Abstract
Metabolic syndrome (MetS) is a major risk factor for cardiovascular and chronic diseases. This study evaluated how genetic predisposition and lifestyle factors contribute to MetS risk. We analysed data from 4,987 Koreans aged ≥ 40 years with at least one ASCVD risk factor (2014–2017). A polygenic risk score for metabolic disease (PRSMetS) using eight metabolic phenotypes was used to categorise participants into high and low PRSMetS groups (top 7% vs. bottom 93%). Lifestyle factors, including obesity, physical activity, smoking, diet, and alcohol consumption, were assessed with a healthy lifestyle score. The associations of PRSMetS and lifestyle factors with MetS risk were evaluated. High PRSMetS was associated with a 1.31-fold higher risk of MetS (adjusted odds ratio [aOR], 1.31; 95% confidence interval [CI], 1.03–1.66). MetS prevalence increased stepwise with worsening lifestyle in both low and high PRSMetS groups. Compared to the ‘low PRSMetS and ideal lifestyle’ group, MetS risk was significantly higher in all other lifestyle groups, with the highest risk observed in the ‘high PRSMetS and poor lifestyle’ (aOR, 9.35; 95% CI, 4.41–19.83) group. Both genetic predisposition and lifestyle factors significantly influenced MetS risk. Maintaining healthy lifestyle habits mitigates MetS risk, even in individuals with high genetic susceptibility.
Similar content being viewed by others
Introduction
Metabolic syndrome (MetS) comprises a cluster of conditions, including abdominal obesity, insulin resistance, dyslipidaemia, and hypertension, all of which increase cardiovascular disease risk1,2,3. Globally, MetS affects approximately 25% of adults4. In South Korea, the prevalence of MetS increased from 28.8% in 2009 to 30.5% in 20135, imposing a substantial burden on healthcare systems6. Thus, early prevention and management of MetS are crucial.
Genome-wide association studies (GWAS) have identified genetic variants contributing to metabolic disorders7including variants in APOA5 (triglycerides), FTO (obesity), and TCF7L2 (glucose regulation)8,9. Polygenic risk scores (PRS) calculated from GWAS summary statistics provide predictive insights into disease susceptibility10and have demonstrated associations with elevated risks of MetS and related cardiovascular outcomes with hazard ratios of 1.1 to 1.2 per standard deviation increase in PRS11. However, the variance in MetS risk explained by PRSs remains relatively modest, up to 5.9% in large-scale studies11,12. This underscores the importance of considering non-genetic factors in understanding and managing MetS risk. Modifiable lifestyle factors, including obesity, physical inactivity, smoking, and unhealthy diets, also contribute significantly to MetS risk, and evidence suggests that targeted lifestyle interventions can substantially reduce these risks13,14,15,16.
Several studies in European populations have shown that individuals with high PRS can reduce their risk of developing MetS and cardiovascular disease through healthy lifestyle behaviours17,18,19. However, data on the interplay between genetic predisposition and lifestyle factors in the development of MetS remain limited in East Asian populations. Therefore, this study aimed to investigate the association between a PRS for metabolic disease (PRSMetS) and MetS risk and to examine the impact of lifestyle factors on the risk of MetS across PRSMetS groups in a Korean general population; thereby, providing evidence to support the importance of integrating genetic risk assessments with lifestyle management in the prevention of MetS.
Methods
Study population
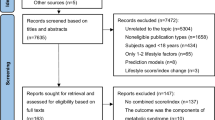
This study utilised data from the Gene-ENvironmental Interaction and phenotypE (GENIE) cohort. This cohort comprised participants who underwent self-referred health check-ups between 2014 and 201720. The GENIE cohort is a prospective subcohort of the Health and Prevention Enhancement (H-PEACE) study20 conducted at the Seoul National University Hospital Healthcare System Gangnam Center. The GENIE cohort specifically includes individuals who provided additional blood samples for genome-wide genotyping between 2014 and 2017. Of the 10,342 people whose GWAS information was first analysed, 8,790 were aged 40 years or older. Among them, 5,110 participants had at least one ASCVD risk factor (old age [≥ 60 years], hypertension, hyperlipidaemia, diabetes mellitus, chronic kidney disease, or current smoking). After excluding participants ≥ 80 years (n = 13) and those who did not have results regarding essential laboratory tests (n = 110), a total of 4,987 individuals without prior history of ASCVD events were included in the final analysis.
