Abstract
Meibomian gland dysfunction (MGD) constitutes the most common cause of dry eye disease (DED), where altering meibum or lipids result in tear film instability. This study compares structural and tear film changes induced by chemical cauterization (CC) and electrocauterization (EC) in establishing a rabbit model of obstructive MGD assessed using non-invasive tear film assessment and histology. Eighteen New Zealand white rabbits were subjected to CC (n = 9) using 1 M NaOH-soaked sponges or EC (n = 9) using electrocautery probes. Non-invasive tear film assessment including tear meniscus height, non-invasive tear film break-up time (NITBUT), and meibography were performed using keratography 5 M (Oculus, Germany) at days 7, 14, 30, 60, and 90 and structural changes were assessed by histopathological, IHC studies, and qPCR. Both groups showed similar clinical features, including increased lid margin vascularity, irregularity, and MG orifice plugging by day 30. NITBUT significantly decreased by day 30 and persisted at day 90 (CC: 9.50 ± 0.37, p = 0.002; EC: 8.34 ± 0.48, p = 0.008), indicating tear film instability. CS scores were higher at day 90, and average meiboscore was 2.0 ± 1.0 (CC, p = 0.03) and 1.7 ± 0.6 (EC, p < 0.01). Histopathology confirmed duct dilatation, inspissated secretions, and acinar atrophy. IHC showed decreased CK-14 expression. Gene expression for proliferation and lipid synthesis was similar between groups. In conclusion, this study demonstrates that CC and EC effectively induce obstructive MGD in rabbits, with measurable changes on non-invasive tear film assessments, meibography, and histology as seen in humans. Non-invasive assessments can provide standardized, objective metrics for studying DED in animal models and evaluating therapeutic interventions.
Similar content being viewed by others
Introduction
Evaporative dry eye (EDE) is the most common type of dry eye disease (DED) seen globally1. It is gaining significant importance lately due to the enormous impact it has on work productivity and quality of lives2,3. With the emergence of digital revolution and increased tech dominance in our day-to-day life, the risk of DED in general and EDE in particular is likely to spike further3,4. Meibomian gland dysfunction (MGD) constitutes the most common cause of EDE, that results from damage to pilosebaceous gland units located in the eyelids called meibomian glands (MG)5,6. These glands secrete meibum which contributes to the lipid layer of the tear film, playing an important role in the maintenance of tear film stability and ocular surface integrity7,8.
While the MGs were first described by Meibum in 1666, various aspects of the involvement of these glands in MGD remain unknown9. The Tear Film and Ocular Surface Society Dry Eye Workshop (TFOS DEWS) report II has also stated that there could be distinct, undiscerned and probably novel risk factors for developing MGD which remain poorly understood3. This emphasizes on the pressing need for extensive research in this area. While human studies have provided valuable insights into the pathophysiology of MGD, there are inherent limitations in exploring various aspects of this condition in humans alone. This has led researchers to study the significance of animal models in elucidating the complex interplay of factors contributing to MGD10.
Animal models have been used for decades to study the pathophysiological mechanisms and benefits of therapeutic interventions of DED7. Rodents and rabbit models have emerged as valuable tools in studying MGD that allow controlled experiments, precise measurements, and assessment of long-term effects of various interventions10,11. However, no single animal model has been able to recapitulate the complete spectrum of MGD as seen in humans7. Additionally, key methods used in humans for objective assessment of tear film, such as non-invasive imaging, have rarely been applied in existing animal models. Rabbits as model animals, allow easy imaging of the ocular surface and the MGs given their larger eyes and ocular surface area11. This enables the use of techniques and instruments in rabbits that are identical to that used in clinics in humans. Thus, this study aims to compare and validate the structural and tear film changes in rabbit models of MGD induced by two methods: chemical cauterization (CC) and electrocauterization (EC) using clinical, non-invasive tear film assessments and histological evaluation. With long-term follow up of these parameters, it seeks to provide deeper insights into the pathophysiology, progression, and impact of obstructive MGD on the ocular surface and tear film. Employing non-invasive imaging modalities and clinical assessment similar to those used in humans in animal models may also prove valuable for identifying opportunities for early diagnosis and treatment, as well as for evaluating the efficacy of new therapeutic agents.
