Abstract
Meniscus-derived stem cells (MeSCs) hold great promise for cytotherapy of the meniscus. Large-scale cell expansion in vitro is usually accompanied by decreased proliferation and migration and increased senescence, leading to a decrease in treatment efficiency. Therefore, the present study aimed to compare the effects of different concentrations of glucose on the proliferation, migration, senescence and protein expression of MeSCs. In this study, human MeSCs were cultured in two types of expansion media: low-glucose (LG) DMEM (5.5 mM glucose) and high-glucose (HG) DMEM (25 mM glucose). At specific passage number, the proliferation rate was evaluated by cell counting, the migration rate was evaluated by a scratch-wound assay, cell senescence was evaluated by β-GAL assays, and protein expression/phosphorylation was evaluated by ELISA. The results showed that a lower concentration of glucose promoted proliferation and migration, reduced cell senescence, and activated PI3K/Akt-related signaling pathways. This study provides a preliminary basis for the use of this medium composition for MeSC expansion.
Similar content being viewed by others
Introduction
The meniscus is a key structure responsible for shock absorption and load transmission. The knee joint relies heavily on the meniscus for absorbing pressure, providing stability, and ensuring proper lubrication. Owing to poor vascularization, especially in the innermost regions of the meniscus, injuries in these areas have a low healing potential1,2. Treatment options for meniscal injuries include meniscal suturing, partial or complete meniscectomy, and meniscal allograft transplantation. However, these treatments have limited efficacy, often leaving patients vulnerable to reinjury and increasing the risk of developing osteoarthritis3,4,5,6.
Meniscus-derived stem cells (MeSCs), located in the outer two-thirds of the meniscus, have a fusiform shape and are often surrounded by a network of collagen fibers. These cells can be expanded in vitro, produce a fibrocartilage matrix, and serve as seed cells for cytotherapy to aid in repairing meniscal injuries. MeSCs are capable of multilineage differentiation and express mesenchymal stem cell (MSC)-specific markers7. MeSCs are hypothesized to adapt better to the microenvironment of meniscal tissues because of their long-term presence in these tissues. Therefore, these cells are predicted to be ideal seed cells for cytotherapy or tissue engineering for meniscal injuries. Furthermore, research indicates that MeSCs have a greater capacity for chondrogenic differentiation than MSCs isolated from other sources8. Consequently, numerous studies have focused on the use of MeSCs in meniscal tissues and cell therapy for meniscal injuries, with favorable outcomes9,10.
MeSCs may be derived from meniscal tissue excised via arthroscopic surgery or meniscectomy. The primary technique for isolating MeSCs involves mechanically fragmenting the meniscus tissue and then subjecting it to enzymatic digestion using collagenase. The tissue is then cultured in vitro by expanding the cells in high-glucose or low-glucose (DMEM), α-MEM or DMEM/F12 medium7,11,12,13,14,15. The primary approach for identifying MeSCs is to evaluate their phenotype and ability to differentiate into three separate lineages, analogous to those observed in MSCs12,13,14,15. Although a significant amount of research has been conducted on MeSCs for cell therapy and tissue engineering, there is a lack of literature concerning improvements in MeSC culture techniques. One of the most significant differences between these media is their glucose concentration. Achieving successful survival and facilitating the expansion of cells from meniscal tissue are challenging for two primary reasons. First, the harvested tissue is on a small scale. Second, the tissue has previously been exposed to pathologically harsh conditions in the tissue microenvironment, which may deleteriously affect the MeSCs. Therefore, modifying the culture media components during in vitro expansion of cells would be beneficial for the treatment of meniscal injuries. For example, in one study, the addition of xanthosine significantly expanded tissue-specific stem cells isolated from the mammary gland16,17.
Glucose in the culture medium is essential for MSC growth. However, exposure to a high-glucose (HG) microenvironment is generally harmful to stem cells, resulting in decreased proliferation, differentiation, migration capacity, and mitochondrial activity18,19,20. Moreover, high-glucose microenvironments may expedite the senescence of MSCs21. Several critical mechanisms are involved, including the formation of advanced glycation end products, activation of protein kinase C (PKC), and dysregulation of the PI3K/Akt or Bcl-2/Bax signaling pathways. These processes lead to increased oxidative stress due to increased levels of reactive oxygen species (ROS), increased production of proinflammatory cytokines, increased levels of growth factors, abnormally high levels of gaseous transmitters, and altered cell bioenergetics, among other effects. Previous results indicate that the intracellular signaling pathways influenced by the high-glucose microenvironment in MSCs include the Akt/mTOR21, PI3K/TSC/mTOR18, PI3K/Akt/PTEN22 and Akt/Bcl-2/Bax23 pathways.
The effect of glucose concentration on MSCs remains a subject of debate. Most studies have shown that high-glucose media can hinder the proliferation capacity, differentiation potential, migration ability, mitochondrial activity and senescence of MSCs24,25. On the other hand, some other studies have indicated that high-glucose media can promote MSC proliferation26. Therefore, investigating the effects of different glucose concentrations on MeSCs cultivated in vitro is imperative. This study aimed to explore how different concentrations of glucose influence the proliferative capacity, senescence, migration and protein expression of human MeSCs. The present study hypothesized that low glucose concentrations may promote the proliferation and migration capacity of MeSCs while delaying cell senescence. These effects may be exerted through the regulation of the expression of specific genes, such as Src, PI3K, Akt, PTEN and Cdk4. The findings of this study indicate that low glucose levels promote the proliferation capacity and migration ability of MeSCs and alleviate their senescence. The PI3K/Akt-related signaling pathways may mediate the effects of low glucose. This study revealed that a lower concentration of glucose significantly promoted proliferation, reduced cell senescence and promoted migration by activating PI3K/Akt-related pathways.
Methods
MeSC culture
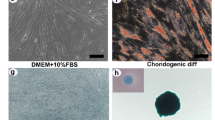
In the present study, MeSCs were isolated from the medial and lateral meniscus blocks of human patients who underwent total knee arthroplasty at Hospital Sultan Abdul Aziz Shah, Universiti Putra, Malaysia, with the approval of the Ethics Committee for Research Involving Human Subject-Universiti Putra Malaysia, Malaysia (Ethics approval No. JKEUPM-2020–26). All human sample isolation procedures adhered to the National and International Ethical Guidelines for Biomedical Research Involving Human Subjects (CIOMS) and the Guideline for Stem Cell Research & Therapy. The cell samples used in the downstream experiments were labeled with Nos. 1 to 6. The surface phenotypes of the cells were tested via flow cytometry, and their capacity to differentiate into adipogenic, osteogenic and chondrogenic lineages was determined. The results confirmed the similarities in their characteristics with those of mesenchymal stem cells and were in agreement with those of other studies12,14,27.
To assess the effects of different concentrations of glucose on MeSCs in terms of their proliferative capacity, and protein expression of certain genes, a fixed density of 1,500 viable cells/cm2 were plated in 6-well plates and cultured for 10 days before every passage.
To evaluate the influence of glucose concentration on MeSC senescence, passage 3 cells were seeded in 6-well plates or T25 flasks, cultured until they reached 80% confluence and detached with trypsin. One well was then subdivided into three wells, and the culture was continued.
The cells were cultured in an incubator (37 °C, 5% CO2) with two commonly employed MSC/MeSC expansion media:
-
1.
DMEM(LG) medium: Low-glucose DMEM (glucose concentration, 5.5 mM) (Nacalai Tesque, Kyoto, Japan), 10% Fetal bovine serum (FBS, Sigma, Sigma, St. Louis, MO, USA), 1% penicillin/streptomycin (Nacalai Tesque, Kyoto, Japan), and 2 mM GlutaMAX (Solarbio, Beijing, China).
-
2.
DMEM(HG) medium: High-glucose DMEM (glucose concentration 25 mM) (Nacalai Tesque, Kyoto, Japan), 10% FBS, 1% penicillin/streptomycin, and 2 mM GlutaMAX (Solarbio, Beijing, China).
Each experimental group was analyzed independently. The culture medium was replaced with fresh medium 24 h after initial seeding and then every three days. To passage the cells, MeSCs were separated from the monolayer using 0.25% trypsin at 37 °C for two minutes. All the assays in this study were performed on the cells from all the six donors.