The study protocol conformed to the ethical guidelines outlined in the Declaration of Helsinki. The institutional review board of our institution approved the GENIE cohort (H-1103-127-357) and this study (H-2305-071-1432). Informed consent for blood storage was obtained during blood sample collection. Waiver of informed consent for this specific study was granted due to its retrospective design.
Evaluation of metabolic syndrome
MetS was determined using standardised criteria established by the International Diabetes Federation and the American Heart Association/National Heart, Lung, and Blood Institute21along with modified WC criteria from the Korean Society for the Study of Obesity22 and specific cut-off values for abdominal obesity in the Asian population21. A diagnosis of MetS was confirmed if an individual exhibited three or more of the following characteristics: an increased WC (≥ 90 cm for men or ≥ 85 cm for women), high BP (≥ 130/85 mmHg or the use of antihypertensive medications), impaired fasting glucose (≥ 100 mg/dL [5.6 mmol/L] or the use of antidiabetic medications), elevated triglyceride levels (≥ 150 mg/dL [1.7 mmol/L] or the use of medications for high triglycerides), and low HDL cholesterol (< 40 mg/dL [1.0 mmol/L] for men or < 50 mg/dL [1.3 mmol/L] for women). MetS burden was defined as the count of MetS components meeting these criteria, ranging from 0 to 5.
Definition of variables
Information on sex, age, physical activity (PA), smoking, alcohol consumption, and diseases was collected through standardised self-reported questionnaires. Anthropometric measurements and test results were collected during health examinations. Trained nurses measured height, weight, waist circumference (WC), and blood pressure (BP). Body mass index (BMI) was calculated by dividing the weight in kilogrammes by the square of the height in metres (kg/m²) and was categorised into < 23 (normal), 23–25 (overweight), and ≥ 25 kg/m² (obese) according to the Asia-Pacific obesity guidelines23. WC was assessed at the midpoint between the lower rib margin and the iliac crest. PA was assessed based on the amount of PA calculated using the short-form International Physical Activity Questionnaire response results24. PA was categorised as inactivity for less than 600 metabolic equivalent of task (MET) min per week, minimally active for 600 MET min to less than 3000 MET min per week, and health-enhancing PA for more than 3000 MET min per week. Smoking status was classified as non-smoker, ex-smoker, and current smoker25. Alcohol consumption was categorised into 14 drinks or less per week and more than 14 drinks per week, according to the US National Institute on Alcohol Abuse and Alcoholism (NIAAA) guideline26. A standard drink contains approximately 14 g (0.6 fluid ounces) of pure alcohol. Diabetes mellitus was defined as fasting glucose ≥ 126 mg/dL or haemoglobin A1c (HbA1c) ≥ 6.5% or treatment with an oral hypoglycaemic agent or insulin. Hypertension was described as systolic BP ≥ 140 mmHg or diastolic BP ≥ 90 mmHg or the use of antihypertensive medications. Hyperlipidaemia was described as low-density lipoprotein (LDL) cholesterol ≥ 160 mg/dL or the use of statins. Chronic kidney disease was defined as an estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m². Fasting glucose, triglycerides, total cholesterol, high-density lipoprotein (HDL) cholesterol, LDL cholesterol, eGFR, HbA1c, and high sensitivity C-reactive protein were evaluated on the day of the health examination.
Evaluation of lifestyle risk factor
To assess lifestyle factors, we considered five components: obesity (BMI ≥ 25 [obese], 23–25 [overweight], < 23 kg/m² [normal]), PA (inactive, minimally active, and health-enhancing PA), smoking (current smoker, ex-smoker, and non-smoker), diet (poor diet, intermediate diet, and ideal diet), and alcohol consumption (> 14 drinks per week, ≤ 14 drinks per week). Each component was assigned a score ranging from 0 to 2 points based on predefined criteria (Supplementary Table S1). Ideal scores were defined as: (1) not overweight, (2) regular PA, (3) no current smoking, (4) ideal diet, and (5) moderate alcohol consumption27. A healthy lifestyle score, representing the sum of scores from these five components, ranged from 0 to 9. Scores were categorised into three groups: 6–9 points for an ideal lifestyle, 3–5 points for an intermediate lifestyle, and 0–2 points for a poor lifestyle.