Materials and methods
This experimental animal study included 18 male New Zealand white rabbits and was approved by the Institutional Animal Ethics Committee (IAEC) of the Centre for Cellular and Molecular Biology, Hyderabad, India (IAEC 13/2021). All the animal handling procedures adhered to the guidelines outlined in the Association for Research in Vision and Ophthalmology (ARVO) statement for the use of animals in Ophthalmic and Vision Research and accordance with ARRIVE guidelines and the Guide for the Care and Use of Laboratory Animals carried out this study. Clinical evaluations and interventions were performed after anesthetising the rabbits by administering intramuscular injection of ketamine (35 mg/kg; Neon Laboratories Limited, Mumbai, India) and xylazine (5 mg/kg; Troy Laboratories, Glendenning, Australia) as per the guidelines outlined in the ‘Tulane University IACUC Guidelines for Rodent and Rabbit Anaesthesia12.
MGD model generation
A Pre-operative (Pre-op) clinical evaluation was conducted on all the rabbits (n = 18), that were subjected to either CC (n = 9) or EC (n = 9) for inducing obstructive MGD. In the CC group, a polyvinyl alcohol sponge spear soaked with 1 M Sodium hydroxide (NaOH) was placed over the MG orifices along the entire length of the everted upper and lower eyelid margin for 30 seconds followed by saline wash (n = 9). A bandage contact lens (Purecon, India) was placed over the cornea and a second sponge was placed adjacent to the soaked sponge to avoid spillage of NaOH onto the ocular surface. In the EC group, both eyelids were everted to expose the MG orifices, which were subjected to electrocautery using a 5-MHz high-frequency electrocauterization unit with an Elman mono-polar cautery probe (KENTAMED RF 100, Bulgaria). Both procedures were performed on the left eye under a stereo zoom dissecting microscope (Carl Zeiss AG, Germany). (Fig. 1; Supplementary video S1.mp4)
Clinical evaluation and imaging
Clinical evaluation, tear film metrics and meibography were assessed on post-operative (post-op) day 7, 14, 30, 60 and 90. Lid margins were assessed for Lid margin vascularity (LMV), Lid margin irregularity (LMI), and Plugging of Gland Orifices (PGO)13. Tear film metrics were assessed by measuring the Non-Invasive Tear Breakup Time (NITBUT)11 and Tear Meniscus Height (TMH) followed by Schirmer’s Test (ST) without local anaesthesia. NITBUT and TMH were captured using keratograph-5 M device (Oculus inc, Wetzlar, Germany) and three blinks were manually performed before capturing the scans. An average of three readings was used for statistical analysis11. The corneal staining was scored according to the National Eye Institute (NEI) scoring scheme14. The structural changes over the MG’s were assessed using infrared meibography images captured using keratograph-5 M device (Fig. 1). The extent of MG alterations was scored using Meiboscore, partial gland loss and complete gland drop outs as per Arita grading system13,15.
Histology and immunohistochemistry studies
The study subjects were euthanized with CO2 at day 7, 30 and 90 (n = 3 at each time point in each group). Eyelid tissues were excised and fixed in either 10% neutral buffer formalin for histology studies or in TRIzol (Invitrogen,15596026) for gene expression analysis.
Paraffin-embedded tissue blocks were cut into 5 μm thick sections in transverse planes using a microtome (Leica RM 2255; Leica, Bannockburn, IL), and were stained with Haematoxylin and Eosin (H&E). For immunofluorescence staining, 5 μm thick paraffin sections were deparaffinised, rehydrated, and subjected to antigen retrieval using sodium citrate buffer (pH 6-6.2). The tissue sections were then blocked in 2.5% BSA for 1 h and immediately incubated in primary antibodies at 4 °C overnight. The primary antibodies used in this study were Cytokeratin (CK)14 (mouse-monoclonal, dilution 1:200, Santa Cruz sc-53253, USA) Subsequently, the tissue samples were incubated in secondary antibody anti-mouse Alexa Flour 488 (A11001, Thermo Fisher, USA) for 45 min at room temperature, The slides were mounted with DAPI (4, 6-diamidino-2-phenylindole; ab104139, Abcam, USA) for nuclear staining.