Cell proliferation
To assess the proliferative capacity of long-term passaged MeSCs, the cells were detached and collected on the 10th day of each passage. Phase-contrast images were taken to document the cell morphology on the 10th day of each passage. The trypan blue exclusion method was used to count the cells. The number of cell population doublings (NCPD) and the cell population doubling time (CPDT) were then calculated using the following equations28,29:
where Nt and Ni are the cell numbers at a specific time point t (Day 10) and at initial seeding (Day 0), respectively. The experiment was performed in triplicate. The NCPD and CPDT at passages 3 and 6 were used to test the proliferation of MeSCs at early and late passages, respectively.
Cell senescence assay
To evaluate the influence of medium components on MeSC senescence, passage 3 cells were seeded on 6-well plates, cultured until they reached 80% confluency and detached with trypsin. One well was subdivided into three wells, and the culture continued until passage 14. SA-β-Gal activity was assessed using commercially available ELISA kits (Abcam, Cambridge, UK) for the cell senescence assay. The tests were conducted following the guidelines provided by the manufacturer. The experiment was performed in triplicate.
Migration assays
A scratch-wound assay was conducted to analyze the migration of MeSCs. The assay was conducted via a previously described methodology19. In brief, passage 3 MeSCs with the density of 5,000 viable cells/cm2 were seeded onto 6-well plates, which were then incubated for 12 h at 37 °C in a 5% CO₂ incubator. Upon reaching 80% confluence, the cells were subjected to starvation by cultured with FBS-free medium for 24 h to inhibit cell proliferation. The cells were cultured with DMEM(LG) medium, DMEM(HG) medium or DMEM(LG)-PI3K inhibitor medium. A scratch was subsequently created on the cell layer utilizing a pipette tip. The surface area of the open wound was measured using an inverted phase-contrast microscope. Images at time zero (time = 0 h) were captured to record the initial area of the wounds, and the recovery of the wounded monolayers due to cell migration toward the denuded area was evaluated after 8, 16 and 24 h (time = Δh) of incubation. ImageJ software (NIH) was used to conduct image analysis. The experiment was performed in triplicate. The migration rate was determined using the following formula:
ELISA
The levels of total Akt (ab126433), phospho-Akt (Ser473) (p-Akt, ab126433), total PTEN (ab206979), total mTOR (ab279869), phospho-mTOR (p-mTOR) (ab279869), total GSK3β (ab279815), phospho-GSK3β (Ser9) (p-GSK3β, ab279815), total Bcl-2 (ab272102), total Bax (ab199080), total p21 (ab214658), total p53 (ab171571), total Caspase-3 (ab285337), total Caspase-9 (ab119508), total CXCR-4 (ab287804), total Cyclin D1 (ab214571), TNF-α (ab181421), TNFSF10 (ab285340), Nanog (ab236720), Oct4 (ab235653), Sox2 (245,707), NESTIN (ab287833), Notch-1 (ab155437) and Ki67 (ab253221) were assessed using commercially available ELISA kits (Abcam, Cambridge, UK). Total PI3K (MBS268899), phospho-PI3K (p-PI3K, MBS167579), nonphospho (Active) β-catenin (MBS167579), total Cdk4 (MBS700490) and Sema3B (MBS761222) were assessed using commercially available ELISA kits (MyBioSource, San Diego, CA, USA). TRAIL/TNFSF10 (DTRL00) was assessed using commercially available ELISA kits (R&D Systems, Inc., Minneapolis, MN, USA). The cells of passage 3 were detached from 6-well plates or T25 flasks with trypsin and washed with phosphate-buffered saline (PBS). Then, 4 × 106 cells were resuspended in 100 μL of cell lysis buffer. The lysates were incubated with shaking at 2–8 °C for 30 min and then centrifuged at 13,000 rpm for 10 min at 2–8 °C. The cell lysates were used for ELISA tests immediately following the guidelines provided by the manufacturer. The absorbance values were measured by microplate reader (Flx 800 Fluorescence Microplate Reader; Agilent, CA, USA) at 450 nm.
The standard solutions with known concentrations which provided by the manufacturer were used to calculate the standard curve. The microplate reader measured the absorbance values and then plotted the concentration of each standard (X-axis) against its corresponding signal intensity (Y-axis) on a chart. Then, the curve fitting method was used to fit the data points to obtain the equation of the standard curve. The concentration of the samples was calculated using the standard curve. The relative expression levels were then calculated for each experimental group relative to the control group for statistical analyses. The experiment was performed in triplicate.
Inhibition of the PI3K signaling pathway
To determine the effect of the PI3K/Akt-related signaling pathway on the influence of low glucose concentrations on the proliferation, migration and protein expression of MeSCs, we used the PI3K inhibitor LY294002 (Sigma, St. Louis, MO, USA) which dissolved in DMSO (dimethyl sulfoxide) (Sigma, St. Louis, MO, USA) was used to block PI3K. To assess the influence of DMSO solvent on MeSCs, the cells were pretreated with DMEM (LG) that contained the same concentration of DMSO for a duration of 24 h. For the proliferation assay, a fixed density of 1,500 viable cells/cm2 of passage 3 MeSCs were plated into 6-well plates and pretreated with the indicated concentration (10 μM) of LY294002 for 24 h. Then, the MeSCs were cultured with DMEM(LG) for 9 days. For the protein expression test, a fixed density of 5.0 × 104 cells/well were seeded into a 6-well plate and treated with the indicated concentration (10 μM) of LY294002 for 48 h. Then, proliferation, migration and protein expression were tested using the methods described in the previous section. The experiment was performed in triplicate.
Results
Low glucose concentrations increased the proliferative capacity of MeSCs
During early passages (passage 3), most MeSCs maintained a spindle shape when cultured in either DMEM(LG) or DMEM(HG), as observed through microscopy. However, at a later passage (passage 6), the cell morphologies diverged from those of the earlier passages, with some cells displaying irregular, flattened shapes and increased size (Fig. 1a,b). By passage 6, both the DMEM(LG) and the DMEM(HG) groups presented significant morphological heterogeneity in the MeSC populations.
Propagation of MeSCs. Panels (a) and (b) show representative micrographs showing the morphology of MeSCs in vitro. Images were taken on Days 7 and 10 to capture the morphological representation of MeSCs that were exposed to either DMEM(LG)- or DMEM(HG)-based culture conditions at passage 3 or passage 6. Scale bars = 500 μm.
Low glucose concentrations increased the proliferative capacity of MeSCs and upregulated proliferation-related genes
The rates of growth at passage 3 and passage 6 were compared between the DMEM(LG) and DMEM(HG) groups. The findings revealed that the mean NCPD for the DMEM(LG) group was 4.39% and 2.10% greater than that of the DMEM(HG) group. The average CPDT for the DMEM(LG) group was 9.31% and 4.18% lower than those for the DMEM(HG) group (Fig. 2a,b). The expression of Ki67 in passage 3 of the DMEM(LG) group was 21.18% greater than that in the DMEM(HG) group (Fig. 2c). These results indicated that a lower concentration of glucose (5.5 mM) promoted the proliferation of MeSCs.
Cell proliferation and Ki67 protein expression results of MeSCs cultured in DMEM(LG) and DMEM(HG). (a) The NCPD of the DMEM(LG) group was significantly greater than that of the DMEM(HG) group at passages 3 and 6. The test was performed on the cells from all the six donors. The experiment was performed in triplicate, and the mean value of every sample was use for group comparisons. Group comparisons were performed using the paired t-test. n = 6. (b) The CPDT of the DMEM(LG) group was significantly lower than that of the DMEM(HG) group at passages 3 and 6. The test was performed on the cells from all the six donors. The experiment was performed in triplicate, and the mean value of every sample was use for group comparisons. Group comparisons were performed using the paired t-test. n = 6. (c) Ki67 expression in passage 3 MeSCs from the DMEM(LG) and DMEM(HG) groups were tested by ELISA. Relative levels were calculated as the relative change from the DMEM(HG) group. The test was performed on the cells from all the six donors. The experiment was performed in triplicate, and the mean value of every sample was use for group comparisons. Group comparisons were performed using the paired t-test. The experiment was performed in triplicate. n = 6.