The evaluation of diet quality relied on a questionnaire developed by the Seoul National University Hospital Healthcare System Gangnam Centre. Components for the dietary evaluation index were derived from the 2020 Korean Nutrient Intake Standards and the dietary composition of Life’s Simple 7 (LS7)28,29. The evaluation included 12 food groups associated with MetS in the Korean population: grains; meat, fish, eggs, and beans; vegetables; fruits; milk and dairy products; fried foods, fat-rich meats; instant and processed foods; salty foods; sweets; beverages; and salt/soy sauce30. Scoring criteria for each component were established based on recommended dietary patterns for Korean adults (Supplementary Table S2). The recommended dietary pattern provides guidelines for intake frequency across different energy levels, ranging from 1,000 to 2,700 kcal, with intake recommendations based on estimated energy requirements. Diet quality was categorised into three levels according to adherence to ideal dietary recommendations: ideal (5 points), intermediate (3 points), and poor (1 point). The total score, calculated by summing the points for each item, had a maximum of 60 points, with higher scores indicating better diet quality. Dietary habits were assessed using a dietary score developed and validated against 24-hour recall nutrient intake data from a subset of 1,624 participants. Participants with higher dietary scores consumed greater amounts of vegetable protein, vegetable fat, and n-3 fatty acids compared to those with lower scores. Based on the total dietary score, participants were classified into three groups: poor (≤ 40 points), intermediate (40 < points < 50), and ideal (≥ 50 points). Details of the scoring criteria and validation results are provided in Supplementary Table S3.
Calculation of the genetically predicted metabolic risk score
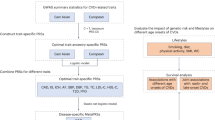
The PRSMetS was calculated as previously described31. We constructed PRSs for eight quantifiable metabolic phenotypes—systolic/diastolic BP, BMI, four blood lipid components, and fasting blood glucose—based on GWAS conducted in two prospective Korean cohorts: the KoGES_Health EXAminee (KoGES_HEXA) cohort from 2006 to 2012 and the KoGES_AnSan and AnSung (KoGES_ASAS) cohort from 2001 to 2003 (n = 37,285). The KoGES cohorts, part of the National Biobank of Korea, is prospective studies that collected comprehensive phenotypic measures and biological samples including genetic data18. Participants were recruited from the national health examinee registry with an age of ≥ 40 at baseline. Anthropometric and clinical data were collected through physical exams, laboratory tests, and interviews about disease status. There is no overlap between participants in the KoGES_HEXA and KoGES_ASAS cohorts (discovery cohorts) and the GENIE cohort (target cohort).
Individuals in the highest PRS quintile demonstrated a 1.4–2.0-fold increased risk of incident hypertension, obesity, hyperlipidaemia, and diabetes. A grid search was conducted to optimise the weighted score of metabolic PRSs for incident ASCVD (PRSMetS). The highest score group was defined as the risk group with a PRSMetS greater than or equal to the seventh percentile of the analysis population. Using the PRSMetS, the high-risk group demonstrated a 3.3-fold (95% confidence interval [CI] 1.7–6.1, p < 0.001) higher risk of incident ASCVD.
Statistical analysis
Frequency and percentage were used to represent categorical variables, whereas continuous variables were expressed as mean and standard deviation. The Chi-square test was used for categorical variables across PRSMetS groups, and the t-test was applied for continuous variables. Multiple logistic regression analysis was employed to evaluate the association between the PRSMetS group and MetS and to compare the risk of MetS across different lifestyle groups within total, low, and high PRSMetS groups. Adjusted odds ratios (ORs) and 95% CIs were reported after adjusting for variables including sex, age, and LDL cholesterol. All statistical analyses were performed using SAS 9.4 statistical software (SAS Institute Inc., Cary, NC, USA). Statistical significance was set at p < 0.05.
Results
General characteristics according to PRSMetS
The mean age of participants was 55.5 ± 8.3 years, with 72.8% being men. Among the participants, 13.8% had diabetes, and 5.4% were on antidiabetic medications. Hypertension was present in 34.8% of participants, with 21.5% using antihypertensive medications. Hyperlipidaemia was observed in 29.6%, with 14.9% taking statins. Chronic kidney disease was reported by 2.0% of participants. The majority had a healthy lifestyle score between 3 and 5 (18.7%, 21.1%, and 21.5%, respectively), whereas scores of 0 (0.5%) or 9 (0.5%) were rare (Table 1).