Quantitative gene expression analysis
The MG tissues were collected and preserved in TRIzol (Invitrogen,15596026) and stored at -80℃ until further use. For total RNA isolation, MG tissues were minced using 15 and 21 no. surgical blades followed by TRIzol RNA isolation protocol as described. The total RNA concentration was quantified by Nanodrop2000 (Thermoscientific, USA) and cDNA was synthesized using 1 µg of total RNA using superscript IV reverse transcriptase kit (18090010, thermoscientific, USA) as per the manufacturer’s protocol. Quantitative real-time polymerase chain reaction (qRT-PCR) was performed in triplicates for specific genes (supplementary table S1); inflammatory marker- CD 4, CD 8, IL-1β; proliferation marker- Ki-67; lipogenic marker- ELOVL, FATP4, PPARG and DyNAmo Color Flash SYBR Green qPCR Kit (F-416 L, Thermo Fisher Scientific, USA) using ABI PRISM®7000 Sequence Detection System (Applied Biosystems, USA). All reactions were performed in technical triplicates to ensure reproducibility and minimize variability. The gene expression was normalized with housekeeping GAPDH gene and the relative fold change was evaluated using the 2^ ΔΔCT.
Results
The study included eighteen male New Zealand white rabbits that weighed between 1.6 and 2.2 kg and aged between 12 and 15 weeks. They were divided into two groups, that underwent CC (n = 9) and EC (n = 9) and were followed up for 7,30 and 90 days (n = 3 in each group) and were sacrificed at the end of follow up period for histological and gene expression study.
Lid margin changes
Lid margin assessment pre and post-op (Fig. 2) revealed that the average LMV in the UL increased from 0 pre-op to 2 ± 0 and 2 ± 0 at D90 in both CC and EC groups respectively (p < 0.001). The average LMI increased from 0 pre-op to 1 ± 0 and 2 ± 0 and LMT increased to 1 ± 0 and 1 ± 0 at D90 in CC and EC group respectively (p < 0.05). The PGO scores increased to 1.3 ± 0.5 and 2 ± 0 at D90 in both CC and EC group (p < 0.001). These changes were not significantly different between CC and EC groups. Greater distortion and irregularity of the lid margin was noted in EC compared to CC group which could partly contribute to tear instability and corneal staining observed in EC models. Figure 3(a-c) and Table 1 depict lid margin changes at various follow ups.
Serial imaging of lid margin alterations: (a) Representative photographic images of rabbits pre and post-op at day 7, 14, 30, 60 and 90 in the chemical cautery group (CC; top row) and electrocautery (EC; bottom row) showing the lid margin changes and progressive closure of meibomian glands orifices by day 90 post-op in both the study groups. Lid margin irregularities were more pronounced in the EC group compared to CC group with few areas of distortion.
Lid margin alterations and tear film metrics: Lid margin vascularity (a), Lid margin irregularity (b), and Plugging of Gland Orifices (c) at pre and post-op in both EC and CC groups show an increase in average score at D30 and D90. Changes in the tear film were assessed by measuring the non-invasive tear breakup time (d) showing a significant decrease from D14 in CC group (p = 0.005) till D90 (p = 0.002) and in the EC group a significant decrease was observed at D30 (p = 0.01) till D90 (p = 0.008). No significant change in tear volume, as assessed by Tear Meniscus Height (e) and Schirmer’s test (f), was observed in either the EC or CC group.
Tear film and ocular surface changes
Average NITBUT changed significantly by D30 (11.43 ± 0.76; p = 0.0006), D60 (9.53 ± 0.40; p = 0.0005) and D90 (9.50 ± 0.37; p = 0.002) in CC group. Similarly, in the EC group, a significant change was observed by D30 (9.73 ± 0.27; p = 0.01), D60 (9.14 ± 0.24; p = 0.008) and D90 (8.34 ± 0.48; p = 0.008) as shown in Fig. 3-d and Table 1. The Schirmer’s test and TMH showed no significant difference compared to pre-op reflecting no significant change in tear volume secreted (Fig. 3-e and -f and Table 1). Corneal staining score showed a significant change in the CC group at D30 (1.17 ± 0.41; p = 0.002), D60 (1.67 ± 0.58; p = 0.019) and D90 (2 ± 0; p = 0.006). In the EC group, a significant change in corneal staining score was observed at D30 (1.17 ± 0.41; p = 0.038), D60 (2.33 ± 0.58; p = 0.006) and D90 (3 ± 0; p = 0.008) as shown in Table 1. Tear film metrics were not significantly different between CC and EC groups during post-op follow-ups.