Low glucose concentrations decelerated the process of MeSC senescence
In the present study, cell senescence was examined using a comparative approach. This was achieved by assessing β-GAL levels in the DMEM(LG) and DMEM(HG) groups. The assessment was performed at passage 14, with cells undergoing a single split into three in each passage. The ELISA results revealed that β-GAL levels in the DMEM(LG) group were 13.46% lower than those in the DMEM(HG) group (Fig. 3). These results provide preliminary evidence that culture media with lower glucose concentrations can decelerate cell senescence.
Effect of glucose on MeSC senescence in the DMEM(LG) and DMEM(HG) groups at passage 14. Compared with those in the DMEM(LG) group, the relative levels of β-GAL in the DMEM(HG) group were significantly greater. Relative levels were calculated as the relative change from the DMEM(HG) group. The test was performed on the cells from all the six donors. The experiment was performed in triplicate, and the mean value of every sample was used for group comparisons. Group comparisons were performed using the paired t-test. n = 6.
Low glucose concentrations increased the migratory capacity of MeSCs
We examined the migration of MeSCs from passage 3 cells in the proliferation test through scratch-wound assays. The results indicated that the migration rates of the DMEM(LG) group were 24.61%, 13.20%, and 10.39% greater than those of the DMEM(HG) group at 8 h, 16 h, and 24 h, respectively. There was no significant difference between the DMEM(LG) group and the DMEM(LG)-DMSO group. These results provide preliminary evidence that culture media with lower glucose concentrations promote the migration ability of MeSCs (Fig. 4).
Scratch-wound assay results for the DMEM (LG), DMEM(HG) and DMEM(LG)-PI3K inhibitor groups. The cells were from passage 3. (a) The percentage migration rate was calculated after 8, 16 and 24 h of incubation. The test was performed on the cells from all the six donors. The experiment was performed in triplicate, and the mean value of every sample was used for group comparisons. Paired t-test was performed to compare differences between two experimental groups. The p value of DMEM(LG) was compared with DMEM(HG) group, the p value of DMEM(LG)-inhibitor was compared with DMEM(LG) group, and the p value of DMEM(LG)-DMSO was compared with DMEM(LG) group. n = 6. (b) Images of the scratch-wound assays showing the migration rate of the MeSCs after 8, 16 and 24 h of incubation. The images were captured at a magnification of 40 × . (b) Images of the scratch-wound assays showing the migration rate of the MeSCs at 8, 16 and 24 h of incubation in the DMEM(LG) group or DMEM(HG) group. The images were captured at a magnification of 40 × .
A PI3K inhibitor blocked PI3K/Akt-related pathways and inhibited the effects of low glucose concentrations on proliferation and migration
To verify the effect of the PI3K/Akt-related signaling pathway on the impact of low glucose concentrations on the proliferation, migration and protein expression of MeSCs, we used a PI3K inhibitor to inhibit PI3K in MeSCs and cultured the MeSCs with DMEM(LG). The findings revealed that the mean NCPD for the DMEM(LG)-PI3K inhibitor group was 3.90% lower than that of the DMEM(LG) group (Fig. 5a). The average CPDT value for the DMEM(LG)-PI3K group was 8.97% greater than that of the DMEM(LG) group (Fig. 5b). We examined the migration of MeSCs from passage 3 cells by scratch-wound assays. The results indicated that the migration rates of the cells in the DMEM(LG)-PI3K inhibitor group were 40.1%, 25.0% and 16.3% lower than those of the cells in the DMEM(LG) group at 8 h, 16 h and 24 h, respectively (Fig. 4a). These findings showed that the PI3K inhibitor blocked the effects of a lower glucose concentration (5.5 mM) on promoting the proliferation and migration capacity of MeSCs.
NCPD and CPDT of DMEM(LG), DMEM(HG) and the DMEM(LG)-PI3K inhibitor at passage 3. (a) The NCPD of the DMEM(LG) group was significantly greater than that of the DMEM(HG) and DMEM(LG)-PI3K inhibitor groups at passage 3. There was no significant difference between the DMEM(LG) group and the DMEM(LG)-DMSO group. (b) The CPDT of the DMEM(LG) group was significantly lower than that of the DMEM(HG) and DMEM(LG)-PI3K inhibitor groups at passage 3. There was no significant difference between the DMEM(LG) group and the DMEM(LG)-DMSO group. All the tests were performed on the cells from all the six donors. The experiment was performed in triplicate, and the mean value of every sample was used for group comparisons. Comparisons between two experimental groups were performed using the paired t-test. n = 6.
Low glucose concentrations activated the PI3K/Akt-related pathways in MeSCs
Given that the glucose concentration in the culture medium was observed to influence MeSC proliferation, migration, and senescence in the experiments above, to investigate the molecular mechanisms underlying these results, the differences in the levels of proteins involved in these processes in passage 4 cells between the DMEM(LG) and DMEM(HG) groups were evaluated using ELISA. In the DMEM(LG) group, the levels of p-PI3K, p-Akt (Ser473), p-GSK3β (Ser9), p-mTOR, β-catenin, Bcl-2, Cdk4, Cyclin D1, CXCR-4 and ki67 increased by 132.52%, 32.39%, 87.72%, 66.32%, and 78.04%, respectively. The levels of total PI3K, total Akt, total GSK3β and total mTOR were not significantly different between the groups (Fig. 6).
PI3K/Akt-related pathway protein analysis in the DMEM(LG) and DMEM(HG) groups. In passage 4 cells, the levels of p-PI3K, p-Akt (Ser473), p-GSK3β (Ser9), p-mTOR, active β-catenin, Bcl-2, Cdk4, CXCR-4 and Cyclin D1 were significantly increased in the DMEM(LG) group, and the level of Bax was significantly decreased in the DMEM(LG) group. Relative levels were calculated as the relative change from the DMEM(HG) group. The test was performed on the cells from all the six donors. The experiment was performed in triplicate, and the mean value of every sample was used for group comparisons. Group comparisons were performed using the paired t-test. n = 6.
Low glucose concentrations activated the Bcl-2/Bax pathway in MeSCs
Research has revealed that high-glucose microenvironments decrease p-Akt and Bcl-2 expression while increasing Bax expression in bone marrow mesenchymal stem cells (BMSCs)23. To verify this phenomenon in MeSCs, the current study tested the differences in the levels of Bcl-2 and Bax in passage 4 cells from DMEM(LG) and DMEM(HG) via ELISA. The level of Bcl-2 increased by 66.03%, and that of Bax decreased by 39.74% in the DMEM(LG) group (Fig. 6j, k.).
Low glucose concentrations increased the levels of proteins involved in the cell cycle-related pathway in MeSCs
High-glucose microenvironments upregulate GSK3β while downregulating Cdk4, CXCR-4 and Cyclin D1 expression in BMSCs19. In the present study, Cdk4, CXCR-4 and Cyclin D1 levels increased by 30.87%, 8.98% and 11.33%, respectively, in the DMEM(LG) group (Fig. 6i, m, and n).
Low glucose concentrations reduced the levels of apoptosis- and senescence-related proteins in MeSCs
Research has revealed that high-glucose microenvironments inhibit proliferation and enhance apoptosis- and senescence-related protein expression in BMSCs23 and pulpous-derived mesenchymal stem cells (NPMSCs)30. In the present study, p21, p53, p16, Caspase-3 and Caspase-9 levels were decreased by 34.50%, 47.7%, 22.47%, 25.40% and 22.97%, respectively, in the DMEM(LG) group (Fig. 7).
Analysis of the levels of proteins involved in apoptosis and cell senescence in the DMEM(LG) and DMEM(HG) groups. In passage 4 cells, the levels of p16, p21, p53, Caspase-3 and Caspase-9 were significantly decreased in the DMEM(LG) group. Relative expression levels were calculated as the relative change from the DMEM(HG) group. The test was performed on the cells from all the six donors. The experiment was performed in triplicate, and the mean value of every sample was used for group comparisons. Group comparisons were performed using the paired t-test. n = 6.
