity and insulin resistance. Second, although some studies have reported a positive correlation between increased plasma visfatin levels and the development of obesity, it has not been clearly confirmed, and the correlation between visfatin and insulin resistance is also still controversial62,64.
Stability is an important factor in developing peptide therapeutics. Therefore, our research team is researching the production of peptides with longer half-lives and higher bioavailability than the original visfatin using the ‘Alanine scanning’ method and ‘fatty acylation’ method to improve the stability of Vis-1 peptide.
In conclusion, these results suggest that Vis-1 peptide has the most effective wound healing ability by inducing angiogenesis in the wound area and the proliferation of keratinocytes and dermal fibroblasts, causing rapid epithelization and contraction of the wound. However, additional nonclinical studies, including pharmacokinetics and safety, are needed for Vis-1 peptide to be developed as a clinical potential therapeutic drugf or wound healing.
Materials and methods
Ethics information
The all animal experimental methods performed followed the ethical principles adopted by the Pusan National University Hospital of Animal Experimentation and approval was obtained by the Ethics Committee on Animal Care and Use of the Home Institution (Approval ID: PNUH-2024-228) prior to the beginning of the experiment. Every effort was made to reduce the number of animals used, and to minimize their suffering. This study is reported in accordance with ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines.
Human dermal fibroblasts (HDFs) were manually isolated from discarded skin tissues obtained during transplant surgery. These clinical procedures and protocols were based on informed consent from all subjects before enrollment and approved by Institute Review Board (IRB) of Pusan National University Hospital (11-2025-014). Research involving human research participants must have been performed in accordance with the Declaration of Helsinki.
Peptide synthesis
The two angiogenic peptides derived from the active site of visfatinwere synthesized usingsolid-phase peptide synthesis (SPPS) methods at HLB PEP (Gwangju, Rep. of Korea). The synthesized peptides were purified using HPLC (high-performance liquid chromatography) system (Shimadzu HPLC LabSolution, Tokyo, Japan) and Tiart C18 column (YMC Co. Ltd., Kyoto, Japan) (5 μm, 30\(\:\times\:\)250 mm). The purity of two peptides was more than 95% and all peptides were lyophilized. The peptides were dissolved in distilled water at a concentration of 1 mM and stored at -20 °C. The sequences of peptides are described in Table 1.
Isolation and cell culture of primary human dermal fibroblasts and human keratinocytes
Skin tissues were collected from nine donors in the department of plastic surgery at Pusan National University Hospital in Yangsan (Rep. of Korea). Among the nine tissues collected, four could not be used because of problems with transportation and storage from the operating room to the laboratory. Among the remaining five, the best was selected for the experiment based on the cell growth rate and morphological characteristics of fibroblasts (large, flat, and spindle-shaped cells).
The isolated skin tissues were sterilized using 70% ethanol and incubated in 25 UI/mL Dispase II solutions (Sigma-Aldrich, St. Louis, MO, USA) for 15 h at 4 °C. Separated dermal tissues were chopped using the scalpel blade and digested in Collagenase/DNase (Collagenase Type I: Gibco, Grand Island, NY, USA and DNase I: Roche, Basel, Switzerland) solution for 90 min at 37 °C under manually shake it every 10 min. The digested samples were filled with the same amount of 10% heat-inactivated fetal bovine serum (FBS) (Gibco) and were filtered through a 70 μm-cell strainer. The suspension was centrifuged for 5 min at 1200 rpm. The cell pellet was resuspended using Dulbecco’s modified eagle’s medium (DMEM) (WELGENE, Gyeongsan, Korea) with 10% FBS and 1% Antibiotics/Antimycotics (Gibco).
Human immortalized keratinocytes (HaCaT) cells were purchased from AddexBio Technologies (San Diego, CA, USA). It was cultured in DMEM supplemented with 10% FBS and 1% Antibiotics/Antimycotics. The cultured cells were maintained at 37 °C in an atmosphere with 5% CO2.
Scratch wound healing assay
HDFs and HaCaT cells were seeded in 6-well plates (2\(\:\times\:\)105 cells and 4\(\:\times\:\)105 cells per well, respectively). When the cells reached 100% confluency, the monolayer of cells was scratched using a 200-µL pipette tip to create a wound. The wounded debris was removed by washing with DPBS (Dulbecco’s phosphate buffered saline) (WELGENE) twice. The cells of each well were treated with DMEM medium containing various concentrations (0.1, 1, and 10 µM) of peptides supplemented with 1% FBS, and the culture was incubated for 12 h (HDFs) or 24 h (HaCaT cells). The scratch closure was observed using a phase-contrast microscope and the scratch area was calculated using ImageJ software (NIH, Bethesda, MD, USA). The wound closure percentage was obtained using the following formula:
A is the scratch area at 0 h, and B is the scratch area at the designated time.