Meibography changes
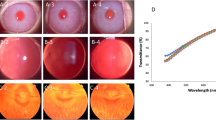
The serial infrared imaging showed structural changes in the MGs from pre-op to post-op in both CC and EC groups appearing as early as Day 30 and increasing by D90 (Fig. 4-a). The average Meiboscore (UL) increased significantly by D30 (1.2 ± 0.4, p < 0.001), D60 (1.7 ± 0.6, p = 0.01) and D90 (2.0 ± 1.0, p = 0.03) in CC group. Similarly in EC group, the score increased by D30 (1.2 ± 0.4, p < 0.001), D60 (1.7 ± 0.6, p < 0.01) and D 90 (1.7 ± 0.6, p < 0.01). There was an increase in partial gland loss scores at D30 (1.5 ± 0.5, p < 0.001), D60 (2 ± 0, p < 0.05) and D90 (2.3 ± 0.6, p = 0.009) in CC and by D30 (1.5 ± 0.5, p < 0.001), D60 (1.7 ± 0.6, p = 0.01) and D90 (2 ± 0, p < 0.001) in EC group. The complete gland dropout scores (UL) were 0.7 ± 1.2 and 1 ± 1 at D90 in CC and EC groups respectively, which were not statistically significant from pre-op. (Fig. 4-b and Table 1). No statistically significant differences were observed on meibography between CC and EC groups postoperatively.
Serial infrared meibography imaging: (a)Representative infrared meibography images of rabbits pre and post-operatively at day 7 to day 90 in the chemical cautery group (CC; top row) and electrocautery (EC; bottom row). Gland dilatation and shortening were observed from day 30 and marked dilatation and complete gland dropouts were observed by day 90 in both groups. (b) Meiboscore, partial gland loss score and gland dropout score (left to right) of the meibomian glands show a significant increase in scores from D30 in both groups indicating morphological changes in the meibomian gland.
Histology and immunohistochemistry examination
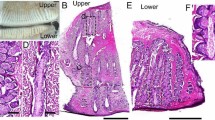
H and E staining revealed duct dilation, central duct obstruction, inspissated secretion and gland blockage in both CC and EC groups at D30 which increased by D90. These changes were more marked in EC group by D30 and similar in both groups at D90 (Fig. 5). The expression of epithelial meibocytes was confirmed by cytokeratin 14 immunostaining which showed decreased expression of CK 14 at D30 and D90 in both the CC and EC groups (Fig. 6-a), confirming the loss of epithelial meibocytes post-operatively in both groups. No reversal of these changes was observed during the experimental period.
Histopathological examination of upper eyelids: Transverse sections of rabbits in control (a & b), chemical cauterization group (at D30 (c-d) and D90 (e-f) and electrocauterization group (at D30 (g & h) and D90 (i & j)). Appearance of structural changes like epithelial hyperplasia, keratinization, duct dilatation (asterisk) with inspissated secretions and atrophy (black arrow) of the adjacent meibomian gland acini appeared by D30 which got established by D90 in both CC and EC groups. (H and E sections, 2x (left) and 10 x (right) magnification). ED = epidermis, D = dermis, MG = meibomian gland, MD = meibomian duct, EF = eyelash follicle. ED marking represents lid margin aspect of the histology section.
Immunohistochemistry of meibomian glands: (a) Expression of cytokeratin 14 (epithelial meibocyte marker) shows reduced expression in both CC and EC group at D30 and D90 post-op. (Magnification; left to right 5x, 20x and 40x) (b) Gene expression analysis of the technical triplicate sample represents increased expression of inflammatory marker (CD4 &CD8) at D7, proliferative marker (Ki67) and lipogenic markers (ELOVL4, FATP4 and PPARG) showing decrease expression at D7 however an increase in expression at D30 is observed.
