Abstract
Elucidating the interactions between piwi-interacting RNAs (piRNAs) and diseases is crucial for diagnosis and treatment. Although several computational approaches have been developed to investigate piRNA-disease associations, sparse datasets present challenges in capturing the complex relationships between piRNAs and diseases. To develop a more accurate prediction model for associations between piRNAs and diseases. We integrated piRNA sequence information, disease-related semantic terms, and existing piRNA-disease association networks to construct a heterogeneous graph. Utilizing the Mamba module, we developed an innovative sequence embedding model, MambaTextCNN, to extract features from piRNA sequences, which we used as node attributes within the heterogeneous graph. A heterogeneous graph convolution method was then applied to identify potential associations between piRNAs and diseases, with cross-attention mechanisms further enhancing node features. Finally, by incorporating positive unlabeled learning techniques, we developed the piRNA-disease association prediction model MambaCAttnGCN+. In 5-fold cross-validation, MambaCAttnGCN + achieved AUCs of 0.94 and 0.953 on two datasets, outperforming seven other state-of-the-art models. Additionally, a comparison of three distinct approaches for representing sequence node features, revealed through ablation experiments that features extracted by MambaTextCNN were the most effective. MambaCAttnGCN + represents a valuable predictive tool for future research on piRNA-disease associations in biomedicine.
Similar content being viewed by others
Introduction
Piwi-interacting RNAs (piRNAs) are a class of small non-coding RNAs (ncRNAs) ranging from 24 to 32 nucleotides in length1. piRNAs were first identified in the testes of Drosophila melanogaster in 20012 and later isolated from the vas deferens of male mice in 20061. To date, piRNAs have been recognized in nearly 44 species3. Although the primary function of piRNAs is to inhibit the transcription of transposons in germ cells and maintain genomic integrity4,5 a growing body of research suggests that piRNAs are involved in diverse biological processes, includding transposon silencing, histone modification, translational regulation, DNA methylation, and immune environment regulation6,7.
An increasing body of evidence is expanding our understanding of piRNAs. Studies have revealed the crucial role of piRNAs in the occurrence and prognosis of multiple human diseases, as well as their potential applications in the diagnosis and treatment of these conditions. This highlights the potential of these ncRNAs as valuable diagnostic markers and therapeutic targets8. For instance, piR-39,980 exerts an inhibitory effect on FDFT1, thereby mitigating tumorigenesis by modulating the proliferation and apoptosis of tongue squamous cell carcinoma in hypoxic environments9. Additionally, hsa_piR_019949 enhances chondrocyte anabolic activity by downregulating the expression of lncRNA NEAT110, while piR-has-23,533 promotes the aggressiveness of head and neck squamous cell carcinoma via USP7 signaling pathways11.
Biological experiments are essential for accurately identifying piRNAs and uncovering their associations with diseases. Numerous hypotheses have been confirmed through such experiments12. However, with the growing number of identified piRNAs and associated diseases, traditional biological experiments such as gene knockout13 and RNA interference14 face challenges, including lengthy experimental timelines, complexity, and high costs15. To address these issues, researchers have developed computational analysis methods to improve the efficiency of piRNA research.
Traditional machine learning and deep learning approaches have been extensively applied in bioinformatics. Using databases such as piRDisease16, ncRPheno17 and MNDR18 a range of computational models have been proposed to elucidate piRNA-disease associations. For instance, iPiDi-PUL19 integrates piRNA and disease features, extracts key attributes through principal component analysis (PCA), and classifies unlabeled associations using machine learning techniques like random forests. It also employs positive unlabeled learning (PUL) strategies to construct reliable negative sample training models. Building on heterogeneous network integration, iPiDA-GCN20 captures complex structural features via graph convolutional networks (GCN); ETGPDA21 uses the attention mechanism-based GCN to derive low-dimensional embedding features for piRNAs and diseases, while developing an embedding transformation module to address inconsistencies in embedding spaces. iPiDA-SWGCN22 mitigates adjacency matrix sparsity through a weighting strategy. PDA-PRGCN23 incorporates feature enhancement techniques, such as subgraph projection and residual scaling, to improve model performance via GCN. GAPDA24 leverages line graphs within heterogeneous networks to dynamically assign weights among nodes, thereby reducing noise effects in interaction networks. Additionally, iPiDA-GBNN25 employs gradient boosting methods with shallow architectures to incrementally construct complex GrowNet frameworks, enhancing model robustness. Compared with traditional machine learning approaches, these advanced methods incorporate inter-node information more effectively.
However, representing sequence features solely through sequence similarity does not sufficiently capture the implicit information within local sub-sequences. To address this limitation, piRDA7 extracts significant and abstract information from the original sequence without relying on feature engineering. It also incorporates a two-step positive-unlabeled learning strategy and guiding techniques to reduce false negatives and bias in positive unlabeled data. Despite its simplicity, robustness, and efficiency, this approach provides limited insights when addressing the complex relationship between piRNA and diseases, resulting in suboptimal model performance. PUTransGCN26 utilizes a heterogeneous graph network to reveal potential piRNA-disease associations by extracting features from the original piRNA sequence using TextCNN as node features within the heterogeneous graph framework. By leveraging an attention mechanism, it automatically adjusts and aggregates weight parameters for heterogeneous node features. This approach integrates PUL strategies to facilitate the identification of reliable negative associations. While effective, TextCNN tends to overlook global information inherent in the original piRNA sequence.
In recent years, state space models and Mamba have made significant advancements27 emerging as promising backbone networks for foundational model selection. Mamba leverages linear complexity and dynamic state modeling to significantly improve the flexibility and accuracy of sequence feature extraction, all while maintaining computational efficiency. This approach shows exceptional promise in DNA sequence analysis28. To develop a more accurate prediction model for piRNA-disease associations, we drew inspiration from prior research and integrated MambaTextCNN, GCN, and Cross Attention to construct our piRNA-disease association prediction framework. Additionally, we employed PUL to generate reliable negative samples for model training. Our contributions are outlined as follows:
-
1.
To address the limitation of TextCNN in capturing the global information inherent in original piRNA sequences, we integrated the Mamba module with CNN to develop the MambaTextCNN model. This model leverages the Mamba module to capture global sequence information while using CNN to enhance local feature extraction.
-
2.
In constructing a more accurate disease association prediction model, we used MambaTextCNN within a piRNA-disease heterogeneous network framework to embed node features from piRNA sequences. By adeptly combining MambaTextCNN, GCN, and Cross Attention, we developed the MambaCAttnGCN + model, achieving effective predictions for unknown piRNA-disease associations.
-
3.
To our knowledge, this is the first application of Mamba in piRNA-disease association prediction (PDA).
Result
Comparison with existing methods
As shown in Tables 1 and 2, MambaCAttnGCN + outperforms other methods across multiple metrics, including ranking index, AUC, and AUPR.
To assess the statistical significance of these improvements, a Mann-Whitney U test was conducted on the scores obtained from these methods. Statistical tests (as shown in supplementary Tables S1 and S2) show that MambaCAttnGCN + significantly outperforms all baseline models (p < 0.05), including ETGPDA; iPiDi-PUL variants based on decision trees (DT), support vector machines (SVM), and random forests (RF); as well as iPiDA-GCN, iPiDA-SWGCN, iPiDA-GBNN, piRDA, and PUTransGCN.
Although no statistically significant difference in the AUPR was observed between MambaCAttnGCN + and PUTransGCN on the piRDisease v1.0 dataset, this result is acceptable given that no additional optimization was performed for this dataset. Moreover, an analysis of six evaluation metrics across both datasets revealed statistically significant differences in five of them, further validating the superior performance of MambaCAttnGCN+.