The prevalence of antihypertensive medication use, hyperlipidaemia, and statin use was significantly higher in the high PRSMetS group compared to the low PRSMetS group. Triglycerides, total cholesterol, and LDL cholesterol were also higher in the high PRSMetS group. Although overall lifestyle risk factors did not differ significantly between groups, the high PRSMetS group showed a higher proportion of intermediate lifestyle scores. Lifestyle patterns also differed depending on the MetS status (Supplementary Table S4). The MetS group had higher rates of obesity, heavy drinking, smoking, poor diets, and lower mean healthy lifestyle scores.
Metabolic syndrome according to PRSMetS and lifestyle
Table 2 summarizes MetS prevalence and its individual components by PRSMetS group. Although MetS was more prevalent in the high PRSMetS group, the difference was not statistically significant (37.3% vs. 32.2%, p = 0.057). Within the components of MetS, the high PRSMetS group showed a significantly higher prevalence of elevated triglycerides compared to the low PRSMetS group (46.5% vs. 36.3%, p = 0.0003). Additionally, the high PRSMetS group had a significantly greater proportion of individuals with multiple MetS components (p = 0.025). MetS prevalence increased significantly with poorer lifestyles in both the low PRSMetS group (p < 0.0001) and the high PRSMetS group (p = 0.004) (Fig. 1; Supplementary Tables S5 and S6).
Risk of metabolic syndrome according to PRSMetS
Adjusted ORs and 95% CIs for the risk of MetS according to PRSMetS groups are detailed in Table 3. In unadjusted analysis, the high PRSMetS group had a 1.26 times higher risk of MetS than the low PRSMetS group, though this association was not statistically significant. However, after adjustment for sex, age and LDL cholesterol (model 2), the high PRSMetS group demonstrated a significantly higher risk of MetS than the low PRSMetS group (OR, 1.31; 95% CI, 1.03–1.66).
Risk of metabolic syndrome according to PRSMetS and lifestyle
Table 3and Fig. 2 present the adjusted ORs and 95% CIs for MetS risk by PRSMetS and lifestyle factors. After adjustment for sex, age and LDL cholesterol (model 2), compared to the ‘low PRSMetS and ideal lifestyle’ group, the risk of MetS was significantly higher in the ‘low PRSMetS and intermediate lifestyle’ (OR, 3.09; 95% CI, 2.55–3.74) and ‘low PRSMetS and poor lifestyle’ groups (OR, 7.47; 95% CI, 5.85–9.53). Using the ‘low PRSMetS and ideal lifestyle’ group as a reference, the ‘high PRSMetS and ideal lifestyle’ group also showed an elevated risk (OR, 1.88; 95% CI, 1.07–3.32), with even greater risks in the ‘high PRSMetS and intermediate lifestyle’ (OR, 4.25; 95% CI, 3.04–5.94) and ‘high PRSMetS and poor lifestyle’ groups (OR, 9.35; 95% CI, 4.41–19.83). Furthermore, an ideal or intermediate lifestyle with high PRSMetS was associated with significantly reduced MetS risk compared to a poor lifestyle with low PRSMetS. Notably, ideal and intermediate lifestyles in the high PRSMetS group reduced MetS risk by 75% (OR, 0.25; 95% CI, 0.14–0.45) and 43% (OR, 0.57; 95% CI, 0.41–0.79), respectively (p < 0.0001), compared to having a poor lifestyle with low PRSMetS.
Bar plot of odds ratios (ORs) and 95% confidence intervals (CIs) for metabolic syndrome risk by PRSMetS and lifestyle categories. Healthy lifestyle scores were categorised as follows: 6–9 points for an ideal lifestyle, 3–5 points for an intermediate lifestyle, and 0–2 points for a poor lifestyle. ORs and 95% CIs were calculated using multiple logistic regression analysis, adjusted for sex, age, and low-density lipoprotein cholesterol levels. PRSMetS, polygenic risk score for metabolic disease.
In each of the PRSMetS groups (Supplementary Tables S7 and S8; Fig. S1), the ‘intermediate lifestyle’ (OR, 3.10; 95% CI, 2.56–3.76) and ‘poor lifestyle’ groups (OR, 7.52; 95% CI, 5.89–9.61) in the low PRSMetS group showed a higher risk of MetS than the ‘low PRSMetS and ideal lifestyle’ group. Similarly, in the high PRSMetS group, the risk of MetS was higher in the ‘intermediate lifestyle’ (OR, 2.20; 95% CI, 1.13–4.31) and ‘poor lifestyle’ groups (OR, 4.52; 95% CI, 1.66–12.29) compared to the ‘high PRSMetS and ideal lifestyle’ group after adjustment for sex, age and LDL cholesterol.
