Abstract
Bloodstream infections (BSIs) caused by multidrug resistant Pseudomonas aeruginosa (MDRPA) and carbapenem resistant Pseudomonas aeruginosa (CRPA) accounted for high morbidity and mortality. This retrospective cohort study aimed to determine risk factors for MDRPA and CRPA BSIs by examining both clinical and laboratory data of inpatients with MDRPA and CRPA BSIs at two tertiary care hospitals in 2017–2021. Generalized linear mixed models were used to identify risk factors for MDRPA and CRPA BSIs. Factors significantly associated with both MDRPA BSIs and CRPA BSIs included central venous catheter, invasive ventilation including duration of use, urinary catheterization, gastric tube insertion, vancomycin use including quantity of usage, imipenem use including quantity of usage, and tigecycline use. Respiratory infection [adjusted odds ratio (aOR) 2.10, 95% confidence interval (95% CI) 1.00–4.42; P = 0.049] was identified as an independent risk factor for MDRPA BSIs. For CRPA BSIs, independent risk factors included the use of invasive ventilation [aOR 2.82, 95% CI 1.36–5.84; P = 0.005] and a history of tigecycline use [aOR 3.34, 95% CI 1.16–9.58; P = 0.025]. Conversely, circulatory system diseases [aOR 0.41, 95% CI 0.22–0.77; P = 0.006] and quantity of piperacillin-tazobactam use [aOR 0.83, 95% CI 0.72–0.96; P = 0.013] were identified as independent protective factors for CRPA BSIs. Inpatients with respiratory infection, invasive ventilation and a history of tigecycline use are at higher risk of MDRPA and CRPA BSIs. More prudent clinical interventions and antimicrobial therapy should be implemented to inpatients with these factors to prevent and control MDRPA and CRPA BSIs.
Similar content being viewed by others
Introduction
Pseudomonas aeruginosa (PA) is a non-fermenting, Gram-negative bacillus and one of the most common pathogens associated with hospital-acquired infections1. As a globally prevalent opportunistic pathogen, PA could cause a range of bacterial infections, including sepsis, bacteremia, pneumonia, endocarditis, urinary tract infections and wound infections2,3. PA is among the top ten bacteria responsible for bloodstream infections (BSIs) worldwide4,5. Mortality rate of Pseudomonas aeruginosa bloodstream infections (PA BSIs) varies in different regions from 7.67% to 31.65%1,6. Our previous research found that the mortality rate of PA BSIs in Central and East China was as high as 17.89%7. The emergence of multidrug resistant Pseudomonas aeruginosa (MDRPA) and carbapenem resistant Pseudomonas aeruginosa (CRPA) has become a significant public health threat, posing substantial clinical challenges8. In May 2024, World Health Organization (WHO) released the 2024 Bacterial Priority Pathogens list in which CRPA was categorized as a high-priority pathogen9. The direct economic burden of CRPA BSIs is at least three times higher than that of carbapenem susceptible PA BSIs10. Our previous research found that mortality rates and healthcare costs of MDRPA BSIs and CRPA BSIs cases were significantly higher than those of non-multidrug-resistant Pseudomonas aeruginosa bloodstream infections (non-MDRPA BSIs) and non-carbapenem resistant Pseudomonas aeruginosa bloodstream infections (non-CRPA BSIs) cases7. Over the past decade, several studies have investigated risk factors for MDRPA BSIs11,12,13,14 and CRPA BSIs6,15,16. To our knowledge, this is the first multicenter study in Central and East China to examine risk factors for MDRPA BSIs and CRPA BSIs. Objectives of this study are as follows: (1) to investigate clinical characteristics of patients with MDRPA BSIs and CRPA BSIs, and (2) to determine risk factors associated with MDRPA BSIs and CRPA BSIs. The findings would help to provide guidance for optimizing clinical interventions and refining infection control measures to prevent MDRPA and CRPA BSIs.