Ablation experiment
To rigorously evaluate the efficacy of the MambaTextCNN approach and to determine the impact of original piRNA sequence characteristics on model performance, we conducted an ablation experiment. We also assessed the influence of different PUL strategies on model outcomes.
This study evaluates three different feature extraction approaches: sequence similarity features, TextCNN, and MambaTextCNN, which are used to generate sequence-based node embeddings within multiple PUL frameworks. We developed a predictive model, SimCatGCN-PUL, which utilizes sequence similarity as the node inputs and integrates GCN with a cross-attention mechanism. The structural schematic of this model is shown in Fig. S5 of Supplementary. For comparison, we constructed CNNCatGCN-PUL, which employs original piRNA sequence features extracted via TextCNN as node embedding and likewise combines GCN with cross-attention mechanism. This model corresponds to the previously described PUTransGCN26 and its schematic is provided in Fig S6 of Supplementary. It is important to note that only difference among these three models lies in their node feature representations; all other architectural components remain consistent.
The evaluated PUL strategies include PU bagging, two-step methods, spy techniques, combinations thereof, and a baseline setting without PUL. By comparing these approaches, we performed a systematic analysis of the individual and combined effects of feature extraction techniques and PUL strategies on the overall predictive performance of the models.
To further contextualize the role of global information modeling in piRNA sequence representation, we considered additional architectures known for their ability to capture long-range dependencies. Recurrent neural networks, such as Long Short-Term Memory (LSTM) networks29 are capable of retaining information over extended sequences through gated memory cells, which makes them suitable for modeling sequential biological data. In contrast, Transformer architectures leverage self-attention mechanisms that allow each token in the sequence to attend to all others simultaneously, thus capturing global contextual information more effectively and efficiently30. These architectures have demonstrated strong performance in various sequence modeling tasks and serve as meaningful baselines when evaluating the potential of the Mamba module as a global information encoder.
Diverse representations of sequence node features
As shown in Tables 3 and 4, under identical PUL conditions, the MambaTextCNN method significantly outperforms TextCNN method in Rank index, AUC, and AUPR across both the MNDRv4.0 and piRDisease v1.0 datasets. For instance, without applying PUL on the MNDRv4.0 dataset, MambaTextCNN achieves improvements of 2.31% in the Rank index, 0.67% in AUC, and 8.23% in AUPR. Similarly, on the piRDisease v1.0 dataset, it yields gains of 1.56%, 1.64%, and 4.09% in the respective metrics. These results underscore the effectiveness of the proposed MambaTextCNN module.
Among 30 evaluation metrics across both datasets, MambaTextCNN demonstrates superior performance in 25 metrics showing comparable results. For example, without PUL on the MNDR v4.0 dataset, MambaTextCNN achieves Rank index and AUC values only 0.1% lower than those of sequence similarity, while improving AUPR by 0.09%. On the piRDisease v1.0 dataset, minor decreases of 0.12% in Rank index, 0.13% in AUC, and 0.57% in AUPR are observed. These findings suggest that MambaTextCNN extracts more comprehensive and informative features from raw piRNA sequences than the sequence similarity method.
Furthermore, as detailed in Tables 3 and 4, and 5, a comparative analysis of the three sequence-based node feature representation methods under the same PUL configurations shows that 91.67% of the scores from MambaTextCNN exceed those of sequence similarity. In contrast, 90% of the sequence similarity scores outperform those from TextCNN. These results further validate the superior capacity of MambaTextCNN in capturing nuanced and discriminative features from piRNA sequences.
Diverse approaches to PUL
As shown in Tables 3 and 4, when sequence node features are held constant, the combination of PU bagging, the two-step method, and spy technique significantly outperforms their individual applications or the absence of PUL. This improvement is evident in both Rank index and AUC metrics across datasets. The degree of enhancement varies by dataset; for instance, when original sequence features are extracted using MambaTextCNN, the spy technique achieves the best performance on the MNDR v4.0 dataset, while the two-step method yields the highest gains on piRDisease v1.0. These findings suggest that integrating multiple PUL strategies enables synergistic benefits by leveraging the strengths of each method to enhance overall model performance.
The proportion of these spies plays a critical role in determining model effectiveness. A higher spy ratio increases the number of presumed negative samples, but also raises the risk of misclassifying true positives. Conversely, a lower spy ratio yields more reliable negatives but limits their quantity, potentially impairing the model’s generalizability. Thus, selecting an optimal spy ratio is essential to balancing reliability and coverage.
As shown in Table 5, MambaTextCNN consistently outperforms TextCNN at every evaluated spy ratio. Notably, when the spy ratio is set to 6%, MambaTextCNN achieves the highest AUC score. Deviations from this optimal value, such as 5% or values above 6%, result in a noticeable decline in AUC performance.
Overall, under equivalent spy ratio settings, MambaTextCNN consistently outperforms both sequence similarity and TextCNN-based methods, further validating the effectiveness of its novel design in enhancing the performance of PUL frameworks.
Different attention mechanisms
To evaluate the impact of different attention mechanisms on model performance, we conducted a comparative analysis between models employing multi-head cross-attention and multi-head self-attention, as shown in Supplementary Table S3. The results demonstrate that multi-head cross-attention consistently outperforms self-attention, with observed improvements of 0.13% in Rank index, 0.12% in AUC, and 1.67% in AUPR. These findings underscore the superior capability of cross-attention in capturing the interdependent relationships between piRNAs and diseases, thereby enhancing overall predictive performance.
Comparison with different PiRNA sequence embedding methods
To evaluate the effectiveness of the MambaTextCNN model in capturing global and local information, we replaced the Mamba module with other commonly used sequence modeling architectures, including Transformer, LSTM, and TextCNN, while keeping all other components fixed. The results are presented in Table S4 of the Supplementary. Among all models, MambaTextCNN achieved the best overall performance. These results highlight the advantage of combining Mamba’s global context modeling with TextCNN ability to extract local sequence patterns, offering a more comprehensive feature representation than models relying solely on either global or local mechanisms.
Parameter evaluation
Using the MNDR4.0 dataset, we conducted a grid search to evaluate the effects of three key hyperparameters, as summarized in Supplementary Tables S5 and S6: (i) convolutional kernel configurations for MambaTextCNN (e.g., (1, 3), (1, 5), (1, 7), (3, 5), (3, 7), (1, 3, 5), (1, 3, 7), (1, 5, 7), and (3, 5, 7)); (ii) the state size of Mamba (14, 16, and 18); and (iii) the dropout rate (0.1, 0.2, and 0.3). The best performance was observed with a kernel configuration of (1, 5, 7), a Mamba state size of 16, and a dropout rate of 0.1.
Generalization experiment
To further evaluate the generalization ability of the proposed model, we applied it to a different type of biological association prediction task: miRNA-disease association (MDA) prediction. We used the Human MicroRNA Disease Database (HMDD v3.2), which is currently the most comprehensive repository of experimentally supported MDAs, containing 15,186 validated associations involving 901 miRNAs and 877 diseases31. To ensure a fair comparison with previous studies, we adopted a benchmark dataset curated and preprocessed by prior work, and compared our model against four representative methods: AMHMDA32, AGAEMD33, MINIMDA34 and MUSCLE35 (supplementary Sect. 2.5 provides an overview of these models).
As shown in supplementary Table S7, our model outperformed AGAEMD in some evaluation metrics, but overall lagged behind the other competing methods. We hypothesize that this result may be attributed to several factors: (1) the proposed model was originally designed for piRNA-disease tasks, and its architecture and feature extraction mechanisms were not tailored for MDA prediction; (2) disease-specific features, which are critical in MDA prediction, were not effectively incorporated into the current model; (3) there are substantial differences in sequence length distributions between miRNAs and piRNAs, yet we did not adjust the embedding-related hyperparameters (e.g., maximum sequence length, embedding dimension) accordingly during this transfer experiment.