Gene expression analysis
The gene expression analysis of total RNA from MG was performed in CC and EC groups at D 7, 30 and 90 (n = 1 in each group). A significant fold change increase or decrease in expression of inflammatory marker; CD4 at D7 (p = 0.07 & 0.03), D30 (p = 0.07 & 0.01) & D90 (p = 0.5 & 0.05), CD8 at D7 (p = 0.002 & 0.02), D30 (p = 0.07 & 0.02) & D90 (p = 0.02 & 0.02) and IL-1β at D7 (p = 0.07 & 0.06), D30 (p = 0.03 & 0.06) & D90 (p = 0.07 & 0.06) in CC and EC group respectively. Proliferative marker- Ki67 was significantly decreased at day 7 in CC (p = 0.01) and EC group (p < 0.001), however, a significant fold change increase was observed at D30 (p = 0.01 &0.03) and D90 (p = 0.03 & 0.004) in both CC and EC group respectively. Lipogenic marker ELOVL4, FATP4 and PPARG showed a significant fold change decrease at D7 in CC (p = 0.001) and EC (p = 0.0001) groups with an increase observed at D30 (p = 0.01) and D90 (P = 0.001) in both EC and CC groups (Fig. 6b).
Discussion
This study aims to compare structural and functional alterations induced by two methods, EC and CC, for inducing obstructive MGD in rabbits. Non-invasive tear film assessments, clinical evaluations, meibography scans, and histological analyses, similar to those used in human studies, were employed to monitor changes over a 12-week follow-up period. Clinical evaluations showed a significant increase in LMV and LMI, with noticeable plugging of gland orifices, while tear film metrics showed reduced NITBUT. The ST and TMH remained unchanged from pre-op levels, with both techniques indicating no change in tear volume. Serial meibography imaging revealed gland dilatation and partial and complete gland loss as early as D30 post-op that increased by D90 and correlated with histopathological and immunohistochemical alterations observed. While alterations in meibography scores were similar with both techniques, histological changes developed more gradually in the CC model compared to EC model, suggesting lesser procedure induced acinar damage and more progressive development of changes in CC group, closely simulating an obstructive pathology. While CC offers a simple, effective, and equipment-independent method for generating an obstructive MGD model, EC may enable quicker model generation, albeit with a learning curve.
With the generation of the first rabbit model of MGD by Jester JV et al. in 1982 using topical epinephrine hydrochloride16numerous groups have generated various rabbit MGD models, expanding our current knowledge on MGD10. MGD models have been generated based on two schools of thought for underlying pathophysiology, first being an obstructive mechanism (top-down approach) with hyperkeratinisation induced obstruction of the gland orifice resulting in acinar changes and secondly a bottom-up approach where acinar atrophy and deficiency in stem cell population results in glandular changes17. A surgically induced MGD rabbit model generated by Gilbard et al., using top-down approach in 1989, showed increased tear film osmolarity with closure of MG orifices by 20 weeks18. In 2017, Eom et al., utilized a 5‑MHz high‑frequency electrosurgical device to coagulate MG orifices in the middle two‑thirds of both eyelid margins in rabbits and showed that closure of MG opening lead to duct enlargement resulting in tear film instability19. However, they did not observe morphological changes on meibography during the 8-week study period19. Bu et al. used an alkali exposure similar to the technique used in this study, to induce MGD in rats, but they targeted only the central two thirds of glands in the upper lid alone with a shorter follow up of 30 days20. However, none of the studies used non-invasive assessment of tear film metrics similar to that used in humans. The current study utilised the top-down approach of obstruction of orifices inducing MGD using CC or EC over the entire length of lid margin revealing significant changes even on non-invasive tear film assessments, including reduced NITBUT and increased meiboscore, while TMH remained unchanged.