Methods
Study design
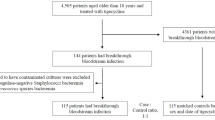
A retrospective cohort study was conducted from January 1, 2017 to December 31, 2021 at two tertiary care hospitals. Xiangya Hospital is a 3500-bed facility located in Hunan Province, Central China and Ruijin Hospital is a 2500-bed facility in Shanghai, East China. All inpatients diagnosed with PA BSIs during the study period were enrolled in this study. Only the first episode of each patient was included and each patient was included only once. Patients with length of hospitalization less than 48 h or incomplete data were excluded from the study.
Definitions
PA BSIs were defined as the presence of PA in blood cultures, accompanied by clinical signs and symptoms of infections17. According to definitions of European Centre for Disease Prevention and Control and U.S. Centers for Disease Control and Prevention, MDRPA is defined as a PA isolate non-susceptible to at least one agent in ≥ 3 antimicrobial classes (aminoglycosides, antipseudomonal carbapenems, antipseudomonal cephalosporins, antipseudomonal penicillins/β-lactamase inhibitors, monobactams, antipseudomonal fluoroquinolones, fosfomycin and polymyxins)11,18. CRPA is defined as a PA isolate that is non-susceptible to imipenem or meropenem19. Blood collection and processing for blood cultures followed international guidelines20. Bacterial identification was performed using MALDI-TOF MS (bioMérieux, Marcy l´Etoile, France or Zybio Inc., Chongqing, China). Antimicrobial susceptibility testing was conducted using the VITEK 2 Compact system (bioMérieux, Marcy l´Etoile, France)21. Antibiotics tested included amikacin, gentamicin, tobramycin, imipenem, meropenem, ceftazidime, cefepime, ciprofloxacin, levofloxacin, piperacillin/tazobactam, cefoperazone/sulbactam, polymyxin B, and aztreonam. Results were interpreted according to the Clinical and Laboratory Standards Institute’s M100-31st edition22. The sensitivity to polymyxin B was double checked using broth microdilution method. Antimicrobial use during the 90-day period preceding the onset of PA BSI was quantified using Defined Daily Doses (DDD) based on the World Health Organization (WHO) ATC/DDD system (2019 version).
Data collection
Data were extracted from hospital electronic information system and laboratory information system. All the data were classified into six categories: (I) demographic characteristics; (II) lifestyle habits; (III) history of healthcare exposure in 90 days prior to PA isolation; (IV) underlying diseases according to admission diagnosis; (V) history of invasive procedures in 90 days prior to PA isolation, and (VI) history of drug usage in 90 days prior to PA isolation. Definition of each variable corresponding to these data was listed in Appendix Table 1. Categories (I)-(VI) were considered as potential risk factors and analyzed accordingly.
Statistical analysis
Data were organized using Excel, and statistical analysis was conducted with R (version 4.2.1). Generalized linear mixed models (GLMM) (Logistic regression with a logit link function) with hospital as a random effect were applied to identify risk factors for MDRPA and CRPA BSIs. First, univariate analysis was performed. Correlation and relevant interactions between variables with P < 0.05 in univariate analysis were checked. After removing variables with high-level correlation (correlation coefficient ≥ 0.70), the remaining variables were considered for inclusion in the multivariate model and selected using lease absolute shrinkage and selection operator (LASSO) penalty (lambda used to choose variables = lambda.1se, the lambda that minimizes cross validation error plus one standard error). The selected variables were included in the final multivariate analysis to determine the independent associations7. Odds ratio (OR) and 95% confidence interval (95% CI) were calculated. A P-value < 0.05 was considered statistically significant. To test the stability of the final multivariate model, variables were removed one by one, and the significance of the remaining variables was assessed23.
Results
Overview of the study
A total of 288 cases were diagnosed with PA BSIs. Two patients who were hospitalized less than 48 h and one patient with incomplete data were excluded and 285 cases were included in the analysis. Among these 285 cases, 75 cases (26.32%) were MDRPA BSIs and 97 cases (34.04%) were CRPA BSIs.