Although the model’s performance on this task was suboptimal, the experiment offers valuable insights into its cross-task generalizability and highlights the need for future adaptations in model structure and training strategies tailored to specific tasks.
Case study
To evaluate the predictive performance of MambaCAttnGCN + in identifying disease-associated piRNAs, we implemented a disease-agnostic validation strategy by removing all known piRNA associations for a target disease, treating it as novel. The model was then used to infer new associations and assign relevance scores, which were used to rank candidate piRNAs. This approach was applied to three representative and clinically significant diseases: renal cell carcinoma (RCC), parkinson’s disease (PD), and cardiovascular disease (CVD).
RCC is the most common form of kidney cancer, accounting for over 175,000 deaths globally each year36. PD ranks as the second most prevalent age-related neurodegenerative disorder after Alzheimer’s disease, with global cases projected to reach 12 million by 205037. CVD remains the leading cause of death worldwide, increasingly affecting younger populations due to lifestyle factors such as poor sleep and diet38. Investigating the role of piRNAs in these diseases offers potential for novel therapeutic strategies.
The top 5 ranked piRNAs for each disease were validated against existing literature using PubMed references. As shown in Table 6 and 15 predicted associations were supported by prior evidence. In RCC, piR-32,637, DQ598675, and piR-4580 were downregulated in tumor tissues, while hsa_piR_004153 and hsa_piR_016735 were upregulated in exosomes36,39,40. In PD, piRNAs including DQ597484, piR-36,772, and others were differentially expressed in both cellular and postmortem samples41. For CVD, PIR58596, PIR32637, PIR36772, and PIR45809 showed reduced expression, while PIR54043 was elevated42.
To assess the model’s predictive capacity, we trained it on the complete dataset and generated predictions across all disease categories. To further evaluate its accuracy, we compared the predicted association scores with previously reported disease-piRNA associations documented in the literature. Predictions were considered putative positive associations if their scores exceeded 0.995 and ranked within the top 10% for a given disease. As detailed in Table S8 of Supplementary, none of the 20 associations reported by PUTransGCN26 were present in the MNDR4.0 dataset used in our study. However, among the 10 associations predicted by our model with scores above 0.995, three ranked within the top 10% of their respective disease categories.
These findings demonstrate that MambaCAttnGCN + can effectively identify novel piRNA-disease associations, highlighting its potential utility in guiding future biological validation and experimental investigations.
Discussion
To advance the prediction of piRNA-disease associations, we developed MambaTextCNN, a novel network for extracting informative features directly from original piRNA sequences. By incorporating GCN and a multi-headed cross-attention mechanism, we further proposed MambaCAttnGCN+, which demonstrated superior predictive performance across two independent benchmark datasets. Ablation studies confirmed that the latent representations learned by MambaTextCNN significantly outperformed those based on conventional sequence similarity, underscoring the importance of sequence-specific features in enhancing piRNA-disease association prediction.
Traditional machine learning approaches, such as those employed by iPiDi-PUL19 have proven effective in reducing experimental costs; however, their performance in feature extraction remains limited. As presented in Tables 1 and 2, models employing deep learning techniques achieved substantially superior results (Rank index: 9–16%, AUC: 80.5–95.5%, AUPR: 32-68.5%) compared to iPiDi-PUL (Rank index: 23–45%, AUC: 56.5-75.85%, AUPR: 9-18.5%). These findings are consistent with previous studies7,20,21,22,25,26 highlighting the advantages of end-to-end deep learning frameworks, which facilitate automated feature extraction and enhance non-linear modeling capabilities. On the MNDR4.0 dataset, MambaCAttnGCN + outperformed iPiDi-PUL (RF) with gains of 14.41% in Rank index, 15.6% in AUC, and 44.38% in AUPR. Comparable improvements were observed on the piRDisease v1.0 dataset, reaffirming the robustness and generalizability of our proposed method.
Numerous researchers have utilized homogeneous or heterogeneous network-based approaches to predict piRNA-disease associations through machine learning and deep learning techniques19,20,21,22,25. While these methods have significantly advanced the field, they often rely on sequence similarity or Gaussian kernel similarity as node features, thereby overlooking the latent information encoded in the original piRNA sequences. Recent studies have underscored the importance of such latent sequence features in enhancing model performance, particularly within ncRNAs research43. For instance, PUTransGCN26 employs TextCNN to extract latent features directly from piRNA sequences, incorporating them as node features within a heterogeneous graph framework. This strategy yields notable performance improvements over traditional similarity-based approaches, as reflected in the metrics presented in Tables 1 and 2. Similarly, PiRDA7 performs association prediction exclusively based on raw piRNA sequences, demonstrating the substantial predictive power inherent in these sequences without the need for extensive feature engineering. Our findings align with these observations. As shown in Tables 3 and 4, and 5, under identical PUL settings, the MambaTextCNN module consistently outperforms or at least matches the performance of traditional sequence similarity-based methods in extracting informative features from raw piRNA sequences. These results underscore the essential role of latent sequence features in constructing accurate and robust piRNA-disease association prediction models. Looking ahead, future studies should prioritize the integration of such latent features to further improve model interpretability and predictive capability, thereby contributing to the ongoing refinement of PDA prediction frameworks.
PUTransGCN26 incorporates a piRNA sequence similarity matrix, a disease similarity mechanism, and a heterogeneous network constructed from piRNA-disease adjacency matrices. It utilizes TextCNN to extract node features from these adjacency matrices. While this approach demonstrates competitive performance, the use of TextCNN is inherently limited in capturing global contextual information within sequences, thereby constraining the model’s ability to fully leverage the latent features embedded in piRNA sequences. To address this limitation, we developed MambaCAttnGCN+, an enhanced model that builds upon the PUTransGCN architecture by integrating Mamba, a novel feature extraction module. In our design, Mamba operates in conjunction with a CNN to form MambaTextCNN, a hybrid mechanism that captures both global dependencies (via Mamba) and local patterns (via CNN). This dual-level feature representation allows for a more comprehensive extraction of sequence information. As shown in Tables 1 and 2, MambaCAttnGCN + achieves consistent improvements over PUTransGCN, with gains of 0.91%, 1.05%, and 1.05% in Rank index, AUC, and AUPR, respectively, on the MNDR4.0 dataset. Comparable enhancements are also observed on the piRDisease v1.0 dataset, further confirming the effectiveness and generalizability of our proposed model.
Additionally, under identical PUL conditions, Tables 3 and 4 reveal that MambaTextCNN outperforms its TextCNN counterpart across multiple metrics. Table 5 further substantiates this trend, demonstrating superior performance even when the spy ratio remains fixed. These findings suggest that MambaTextCNN is a promising alternative to conventional CNN-based extractors in DNA and RNA sequence analysis, offering a novel and effective framework for capturing both local and global features inherent in biological sequences.
While our work centers on piRNA–disease associations, extensive efforts have been devoted to miRNA–disease prediction using methods such as matrix factorization and GCN (e.g., IMIPMF44, NCMD45, SMAP46, GCNCF47). These models achieve high accuracy but often rely on similarity networks rather than modeling RNA sequences directly. In contrast, we focus on piRNAs (an underexplored class of ncRNAs) and introduce a novel state space framework, MambaTextCNN, to capture both local and global sequence features. This hybrid approach, when integrated with GCN and cross-attention mechanisms, consistently outperforms similarity- and CNN-based baselines. Our findings highlight the potential of sequence-level modeling in piRNA research and suggest opportunities for multi-class small RNA frameworks that incorporate both piRNA and miRNA data.