Preparation of full-thickness excision wound healing mice and treatment
Full-thickness excision wound healing mice models were created to evaluate the regenerative effects of Vis-1 peptide in vivo.
Twenty male ICR mice 7-week old were purchased from KOATECH (Pyeongtaek, Korea). At the time of the wound healing test the mice were 8 weeks old and had body weights of 38.2 ± 2.5 g (mean ± SEM). The mice were kept in individual cages and provided with food and water ad libitum under 12 h light/dark cycles at a temperature of 21 ± 2 °C and relative humidity of 55 ± 10%.
The mice adapted after a week were anesthetized using isoflurane (inhalant anesthesia) and the back of the mice was shaved with an electric clipper. The dorsal skin was disinfected with alcohol, and full-thickness skin wounds were made using an 8-mm biopsy punch. After surgery, mice were randomly divided into four groups as follows;
(1) DPBS treated (Control) group (n = 5);
(2) 20 µM hEGF treated (Positive control) group (n = 5);
(3) 1 µM Vis-1 treated group (n = 5);
(4) 20 µM Vis-1 treated group (n = 5).
The mice with full-thickness wounds were treated with 20 µL of DPBS, 20 µM hEGF, 1 µM, or 20 µM Vis-1 applied directly to the wound area twice daily for 10 days. The day when wounds were created was designated as Day 0, and the wounds were photographed at intervals of 2 days. Wound areas were analyzed using ImageJ software and the wound closure rate was calculated according to the following formula:
A is the wound area on Day 0, and B is the wound area on the designated day.
Histological analysis
Mice were sacrificed using CO2 gas on Day 4, Day 8, and Day 10, and wound tissues were isolated from mice. The skin specimens were immediately fixed in 4% paraformaldehyde (Biosesang, Yongin, Korea) and dehydrated using a series of ethanol, cleared in xylene, and embedded in paraffin. The tissues of paraffin blocks were cut to 5 μm thickness and put on the silane coated slides. The sections were stained with H&E (Hematoxylin and Eosin) to monitor the wound healing process57. Deparaffinized sections were stained with Harris Hematoxylin for 2 min. And then, the slides were decolorized using 1% acid alcohol and 2% potassium acetate and stained with Eosin for 2 min. Masson’s trichrome method was used to identify the collagen fibers58. The sections of deparaffinization were submerged in Bouin’s solutions and washed using tap water until the yellow color in the sample. The samples were stained with modified Weigert’s hematoxylin for 8 min and treated with phosphomolybdic acid solution. The last step was collagen staining with methyl blue solution for 5 min. Mounting sections were photographed using a light microscope and Aperio ImageScope software (Leica Biosystem, Wetzlar, Germany).
Western blot
HDFs and HaCaT cells were seeded in 100-mm dishes at each density of 5\(\:\times\:\)105 cells and 1\(\:\times\:\)106 cells per dish, respectively. When the cells reached 80% confluency, the cultured cells were treated with various concentrations (0.1, 1, and 10 µM) of Vis-1 peptide for 12 h (HDFs)or24 h (HaCaT cells). The cells were lysed with ProEX™ CETi Lysis Buffer with Inhibitor (TransLab, Daejeon, Korea) and the protein concentration was measured using Pierce™ BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions.
The protein lysates were separated by SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis) and transferred onto PVDF (polyvinylidene difluoride) membranes. The membranes were blocked with 1X Phospho-Block Solution (TransLab) for 1 h at room temperature and then incubated with the suitable primary antibody at 4 °C overnight. The used primary antibodies were β-Actin (1:1000, Santa Cruz Biotechnology, Dallas, TX, USA), β-Catenin (1:1000, Santa Cruz Biotechnology), p-ERK 1/2 (1:1000, Santa Cruz Biotechnology), p-JNK (1:1000, Cell Signaling Technology, Danvers, MA, USA) and p-p38 (1:1000, Cell Signaling). The next day, the membranes were washed with 1X TBST buffer (TransLab) for five times and incubated with the corresponding secondary antibody for 1 h at room temperature. HRP-conjugated Goat anti-Rabbit IgG (1:1000, ABclonal, Woburn, MA, USA) and HRP-conjugated Goat anti-Mouse IgG (1:1000, ABclonal) were used as the secondary antibodies. The signals were detected using SMARTGENE ECL solution (Daejeon, Korea) and ATTO Ez-Capter II (ATTO Technology, Amherst, NY, USA). The results were analyzed using ImageJ software and normalized to the expression of β-Actin.