Clinical characteristics of MDRPA BSIs cases
The median age of the 75 MDRPA BSIs cases was 52 years, with a higher proportion of male cases (56/75, 74.67%) (Table 1). A history of ICU admission was noted in 30.67% of the patients. The most common underlying conditions were respiratory diseases (31/75, 41.33%) and circulatory system diseases (29/75, 38.67%) (Table 1). The most common invasive procedures included central venous catheterization (CVC) (53/75, 70.67%), urinary catheterization (51/75, 68.00%), and surgery (40/75, 53.33%) (Table 1). The majority of these patients had a history of antibiotic use (65 /75, 86.67%) and corticosteroid use (46/75, 61.33%) (Table 1).
Risk factors for MDRPA BSIs
Univariate analysis indicated that respiratory diseases, respiratory infection, CVC, invasive ventilation and its duration, urinary catheterization and its duration, gastric tube insertion and its duration, vancomycin use and its quantity, aminoglycosides use, imipenem use and its quantity, quantity of meropenem use, cefoperazone-sulbactam and tigecycline were associated with MDRPA BSIs (Table 1). Multivariate analysis revealed that respiratory infection [adjusted odds ratio (aOR) 2.10, 95% confidence interval (95% CI) 1.00–4.42; P = 0.049] was an independent risk factor for MDRPA BSIs (Table 1).
Clinical characteristics of CRPA BSIs cases
Among the 97 CRPA BSIs cases, the median age was 55 years, with a higher proportion of male patients (69/97, 71.13%) (Table 2). A history of ICU admission was present in 30.93% of the patients. The most common underlying conditions were circulatory system diseases (34/97, 35.05%) and respiratory system diseases (29/97, 29.90%) (Table 2). The most frequent invasive procedures included CVC (68/97, 70.10%), urinary catheterization (67/97, 69.07%), and surgery (53 /97, 54.64%) (Table 2). Most of these patients had a history of antibiotic use (85/97, 87.63%) and corticosteroid use (59/97, 60.82%) with in the 90 days prior to the onset of PA BSIs (Table 2).
Risk factors for CRPA BSIs
Univariate analysis indicated that CVC, invasive ventilation and its duration, urinary catheterization, gastric tube insertion, vancomycin use and its quantity, linezolid use and its quantity, imipenem use and its quantity, quantity of meropenem, piperacillin-tazobactam and tigecycline were associated with CRPA BSIs (Table 2). Multivariate analysis revealed that invasive ventilation [aOR: 2.82, (95% CI) 1.36–5.84; P = 0.005] and a history of tigecycline use [aOR: 3.34, (95% CI) 1.16–9.58; P = 0.025] were independent risk factors for CRPA BSIs, while circulatory system diseases [aOR: 0.41, (95% CI) 0.22–0.77; P = 0.006] and quantity of piperacillin-tazobactam [aOR: 0.83, (95% CI) 0.72–0.96; P = 0.013] were independent protective factors against CRPA BSIs (Table 2).
Discussion
This study conducted a detailed risk factor analysis of PA BSIs at individual level. Our preliminary research revealed that the annual incidence rate of PA BSIs fluctuated between 2.37 and 3.51 cases per 100,000 patient-days between 2017 and 20217. The 30-day mortality rate of PA BSIs was 17.89%7. We also found that MDRPA or CRPA BSIs are associated with significant higher healthcare costs and lower crude 30-day survival7. Moreover, proportion of MDRPA BSIs (26.32%) was significantly higher than that reported in Northern China (13.0%)24 while proportion of CRPA BSIs (34.04%) was significantly higher than that reported in Taiwan (15.95%)6. Understanding local risk factors for MDR/CRPA BSIs is crucial and could help to make more targeted and effective surveillance programmes and infection prevention and control measures.