Notably, our case study revealed two novel piRNA-disease associations not previously documented in public databases: hsa_piR_004153 and hsa_piR_016735 were both found to be consistently upregulated in exosomes derived from Renal Cell Carcinoma (RCC) samples compared to normal renal tissue. This observation suggests potential involvement of these piRNAs in RCC pathogenesis, particularly in the extracellular signaling milieu.
For hsa_piR_004153, prior studies have reported downregulation in colorectal cancer tissues and serum48 but upregulation in plasma extracellular vesicles (EVs) from smokers49 and patients with Parkinson’s disease50 suggesting a context-dependent expression profile. These divergent patterns highlight a possible link between elevated extracellular piR_004153 and inflammatory processes, which are frequently observed in smoking-related disorders, neurodegeneration, and renal carcinogenesis36. Consistently, our mining of the exRNA-disease database51 revealed elevated piR_004153 levels in patients with subarachnoid hemorrhage and gastric cancer, both conditions associated with systemic inflammation (As shown in the supplementary Fig. S1 and S2).
Similarly, hsa_piR_016735 was also upregulated in RCC-derived exosomes and has been previously reported to exhibit downregulation in breast cancer tumors52 but elevated expression in rheumatoid arthritis synovial fibroblasts53 and in the plasma EVs of smokers49 and Parkinson’s patients50. These findings again point toward a possible role in chronic inflammation or immune modulation. Data from exRNAdisease51 further demonstrate shifts in expression across inflammatory and neoplastic contexts, including subarachnoid hemorrhage and gastric cancer (As shown in the supplementary Fig. S3 and S4).
Together, these findings suggest that hsa_piR_004153 and hsa_piR_016735 may represent previously unrecognized extracellular regulators in RCC, potentially acting through inflammation-related pathways. Their consistent enrichment in RCC exosomes underscores their promise as non-invasive biomarkers, while their broader expression across inflammatory pathologies hints at conserved molecular mechanisms linking chronic inflammation and tumor progression. Functional studies are warranted to elucidate their roles and targets within the tumor microenvironment.
In recent years, the Transformer architecture has become a dominant framework across a wide range of applications. Its attention mechanism enables models to focus selectively on relevant portions of the input sequence, thereby improving contextual understanding54,55. However, a major limitation of this architecture lies in its computational inefficiency—specifically, the attention mechanism scales quadratically with input length, posing challenges for processing very long sequences. In contrast, Mamba has recently emerged as a promising alternative, designed to efficiently model complex dependencies in sequential data with lower computational overhead27. In this work, we incorporated Mamba not as a standalone global encoder, but in combination with TextCNN to form a hybrid architecture MambaTextCNN. This design integrates global context modeling with local pattern extraction, enabling a more comprehensive and efficient representation of piRNA sequences.
While our model demonstrated improved performance in predicting piRNA–disease associations, several limitations remain. First, the number of experimentally validated associations is currently limited, although we anticipate that the volume of available data will increase in the future. Second, our work to date has primarily focused on leveraging Mamba’s potential for extracting features from original sequences. In future studies, we plan to explore replacing the cross-attention classifier with Mamba to reduce computational costs and further harness its capabilities in disease association prediction. Finally, experimental validation of the predicted associations would enhance the reliability and biological significance of our findings.
Conclusion
The MambaTextCNN model proposed in this study demonstrates strong capability in extracting features from original piRNA sequences. By integrating GCN with cross-attention mechanisms, the enhanced MambaTextCNN + model significantly improves the accuracy of piRNA–disease association predictions. This advancement not only contributes to identifying piRNA-related diseases in biological research, but also enables a more comprehensive investigation into the role of piRNAs in disease progression. Furthermore, it deepens our understanding of disease mechanisms and may inform the development of more effective therapeutic strategies.
Dataset
In line with recommendations from previous studies26 we evaluated our model using two benchmark datasets: MNDR4.018 and piRDisease v1.016. MNDR v4.0 is a comprehensive database of ncRNA-disease associations, encompassing 18 RNA types, 117 species, and 4090 diseases, with a total of 3,428,058 associations. From this resource, we extracted the piRNA-disease subset and removed duplicate entries and ambiguous entries, resulting in a dataset comprising 8,205 piRNAs and 15 diseases, including 9616 experimentally verified associations. In contrast, piRDisease v1.0 is a dedicated database for piRNA-disease associations, containing 7,939 validated associations involving 4796 piRNAs and 28 diseases. After filtering out duplicates, we retained a refined dataset comprising 4,350 piRNAs, 21 diseases, and 5,002 verified associations.
Methods
Mamba and MambaTextCNN
Mamba
The Mamba module27 consists of a combination of a selective Structured State-Space Model (SSM) sequence transformation (A detailed introduction to the SSM module is provided in Supplementary Sect. 2.1) and a gated MLP mechanism. As shown in the leftmost schematic diagram in Fig. 1, the input sequence is duplicated and projected to twice its original dimension, followed by causal convolution and subsequent application of the SiLU/Swish nonlinear activation function56 which culminates in the selective SSM process. Alternatively, a separate path applies SiLU nonlinearity to the input before gating the output from the SSM. Finally, this gated representation is projected back to its original dimension, d.
MambaTextCNN
As illustrated in Fig. 2, the MambaTextCNN model architecture represents a nuanced variation of the TextCNN framework57. Let \(\:{x}_{i}\in\:{\mathbb{R}}^{k}\) denote the k-dimensional vector corresponding to the i-th subsequence within the RNA sequence. A RNA of length n is expressed as:
.
Where \(\:⨁\) serves as the concatenation operator. More formally, let \(\:{x}_{i:i+j}\) represent the concatenation of the subsequence comprising \(\:{x}_{i},{x}_{i+1},\cdots\:,{x}_{i+j}\).
The Mamba module performs feature extraction on the vector representations of RNA subsequences:
.
The convolution operation involves a filter \(\:W\in\:{\mathbb{R}}^{hk}\), which is applied to the window of h sub-sequences to yield a novel feature. For example, the feature \(\:{c}_{i}\) is derived from the window of the sub-sequence \(\:{X}_{i:i+h-1}\).
.
Where \(\:b\in\:\mathbb{R}\) represents a bias term, while f denotes a nonlinear activation function, such as ReLU. The filter is systematically applied to each potential subsequence window within the RNA sequence \(\:\{{x}_{1:h},{x}_{2:h+1},\cdots\:,{x}_{n-h+1:n}\}\) to generate a feature map.
.
\(\:C\in\:{\mathbb{R}}^{n-h+1}\). We apply a max pooling operation58 on the feature map, designating the maximum value \(\:\widehat{c}=\text{m}\text{a}\text{x}\left\{c\right\}\) as the feature associated with this specific filter. This process aims to capture the most salient feature for each feature map the one exhibiting the highest value.
The extraction method for each feature from individual filters has been detailed. This architecture employs filters with varying window sizes to generate distinct activations. These transformed outputs make up the penultimate layer before being processed through a fully connected softmax layer that outputs categorical likelihood predictions.
MambaCAttnGCN+
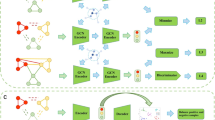
We propose a predictor, termed MambaCAttnGCN+, which integrates MambaTextCNN, GCN, and a cross-attention mechanism to predict piRNA-disease associations. To minimize the impact of positive samples on model performance during training, we employed PUL. The workflow of MambaCAttnGCN+, illustrated in Fig. 3, comprises three main steps: (a) generating embeddings for piRNA sequences using MambaTextCNN; (b) extracting piRNAs and disease features from the heterogeneous network via GCN; and (c) predicting association scores through the cross-attention mechanism.