Statistical analysis
In vitro and in vivo results are presented as mean ± SEM (standard error of the mean). The results were analyzed using an unpaired t-test in GraphPad Prism (GraphPad Software Inc., CA, USA). P < 0.05 was considered statistically significant.
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
References
Irfan-Maqsood, M. Classification of wounds: know before research and clinical practice. Genes Cells. 4, 1–4 (2018).
Rahimnejad, M., Derakhshanfar, S. & Zhongm, W. Biomaterials and tissue engineering for Scar management in wound care. Burns Trauma. 5, 1–9 (2017).
Spiwak, R., Sareen, S. & Logsetty, S. Techniques to assess long-term outcomes after burn injuries. Eur. Burn J. 3, 328–339 (2022).
Gonalez, A. C. O., Andrade, Z. A., Costa, T. F. & Medrado, A. R. A.P. Wound healing - A literature review. Bras. Dermatol. 91, 614–620 (2016).
Fu, S. et al. Mechanotransduction in wound healing: from the cellular molecular levels to the clinic. Adv. Skin. Wound Care. 34, 67–74 (2021).
Canedo-Dorantes, L. & Canedo-Ayala, M. Skin acute wound healing: acomprehensive review. Int. J.Inflam. 3706315 (2019).
Toma, A. I., Fuller, J. M., Willett, N. J. & Goudy, S. L. Oral wound healing models and emerging regenerative therapies. Transl Res. 236, 17–34 (2021).
Hade, M. D., Suire, C. N. & Suo, Z. Mesenchymal stem cell-derived exosomes: applications in regenerative medicine. Cells 10, 1959 (2021).
Kaur, G., Narayanan, G., Garg, D., Sachdev, A. & Matai, I. Biomaterials-based regenerative strategies for skin tissue wound healing. ACS Appl. Bio Mater. 5, 2069–2106 (2022).
Safina, I., Childress, L. T., Myneni, S. R., Vang, K. B. & Biris, A. S. Cell-biomaterial constructs for wound healing and skin regeneration. Drug Metab. Rev. 54, 63–94 (2022).
Reddy, L. V. K., Murugan, D., Mullick, M., Begum Moghal, E. T. & Sen, D. Recent approaches for angiogenesis in search of successful tissue engineering and regeneration. Curr. Stem Cell. Res. Ther. 15, 111–134 (2020).
Lu, C. C. et al. Dracorhodin perchlorate enhances wound healing via β-catenin, ERK/p38, and AKT signaling in human HaCaT keratinocytes. Exp. Ther. Med. 22, 822 (2021).
Bao, P. et al. The role of vascular endothelial growth factor in wound healing. J. Surg. Res. 153, 347–358 (2009).
Le, T. H. V. & &Kwon, S. M. Vascular endothelial growth factor biology and its potential as a therapeutic target in rheumatic disease. Int. J. Mol. Sci. 22, 5387 (2021).
Adya, R., Tan, B. K., Punn, A., Chen, J. & &Randeva, H. S. .Visfatin induces human endothelial VEGF and MMP-2/9 production via MAPK and PI3K/Akt signaling pathways: novel insights into visfatin-induced angiogenesis. Cardiovasc. Res. 78, 356–365 (2008).
Chen, D. et al. Visfatin promotes angiogenesis of RF/6A cells through upregulation of VEGF/VEGFR-2 under high-glucose conditions. Exp. Ther. Med. 21, 389 (2021).
Lee, B. C., Song, J., Lee, A., Cho, D. & Kim, T. S. Visfatin promotes wound healing through the activation of ERK1/2 and JNK1/2 pathway. Int. J. Mol. Sci. 19, 3642 (2018).
Farhadi, T. & Hashemian, S. M. Computer-aided design of amino acid-based therapeutics: a review. Drug Des. Devel Ther. 12, 1239–1254 (2018).
Pandya, A. K. & &Patravale, V. B. Computational avenues in oral protein and peptide therapeutics. Drug Discov Today. 26, 1510–1520 (2021).
Choi, J. M. et al. Computer simulation approach to the identification of visfatin-derived angiogenic peptides. Plus One. 18, e0287577 (2023).
Lee, K., Silva, E. A. & Mooney, D. J. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J. R Soc. Interface. 8, 153–170 (2011).