Low glucose concentrations have no effect on the levels of PTEN, Sema3B or TNF family proteins in MeSCs
Research has revealed that high-glucose microenvironments influence the expression of PTEN22, Sema3B20 and TNF family genes31. In the present study, the levels of PTEN, Sema3B, TNF-α and TNFSF10 did not significantly differ between the groups (Fig. 8).
Protein analysis of PTEN, Sema3B, TNF-α and TNFSF10 in the DMEM(LG) and DMEM(HG) groups. In passage 4 cells, the levels of PTEN, Sema3B, TNF-α and TNFSF10 were not significantly different between the DMEM(LG) group and the DMEM(HG) group. Relative expression levels were calculated as the relative change from the DMEM(HG) group. The test was performed on the cells from all the six donors. The experiment was performed in triplicate, and the mean value of every sample was used for group comparisons. Group comparisons were performed using the paired t-test. n = 6.
Low glucose concentrations reduced the levels of proteins related to stemness in MeSCs from one donor
Research has revealed that high-glucose microenvironments accelerate cell senescence and increase the expression of the pluripotent markers Nanog, Oct4, and Sox2 in adipose-derived stem cells (ADSCs)32. The MeSCs from all the donors showed reduced levels of Nanog, Oct4, Sox2, NESTIN and Notch-1 (Fig. 9a–e.) with the exception of those from donor No. 5 (Fig. 9f–j.).
Analysis of the levels of proteins involved in stemness in the DMEM(LG) and DMEM(HG) groups. In passage 4 cells, MeSCs from donors No. 1 to 4 and No. 6 did not significantly differ in the levels of Nanog (a), Oct4 (b), Sox2 (c), NESTIN (d) or Notch-1 (e) between the DMEM(LG) and DMEM(HG) groups. Relative expression levels were calculated as the relative change from the DMEM(HG) group. The test was performed on the cells from all the six donors. The experiment was performed in triplicate, and the mean value of every sample was used for group comparisons. Group comparisons were performed using the paired t-test. n = 5. The levels of Nanog (f), Oct4 (g), Sox2 (h), NESTIN (i) and Notch-1 (j) were significantly increased in the DMEM(LG) group of MeSCs from donor No. 5. Relative expression levels were calculated as the relative change from the DMEM(HG) group. The experiment was performed six times for each donor samples, and the mean value of every sample was used for group comparisons. Group comparisons were performed using the Mann–Whitney non-parametric. n = 6.
A PI3K inhibitor blocked the up-/downregulation of PI3K/Akt-related genes/proteins
To verify the effect of the PI3K/Akt-related signaling pathway on the influence of low glucose concentrations on the proliferation, migration and protein expression of MeSCs, we used a PI3K inhibitor to inhibit PI3K in MeSCs and cultured the MeSCs with DMEM(LG). The differences in protein expression among passage 4 cells in the DMEM(LG), DMEM(HG) and DMEM(LG)-PI3K inhibitor groups were measured using ELISA. In the DMEM(LG)-PI3K inhibitor group, p-Akt (Ser473), p-mTOR, β-catenin, Bcl-2, Cdk4, Cyclin D1, and CXCR-4 expression was lower than that in the DMEM(LG) group (Fig. 10); the expression of p-GSK3β (Ser9) was lower than that in the DMEM(LG) group (Fig. 10 b). The Bax, p21, p53, p16, Caspase-3 and Caspase-9 expression levels were greater than those in the DMEM (LG) group (Fig. 10). All the protein levels between the DMEM(LG) groups and the DMEM(LG)-DMSO group showed no significant differences. These results indicated that the PI3K inhibitor blocked the effect of low glucose on PI3K/Akt-related pathways and further inhibited the downstream Bcl-2/Bax, Cdk4/Cyclin D1/CXCR-4 and apoptosis/senescence signaling pathways. Combined with the results of the proliferation and migration assays, we can preliminarily confirm that the lower glucose concentration promoted the proliferation and migration ability of MeSCs by activating PI3K/Akt-related pathways.
Protein analysis in the DMEM(LG), DMEM(HG) and DMEM(LG)-PI3K inhibitor groups. Relative expression levels were calculated as the relative change from the DMEM(HG) group. The test was performed on the cells from all the six donors. The experiment was performed in triplicate for each donor sample, and the mean value of every sample was used for group comparisons. Group comparisons were performed using the paired t-test. n = 6.
Discussion
Despite positive results on the use of MeSCs in meniscus repair and tissue engineering8,33,34, the influence of culture medium constituents on MeSCs has yet to be investigated. The basic media used for MeSC culture include high-glucose or low-glucose (DMEM), α-MEM and DMEM/F12; the choice of culture medium for MeSCs has not been uniform. The ideal composition of the medium has been shown to increase the proliferation, differentiation, and migration abilities of MSCs and maintain their stemness. A substantial body of research has demonstrated that the glucose concentration in the culture medium significantly influences the proliferation, differentiation, and migration capabilities of MSCs19,22,31,35. Because MeSCs are a special type of MSC that is more suitable for the cytotherapy of meniscal injury, this study sought to optimize the medium composition specifically for MeSCs by investigating the effect of the glucose concentration in the medium on MeSCs. In this study, MeSCs from six donors were cultured in media with two different glucose concentrations: 5.5 mM (low) and 25 mM (high). The proliferative and migratory capacities of the MeSCs were assessed by cell viability and wound-healing assays. Cell senescence was evaluated by a β-GAL assay, whereas protein level and phosphorylation was evaluated using ELISA. A previous study suggested that cells might only exhibit harmful effects after prolonged exposure to high-glucose culture conditions, as they can tolerate short-term exposure36. In this study, MeSCs were cultured for 10 days at each passage, the proliferation at passages 3 and 6 was tested, and passage 4 cells were used as ELISA samples. Compared with those in the DMEM (HG) group, the proliferative capacity and migration ability in the DMEM(LG) group were significantly greater (Figs. 2 and 3).
The results of the cell propagation experiment revealed that DMEM(LG) effectively promoted the proliferation of MeSCs during both passage 3 and passage 6) (Fig. 2). Significant decreases in the NCPD and CPDT were observed in both the DMEM(LG) and the DMEM(HG) groups at passage 6 (Fig. 2). Compared with those at passage 3, the differences in NCPD and CPDT between the DMEM(LG) and DMEM(HG) groups were smaller at passage 6; in other words, the DMEM(HG) group maintained relatively stable NCPD and CPDT throughout the passages. These findings are consistent with prior research on human BMSCs19,28. Protein expression analysis revealed higher levels of the cell proliferation marker Ki67 in passage 4 cells from the DMEM(LG) group than in those from the DMEM(HG) group (Fig. 6).
The ELISA results demonstrated greater activity of the PI3K/Akt signaling pathway in the DMEM(LG) group than in the DMEM(HG) group (Fig. 6). This pathway is essential for regulating essential cellular processes, including cell proliferation, differentiation, apoptosis, and migration37. The primary downstream target of PI3K is Akt, which triggers responses from various downstream proteins to regulate cellular functions. Akt activation can also increase the levels of the antiapoptotic protein Bcl-2 via the mammalian target of the rapamycin (mTOR) pathway, supporting cell survival38,39. Akt also inhibits GSK3β, further supporting cell survival and promoting cell proliferation40. As a result, tissue engineering approaches and various stem cell therapies have aimed to increase MSC function and support tissue and organ repair by activating the PI3K/Akt pathway41. Phosphorylation of Akt at Ser473 represents a common mode of Akt activation induced by PI3K upstream of the PI3K/Akt pathway41,42. Activated Akt can inhibit the activity of GSK3β (Ser9 site) and activate mTOR (Ser2448 site) through phosphorylation, thereby promoting MSC proliferation and differentiation41,42,43. The findings of this study demonstrated that MeSCs cultured in the DMEM(LG) group presented higher expression levels and increased p-Akt (Ser473) levels than those cultured in the DMEM(HG) group did, whereas the total Akt concentration remained relatively unchanged. Compared with those in the DMEM (LG) and DMEM (HG) groups, the expression of p-Akt in MeSCs pretreated with a PI3K inhibitor in DMEM (LG) was lower (Fig. 6). The rate of cell proliferation was also reduced in parallel (Fig. 2). Therefore, lower glucose concentrations activated the PI3K/Akt pathway by upregulating PI3K expression, which in turn promoted the proliferation of MeSCs. These findings are in accordance with those previously reported for in vitro cultured BMSCs21,22,23.