We used GLMM to identify risk factors for MDRPA and CRPA BSIs in this study. First, GLMM combines the generalized linear model and the mixed effects model, and allows the response variable to follow any distribution that is consistent with generalized linear model which is a generalization of the classical linear regression model. Second, previous study specified that patients clustered within the same hospital were more likely to be similar to each other than similar to patients from another hospital, and medical conditions varied across different hospitals in different regions25. Given all the above factors, GLMM with the hospital where the patient with MDRPA and CRPA BSIs was treated being a random effect was fitted to account for differences in study setting and geographic location.
Similar to findings of other studies, the majority of MDRPA and CRPA BSIs cases were male12,13,14,15,26. However, gender is not associated with adverse clinical outcomes7. Respiratory infection was identified as an independent risk factor for MDRPA BSIs (Table 1). PA is a common cause of respiratory infection27 and prone to form biofilms on the surface of equipment or implants16,28, making them more prone to colonization. Empirical therapy for respiratory infections frequently employs broad-spectrum antibiotics, which readily select for resistant strains such as MDRPA29. Furthermore, these patients often require invasive procedures including endotracheal intubation and sputum suction30. Such interventions may directly introduce colonized resistant pathogens into the bloodstream or cause mucosal damage, potentially leading to MDRPA BSIs. Interestingly, circulatory system diseases acted as an independent protective factor against CRPA BSIs. Some therapeutic drugs for patients with circulatory system diseases, such as aspirin, may inhibit PA quorum sensing (QS) systems31. Since QS inhibitors disrupt bacterial communication rather than exerting direct bactericidal effects, they could potentially slow the development of antibiotic resistance32. This might explain the observed protective association specifically against CRPA (but not susceptible PA) BSIs, and further research is needed to elucidate the specific mechanisms. Our study identifies that MDRPA BSIs were associated with respiratory diseases, respiratory infection, CVC, invasive ventilation (and duration), urinary catheterization (and duration), and gastric tube insertion (and duration), while CRPA BSIs were associated with CVC, invasive ventilation (and duration), urinary catheterization, and gastric tube insertion in univariable analysis. However, in multivariable analysis, only respiratory infection was identified as an independent risk factor for MDRPA BSIs and invasive ventilation remained significantly associated with CRPA BSIs (Tables 1 and 2). These invasive devices and procedures have been widely studied as risk factors for infections caused by MDRPA and CRPA, as they bypass the host’s innate mechanical defenses and create an ecological niche conducive to microbial colonization and infection13,16,26. Moreover, invasive ventilation has been identified as an independent risk factor for CRPA BSIs (Table 2). This association can be attributed to two key mechanisms. First, critically ill patients requiring mechanical ventilation often receive broad-spectrum antibiotics, which selectively promote the growth of CRPA strains. Second, endotracheal intubation or tracheostomy disrupts the natural airway barriers, allowing colonizing CRPA to invade the bloodstream more easily.