Sridhar, K., Inbaraj, B. S. & Chen, B. H. Recent developments on production, purification and biological activity of marine peptides. Food Res. Int. 147, 110468 (2021).
Muttenthaler, M., King, G. F., Adams, D. J. & Alewood, P. F. Trends in peptide drug discovery. Nat. Rev. Drug Discov. 20, 309–325 (2021).
Sheng, Y. et al. Eighteen novel bioactive peptides from Monkfish (Lophius litulon) swim bladders: production, identification, antioxidant activity, and stability. Mar. Drugs. 21, 169 (2023).
Otvos, L. The latest trends in peptide drug discovery and future challenges. Expert Opin. Drug Discov. 19, 869–872 (2024).
Gomes, A., Teixeira, C., Ferraz, R. & Prudencio, C. & Gomes. P. Wound-healing peptides for treatment of chronic diabetic foot ulcers and other infected skin injuries. Molecules 22, 1743 (2017).
He, X. et al. A frog-derived Immunomodulatory peptide promotes cutaneous wound healing by regulating cellular response. Front. Immunol. 10, 2421 (2019).
Wang, S. et al. A novel peptide from the skin of amphibian Ranalimnocharis with potency to promote skin wound repair. Nat. Prod. Res. 35, 3514–3518 (2021).
Fu, S. et al. A novel peptide from Polypedatesmegacephalus promotes wound healing in mice. Toxins14, 753 (2022).
Werner, S., Krieg, T. & Smola, H. Keratinocyte-fibroblast interactions in wound healing. J. Invest. Dermatol. 127, 998–1008 (2007).
Piipponen, M., Li, D. & Landén, N. X. The immune functions of keratinocytes in skin wound healing. Int. J. Mol. Sci. 21, 8790 (2020).
Liu, M. et al. Accelerated wound healing induced by a novel amphibian peptide (OA-FF10). Protein Pept. Lett. 26, 261–270 (2019).
Henrot, P. et al. A method for isolating and culturing skin cells: application to endothelial cells, fibroblasts, keratinocytes, and melanocytes from punch biopsies in systemic sclerosis skin. Front. Immunol. 11, 1–12 (2020).
Supp, D. M. et al. Isolation and feeder-free primary culture of four cell types from a single human skin sample. STAR. Protocols. 3, 1–33 (2022).
Micallef, L. et al. Effects of extracellular calcium on the growth-differentiation switch in immortalized keratinocyte HaCaT cells compared with normal human keratinocytes. Exp. Dermat. 18, 143–151 (2008).
Colombo, I. et al. HaCaTcells as a reliable in vitro differentiation model to dissect the inflammatory/repair responses of human keratinocytes.Mediat. Inflamm. 1–12 (2017).
Wu, J. et al. A frog Cathelicidin peptide effectively promotes cutaneous wound healing in mice. Biochem. J. 475, 2785–2799 (2018).
Cao, X. et al. Cathelicidin-OA1, a novel antioxidant peptide identified from an amphibian, accelerates skin wound healing. Sci. Rep. 8, 943 (2018).
Kotian, S. R. et al. Influence of traditional medicines on the activity of keratinocytes in wound healing: an in vitro study. Anat. Cell. Biol. 52, 324–332 (2019).
Cai, S. et al. Derivatives of gecko cathelicidin-related antioxidant peptide facilitate skin wound healing. Eur. J. Pharm. 890, 173649 (2021).
Krizanova, O. et al. Signaling pathways in cutaneous wound healing. Front. Physiol. 13, 1030851 (2022).
Gumede, D. B., Abrahamse, H. & &Houreld, N. N. Targeting Wnt/β-catenin signaling and its interplay with TGF-β and Notch signaling pathways for the treatment of chronic wounds. Cell. Commun. Signal. 22, 244 (2024).
Lindley, L. E., Stojadinovic, O. & Pastar, I. Tomic–Canic, M. Biology and biomarkers for wound healing. Plast. Reconstr. Surg. 138, S18–S28 (2016).
Choi, S., Yoon, M. & Choi, K. Y. Approaches for regenerative healing of cutaneous wound with an emphasis on strategies activating the Wnt/β-Catenin pathway. Adv. Wound Care. 11, 70–86 (2022).
Thuraisingam, T. et al. MAPKAPK-2 signaling is critical for cutaneous wound healing. J. Invest. Dermatol. 130, 278–286 (2010).
Pereira Beserra, F. et al. Lupeol, a pentacyclic triterpene, promotes migration, wound closure, and contractile effect in vitro: Pssible involvement of PI3K/Akt and p38/ERK/MAPK Pthways. Molecules 23, 2819 (2018).