mTOR is a conserved protein kinase belonging to the PI3K-associated kinase family and responds to stimuli such as stress, starvation, and hypoxia44. mTOR regulates cell proliferation and metabolism as well as other biological functions in a wide range of cell types; for example, it promotes the translation and synthesis of proteins responsible for regulating proliferation44,45. The activation of mTOR can also induce cell growth, which is achieved by promoting protein transcription and translation and inhibiting autophagy44,45. The PI3K/Akt/mTOR pathway is a key intracellular signaling pathway that is activated by nutrient availability, hormones, and growth factors and is well recognized for its role in regulating proliferation and cell growth46. Akt activates mTOR via the phosphorylation of mTOR at the Ser 2448 (S2448) and Ser 2481 (S2481) sites, as it is positioned upstream of mTOR46. This study revealed no significant difference in overall mTOR expression between the DMEM(LG) and DMEM(HG) groups. However, the level of p-mTOR (Ser2488) was notably greater in the DMEM(LG) group. Furthermore, the levels of p-mTOR, NCPD, and CPDT were lower in the DMEM(LG)-PI3K inhibitor group than in both the DMEM(LG) and the DMEM(HG) groups. These findings underscore the critical role of the PI3K/Akt/mTOR pathway in enhancing MeSC proliferation under low-glucose conditions. In contrast to the results obtained in this study, previous findings concerning the effect of glucose concentration on the PI3K/Akt/mTOR signaling pathway are controversial. A study on rat MSCs demonstrated that high glucose levels upregulated p-Akt and p-mTOR21. Conversely, research on human adipose tissue-derived MSCs (hAD-MSCs) revealed that high glucose levels suppressed the PI3K/Akt/mTOR signaling pathway18.
Glycogen synthase kinase 3β (GSK3β) is an important serine and threonine protein kinase and an important target of Akt. The PI3K/Akt/GSK3β pathway is crucial for regulating cell proliferation, metabolism, and apoptosis. Additionally, GSK3β is involved in MSC migration47. GSK3β activity acts as a central regulator that integrates signaling inputs and controls the stability and phosphorylation of β-catenin in Wnt signaling. The Wnt/β-catenin axis includes a series of proteins that are closely involved in embryonic development and regulating homeostasis in adult tissues48,49. Inhibiting GSK3β can promote MSC migration by increasing CXCR-4 and β-catenin expression. β-catenin is a multifunctional protein involved in gene regulation and cell adhesion. In humans, the CTNNB1 gene encodes the β-catenin protein48,50. Numerous studies have demonstrated that the β-catenin-related signaling pathway in stem cells, which is activated by factors such as drugs or cytokines, disrupts the destruction complex comprising Axin, APC, CK1, and GSK3β. Once liberated, β-catenin accumulates in the cytoplasm and subsequently translocates to the nucleus50,51,52,53. A study on MSC migration revealed that hepatocyte growth factor (HGF) activates β-catenin signaling in MSCs through two primary mechanisms: phosphorylation of LRP6 or inhibition of GSK3β phosphorylation via the ERK1/2 and Akt pathways54. This activation is further supported by increased expression and nuclear translocation of TCF4, leading to Nedd9 upregulation, which is crucial for enhancing focal adhesion generation and turnover, as well as F-actin polymerization, thereby promoting chemotactic migration54. In this study, the results of the scratch-wound migration assay revealed that DMEM(LG) effectively promoted MeSC migration (Fig. 4). The migration rate of cells in the DMEM(LG)-PI3K inhibitor group was lower than that in both the DMEM(LG) and the DMEM(HG) groups. The ELISA results revealed no significant difference in the total expression of GSK3β between the DMEM(LG) and DMEM(HG) groups. However, the levels of p-GSK3β (Ser9) and β-catenin were significantly greater in the DMEM(LG) group. In brief, the p-GSK3β, β-catenin, NCPD, CPDT, and migration rate in the DMEM(LG)-PI3K inhibitor group were lower than those in the DMEM(LG) and DMEM(HG) groups. These findings indicate that the PI3K/Akt/GSK3β pathway is crucial for the low glucose-induced proliferation and migration of MeSCs. Prior studies have indicated that high glucose can inhibit the proliferation and migration of human BMSCs through the activation of GSK3β and the inhibition of β-catenin19, which is consistent with the current results.
The chemokine receptor CXCR4 promotes cell growth by inducing the expression of Cyclin D1 and facilitating cell cycle progression by β-catenin and PI3K signaling55,56. Cyclin D1, a target of the canonical Wnt pathway, plays an essential role in regulating the transition from G1 to S phase during cell proliferation. Cyclin D1 regulates the cell cycle and proliferation in association with CDK4/655,57. Previous studies have shown that inhibition (phosphorylation at the Ser9 site) of GSK3β promotes MSC migration by increasing the expression of CXCR-4 and Cyclin D119. In this study, the ELISA results revealed that the levels of p-GSK3β, β-catenin, Cyclin D1 and CXCR-4 were markedly greater in the DMEM(LG) group than in the DMEM(HG) group. Low glucose inhibited GSK3β by phosphorylating it but activated the expression of CXCR-4 and Cyclin D1. In brief, the p-GSK3β, β-catenin, CXCR-4, and Cyclin D1 levels and the rates of migration in the DMEM(LG)-PI3K inhibitor group were lower than those in the DMEM(LG) and DMEM(HG) groups. These findings indicate that the PI3K/GSK3β/β-catenin/CXCR-4/Cyclin D1 pathway is crucial for the low glucose-induced migration of MeSCs. A previous study also demonstrated that high glucose could inhibit the proliferation and migration of human BMSCs through the suppression of CXCR-4, Cyclin D1 and Cyclin D119, which was consistent with the results of the present study.
Bcl-2 and Bax, key members of the Bcl family, are essential regulators of apoptosis, influencing the permeability of the mitochondrial membrane, cytochrome c release, and mitochondrial function58,59. It is believed that Bcl-2 prevents cytochrome C release by counteracting Bax, thereby inhibiting the activation of apoptotic pathways and promoting cell survival60,61. The results of the present study support this hypothesis, as lower glucose concentrations activated the Bcl-2/Bax pathway. The results obtained from this study were similar to those of another study, which showed that high glucose downregulated the expression of Bcl-2 and upregulated the expression of BAX.
Sema3B has been implicated in promoting the osteogenic differentiation and proliferation of BMSCs through the Akt pathway under high-glucose conditions, where high glucose levels reduce Sema3B expression and inhibit MSC proliferation, Sema3B significantly increased Akt phosphorylation20. However, pretreatment with an Akt inhibitor prevented the increase in ALP activity and proliferation of BMSCs in a high-glucose environment, suggesting that Sema3B supports osteogenic differentiation by modulating the Akt pathway under such conditions20. This effect, however, was not evident in the current study. The only in vitro study investigating the downregulation of Sema3B expression by high glucose was conducted using mouse BMSCs and and did not use inhibitors to validate the effect of Sema3B on the Akt pathway20. We propose that the differences between the results of the present study and those of previous studies may arise from the distinct molecular mechanisms through which MSCs from different species, or that high glucose levels do not influence the Akt pathway via Sema3B. However, these specific molecular mechanisms need to be validated using other human MSCs.