Many studies have identified a history of carbapenem use and quantity of their usage as critical risk factors for CRPA BSI33,34,35. However, our study demonstrated that prior tigecycline exposure (rather than carbapenems) emerged as an independent risk factor for CRPA BSI. Tigecycline usage is reported to be associated with carbapenem-resistant Enterobacteriaceae (CRE) BSIs36. Although tigecycline has limited activity against PA, it is frequently employed to treat severe CRE infections37. These patients often exhibit immunocompromised status, prolonged hospitalization, or require invasive interventions (e.g., mechanical ventilation). Also, last-line resort antibiotics like tigecycline and vancomycin are empirically used to cover both gram-positive and gram-negative resistant bacterial infections for severe ill patients. Furthermore, CRE may promote the horizontal transfer of plasmid-encoded resistance determinants (e.g., carbapenemase genes blaKPC or blaNDM) to co-colonizing PA strains, thereby conferring carbapenem resistance38. This process could increase the prevalence of CRPA within colonized PA populations, ultimately elevating the risk of CRPA BSIs. Similar to tigecycline, colistin is frequently employed for critically ill patients with carbapenem-resistant bacterial infections39,40. In vitro studies have demonstrated that colistin exhibits synergistic antibacterial effects when combined with carbapenems, tigecycline, fosfomycin, rifampin, or aminoglycosides41. Notably, recent literature reports that triple combination therapy (colistin plus rifampin with either carbapenems or ceftazidime-avibactam) demonstrates enhanced synergy and superior bactericidal activity against CRPA41, offering a promising therapeutic option for CRPA BSIs. However, in our study cohort, the number of patients with prior colistin exposure before developing BSIs was insufficient to evaluate potential associations between colistin use and MDRPA or CRPA BSIs, further research is needed to investigate the associations. In addition to tigecycline, our investigation into the role of antibiotic exposure history in CRPA BSIs revealed that the cumulative dose of prior piperacillin-tazobactam use served as an independent protective factor against CRPA BSIs. As a β-lactam/β-lactamase inhibitor combination, piperacillin-tazobactam retains potent activity against most non-CRPA. Previous studies have demonstrated that patients receiving extended-infusion piperacillin-tazobactam therapy exhibited significantly lower 14-day mortality rates and shorter median post-culture hospitalization durations compared to those on intermittent-infusion regimens despite identical cumulative doses (3.375 g in both groups)42. However, the causal relationship between high-dose piperacillin-tazobactam exposure and reduced CRPA BSIs risk remains unclear, warranting further investigation into its underlying mechanisms.
This multicenter study conducted a comprehensive investigation into risk factors for MDRPA and CRPA BSIs. However, there are several limitations. First, antibiotics tested in antimicrobial susceptibility testing are not entirely same in the two hospitals. Second, the study lacks data on molecular diagnostic methods, such as resistance and virulence genes. Toxin-antitoxin (TA) genes in PA isolates are not only related to antibiotic resistance but also play important roles in bacterial pathogenesis and virulence. Further molecular studies are necessary to illustrate the role of TA genes in the pathogenesis of PA and to elucidate their association with antibiotic resistance43. Future research should focus on more in-depth bioinformatics analyses and phylogenetic studies of bacterial strains.
Our study identifies high-risk patients for MDRPA and CRPA BSIs in Central and East China, with significant implications for clinical practice and public health strategies. First, these findings enable clinicians to proactively identify hospitalized patients at high risk of MDRPA or CRPA BSIs (e.g., respiratory infection, invasive ventilation), facilitating timely implementation of targeted infection control measures like preemptive isolation and active surveillance. Second, the results underscore the critical need to strengthen patient and healthcare staff education on hand hygiene compliance, adherence to treatment regimens, and judicious antibiotic use (e.g., tigecycline, vancomycin and piperacillin-tazobactam). Finally, in high-incidence settings such as ICUs, enhanced infection prevention protocols should be prioritized, including strict environmental and equipment decontamination (e.g., daily chlorhexidine disinfection) and contact isolation measures.
Conclusions
Inpatients with respiratory infection, invasive ventilation and a history of tigecycline use are at higher risk of MDRPA and CRPA BSIs. More prudent clinical interventions and antimicrobial therapy should be implemented to inpatients with these factors to prevent and control MDRPA and CRPA BSIs.
Data availability
Data is provided within the manuscript or supplementary information files. The datasets used and/or analyzed during the current study are also available from the corresponding author on reasonable request.
References
Hong, D. J. et al. Epidemiology and characteristics of metallo-β-lactamase-producing Pseudomonas aeruginosa. Infect. Chemotherapy 47(2), 81–97 (2015).
Li, J. et al. Characterization of clinical extensively drug-resistant Pseudomonas aeruginosa in the Hunan province of China. Ann. Clin. Microbiol. Antimicrob. 15(1), 35 (2016).