Discussion
This study explored the association between PRSMetS and the risk of MetS and investigated the impact of lifestyle risk factors on MetS risk in the Korean general population. The main findings were as follows: (i) individuals with high PRSMetS had a 1.31-fold increased risk of MetS; (ii) regardless of PRSMetS level, those with poorer lifestyles showed a higher prevalence of MetS in a dose-dependent manner; and (iii) individuals with high PRSMetS who maintained ideal or intermediate lifestyles had a lower MetS risk compared to those with low PRSMetS and poor lifestyles. These results highlight the significant impact of both genetic predisposition and lifestyle on MetS risk, underscoring the importance of maintaining an ideal lifestyle for MetS prevention, even in those with high genetic susceptibility among Korean adults.
MetS is a complex metabolic disorder characterised by interrelated physiological, biochemical, and clinical factors, such as insulin resistance, visceral fat accumulation, endothelial dysfunction, and dyslipidaemia32. The prevalence of MetS has risen from 27.1% in 2001 to 33.2% in 202033. This has been attributed to a combination of clinical factors and lifestyle changes34. A study in Australian adults linked a Western dietary pattern (rich in meat, refined grains, and processed foods) to higher MetS risk, insulin resistance, abnormal β-cell function, and reduced insulin sensitivity. In contrast, a healthy dietary pattern (rich in fruits, vegetables, and whole grains) showed protective effects35. The Western diet al.so promotes pro-inflammatory cytokines36causing inflammation that leads to LDL accumulation in arterial walls and endothelial dysfunction, a major factor in cardiovascular diseases37. Alcohol intake also exacerbates MetS by raising triglycerides and blood pressure38. Conversely, PA has demonstrated a protective effect against MetS. Korean adults who engaged in moderate PA, walking, or flexibility exercises had significantly lower MetS prevalence than those who were inactive39. Regular exercise reduces body fat, increases muscle mass, and improves insulin sensitivity, metabolic health, and vascular function; thus, lowering the risks of cardiovascular and cerebrovascular diseases40. Overall, the roles of lifestyle factors, including diet, alcohol, and PA, are crucial in the risk of developing MetS.
Evidence underscores the importance of adopting healthy lifestyle habits to lower mortality rates and enhance metabolic and cardiovascular health outcomes. Among patients with ischemic heart disease, smoking was associated with elevated mortality rates, whereas vigorous PA significantly reduced mortality risk. Adherence to the AHA Step 2 diet was associated with lower mortality rates41. In this regard, the AHA proposed LS7 in 2010, defining ideal cardiovascular health based on seven modifiable risk factors through lifestyle changes23,42. This framework was continuously refined and, in 2022, expanded into Life’s Essential 8, comprising diet, PA, nicotine exposure, sleep health, BMI, blood lipids, blood glucose, and BP43. Several studies have confirmed a strong and stepwise inverse association between these metrics and total cardiovascular disease, cardiovascular mortality, and all-cause mortality43. Assessing cardiovascular risks, including MetS, and implementing active management strategies in adults remain essential, as lifestyle modifications significantly influence the prevention and management of MetS and cardiovascular diseases.
PRS, derived from the genome-wide association study data, evaluates disease risk based on genetic variants within an individual’s genome44. Higher scores generally indicate an elevated risk44. For coronary artery disease, combining multiple genomic risk scores (GRS) into a metaGRS significantly enhances risk prediction compared to individual GRS45,46. The metaGRS also achieved a superior predictive ability compared to traditional risk factors like BMI, diabetes, hypertension, smoking, family history of heart disease, and high cholesterol46. These findings highlight the value of integrating metaGRS with traditional risk factors for improved prediction. Building on this concept, the present study utilised the PRSMetS that incorporates individual components of the genetic predisposition to various metabolic diseases.
Recent studies have reported the utility of integrating genetic risk with lifestyle interventions. In American adults, individuals with high PRS and ideal LS7 scores lived 20.2 years longer without coronary heart disease than those with low LS7 scores28. Khera et al. demonstrated that low genetic risk paired with unhealthy lifestyles led to higher coronary event rates than healthy lifestyles17. Consistent with these findings, our study showed that among individuals with high PRSMetS, lifestyle factors substantially modified the risk of metabolic syndrome. Specifically, individuals with a poor lifestyle in the high PRSMetS group had a 4.52-fold higher risk of MetS (95% CI: 1.66–12.29) compared to those with an ideal lifestyle in the same genetic risk group. Notably, in the low PRSMetS group, poor lifestyle habits were also associated with a 7.52-fold increased risk of MetS (95% CI: 5.89–9.61). These findings highlight that lifestyle factors can strongly influence MetS risk, regardless of an individual’s genetic risk level.