Flowchart of MambaCAttnGCN+. (A) Establishment of the piRNA-Disease Heterogeneity Network: Each piRNA sequence is segmented into multiple 3-mers, with feature vectors generated by word2vec substituting these 3-mers. Subsequently, the features of piRNA nodes are embedded using MambaTextCNN. (B) Feature extraction utilizing GCN: A heterogeneous network is constructed by integrating similarity matrices for piRNAs and diseases, along with their adjacency matrix. Graph convolutional networks (GCNs) are then applied to extract features for both node types. (C) Score prediction utilizing the cross-attention mechanism: In the encoder designed to extract piRNA features, the query (Q) matrix encodes piRNA representations, while the key (K) and value (V) matrices correspond to disease features; in the encoder for disease feature extraction, this assignment is reversed.
Embedding of PiRNA sequences for enhanced representation
Inspired by the DeepLncLoc model59 we utilized k-mers in combination with MambaTextCNN to achieve piRNA embedding. The workflow for piRNA embedding is illustrated in Fig. 3(A), and the detailed steps are presented below:
-
1.
Subsequence Division: Each piRNA sequence is divided into \(\:m\) consecutive k-mer subsequences. The piRNA sequence representation is as follows:
Here, \(\:{\tilde{P}}_{i}\) represents the \(\:i\)-th subsequence of the piRNA sequence. The value of k is set to 3, with a stride window of 1. For example, the sequence “ACCAATG” is segmented into {ACC, CCA, CAA, AAT, ATG}.
-
2.
Subsequence Vectorization: We utilize Word2vec60 with a skip-gram architecture to pre-train embeddings for all 3-mer subsequences derived from piRNA sequences, thereby representing each 3-mer as a dense feature vector.
-
3.
Subsequence Vector Encoding: Each piRNA sequence is divided into 31 overlapping 3-mers, which are mapped to 128-dimensional pre-trained embeddings. This process yields a matrix of shape (31, 128) per sequence. When processed in batches, the resulting tensor has shape (N, 31, 128), where N is the number of piRNA sequences.
-
4.
Advanced Sequence Feature Extraction using MambaTextCNN: To capture both global and local dependencies within the piRNA sequences, we employ a multi-branch architecture consisting of three parallel pathways. Each pathway begins with a Mamba module configured with a state size of 16 and a convolution kernel size of 4. Following the Mamba module, each branch includes a 1D convolution layer with a kernel size of 1, 5, or 7, respectively, designed to extract local features at different granularities. A max pooling operation is then applied to each convolutional output to reduce dimensionality and emphasize the most salient features. Finally, the outputs from the three branches are concatenated to form a unified representation of shape (N, 384), where N is the number of piRNA sequences in the batch.
Heterogeneous piRNA-disease graph construction and GCN-based feature extraction
Establishment of the piRNA-disease heterogeneity network
We developed a heterogeneous piRNA-disease network, consisting of three types of edges (piRNA-disease edges, piRNA-piRNA edges, and disease-disease edges, denoted as \(\:{E}_{piRNA-disease}\), \(\:{E}_{piRNA-piRNA}\), and \(\:{E}_{disease-disease}\), respectively. Here, \(\:{E}_{piRNA-disease}\) is derived from the piRNA-disease associations in the original database MNDRv 4.0, while the other two edge types are based on similarities among homogeneous biological entities. The network comprises two categories of nodes (piRNAs and diseases, represented as \(\:{V}_{piRNA}\) and \(\:{V}_{disease}\), respectively). The piRNA-disease heterogeneous network can be formally expressed as:
.
In this equation, \(\:{S}_{p}\in\:{\mathbb{R}}^{m\times\:m}\) denotes the piRNA similarity matrix, \(\:{S}_{d}\in\:{\mathbb{R}}^{n\times\:n}\) signifies the disease similarity matrix, and \(\:A\in\:{\mathbb{R}}^{m\times\:n}\) represents the piRNA-disease adjacency matrix. Here, \(\:m\) indicates the number of piRNAs, while \(\:n\) denotes the number of diseases.
PDA matrix
The adjacency matrix \(\:{A}_{PD}\) characterizes the interrelationship between each piRNA and its corresponding disease:
.
If the \(\:i\)-th piRNA is associated with the \(\:j\)-th disease, then \(\:{a}_{ij}=1\); otherwise, \(\:{a}_{ij}=0\).
PiRNA similarity matrix
The piRNA similarity matrix based on sequence and adjacency information is denoted as \(\:{S}_{p}^{seq+GIP}\in\:{\mathbb{R}}^{m\times\:m}\). Assuming that piRNAs with similar sequences tend to share functional roles, we apply the Smith-Waterman alignment algorithm61 to quantify pairwise similarity. Sequence data are obtained from piRBase v3.03. The normalized sequence similarity is calculated as:
.
Here, \(\:SW({p}_{i},{p}_{j})\in\:{\mathbb{R}}^{m\times\:m}\) denotes the sequence alignment score between the \(\:i\)-th and \(\:j\)-th piRNA. Additionally, Gaussian Interaction Profile (GIP) kernel similarity is computed based on the assumption that piRNAs with analogous disease interaction patterns are likely to be functionally related19,21,24,62,63, defined as64:
.
Where \(\:{A}_{PD}\left({p}_{i},\right)\) and \(\:{A}_{PD}({p}_{j},)\) denote the i-th and j-th row vectors of the adjacency matrix \(\:{A}_{PD}\), respectively, while \(\:{\lambda\:}_{p}\) represents the kernel width coefficient, defined as:
.
The piRNA similarity matrix is then constructed based on both sequence similarity and GIP kernel similarity, as follows:
.
Disease similarity matrix
Disease similarity is derived from the Disease Ontology (DO), which provides a standardized framework for capturing topological relationships among diseases65 in a directed acyclic graph (DAG). The semantic similarity between the \(\:i\)-th and \(\:j\)-th diseases is calculated as follows:
Where \(\:{T}_{i}\) represents the directed acyclic graph (DAG) of the \(\:i\)-th disease, while \(\:{S}_{{d}_{i}}\) reflects the semantic influence of disease \(\:t\in\:{T}_{i}\) on the \(\:i\)-th disease. The calculation methodology is outlined as follows:
.
Following prior studies26 we set the attenuation parameter \(\:\theta\:\) is established at 0.5. Similarly, the GIP kernel similarity among diseases is calculated as64:
.
Where \(\:{A}_{PD}\left(,{\:d}_{i}\right)\) and \(\:{A}_{PD}\left({,\:\:d}_{j}\right)\) denote the \(\:i\)-th and \(\:j\)-th column vectors of the adjacency matrix \(\:{A}_{PD}\), respectively, while \(\:{\:\lambda\:}_{d}\) represents the kernel width coefficient, defined as follow:
.
The disease similarity matrix is then constructed based on both disease semantic similarity and GIP kernel similarity, as follows:
.
Feature extraction utilizing GCN
GCN are widely used for aggregating information from adjacent nodes and uncovering latent network structures20,21,22,26 in network analysis tasks. Here, GCN is employed to extract piRNA-disease association features from heterogeneous graph networks. As illustrated in Fig. 3(B), the input relationship for GCN is synthesized using the similarity matrices of piRNA and diseases. Let \(\:{H}^{l}\in\:{R}^{d}\) represent the node embedding at the \(\:l\)-th layer of GCN; thus, the computation of the node embedding \(\:{H}^{l+1}\in\:{R}^{d}\) is computed as:
.