The function of PTEN is dependent on the cell type, particularly with respect to cellular proliferation62. Hyperglycemia has been shown to increase PTEN activation and inhibit Akt phosphorylation, leading to insulin resistance in atrial biopsies and cellular apoptosis in renal tubular cells62,63,64. Exposure of mouse bone marrow-derived mesenchymal stem cells (BMSCs) to high glucose levels has been associated with decreased miR-32-5p expression, increased PTEN expression, and PI3K/Akt signaling pathway inhibition22. In contrast, miR-32-5p overexpression resulted in the downregulation of PTEN expression, alleviated the inhibitory effects of high glucose on the PI3K/Akt signaling pathway, and accelerated cell cycle progression from the G0/G1 phase to the G2/M and S phases22. Based on these clues, the current study explored PTEN expression in MeSCs at varying glucose concentrations. However, this effect was not observed in the present study. The only in vitro study investigating the downregulation of PTEN expression by high glucose was conducted using mouse BMSCs22. We propose that the differences between the results of the present study and those of previous studies may arise from the distinct molecular mechanisms through which MSCs from different species or tissues respond to varying glucose concentrations. However, these specific molecular mechanisms need to be validated using other human MSCs.
A previous study revealed that high-glucose conditions led to increased stemness protein expression in adipose-derived stem cells (ADSCs) treated with high glucose32. In the present study, only the cells from donor No. 5 presented upregulated expression of stemness genes (Fig. 9). No notable specificity was identified in the patient examination or diagnostic information of donor No. 5. Therefore, this phenomenon is attributed to individual differences.
Tumor necrosis factor-α (TNF-α) is a key regulator of MSC function and fate65,66. As a member of the TNF superfamily, which accounts for 70–95% of the activity of the TNF superfamily, TNF-α plays a critical role in inflammation, carcinogenesis, and the immune response. It is an essential signaling molecule that orchestrates inflammatory and immune responses and can trigger apoptosis and programmed necrosis65. Previous studies have suggested that members of the TNF family, particularly TNF and TNFSF10, are significantly upregulated in gingival MSCs under high-glucose conditions31. However, this effect was not observed in the present study. The only study examining the impact of glucose concentration on the expression of TNF family genes in MSCs utilized Gingival Mesenchymal Stem Cells (GMSCs)31. It employed higher glucose concentrations (50 mM and 75 mM) compared to the HG group in the current research. The differences in findings between the present study and previous research might be attributed to the varying glucose concentrations used or the distinct tissue origins of the MSCs.
The results of the β-GAL assay demonstrated the efficacy of DMEM(LG) in alleviating the senescence of MeSCs (Fig. 3). Glucose plays an essential role in regulating cellular senescence, and diabetes has been shown to alter the expression of chemokines in MSCs67. Culturing MSCs in high-glucose medium has been shown to accelerate cell senescence, genomic instability, and telomere alterations52,68. An in vitro study on BMSCs revealed that high glucose increased the number of SA-β-Gal-positive cells and increased the expression of p53, p16, and p2121. In contrast, Akt inhibition was shown to counteract the stimulatory impact of high glucose on BMSC senescence21. In this study, the expression of proteins associated with cell senescence, including Caspase-3, p21, p16, and p53, was reduced in the DMEM(LG) group but elevated in the DMEM(LG)-PI3K inhibitor group compared with the DMEM(LG) and DMEM(HG) groups. These findings suggest that low glucose mitigated MeSC senescence via the PI3K/Akt signaling pathway. However, the protein expression profile observed in this study differs from that reported in previous studies on BMSCs. While high glucose was reported to suppress BMSC proliferation and accelerate cellular senescence predominantly through the Akt/mTOR signaling pathway, no significant changes in p-GSK3β or β-catenin expression were detected21. At this time, the reason for this discrepancy remains unclear.
The study has several limitations. This study chose the most commonly used concentrations in the basal medium (5.5 mM and 25 mM) to compare the effect of low and high glucose, which may not include the optimal glucose concentration for MeSCs. This study focused primarily on the effects of medium composition on MeSCs, and did not confirm the impact of glucose on the chondrogenic differentiation potential of MeSCs, which is a key point of cytotherapy for meniscal injury. Previous findings have demonstrated that a high-glucose microenvironment enhances the lipogenic differentiation potential of BMSCs cultured in vitro by upregulating the expression of the adipogenic genes PPARγ and LPL69; promotes the osteogenic differentiation of BMSCs by upregulating RUNX2, OSX69 and Sema3B20; and can promote the chondrogenic differentiation of BMSCs70. Another study of umbilical cord mesenchymal stem cells (UCMSCs) showed that a high glucose microenvironment inhibits the chondrogenic differentiation potential of the cells71. Other studies reported that high glucose exposure on human MSCs derived from the bone marrow and various gestational tissues, including chorionic villi, placenta, and umbilical cord could inhibit osteogenic differentiation69. Altogether, these findings indicate that glucose concentration influences the differentiation potential of MSCs, and thus future studies to investigate the chondrogenicity of MeSCs will be highly valuable.
Furthermore, research has demonstrated that the components of culture medium can affect the positivity rate of MSC-specific phenotypes, such as CD73, CD90, and CD10572,73. However, these studies have not explored the molecular mechanisms that lead to changes in MSC-specific phenotypes, and there is a lack of research on the impact of glucose concentration on these phenotypes.
Noteworthy, the cells used were from aged donors with meniscal injuries, which may have resulted in lower cell viability than those from younger, healthier donors. The current study did not collect meniscus tissue blocks from young donors because this would require donors who have experienced severe acute meniscus injury, which are challenging to find. Furthermore, stem cells cultivated in 2D and 3D environments may exhibit divergent characteristics, which may consequently influence the results of experimental studies. To obtain more comprehensive and reliable results, it is necessary to isolate and culture young donor-derived MeSCs in the future and test their response to different glucose concentrations in 2D and 3D culture environments.
Several other studies have reported the effects of glucose concentration on various signaling pathways. One particular study indicated that high glucose levels promoted the proliferation of BMSCs by activating the Ca2+/PKC/MAPKs signaling pathway74. However, this finding contradicted the conclusions of other studies, this signaling pathway was not investigated in this study. A high glucose microenvironment has been shown to enhance cell migration by increasing ROS in human umbilical cord blood-derived mesenchymal stem cells (hUCB-MSC)75. ROS can regulate cellular functions through various signaling pathways. Therefore, the effect of glucose on Ca2+/PKC/MAPKs or other signaling pathway and ROS in MeSCs is a crucial area that needs further exploration in future MeSC studies. Despite that, the findings from the current study could offer valuable insights for enhancing the application of MeSCs in tissue engineering and cell therapy.
Conclusion
This study investigated the impact of glucose concentration on MeSCs and the underlying molecular mechanisms, aiming to establish a foundation for advancing improved techniques for applying MeSCs in tissue engineering and cell therapy. These findings demonstrated that high glucose levels significantly inhibited MeSC proliferation and migration and aggravated the senescence of MeSCs. The effects of glucose were found to be mediated by PI3K/Akt-related signaling pathways. Additionally, notable morphological changes were observed between cells from early and later passages. These results indicate that cell therapy and tissue engineering using MeSCs should prioritize the use of cells from early passages. These findings provide a theoretical basis for optimizing methods for the application of MeSCs in tissue engineering and cell therapy.
Statistical analysis
Statistical analysis was conducted using SPSS 27 (IBM, Chicago, IL, US). Data are presented as mean ± SEM. Group comparisons were performed using either the paired T test or Mann–Whitney non-parametric test, as applicable. A p-value of < 0.05 was considered statistically significant.
Data availability
Data generated and analyzed in this study can be obtained from the corresponding author upon request.
References
Garcia, J. R. et al. Diagnosis and treatment strategies of meniscus root tears: A scoping review. Orthop. J. Sport. Med. 12, 23259671241283960 (2024).
Hantouly, A. T. et al. Meniscus root tears: State of the art. Int. Orthop. 48, 955–964 (2024).
Popper, H. R., Fliegel, B. E., Elliott, D. M. & Su, A. W. Surgical management of traumatic meniscus injuries. Pathophysiology 30, 618–629. https://doi.org/10.3390/pathophysiology30040044 (2023).
Ishii, Y. et al. Unique patterns of medial meniscus extrusion during walking and its association with limb kinematics in patients with knee osteoarthritis. Sci. Rep. 13, 12513 (2023).
Deviandri, R. et al. Health-economic evaluation of meniscus tear treatments: A systematic review. Knee Surg. Sports Traumatol. Arthrosc. 31, 3582–3593 (2023).