Non-invasive tear film assessment like NITBUT, TMH and meibography are preferred for tear film evaluation in humans due to the objective nature of assessment21. Tear breakup time has been reported to positively correlate with meibography and lipid layer thickness (LLT) in humans with normal NITBUT and LLT protecting the ocular surface by reducing the tear film evaporation2,22,23. A study by Singh et al. investigated the repeatability of using non- invasive tear film assessment in healthy rabbit eyes and reported a mean NIBUT of 14.3 ± 5.8 and 12.3 ± 5.8 s and mean TMH of 0.43 ± 0.23 and 0.33 ± 0.14 mm in right and left eye respectively. This study also demonstrated good repeatability of these parameters in rabbits using Oculus Keratograph 5M11. However, studies assessing tear film changes using non-invasive imaging in animal models for DED are lacking, highlighting a gap in literature. Utilising non-invasive tear film assessments to establish baseline and disease-induced tear film characteristics in animal models could enhance insights into pathophysiology of DED and ensure uniformity across studies. The current study revealed reduced NITBUT and MG alterations with both techniques, which can serve as surrogate measures for altered tear film stability and LLT24. The changes in tear film metrics and meibography were consistent with changes seen on histopathology and immunohistochemistry.
Histopathological evaluation confirmed changes suggestive of MGD, similar to those reported in various other MGD models, including duct dilation, obstruction of the central ductule, inspissated secretions, and acinar atrophy at day 30 with changes becoming more prominent by day 90 in both the study groups8,25. Additionally, a significant reduction in the CK-14 was also observed, which suggests the loss of acinar cells secondary to gland obstruction post-surgery in both groups. Representative gene expression data of technical triplicates showed increased inflammatory markers (CD4,CD8 and IL-1β) in both groups, and an initial decrease followed by an increase in lipogenic markers, ELOVL4, FATP4, and PPARG. These changes resemble those observed in a decussating DED model, which also showed an initial reduction in lipid synthesis in meibocytes, followed by an increase possibly due to a compensatory response from residual functional acini17. However, the representative sample size is too small to draw meaningful conclusions.
The key strengths of the study include its larger sample size, objective assessment of the tear film using non-invasive metrics, long-term serial follow-up, and a comparative analysis between EC model and CC model. Both chemical cauterization (CC) and electrocauterization (EC) techniques effectively induce features of obstructive meibomian gland dysfunction in rabbits; however, we observed key differences between the two methods. CC is a relatively simple, cost-effective, and widely accessible technique that does not require specialized equipment and may have a faster learning curve. However, care needs to be taken to avoid spillage of the agent onto adjacent structures that may result in superficial tissue damage extending beyond the target glands, potentially introducing variability in inflammation and healing responses.
In contrast, EC enables more localized and controlled damage, leading to consistent gland obstruction. This precision may improve model reproducibility but requires specialized equipment and technical expertise, which may limit its feasibility in resource-limited settings. Additionally, we observed higher degree of lid margin distortion in the EC model, that may impact tear film assessments. These distinctions are critical when selecting a model. While neither method alone captures the full complexity of human MGD pathogenesis, both the CC and EC model offer valuable insights into the changes following gland obstruction and provide a platform for testing potential new therapeutic agents.
One of the limitations to be discussed is that the Harderian glands, reported to contribute to tear film secretions including lipids in rabbits, were not excised in the current model26. However, despite not excising these accessory glands, a reduction in NITBUT was noted likely due to damage to MGs across the entire length of the lid margins, significantly reducing the source of lipids from MGs inducing tear instability. We believe that removal of Harderian glands would further bring down the NITBUT values and increase impact on the ocular surface. In this study, the tear film assessments were performed under general anesthesia which is expected to reduce tear production27. Additionally, the blinks were performed manually before capturing the images on keratography 5 M as the rabbits were anesthetised. However, the objective measurements obtained may still be comparable as similar protocols were followed for both pre and post operative follow ups. Key limitations of the study include the limited availability of rabbit-specific antibodies, restricting molecular validation, and the lack of assessment of contralateral eyes as internal controls for systemic and compensatory effects. Additionally, eyelid fat analysis, Oil Red O staining and lipid tear thickness (LTT) measurements were not performed, which could have further validated model effectiveness.