Where \(\:{A}_{h}\) is obtained from Eq. 6. The matrix \(\:{\tilde{A}}_{h}\) denotes the adjacency matrix corresponding to the entire network, while \(\:\tilde{D}\) represents the degree matrix of \(\:{\tilde{A}}_{h}\). \(\:I\) represents the identity matrix, \(\:{H}^{l}\) refers to the node embedding at the \(\:l\)-th layer, and \(\:{W}^{l}\) indicates the trainable parameter matrix. Additionally, \(\:\sigma\:\) denotes the nonlinear activation function ReLU.
The initial embedding \(\:{H}^{0}\) is formed by concatenating the piRNA embeddings obtained through MambaTextCNN with the disease embeddings \(\:{S}_{d}^{sem}\) derived from the disease semantic similarity matrix. The first m rows of \(\:{H}^{l}\) correspond to the piRNA embeddings, while the final n rows represent the disease embeddings, where m and n denote the respective quantities of piRNAs and diseases.
Score prediction utilizing the cross-attention mechanism
To better capture the complex relationship between piRNAs and diseases, a cross-attention mechanism is implemented (As shown in Fig. 4)30. This mechanism enables the model to concentrate on relevant piRNA-disease pairs by integrating features from both piRNA and diseases. The input to the feature cross-attention module consists of the tRNA embedding and disease embedding obtained from the comprehensive view embedding \(\:{H}^{l+1}\), as defined in Eq. 17.
When extracting piRNA features, the encoder’s Q matrix input comprises piRNA embeddings, while the K and V matrices contain disease embeddings, and vice versa when extracting disease features. Scaled dot-product attention is calculated as follows:
.
Where \(\:{\overline{W}}^{Q}\),\(\:{\overline{W}}^{K}\),\(\:{\overline{W}}^{V}\) are trainable parameter matrices, and \(\:{d}_{k}\) as the feature vector dimension (\(\:{d}_{k}\) = 256). The multi-head cross-attention mechanism aggregates information from multiple attention heads, allowing the model to capture diverse interaction patterns. The final correlation score for each piRNA-disease pair is computed as the inner product of their respective feature vectors derived from the model output.
.
Here, h denotes the number of heads, with a default configuration set to 2.
The skip connection and normalization block that follows the MultiHead operation are designed to integrate both the original features and the aggregated features within the attention mechanism. Ultimately, the correlation score between each piRNA and disease is derived by computing the inner product of their respective feature vectors obtained from the model output.
Utilize PUL to identify reliable negative associations
In our dataset, due to factors such as cost and technical limitations, we only have ‘positive data’ validated through biological experiments and ‘unlabeled data’ that remains unverified. Unlabeled associations may contain hidden positives, so treating all unlabeled samples as negative could introduce bias, distort the dataset’s distribution, and ultimately degrade the model’s performance.
To address this issue, we applied the PUL to identify reliable negative associations66. PUL has proven effective in tasks with substantial unlabeled data and a limited proportion of positive instances, such as data stream classification and information retrieval. In the context of piRNA-disease association prediction, several methods have employed bagging strategies to identify high-quality negative associations7,19,62, while PUTransGCN26 combines three distinct methods. Building on prior research, we employed a combination of these three methods to construct reliable negative samples.
The core idea of the spy technique is to embed known positive instances as ‘undercover agents’ within unlabeled associations, and then train an initial classifier under the assumption that all unlabeled examples are negative. For all unconfirmed negative associations, those with posterior probabilities lower than any undercover agent are classified as reliable negative associations. PU bagging involves classifying unlabeled associations using multiple relatively accurate, smaller classifiers, with associations that produce lower average scores across these classifiers being deemed reliable negative associations. The two-step approach iteratively updates the model to enhance classification accuracy and reliability. Detailed procedures are available in Supplementary Sect. 2.2.
Performance evaluation
We used 5-fold cross-validation to evaluate model performance. The results were obtained by calculating the average and variance of the final five iterations across all folds. The verified associations set \(\:{\dot{\mathbb{P}}}^{+}\) and the unlabeled association sset \(\:{\dot{\mathbb{P}}}^{U}\) are partitioned into five equally sized subsets as follows:
.
The delineation of the dataset is as follows:
.
Here, i∈[1,2,3,4,5], where \(\:\complement\:\) represents the complement operation, and PUL refers to the selection of reliable negative samples. It is important to note that for each cross-validation iteration, the GIP kernel similarity matrices \(\:{S}_{p}^{GIP}\) and \(\:{S}_{d}^{GIP}\) must be recalculated based on a newly defined training set.
To tackle the issue of modeling with positive and unlabeled data, we incorporated three established PUL strategies (Spy, PU Bagging, and Two-Step) into the training pipeline of the MambaCAttnGCN + model. Specifically, during each fold of five-fold cross-validation, the dataset was divided into training and test sets. From the training set, we applied these PUL methods to identify a set of reliable negative samples from the unlabeled portion. These negative samples were then combined with the known positive samples to construct a training dataset \(\:{\dot{\mathbb{P}}}_{i}^{train}\).
Importantly, the test set \(\:{\dot{\mathbb{P}}}_{i}^{test}\)contained both known positive samples and the remaining unlabeled data, which were not processed by any PUL method. This setup ensures that the model’s generalization performance is evaluated under a realistic positive-unlabeled setting. The incorporation of PUL in this manner enables the model to benefit from supervision while mitigating label noise and class imbalance.
To facilitate a more robust comparison with existing research approaches12,14,16,19,20, we evaluated the performance of the predictive model using the area under the receiver operating characteristic curve (AUC), the area under the precision-recall curve (AUPR), and the ranking of positive predictions19. A lower rank index value indicates superior model performance. The formula for calculating the ranking index is as follows:
.
Where \(\:\left|{\dot{\mathbb{T}}}_{test}^{+}\right|\) denotes the total number of known PDAs within the test subset \(\:{\dot{\mathbb{T}}}_{test}^{+}\), while \(\:\left|{\dot{\mathbb{T}}}_{test}\right|\) signifies the overall count of piRNA-disease pairs present in the same test subset. An association within the positive test subset \(\:{\dot{\mathbb{T}}}_{test}^{+}\) is represented by \(\:a\), and \(\:{\gamma\:}_{a}\) indicates its rank position among all associations in this positive test set.
Implementation details
Our methodology was implemented using Python 3.10 and PyTorch 2.1, with all experiments conducted on a single NVIDIA RTX 4090 GPU equipped with 24GB of memory. The model was trained for a maximum of 220 epochs, during which all trainable parameters were optimized using the Adam optimizer at a learning rate of 0.001. A comprehensive summary of all hyperparameters is provided in Supplementary Table S9.
We performed a comparative analysis of the performance of MambaCAttnGCN + against seven state-of-the-art prediction models across two datasets. These models include ETGPDA21, iPiDi-PUL19, iPiDA-GCN20, iPiDASWGCN22, iPiDA-GBNN25, piRDA7 and PUTransGCN26. The study referenced as26 provided a comprehensive reproduction of the performance metrics for these models under configuration conditions to ours and included replicable code. Therefore, we utilized these results directly. This comparative evaluation aims to provide a clearer understanding of the effectiveness and advantages of our proposed model.
Data availability
The source code and data used to produce the results and analyses presented in this manuscript are available from the GitHub repository: https://github.com/xiangkui9e/MambaCAttnGCN-.
References
Aravin, A. et al. A novel class of small RNAs bind to MILI protein in mouse testes. Nature 442, 203–207. https://doi.org/10.1038/nature04916 (2006).
Aravin, A. A. et al. Double-stranded RNA-mediated Silencing of genomic tandem repeats and transposable elements in the D. melanogaster germline. Curr. Biol. 11, 1017–1027. https://doi.org/10.1016/s0960-9822(01)00299-8 (2001).