Ren, X. et al. The safety, efficacy, and functional outcomes on arthroscopic fixation of posterior cruciate ligament avulsion fracture by a bio-absorbable anchor or traditional pull-out technique: A prospective cohort study. Front. Bioeng. Biotechnol. 10, 1055176 (2022).
Korpershoek, J. V. et al. Selection of highly proliferative and multipotent meniscus progenitors through differential adhesion to fibronectin: A novel approach in meniscus tissue engineering. Int. J. Mol. Sci. 22, 64 (2021).
Ding, Z. & Huang, H. Mesenchymal stem cells in rabbit meniscus and bone marrow exhibit a similar feature but a heterogeneous multi-differentiation potential: Superiority of meniscus as a cell source for meniscus repair evolutionary developmental biology and morphology. BMC Musculoskelet. Disord. 16, 1–14 (2015).
Tong, J. B. et al. Current advances in the development of meniscus tissue engineering: narrative review. Med. J. Malaysia 78, 534–540 (2023).
Bian, Y. et al. Advances in meniscus tissue engineering: Towards bridging the gaps from bench to bedside. Biomaterials 312, 122716 (2025).
Simson, J. A., Strehin, I. A., Allen, B. W. & Elisseeff, J. H. Bonding and fusion of meniscus fibrocartilage using a novel chondroitin sulfate bone marrow tissue adhesive. Tissue Eng. Part A 19, 1843–1851 (2013).
Verdonk, P. C. M. et al. Characterisation of human knee meniscus cell phenotype. Osteoarthr. Cartil. 13, 548–560 (2005).
Shen, W. et al. Intra-articular injection of human meniscus stem/progenitor cells promotes meniscus regeneration and ameliorates osteoarthritis through stromal cell-derived factor-1/CXCR4-mediated homing. Stem Cells Transl. Med. 3, 387–394 (2014).
Chahla, J. et al. Assessing the resident progenitor cell population and the vascularity of the adult human meniscus. Arthroscopy 37, 252–265 (2021).
Shen, W. et al. Osteoarthritis prevention through meniscal regeneration induced by intra-articular injection of meniscus stem cells. Stem Cells Dev. 22, 2071–2082 (2013).
Choudhary, R. K. & Capuco, A. V. In vitro expansion of the mammary stem/progenitor cell population by xanthosine treatment. BMC Cell Biol. 13, 14 (2012).
Choudhary, S. et al. Examination of the xanthosine response on gene expression of mammary epithelial cells using RNA-seq technology. J. Anim. Sci. Technol. 60, 18 (2018).
Abu-El-Rub, E. et al. Human mesenchymal stem cells exhibit altered mitochondrial dynamics and poor survival in high glucose microenvironment. World J. Stem Cells 15, 1093–1103 (2023).
Zhang, B. et al. High glucose microenvironments inhibit the proliferation and migration of bone mesenchymal stem cells by activating GSK3β. J. Bone Miner. Metab. 34, 140–150 (2016).
Xing, Q., Feng, J. & Zhang, X. Semaphorin3B promotes proliferation and osteogenic differentiation of bone marrow mesenchymal stem cells in a high-glucose microenvironment. Stem Cells Int. 2021, 6637176 (2021).
Zhang, D. et al. High glucose induces the aging of mesenchymal stem cells via Akt/mTOR signaling. Mol. Med. Rep. 16, 1685–1690 (2017).
Zhu, G., Chai, J., Ma, L., Duan, H. & Zhang, H. Downregulated microRNA-32 expression induced by high glucose inhibits cell cycle progression via PTEN upregulation and Akt inactivation in bone marrow-derived mesenchymal stem cells. Biochem. Biophys. Res. Commun. 433, 526–531 (2013).
Kong, Y. et al. Norepinephrine protects against apoptosis of mesenchymal stem cells induced by high glucose. J. Cell. Physiol. 234, 20801–20815 (2019).
Keats, E. & Khan, Z. A. Unique responses of stem cell-derived vascular endothelial and mesenchymal cells to high levels of glucose. PLoS ONE 7, e38752 (2012).
Lo, T., Ho, J. H., Yang, M.-H. & Lee, O. K. Glucose reduction prevents replicative senescence and increases mitochondrial respiration in human mesenchymal stem cells. Cell Transplant. 20, 813–825 (2011).
Al-Qarakhli, A. M. A., Yusop, N., Waddington, R. J. & Moseley, R. Effects of high glucose conditions on the expansion and differentiation capabilities of mesenchymal stromal cells derived from rat endosteal niche. BMC Mol. cell Biol. 20, 51 (2019).
Lee, J., Jang, S., Kwon, J., Oh, T. I. & Lee, E. Comparative evaluation of synovial multipotent stem cells and meniscal chondrocytes for capability of fibrocartilage reconstruction. Cartilage 13, 980S-990S (2021).
Yang, Y.-H.K., Ogando, C. R., Wang See, C., Chang, T.-Y. & Barabino, G. A. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res. Ther. 9, 131 (2018).
Oh, M. S. et al. Transcriptomic changes in human tonsil-derived mesenchymal stem cells across culture passages. Genes 15, 1626. https://doi.org/10.3390/genes15121626 (2024).
Liu, Y. et al. The effect of high glucose on the biological characteristics of nucleus pulposus-derived mesenchymal stem cells. Cell Biochem. Funct. 38, 130–140 (2020).
Junaid, R., Wahid, M., Waseem, F. S., Habib, R. & Hasan, A. Effect of glucose mediated oxidative stress on apoptotic gene expression in gingival mesenchymal stem cells. BMC Oral Health 21, 653 (2021).
Cheng, N.-C., Hsieh, T.-Y., Lai, H.-S. & Young, T.-H. High glucose-induced reactive oxygen species generation promotes stemness in human adipose-derived stem cells. Cytotherapy 18, 371–383 (2016).
Angele, P., Kujat, R., Koch, M. & Zellner, J. Role of mesenchymal stem cells in meniscal repair. J. Exp. Orthop. 1, 12 (2014).
Sun, H. et al. Single-cell RNA-seq analysis identifies meniscus progenitors and reveals the progression of meniscus degeneration. Ann. Rheum. Dis. 79, 408–417 (2020).
Mateen, M. A., Alaagib, N. & Haider, K. H. High glucose microenvironment and human mesenchymal stem cell behavior. World J. Stem Cells 16, 237–244. https://doi.org/10.4252/wjsc.v16.i3.237 (2024).
Weil, B. R., Abarbanell, A. M., Herrmann, J. L., Wang, Y. & Meldrum, D. R. High glucose concentration in cell culture medium does not acutely affect human mesenchymal stem cell growth factor production or proliferation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R1735–R1743 (2009).
Ramazzotti, G. et al. Phosphoinositide 3 kinase signaling in human stem cells from reprogramming to differentiation: A tale in cytoplasmic and nuclear compartments. Int. J. Mol. Sci. 20, 2026 (2019).
Zhu, Z. et al. BCL2 enhances survival of porcine pluripotent stem cells through promoting FGFR2. Cell Prolif. 54, e12932 (2021).
Liu, R. et al. PI3K/AKT pathway as a key link modulates the multidrug resistance of cancers. Cell Death Dis. 11, 797 (2020).
Guo, J. et al. Evaluation of the effect of GSK-3β on liver cancer based on the PI3K/AKT pathway. Front. cell Dev. Biol. 12, 1431423 (2024).
Chen, J., Crawford, R., Chen, C. & Xiao, Y. The key regulatory roles of the PI3K/Akt signaling pathway in the functionalities of mesenchymal stem cells and applications in tissue regeneration. Tissue Eng. Part B. Rev. 19, 516–528 (2013).
Vincent, E. E. et al. Akt phosphorylation on Thr308 but not on Ser473 correlates with Akt protein kinase activity in human non-small cell lung cancer. Br. J. Cancer 104, 1755–1761 (2011).
Figueiredo, V. C., Markworth, J. F. & Cameron-Smith, D. Considerations on mTOR regulation at serine 2448: Implications for muscle metabolism studies. Cell. Mol. Life Sci. 74, 2537–2545 (2017).
Panwar, V. et al. Multifaceted role of mTOR (mammalian target of rapamycin) signaling pathway in human health and disease. Signal Transduct. Target. Ther. 8, 375 (2023).