Our study used the PRSMetS for eight individual metabolic phenotypes, designed to optimally predict ASCVD31. Individuals with high PRSMetS had a 1.31-fold increased risk of MetS (95% CI: 1.03–1.66), consistent with previous reports showing hazard ratios of approximately 1.10–1.20 per standard deviation increase in PRSMetS28. Importantly, an ideal or intermediate lifestyle in the high PRSMetS group reduced MetS risk by 75% and 43%, respectively, compared to having a poor lifestyle with low PRSMetS. This suggests that, while genetic risk contributes to MetS susceptibility, its effects can be substantially mitigated by maintaining healthy lifestyle habits. Using PRSMetS to assess genetic risk, alongside promoting regular PA, smoking cessation, and healthy eating, is expected to reduce both MetS and ASCVD risks. These findings underscore the importance of integrating genetic risk assessments with lifestyle interventions in clinical and public health strategies for MetS prevention, providing valuable insights for personalised health strategies and public health policy development. It also suggests that while genetic risk is an important consideration, public health interventions should prioritize modifiable lifestyle factors to reduce MetS risk. Clinicians should emphasize dietary counseling, increased physical activity, and smoking cessation, even in individuals with high genetic risk.
This study had several limitations. Firstly, the data from the GENIE cohort were collected through a cross-sectional study conducted within a limited time frame. Although associations were identified, determining causal relationships was not possible. Secondly, this study relied on self-reported data, which could have introduced inaccuracies in measuring lifestyle risk factors. Thirdly, the thresholds for lifestyle risk factors were based on the criteria adopted by the GENIE cohort, potentially limiting the generalisability of the findings to other settings or populations. Fourthly, although the present study was conducted in a general population setting, our cohort included individuals with at least one ASCVD risk factor. Therefore, caution is warranted when extrapolating these findings to populations without ASCVD risk factors or younger age groups. Fifthly, a potential collider bias could have been introduced by selecting individuals with ASCVD risk factors. Therefore, our findings should be validated in future studies using general population cohorts without ASCVD risk factors to confirm the robustness and generalizability of our results. Finally, certain indicators were difficult to measure as continuous values. Given the nonlinear relationship between some indicators and health, applying a ranking score system for each indicator, such as a scoring method ranging from 0 to 9 with categories like ideal, intermediate, and poor, would be more appropriate43. Although somewhat subjective, this method is considered effective for capturing individual differences and tracking changes in cardiovascular health over time43.
In conclusion, this cohort study of Korean adults suggested that individuals with high PRSMetS were associated with an increased risk of MetS. However, maintaining healthy lifestyle habits, including regular PA, a healthy diet, and smoking cessation, reduced the risk of MetS, even among individuals with high PRSMetS. These findings highlight the need for personalised health strategies that utilise PRSMetS to identify high-risk groups for MetS and alleviate the impact of genetic predisposition on MetS development.
Data availability
The data underlying this article will be shared upon reasonable request to the corresponding author.
References
Mohamed, S. M. et al. Metabolic syndrome: risk factors, diagnosis, pathogenesis, and management with natural approaches. Food Chem. Adv. 3, 100335 (2023).
Tian, X. et al. Insulin resistance mediates obesity-related risk of cardiovascular disease: a prospective cohort study. Cardiovasc. Diabetol. 21 (1), 289 (2022).
Wang, M. C. & Lloyd-Jones, D. M. Cardiovascular risk assessment in hypertensive patients. Am. J. Hypertens. 34 (6), 569–577 (2021).
O’Neill, S. & O’Driscoll, L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes. Rev. 16 (1), 1–12 (2015).
Lee, S. E. et al. Trends in the prevalence of metabolic syndrome and its components in South korea: findings from the Korean National health insurance service database (2009–2013). PLoS One 13(3), e0194490 (2018).
Chong, K. S. et al. Longitudinal economic burden of incident complications among metabolic syndrome populations. Cardiovasc. Diabetol. 23 (1), 246 (2024).