Wang, J. J. et al. PiRBase: integrating PiRNA annotation in all aspects. Nucleic Acids Res. 50, D265–D272. https://doi.org/10.1093/nar/gkab1012 (2022).
Wang, X., Ramat, A., Simonelig, M. & Liu, M. F. Emerging roles and functional mechanisms of PIWI-interacting RNAs. Nat. Rev. Mol. Cell. Bio. 23, 771–771. https://doi.org/10.1038/s41580-022-00548-w (2022).
Ozata, D. M., Gainetdinov, I., Zoch, A., O’Carroll, D. & Zamore, P. D. PIWI-interacting rnas: small RNAs with big functions. Nat. Rev. Genet. 20, 89–108. https://doi.org/10.1038/s41576-018-0073-3 (2019).
Wu, W., Lu, B. F., Jiang, R. Q. & Chen, S. The function and regulation mechanism of PiRNAs in human cancers. Histol. Histopathol. 36, 807–816. https://doi.org/10.14670/Hh-18-323 (2021).
Ali, S. D., Tayara, H. & Chong, K. T. Identification of PiRNA disease associations using deep learning. Comput. Struct. Biotechnol. 20, 1208–1217. https://doi.org/10.1016/j.csbj.2022.02.026 (2022).
Wang, K. et al. Emerging functions of piwi-interacting RNAs in diseases. J. Cell. Mol. Med. 25, 4893–4901. https://doi.org/10.1111/jcmm.16466 (2021).
Chattopadhyay, T. & Mallick, B. FDFT1 repression by piR-39980 prevents oncogenesis by regulating proliferation and apoptosis through hypoxia in tongue squamous cell carcinoma. Life Sci. 329, 54. https://doi.org/10.1016/j.lfs.2023.121954 (2023).
Zhang, X. H. et al. PiRNA hsa_pir_019949 promotes chondrocyte anabolic metabolism by inhibiting the expression of LncRNA NEAT1. J. Orthop. Surg. Res. 19, 1. https://doi.org/10.1186/s13018-023-04511-z (2024).
Hu, H. L. et al. PiR-hsa-23533 promotes malignancy in head and neck squamous cell carcinoma via USP7. Transl Oncol. 45, 90. https://doi.org/10.1016/j.tranon.2024.101990 (2024).
Guo, C., Wang, X. L. & Ren, H. Databases and computational methods for the identification of piRNA-related molecules: A survey. Comput. Struct. Biotechnol. 23, 813–833. https://doi.org/10.1016/j.csbj.2024.01.011 (2024).
Ernst, C., Odom, D. T. & Kutter, C. The emergence of PiRNAs against transposon invasion to preserve mammalian genome integrity. Nat. Commun. 8, 7. https://doi.org/10.1038/s41467-017-01049-7 (2017).
Thakker, D. R. et al. Neurochemical and behavioral consequences of widespread gene knockdown in the adult mouse brain by using nonviral RNA interference. Proc. Natl. Acad. Sci. USA. 101, 17270–17275. https://doi.org/10.1073/pnas.0406214101 (2004).
Chen, X. et al. Computational models for LncRNA function prediction and functional similarity calculation. Brief. Funct. Genomics. 18, 58–82. https://doi.org/10.1093/bfgp/ely031 (2019).
Muhammad, A., Waheed, R., Khan, N. A., Jiang, H. & Song, X. Y. PiRDisease v1.0: a manually curated database for PiRNA associated diseases. Database-Oxford https://doi.org/10.1093/database/baz052 (2019).
Zhang, W. L. et al. NcRPheno: a comprehensive database platform for identification and validation of disease related noncoding RNAs. Rna Biol. 17, 943–955. https://doi.org/10.1080/15476286.2020.1737441 (2020).
Chen, J. et al. RNADisease v4.0: an updated resource of RNA-associated diseases, providing RNA-disease analysis, enrichment and prediction. Nucleic Acids Res. 51, D1397–D1404. https://doi.org/10.1093/nar/gkac814 (2023).
Wei, H., Xu, Y. & Liu, B. iPiDi-PUL: identifying Piwi-interacting RNA-disease associations based on positive unlabeled learning. Brief. Bioinform. 22, 58. https://doi.org/10.1093/bib/bbaa058 (2021).
Hou, J. L., Wei, H., Liu, B. & iPiDA-GCN Identification of piRNA-disease associations based on graph convolutional network. PLoS Comput. Biol. 18, 71. https://doi.org/10.1371/journal.pcbi.1010671 (2022).
Meng, X. H. et al. ETGPDA: identification of piRNA-disease associations based on embedding transformation graph convolutional network. BMC Genom. 24, 8. https://doi.org/10.1186/s12864-023-09380-8 (2023).
Hou, J. L., Wei, H., Liu, B. & iPiDA-SWGCN Identification of piRNA-disease associations based on supplementarily weighted graph convolutional network. PLoS Comput. Biol. 19, 42. https://doi.org/10.1371/journal.pcbi.1011242 (2023).
Zhang, P. et al. PDA-PRGCN: identification of Piwi-interacting RNA-disease associations through subgraph projection and residual scaling-based feature augmentation. BMC Bioinform. 24, 3. https://doi.org/10.1186/s12859-022-05073-3 (2023).
Zheng, K. et al. Line graph attention networks for predicting disease-associated Piwi-interacting RNAs. Brief. Bioinform. 23, 393. https://doi.org/10.1093/bib/bbac393 (2022).
Qian, Y., He, Q. & Deng, L. IEEE International Conference on Bioinformatics and Biomedicine (BIBM) (2021).
Chen, Q. H., Zhang, L. Y., Liu, Y. J., Qin, Z. H. & Zhao, T. Y. PUTransGCN: identification of piRNA-disease associations based on attention encoding graph convolutional network and positive unlabelled learning. Brief. Bioinform. 25, 144. https://doi.org/10.1093/bib/bbae144 (2024).
Dao, T. & Gu, A. Transformers are SSMs: Generalized models and efficient algorithms through structured state space duality. Preprint at http://arXiv.org/2405.21060 (2024).
Schiff, Y. et al. Caduceus: Bi-directional equivariant long-range Dna sequence modeling. Preprint at http://arXiv.org/2403.03234 (2024).
Schuster, M. & Paliwal, K. K. Bidirectional recurrent neural networks. IEEE Trans. Signal Process. 45, 2673–2681 (1997).
Vaswani, A. et al. Proceedings of the 31st International Conference on Neural Information Processing Systems 6000–6010 (Curran Associates Inc., 2017).
Huang, Z. et al. HMDD v3.0: a database for experimentally supported human microRNA-disease associations. Nucleic Acids Res. 47, D1013–D1017. https://doi.org/10.1093/nar/gky1010 (2019).
Ning, Q. et al. AMHMDA: attention aware multi-view similarity networks and hypergraph learning for miRNA-disease associations identification. Brief. Bioinform. 24, 94. https://doi.org/10.1093/bib/bbad094 (2023).
Zhang, H. Z. et al. Predicting miRNA-disease associations via node-level attention graph auto-encoder. IEEE Acm Trans. Comput. Biol. 20, 1308–1318. https://doi.org/10.1109/Tcbb.2022.3170843 (2023).
Lou, Z. Z. et al. Predicting miRNA-disease associations via learning multimodal networks and fusing mixed neighborhood information. Brief. Bioinform. 23, 159. https://doi.org/10.1093/bib/bbac159 (2022).
Ji, B. Y., Zou, H. T., Xu, L. W., Xie, X. L. & Peng, S. L. MUSCLE: multi-view and multi-scale attentional feature fusion for microRNA-disease associations prediction. Brief. Bioinform. 25, 167. https://doi.org/10.1093/bib/bbae167 (2024).