Liu, G. Y. & Sabatini, D. M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 21, 183–203 (2020).
Zhang, H.-P. et al. PI3K/AKT/mTOR signaling pathway: An important driver and therapeutic target in triple-negative breast cancer. Breast Cancer 31, 539–551 (2024).
Romorini, L. et al. AKT/GSK3β signaling pathway is critically involved in human pluripotent stem cell survival. Sci. Rep. 6, 35660 (2016).
Hu, L., Chen, W., Qian, A. & Li, Y.-P. Wnt/β-catenin signaling components and mechanisms in bone formation, homeostasis, and disease. Bone Res. 12, 39 (2024).
de Nys, R. et al. Proteomic analysis of the developing mammalian brain links PCDH19 to the Wnt/β-catenin signalling pathway. Mol. Psychiatry https://doi.org/10.1038/s41380-024-02482-z (2024).
Yang, C.-M. et al. β-Catenin promotes cell proliferation, migration, and invasion but induces apoptosis in renal cell carcinoma. Onco. Targets. Ther. 10, 711–724 (2017).
Wang, W. et al. Involvement of Wnt/β-catenin signaling in the mesenchymal stem cells promote metastatic growth and chemoresistance of cholangiocarcinoma. Oncotarget 6, 42276–42289 (2015).
Ren, J. et al. Time association study on a sub-acute mouse model of Parkinson’s disease. Heliyon 10, e34082 (2024).
Liu, J. et al. Wnt/β-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 7, 3 (2022).
Hu, Y. et al. Hepatocyte growth factor facilitates the repair of spinal cord injuries by driving the chemotactic migration of mesenchymal stem cells through the β-catenin/TCF4/Nedd9 signaling pathway. Stem Cells 42, 957–975 (2024).
Mo, W. et al. CXCR4/CXCL12 mediate autocrine cell- cycle progression in NF1-associated malignant peripheral nerve sheath tumors. Cell 152, 1077–1090 (2013).
Zhou, M. et al. Cell surface patching via CXCR4-targeted nanothreads for cancer metastasis inhibition. Nat. Commun. 15, 2763 (2024).
Montalto, F. I. & De Amicis, F. Cyclin D1 in cancer: A molecular connection for cell cycle control, adhesion and invasion in tumor and stroma. Cells 9, 2648 (2020).
Wang, Q. et al. The relationship between the Bcl-2/Bax proteins and the mitochondria-mediated apoptosis pathway in the differentiation of adipose-derived stromal cells into neurons. PLoS ONE 11, e0163327 (2016).
Peña-Blanco, A. & García-Sáez, A. J. Bax, Bak and beyond-mitochondrial performance in apoptosis. FEBS J. 285, 416–431 (2018).
Qian, S. et al. The role of BCL-2 family proteins in regulating apoptosis and cancer therapy. Front. Oncol. 12, 985363 (2022).
Zhang, M., Zheng, J., Nussinov, R. & Ma, B. Release of Cytochrome C from Bax Pores at the Mitochondrial Membrane. Sci. Rep. 7, 2635 (2017).
Li, Y., Ma, R. & Hao, X. Therapeutic role of PTEN in tissue regeneration for management of neurological disorders: stem cell behaviors to an in-depth review. Cell Death Dis. 15, 268 (2024).
Rane, M. J. et al. Interplay between Akt and p38 MAPK pathways in the regulation of renal tubular cell apoptosis associated with diabetic nephropathy. Am. J. Physiol. Renal Physiol. 298, F49-61 (2010).
Wang, B. et al. Differences in myocardial PTEN expression and Akt signalling in type 2 diabetic and nondiabetic patients undergoing coronary bypass surgery. Clin. Endocrinol. (Oxf) 74, 705–713 (2011).
Li, W., Liu, Q., Shi, J., Xu, X. & Xu, J. The role of TNF-α in the fate regulation and functional reprogramming of mesenchymal stem cells in an inflammatory microenvironment. Front. Immunol. 14, 1074863 (2023).
Arias, A. A. et al. Tuberculosis in otherwise healthy adults with inherited TNF deficiency. Nature 633, 417–425 (2024).
Kočí, Z. et al. Characterization of human adipose tissue-derived stromal cells isolated from diabetic patient’s distal limbs with critical ischemia. Cell Biochem. Funct. 32, 597–604 (2014).
Stolzing, A., Coleman, N. & Scutt, A. Glucose-induced replicative senescence in mesenchymal stem cells. Rejuvenation Res. 9, 31–35 (2006).
Hankamolsiri, W. et al. The effects of high glucose on adipogenic and osteogenic differentiation of gestational tissue-derived MSCs. Stem Cells Int. 2016, 9674614 (2016).
Zuncheddu, D. et al. Effect of glucose depletion and fructose administration during chondrogenic commitment in human bone marrow-derived stem cells. Stem Cell Res. Ther. 13, 533 (2022).
Qi, L. et al. Umbilical cord mesenchymal stem cell conditioned medium restored the expression of collagen II and aggrecan in nucleus pulposus mesenchymal stem cells exposed to high glucose. J. Bone Miner. Metab. 37, 455–466 (2019).
Fitzgerald, J. C., Shaw, G., Murphy, J. M. & Barry, F. Media matters: Culture medium-dependent hypervariable phenotype of mesenchymal stromal cells. Stem Cell Res. Ther. 14, 363 (2023).
Kusnanto, P., Purwanto, B., Wasita, B. & Widyaningsih, V. Phenotype and conditioning medium on umbilical cord-mesenchymal stem cell (UC-MSC). Indones. J. Gastroenterol. Hepatol. Dig. Endosc. 24, 127–131 (2023).
Ryu, J. M., Lee, M. Y., Yun, S. P. & Han, H. J. High glucose regulates cyclin D1/E of human mesenchymal stem cells through TGF-beta1 expression via Ca2+/PKC/MAPKs and PI3K/Akt/mTOR signal pathways. J. Cell. Physiol. 224, 59–70 (2010).
Oh, J. Y. et al. High glucose-induced reactive oxygen species stimulates human mesenchymal stem cell migration through snail and EZH2-dependent E-cadherin repression. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 46, 1749–1767 (2018).
Acknowledgements
This work was funded by the National Key R&D Program of China (2023YFC2308200), Natural Science Foundation of Inner Mongolia Autonomous Region (2025LHMS08003), Deanship of Graduate Studies and Scientific Research at Jouf University (DGSSR-2025-01-01138) and Putra Grants of Universiti Putra Malaysia (GP-IPS/2022/9718000 and GPI/2024/9798600).
Funding
This research was funded by the National Key R&D Program of China (2023YFC2308200), Natural Science Foundation of Inner Mongolia Autonomous Region (2025LHMS08003), Deanship of Graduate Studies and Scientific Research at Jouf University (DGSSR-2025-01-01138), and Putra Grants of Universiti Putra Malaysia (GP-IPS/2022/9718000 and GPI/2024/9798600).
Author information
Authors and Affiliations
Contributions
T.JB Conceptualization; Methodology; Formal analysis; Investigation; Writing-original draft; Data curation; Project administration; Funding acquisition. B.A. Methodology; Formal analysis; Investigation; Project administration; Funding acquisition. S.K.S. Resources. S.R. Resources. R.R. Resources. M.ZJ. Funding acquisition. W.XY. Funding acquisition. M.P.L. Conceptualization; Writing-review and editing; Data curation; Project administration; Funding acquisition.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical consideration
All human sample isolation procedures adhered to the National and International Ethical Guidelines for Biomedical Research Involving Human Subjects (CIOMS) and the Guideline for Stem Cell Research & Therapy. Approval was obtained from the Ethics Committee for Research Involving Human Subjects at Universiti Putra Malaysia (Ethics Approval No.: JKEUPM-2020-26). Informed consent was obtained from all participants.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tong, J., Alzahrani, B., Subbiah, S.K. et al. Effects of low glucose microenvironment on the proliferation, migration and senescence of meniscus-derived stem cells. Sci Rep 15, 23748 (2025). https://doi.org/10.1038/s41598-025-07642-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-07642-x