Fesinmeyer, M. D. et al. Genetic variants associated with fasting glucose and insulin concentrations in an ethnically diverse population: results from the population architecture using genomics and epidemiology (PAGE) study. BMC Med. Genet. 14, 98 (2013).
Willer, C. J. et al. Discovery and refinement of loci associated with lipid levels. Nat. Genet. 45 (11), 1274–1283 (2013).
Scott, R. A. et al. Large-scale association analyses identify new loci influencing glycemic traits and provide insight into the underlying biological pathways. Nat. Genet. 44 (9), 991–1005 (2012).
Lewis, C. M. & Vassos, E. Polygenic risk scores: from research tools to clinical instruments. Genome Med. 12, 44 (2020).
Park, S. et al. Multivariate genomic analysis of 5 million people elucidates the genetic architecture of shared components of the metabolic syndrome. Nat. Genet. 56 (11), 2380–2391 (2024).
van Walree, E. S. et al. Disentangling genetic risks for metabolic syndrome. Diabetes 71 (11), 2447–2457 (2022).
Park, S. et al. Smoking, development of or recovery from metabolic syndrome, and major adverse cardiovascular events: a nationwide population-based cohort study including 6 million people. PLoS One 16(1), e0241623 (2021).
Rodríguez-Monforte, M., Sánchez, E., Barrio, F., Costa, B. & Flores-Mateo, G. Metabolic syndrome and dietary patterns: a systematic review and meta-analysis of observational studies. Eur. J. Nutr. 56 (3), 925–947 (2017).
Suliga, E., Ciesla, E., Lelonek, M., Piechowska, A. & Gluszek, S. Lifestyle elements and risk of metabolic syndrome in adults. PLoS One 17(9), e0275510 (2022).
Zhang, M. et al. How do lifestyle factors modify the association between genetic predisposition and obesity-related phenotypes? A 4-way decomposition analysis using UK biobank. BMC Med. 22 (1), 230 (2024).
Khera, A. V. et al. Genetic risk, adherence to a healthy lifestyle, and coronary disease. N Engl. J. Med. 375 (24), 2349–2358 (2016).
Kim, Y., Han, B. G. & KoGES Group. Cohort profile: the Korean genome and epidemiology study (KoGES) consortium. Int. J. Epidemiol. 46(2), e20 (2017).
Yun, J. S. et al. Polygenic risk for type 2 diabetes, lifestyle, metabolic health, and cardiovascular disease: a prospective UK biobank study. Cardiovasc. Diabetol. 21 (1), 131 (2022).
Lee, C. et al. Health and prevention enhancement (H-PEACE): a retrospective, population-based cohort study conducted at the Seoul National university hospital Gangnam center, Korea. BMJ Open. 8(4), e019327 (2018).
Alberti, K. G., the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association. & et al Harmonizing the metabolic syndrome: a joint interim statement of. Circulation 120 (16), 1640–1645 (2009). World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity.
Seo, M. H. et al. 2018 Korean society for the study of obesity guideline for the management of obesity in Korea. J. Obes. Metab. Syndr. 28 (1), 40–45 (2019).
WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 363 (9403), 157–163 (2004).
Oh, J. Y., Yang, Y. J., Kim, B. S. & Kang, J. H. Validity and reliability of Korean version of international physical activity questionnaire (IPAQ) short form. Korean J. Fam Med. 28 (7), 532–541 (2007).
Sohn, S. & Savova, G. K. Mayo clinic smoking status classification system: extensions and improvements. AMIA Annu. Symp. Proc. 619–623 (2009). (2009).
National Institute on Alcohol Abuse and Alcoholism. What is a standard drink? (2024). https://www.niaaa.nih.gov/alcohols-effects-health/what-standard-drink
Lloyd-Jones, D. M. et al. Defining and setting National goals for cardiovascular health promotion and disease reduction: the American heart association’s strategic impact goal through 2020 and beyond. Circulation 121 (4), 586–613 (2010).
Hasbani, N. R. et al. American heart association’s life’s simple 7: lifestyle recommendations, polygenic risk, and lifetime risk of coronary heart disease. Circulation 145 (11), 808–818 (2022).
Ministry of Health and Welfare & The Korean Nutrition Society. Dietary reference intakes for Koreans 2020. Ministry Health Welf. (2020). https://www.kns.or.kr/FileRoom/FileRoom_view.asp?idx=108&BoardID=Kdr
Baik, I., Lee, M., Jun, N. R., Lee, J. Y. & Shin, C. A healthy dietary pattern consisting of a variety of food choices is inversely associated with the development of metabolic syndrome. Nutr. Res. Pract. 7 (3), 233–241 (2013).