Abdelaziz, M. A., El-Aziz, A. M. A., El-Sokkary, M. M. A. & Barwa, R. Characterization and genetic analysis of extensively drug-resistant hospital acquired Pseudomonas aeruginosa isolates. BMC Microbiol. 24(1), 225 (2024).
Diekema, D. J. et al. The microbiology of bloodstream infection: 20-year trends from the SENTRY antimicrobial surveillance program. Antimicrob. Agents Chemotherapy 63(7), 10–1128 (2019).
Peirano, G., Matsumara, Y., Nobrega, D., Church, D. & Pitout, J. D. D. Population-based genomic surveillance of Pseudomonas aeruginosa causing bloodstream infections in a large Canadian health region. Eur. J. Clin. Microbiol. Infect. Dis. 43(3), 501–510 (2024).
Tsao, L. H. et al. Risk factors for healthcare-associated infection caused by carbapenem-resistant Pseudomonas aeruginosa. J. Microbiol. Immunol. Infect. 51(3), 359–366 (2018).
Xiao, S., Liang, X., Han, L. & Zhao, S. Incidence, antimicrobial resistance and mortality of Pseudomonas aeruginosa bloodstream infections among hospitalized patients in China: A retrospective observational multicenter cohort study from 2017 to 2021. Front. Public Health 11, 1294141 (2024).
Abd El-Baky, R. M. et al. Prevalence and some possible mechanisms of colistin resistance among multidrug-resistant and extensively drug-resistant Pseudomonas aeruginosa. Infect. Drug Res. 13, 323–332 (2020).
Jesudason, T. WHO publishes updated list of bacterial priority pathogens. Lancet Microbe. 5(9), 100940 (2024).
Yang, K. et al. Socio-economic burden of bloodstream infections caused by carbapenem-resistant and carbapenem-susceptible Pseudomonas aeruginosa in China. J. Global Antimicrob. Res. 26, 101–107 (2021).
Herrera, S., Bodro, M. & Soriano, A. Predictors of multidrug resistant Pseudomonas aeruginosa involvement in bloodstream infections. Curr. Opin. Infect. Dis. 34(6), 686–692 (2021).
Viasus, D. et al. Predictors of multidrug-resistant Pseudomonas aeruginosa in neutropenic patients with bloodstream infection. Clin. Microbiol. Infect. 26(3), 345–350 (2020).
Defez, C. et al. Risk factors for multidrug-resistant Pseudomonas aeruginosa nosocomial infection. J. Hosp. Infect. 57, 209–216 (2004).
Trecarichi, E. M. et al. Multidrug resistant Pseudomonas aeruginosa bloodstream infection in adult patients with hematologic malignancies. Haematologica 96(1), e1–e3 (2011).
Yuan, F., Li, M., Wang, X. & Fu, Y. Risk factors and mortality of carbapenem-resistant Pseudomonas aeruginosa bloodstream infection in haematology department: A 10-year retrospective study. J. Global Antimicrob. Res. 37, 150–156 (2024).
Li, L., Huang, Y., Tang, Q. & Zheng, Y. Risk factors for carbapenem-resistant Pseudomonas aeruginosa infection in children. Pediatric Infect. Dis. J. 41(8), 642–647 (2022).
Garner, J. S., Jarvis, W. R., Emori, T. G., Horan, T. C. & Hughes, J. M. CDC definitions for nosocomial infections 1988. Am. J. Infect. Control. 16(3), 128–140 (1988).
Magiorakos, A. P. et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 18(3), 268–281 (2012).
Han, R. et al. Dissemination of carbapenemases (KPC, NDM, OXA-48, IMP, and VIM) among carbapenem-resistant enterobacteriaceae isolated from adult and children patients in China. Front. Cell. Infect. Microbiol. 10, 314 (2020).
Baron, E. J. et al. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2013 recommendations by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM)(a). Clin. Infect. Dis. 57(4), e22–e121 (2013).
Patil, S. et al. Resistance genomics and molecular epidemiology of high-risk clones of ESBL-producing Pseudomonas aeruginosa in young children. Front. Cell. Infect. Microbiol. 13, 1168096 (2023).