In conclusion, this study demonstrated the feasibility of using CC and EC to induce obstructive MGD in rabbits, with observed changes in non-invasive tear film assessments, meibography, and histopathology. Using non-invasive tear film assessments in animal studies related to DED can facilitate objective, quantitative, and standardized measurements across studies, similar to those used in humans, enhancing our understanding of the impact of MG alterations on the ocular surface and tear film. This approach can also be used in future studies for evaluating the therapeutic effects of novel interventions and treatments targeting DED.
Statistical analysis
The statistical analysis was performed using GraphPad Prism 10.2.0. Unless otherwise mentioned, all the experiments were conducted in triplicates. Data were analysed in a masked fashion and data are presented as mean ± standard deviation.
Data availability
The datasets used and analysed during the current study available from the corresponding author on reasonable request. All data generated or analysed during this study are included in this published article and its supplementary information files.
Abbreviations
- MGD:
-
Meibomian Gland Dysfunction
- CC:
-
Chemical Cauterization
- EC:
-
Electro Cauterization
- MG:
-
Meibomian Gland
- TMH:
-
Tear Meniscus Height
- NITBUT:
-
Non-Invasive Tear film Break-up Time
- CS:
-
Corneal Staining
- HPE:
-
Histopathological Examination
- EDE:
-
Evaporative dry eye
- DED:
-
Dry Eye Disease
- Pre-op:
-
Pre-operative
- Post-op:
-
Post-operative
- LMV:
-
Lid Margin Vascularity
- LMI:
-
Lid Margin Irregularity
- LMT:
-
Lid Margin Telangiectasia
- UL:
-
Upper Lid
- LL:
-
Lower Lid
- PGO:
-
Plugging of Gland Orifices
- CS:
-
Corneal Staining
- ST:
-
Schirmer’s Test
References
Nelson, J. D. et al. The International Workshop on Meibomian Gland Dysfunction: Report of the Definition and Classification Subcommittee. 52, 1930–1937 (2011).
Bron, A. J., Tiffany, J. M., Gouveia, S. M. & Yokoi, N. Voon, L. W. Functional aspects of the tear film lipid layer. Exp. Eye Res. 78, 347–360 (2004).
Stapleton, F. et al. The ocular surface TFOS DEWS II epidemiology report. Ocular Surf. 15, 334–365 (2017).
Donthineni, P. R. et al. Incidence, demographics, types and risk factors of dry eye disease in india: electronic medical records driven big data analytics report I. Ocular Surface 17, 250-256 (2019).
Moon, J. H., Kim, K. W. & Moon, N. J. Smartphone use is a risk factor for pediatric dry eye disease according to region and age: a case control study. BMC Ophthalmol 16, 118 (2016).
Uchino, M. et al. Prevalence of dry eye disease and its risk factors in visual display terminal users: the Osaka study. Am J. Ophthalmol 156, 759-766 (2013).
Sun, M., Moreno, I. Y., Dang, M. & Coulson-Thomas, V. J. Meibomian gland dysfunction: what have animal models taught us?? Int J. Mol. Sci 21, 8822 (2020).
Han, J. Y. et al. Safety and efficacy of a low-level radiofrequency thermal treatment in an animal model of obstructive meibomian gland dysfunction. Lasers Med. Sci. 37, 2907–2915 (2022).
Knop, E., Knop, N., Millar, T., Obata, H. & Sullivan, D. A. The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Invest. Ophthalmol. Vis. Sci. 52, 1938–1978 (2011).
Prasad, D. et al. A review of rabbit models of meibomian gland dysfunction and scope for translational research. Indian J. Ophthalmol. 71, 1227–1236 (2023).
Singh, S. et al. Repeatability of Non-invasive tear film evaluation in healthy rabbit eyes. Curr. Eye Res. https://doi.org/10.1080/02713683.2023.2200915 (2023).
Tulane University. (n.d.). Institutional Animal Care & Use Committee Office. https://research.tulane.edu/iacuc
Arita, R. et al. Development of definitive and reliable grading scales for meibomian gland dysfunction. Am. J. Ophthalmol. 169, 125–137 (2016).
Report of the National Eye Institute/Industry workshop on Clinical Trials in Dry. Eyes - PubMed. https://pubmed.ncbi.nlm.nih.gov/8565190/
Arita, R., Itoh, K., Inoue, K. & Amano, S. Noncontact infrared meibography to document age-related changes of the meibomian glands in a normal population. Ophthalmology 115, 911–915 (2008).