Song, H. et al. Prediction of incident atherosclerotic cardiovascular disease with polygenic risk of metabolic disease: analysis of 3 prospective cohort studies in Korea. Atherosclerosis 348, 16–24 (2022).
Fahed, G. et al. Metabolic syndrome: updates on pathophysiology and management in 2021. Int. J. Mol. Sci. 23 (2), 786 (2022).
Park, D. et al. 20-year trends in metabolic syndrome among Korean adults from 2001 to 2020. JACC Asia. 3 (3), 491–502 (2023).
Lim, S. et al. Components of metabolic syndrome in Korean adults: a hospital-based cohort at Seoul National university Bundang hospital. J. Obes. Metab. Syndr. 28 (2), 118–128 (2019).
Ushula, T. W. et al. Dietary patterns and the risks of metabolic syndrome and insulin resistance among young adults: evidence from a longitudinal study. Clin. Nutr. 41 (7), 1523–1531 (2022).
Christ, A., Lauterbach, M. & Latz, E. Western diet and the immune system: an inflammatory connection. Immunity 51 (5), 794–811 (2019).
Ley, K., Gerdes, N. & Winkels, H. ATVB distinguished scientist award: how costimulatory and coinhibitory pathways shape atherosclerosis. Arterioscler. Thromb. Vasc Biol. 37 (5), 764–777 (2017).
Lee, S. R. et al. Increased prevalence of metabolic syndrome among hypertensive population: ten years’ trend of the Korean National health and nutrition examination survey. Int. J. Cardiol. 166 (3), 633–639 (2013).
Lee, J., Kim, Y. & Jeon, J. Y. Association between physical activity and the prevalence of metabolic syndrome: from the Korean National health and nutrition examination survey, 1999–2012. Springerplus 5 (1), 1870 (2016).
Chomiuk, T., Niezgoda, N., Mamcarz, A. & Śliż, D. Physical activity in metabolic syndrome. Front. Physiol. 15, 1365761 (2024).
Maron, D. J. et al. Healthy behavior, risk factor control, and survival in the COURAGE trial. J. Am. Coll. Cardiol. 72 (19), 2297–2305 (2018).
Isiozor, N. M. et al. American heart association’s cardiovascular health metrics and risk of cardiovascular disease mortality among a middle-aged male Scandinavian population. Ann. Med. 51 (5–6), 306–313 (2019).
Lloyd-Jones, D. M. et al. Life’s essential 8: updating and enhancing the American heart association’s construct of cardiovascular health: a presidential advisory from the American heart association. Circulation 146 (5), e18–e43 (2022).
Collister, J. A., Liu, X. & Clifton, L. Calculating polygenic risk scores (PRS) in UK biobank: a practical guide for epidemiologists. Front. Genet. 13, 818574 (2022).
Abraham, G. et al. Genomic risk score offers predictive performance comparable to clinical risk factors for ischaemic stroke. Nat. Commun. 10, 5819 (2019).
Inouye, M. et al. Genomic risk prediction of coronary artery disease in 480,000 adults: implications for primary prevention. J. Am. Coll. Cardiol. 72 (16), 1883–1893 (2018).
Acknowledgements
None.
Funding
This research was supported by the SNU Office of Research Affairs, R&DB Foundation (04601-20220035) and Daedong Inc. The supporters had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
Authors’ contributions to the article were as follows: Concept and design: S.-Y.C., C.-S.K.; data acquisition: S.-Y.C., H.L., T.-M.R., S.Y.H.; data analysis and interpretation: S.-Y.C., C.-S.K., H.L., T.-M.R., S.Y.H., H.S., Y.K., S.-P.L.; statistical analysis: S.Y.H., S.-Y.C., C.-S.K.; drafting and finalizing the paper: S.Y.H., T.-M.R., S.-Y.C.; critical revision of the paper for important intellectual content: S.-Y.C., C.-S.K., T.-M.R., H.L., H.S., Y.K., S.-P.L.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hwang, S.Y., Rhee, TM., Kim, CS. et al. Influence of lifestyle risk factors and genetic predisposition on metabolic syndrome risk in Korean adults. Sci Rep 15, 24060 (2025). https://doi.org/10.1038/s41598-025-07369-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-07369-9