Boguslawska, J. et al. Non-coding RNAs secreted by renal cancer include pir_004153 that promotes migration of mesenchymal stromal cells. Cell. Commun. Signal. 23, 1. https://doi.org/10.1186/s12964-024-02001-1 (2025).
Romano, S. et al. Machine learning-based meta-analysis reveals gut Microbiome alterations associated with parkinson’s disease. Nat. Commun. 16, 4227. https://doi.org/10.1038/s41467-025-56829-3 (2025).
Bai, H. M. Y. et al. The significance of small noncoding RNAs in the pathogenesis of cardiovascular diseases. Genes Dis. 12, 342. https://doi.org/10.1016/j.gendis.2024.101342 (2025).
Li, Y. P. et al. Piwi-interacting RNAs (piRNAs) are dysregulated in renal cell carcinoma and associated with tumor metastasis and cancer-specific survival. Mol. Med. 21, 381–388. https://doi.org/10.2119/molmed.2014.00203 (2015).
Busch, J. et al. Piwi-interacting RNAs as novel prognostic markers in clear cell renal cell carcinomas. J. Exp. Clin. Cancer Res. 34, 61. https://doi.org/10.1186/s13046-015-0180-3 (2015).
Schulze, M. et al. Sporadic parkinson’s disease derived neuronal cells show disease-specific mRNA and small RNA signatures with abundant deregulation of PiRNAs. Acta Neuropathol. Commun. 6, 58. https://doi.org/10.1186/s40478-018-0561-x (2018).
Mick, E. et al. Stroke and Circulating extracellular RNAs. Stroke 48, 828–834. https://doi.org/10.1161/Strokeaha.116.015140 (2017).
Tan, J. J., Li, X. Y., Zhang, L. & Du, Z. L. Recent advances in machine learning methods for predicting LncRNA and disease associations. Front. Cell. Infect. Mi. 12, 72. https://doi.org/10.3389/fcimb.2022.1071972 (2022).
Ha, J., Park, C., Park, C. & Park, S. I. M. I. P. M. F. Inferring miRNA-disease interactions using probabilistic matrix factorization. J. Biomed. Inf. 102, 58. https://doi.org/10.1016/j.jbi.2019.103358 (2020).
Ha, J. & Park, S. N. C. M. D. Node2vec-based neural collaborative filtering for predicting MiRNA-disease association. IEEE ACM Trans. Comput. Biol. 20, 1257–1268. https://doi.org/10.1109/Tcbb.2022.3191972 (2023).
Ha, J. S. M. A. P. Similarity-based matrix factorization framework for inferring miRNA-disease association. Knowl-Based Syst. 263, 95. https://doi.org/10.1016/j.knosys.2023.110295 (2023).
Ha, J. Graph convolutional network with neural collaborative filtering for predicting miRNA-disease association. Biomedicines 13, 36. https://doi.org/10.3390/biomedicines13010136 (2025).
Qu, A. L. et al. A serum PiRNA signature as promising non-invasive diagnostic and prognostic biomarkers for colorectal cancer. Cancer Manag Res. 11, 3703–3720. https://doi.org/10.2147/Cmar.S193266 (2019).
Sundar, I. K., Li, D. M. & Rahman, I. Small RNA-sequence analysis of plasma-derived extracellular vesicle MiRNAs in smokers and patients with chronic obstructive pulmonary disease as Circulating biomarkers. J. Extracell. Vesicles. 8, 16. https://doi.org/10.1080/20013078.2019.1684816 (2019).
Aguilar, M. A. et al. Neuronally enriched microvesicle RNAs are differentially expressed in the serums of parkinson’s patients. Front. Neurosci-Switz. 17, 23. https://doi.org/10.3389/fnins.2023.1145923 (2023).
Zhao, L., Ning, Q. Q., Zheng, G. T., Luo, J. & Dong, D. ExRNAdisease: an extracellular RNA transcriptome atlas in human diseases. Gene 836, 62. https://doi.org/10.1016/j.gene.2022.146662 (2022).
Krishnan, P. et al. Piwi-interacting RNAs and PIWI genes as novel prognostic markers for breast cancer. Oncotarget 7, 37944–37956. https://doi.org/10.18632/oncotarget.9272 (2016).
Plestilová, L. et al. Expression and regulation of PIWIL-proteins and PIWI-interacting RNAs in rheumatoid arthritis. PLoS ONE. 11, 920. https://doi.org/10.1371/journal.pone.0166920 (2016).
Aleissaee, A. A. et al. Transformers in remote sensing: A survey. Remote Sens-Basel. 15, 60. https://doi.org/10.3390/rs15071860 (2023).
Han, K. et al. A survey on vision transformer. IEEE Trans. Pattern Anal. 45, 87–110. https://doi.org/10.1109/Tpami.2022.3152247 (2023).
Ramachandran, P., Zoph, B. & Le, Q. V. Searching for activation functions. Preprint http://arXiv.org/1710.05941 (2017).
Kim, Y. Proceedings of the 2014 Conference on Empirical Methods in Natural Language Processing (EMNLP) (eds Alessandro, M. et al.) 1746–1751.
Collobert, R. et al. Natural Language processing (almost) from scratch. J. Mach. Learn. Res. 12, 2493–2537 (2011).
Zeng, M. et al. DeepLncLoc: a deep learning framework for long non-coding RNA subcellular localization prediction based on subsequence embedding. Brief. Bioinform. 23, 360. https://doi.org/10.1093/bib/bbab360 (2022).
Řehůřek, R. Proceedings of the LREC 2010 Workshop on New Challenges for NLP Frameworks 45.
Smith, T. F. & Waterman, M. S. Identification of common molecular subsequences. J. Mol. Biol. 147, 195–197 (1981).
Wei, H., Ding, Y. X., Liu, B. & iPiDA-sHN Identification of Piwi-interacting RNA-disease associations by selecting high quality negative samples. Comput. Biol. Chem. 88, 361. https://doi.org/10.1016/j.compbiolchem.2020.107361 (2020).
Zheng, K. et al. SPRDA: a link prediction approach based on the structural perturbation to infer disease-associated Piwi-interacting RNAs. Brief. Bioinform. 24, 98. https://doi.org/10.1093/bib/bbac498 (2023).
van Laarhoven, T., Nabuurs, S. B. & Marchiori, E. Gaussian interaction profile kernels for predicting drug-target interaction. Bioinformatics 27, 3036–3043. https://doi.org/10.1093/bioinformatics/btr500 (2011).
Kibbe, W. A. et al. Disease ontology 2015 update: an expanded and updated database of human diseases for linking biomedical knowledge through disease data. Nucleic Acids Res. 43, D1071–D1078. https://doi.org/10.1093/nar/gku1011 (2015).
Bekker, J. & Davis, J. Learning from positive and unlabeled data: a survey. Mach. Learn. 109, 719–760. https://doi.org/10.1007/s10994-020-05877-5 (2020).
Funding
This work was supported in part by the National Natural Science Foundation of China (Grant No. 62172128).
Author information
Authors and Affiliations
Contributions
DJY designed the study, XKL and DJY participated in the acquisition of data. XKL wrote the article, XJZ, BZ and JZ critically reviewed it. All authors assisted in the revision process, and gave approval for the final version of the article to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yao, D., Li, X., Zhan, X. et al. MambaCAttnGCN+: a comprehensive framework integrating MambaTextCNN, cross-attention and graph convolution network for piRNA-disease association prediction. Sci Rep 15, 25058 (2025). https://doi.org/10.1038/s41598-025-07641-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-07641-y