Jester, J. V. et al. In vivo biomicroscopy and photography of meibomian glands in a rabbit model of meibomian gland dysfunction. Invest Ophthalmol. Vis. Sci 22, 660-667 (1982).
Jester, J. V., Parfitt, G. J. & Brown, D. J. Meibomian gland dysfunction: hyperkeratinization or atrophy? BMC Ophthalmol. 15, 156 (2015).
Gilbard, J. P., Rossi, S. R. & Gray, K. L. A new rabbit model for keratoconjunctivitis Sicca. Invest. Ophthalmol. Vis. Sci. 28, 225–228 (1987).
Eom, Y. et al. Meibomian glands and ocular surface changes after closure of meibomian gland orifices in rabbits. Cornea 37, 218-226 (2018).
Bu, J. et al. Transitory alkali exposure on meibomian gland orifices induces meibomian gland dysfunction. Ocul Surf. 29, 406–415 (2023).
Wolffsohn, J. S. et al. The ocular surface TFOS DEWS II diagnostic methodology report. Ocular Surf. 15, 539–574 (2017).
Bron, A. J., Benjamin, L. & Snibson, G. R. Meibomian gland disease. Classification and grading of lid changes. Eye (Basingstoke) 5, 395-411 (1991).
Bron, A. J. & Tiffany, J. M. The meibomian glands and tear film lipids: structure, function, and control. Adv Exp. Med. Biol 438, 281-295 (1998).
Mizoguchi, S. et al. Ocular surface alkali injury damages meibomian glands in mice. Ocul Surf. 15, 713–722 (2017).
Miyake, H., Oda, T., Katsuta, O., Seno, M. & Nakamura, M. A novel model of meibomian gland dysfunction induced with complete freund’s adjuvant in rabbits. Vision (Switzerland) 1, 10 (2017).
Polans, A. S. et al. Pilot study of a ‘Large-Eye,’ surgically induced dry eye rabbit model by selective removal of the harderian, lacrimal, and meibomian glands. Ocul Oncol. Pathol. 4, 52–56 (2017).
Cross, D. A. & Krupin, T. Implications of the effects of general anesthesia on basal tear production. Anesth. Analg. 56, 35–37 (1977).
Acknowledgements
The authors would like to thank Anirudh Reddy and Mohd Salman for his help with animal handling for this study, Tirupathi Rao, Krothapalli Ravindranath Ophthalmic Research Biorepository, L V Prasad Eye Institute for providing histology sections and Centre for Cellular and Molecular Biology (CCMB) for providing the Animal House facility to conduct the study.
Funding
This work was funded by the Hyderabad Eye Research Foundation, Hyderabad, India. Financial support has also been received from the Indian Council of Medical Research (ICMR) for Senior Research Fellowship to DP (2021– 12785).
Author information
Authors and Affiliations
Contributions
D.P- Data curation, Formal analysis, Writing – original draft, Writing – review and editing. S.J – Resources, Formal analysis, Validation. K.K.B– Resources, Project administration, Writing – review and editing. S.B – Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review and editing. V.S – Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review and editing. P.R.D– Conceptualization, Formal analysis, Investigation, Methodology, Supervision, Validation, Writing – review and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
There are no conflicts of interest.
Disclosures
The authors presented a part of this work at the Association for Research in Vision and Ophthalmology (ARVO) annual meeting in April 2023, held in New Orleans, LA, USA. The corresponding abstract was published in the Investigative Ophthalmology & Visual Science (IOVS) journal as an ARVO annual meeting abstract. Additionally, a segment of this work was presented at the ARVO annual meeting in May 2024, in Seattle, USA. The abstract was published in IOVS as an ARVO annual meeting abstract.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 2
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Prasad, D., Jakati, S., Bokara, K.K. et al. Comparative assessment of structural and tear film alterations in rabbit meibomian gland dysfunction models using chemical and electrocauterization techniques. Sci Rep 15, 23494 (2025). https://doi.org/10.1038/s41598-025-06923-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-06923-9