Chung, J. G. Newly synthesized Quinazolinone HMJ–38 suppresses angiogenetic responses and triggers human umbilical vein endothelial cell apoptosis through p53–modulated fas/death receptor signaling. Toxicol. Appl. Pharmacol. 269, 150–162 (2013).
Li, F., Jiang, T., Liu, W., Hu, Q. & Yin, H. The angiogenic effect of Dracorhodin perchlorate on human umbilical vein endothelial cells and its potential mechanism of action. Mol. Med. Rep. 14, 1667–1672 (2016).
Lu, Y. et al. Autocrine and Paracrine Effects of vascular endothelial cells promote cutaneous wound healing. Biomed. Res. Int. 6695663 (2021).
Cialdai, F., Colciago, A., Pantalone, D., Rizzo, A. M. & &Zava, S. Effect ofunloading condition on the healing process and effectiveness ofplatelet rich plasma as a countermeasure: study on in vivo and invitro wound healing models. Int. J. Mol. Sci. 21, 407 (2020).
Sarian, M. N., Zulkefli, N., Zain, C. & Maniam, M. S. Fakurazi. S. A review with updated perspectives on in vitro and in vivo wound healing models. Turk. J. Biol. 47, 236–246 (2023).
Stamm, A. et al. In vitro wound healing assays–state of the Art. BioNanoMat 17, 79–87 (2016).
Grada, A., Otero-Vinas, M., Prieto-Castrillo, F. & Obagi, Z. &Falanga v.research techniques made simple: analysis of collective cell migration using the wound healing assay. J. Invest. Dermatol. 137, e11–e16 (2017).
Wahedi, H. M. et al. Aloesin from Aloe vera accelerated skin wound healing by modulating mapk/rho and Smad signaling pathways in vitro and in vivo. Phytomedicine 28, 19–26 (2017).
Jian, K. et al. PDGF-BB-derived supramolecular hydrogel for promoting skin wound healing. Nanobiotechnology20, 201 (2022).
Ahmad N.In vitro and in vivo characterization methodsfor evaluation of modern wound dressings.Pharmaceutics15, 42 (2022).
Madigan, M. C. et al. Xanthine oxidoreductase function contributes to normal wound healing. Mol. Med. 21, 313–322 (2015).
Masson-Meyers, D. S. et al. Experimental models and methods for cutaneous wound healing assessment. Int. J. Exp. Pathol. 101, 21–37 (2020).
Takahashi, M. et al. The antimicrobial peptide human β-Defensin-3 accelerates wound healing by promoting angiogenesis, cell migration, and proliferation through the FGFR/JAK2/STAT3 signaling pathway. Front. Immunol. 12, 712781 (2021).
Li, J. et al. PR39, a peptide regulator of angiogenesis. Nat. Med. 6, 49–55 (2000).
Griffioen, A. W. et al. Anginex, a designed peptide that inhibits angiogenesis. Biochem. J. 354, 233–242 (2001).
Khanna, D., Khanna, S., Khanna, P., Kahar, P. & Patel, B. M. Obesity: A Chronic Low-Grade Inflammation and Its Markers. Cureus 14, e2271 (2022).
Abdalla, M. M. I. Role of visfatin in obesity-induced insulin resistance. World J. Clin. Cases. 10, 10840–10851 (2023).
Ren, X., Zhao, M., Lash, B., Martino, M. M. & Julier, Z. Growth factor engineering strategies for regenerative medicine applications. Front. Bioeng. Biotechnol. 7, 469 (2020).
Stastny, J. et al. Visfatin and its role in obesity development. Diabetes Metabolic Syndrome: Clin. Res. Rei. 6, 120–124 (2012).
Acknowledgements
This study was supported by the National Research Foundation of Korea(NRF) grant funded by the Korea government(MSIT) (No. 2021R1G1A1095262).
Author information
Authors and Affiliations
Contributions
Bo Sun Joo and Ju-Hwa Baekwrote the main manuscript text. Bo Sun Joo also contributed to the study conception and design. Ju-Hwa Baek also Figs. 1, 2 and 3 and Min Jung Park prepared Figs. 4, 5 and 6. Hyunseok Choi and Ji Myung Choi contributed to the peptide synthesis and the establishment of animal model. Jae Woo Lee supervised the whole project and edited the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Joo, B.S., Baek, JH., Park, M.J. et al. Effects of peptides derived from active sites of visfatin on wound healing. Sci Rep 15, 22169 (2025). https://doi.org/10.1038/s41598-025-06751-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-06751-x