Pa, W. Clinical and laboratory standards institute (CLSI): Performance standards for antimicrobial susceptibility testing. 31st ed. Wayne PA. 2021;CLSI supplement M100 (2021).
Zhao, S. et al. Risk factors for carbapenemase-producing organisms among inpatients in Scotland: A national matched case-control study. Infect. Control Hosp. Epidemiol. 42(8), 968–977 (2021).
Zhao, Y. et al. Risk factors and outcomes of antibiotic-resistant Pseudomonas aeruginosa bloodstream infection in adult patients with acute Leukemia. Clin. Infect. Dis. 71(Suppl 4), s386–s393 (2020).
Khadem, T. et al. Risk factors for carbapenem-nonsusceptible Pseudomonas aeruginosa: Case-control study. Diagn. Microbiol. Infect. Dis. 89, 146–150 (2017).
Rodríguez, N. E. G. et al. Pseudomonas aeruginosa bloodstream infections in children and adolescents: Risk factors associated with carbapenem resistance and mortality. J. Hosp. Infect. 149, 56–64 (2024).
Zupetic, J. et al. Elastase activity from Pseudomonas aeruginosa respiratory isolates and ICU mortality. Chest Infect. Original Res. 160(5), 1624–1633 (2021).
Veerachamy, S., Yarlagadda, T., Manivasagam, G. & Yarlagadda, P. K. Bacterial adherence and biofilm formation on medical implants: a review. Proc. Inst. Mech. Eng. [H] 228(10), 1083–1099 (2014).
Kuijpers, S. M. E. et al. The evidence base for the optimal antibiotic treatment duration of upper and lower respiratory tract infections: An umbrella review. Lancet Infect Dis. 25(1), 94–113 (2025).
Martin-Loeches, I. et al. ERS/ESICM/ESCMID/ALAT guidelines for the management of severe community-acquired pneumonia. Intensive Care Med. 49(6), 615–632 (2023).
El-Mowafy, S. A., Abd El Galil, K. H., El-Messery, S. M. & Shaaban, M. I. Aspirin is an efficient inhibitor of quorum sensing, virulence and toxins in Pseudomonas aeruginosa. Microb. Pathog. 74, 25–32 (2014).
Chen, H. et al. From quorum sensing inhibition to antimicrobial defense: The dual role of eugenol-gold nanoparticles against carbapenem-resistant Pseudomonas aeruginosa. Colloids Surf B Biointerfaces 247, 114415 (2025).
Zhang, D. et al. Risk factors for carbapenem-resistant Pseudomonas aeruginosa infection or colonization in a Chinese teaching hospital. J. Infection Dev. Countries 12(08), 642–648 (2018).
Wei, X. et al. Risk factors and outcomes of patients with carbapenem-resistant Pseudomonas aeruginosa bloodstream infection. Infect. Drug Res. 16, 337–345 (2023).
Zhao, Y. et al. Risk factors and outcomes of antibiotic-resistant Pseudomonas aeruginosa bloodstream infection in adult patients with acute Leukemia. Clin. Infect. Dis. 71(Supplement_4), S386–S393 (2020).
Li, Y. et al. Five-year change of prevalence and risk factors for infection and mortality of carbapenem-resistant Klebsiella pneumoniae bloodstream infection in a tertiary hospital in North China. Antimicrob. Res. Infect. Control 9(1), 79 (2020).
Ku, K. et al. Retrospective evaluation of colistin versus tigecycline for the treatment of Acinetobacter baumannii and/or carbapenem-resistant enterobacteriaceae infections. Am. J. Infect. Control 40(10), 983–987 (2012).
Macesic, N., Uhlemann, A. C. & Peleg, A. Y. Multidrug-resistant Gram-negative bacterial infections. Lancet 405(10474), 257–272 (2025).
Katip, W. et al. Comparative effectiveness and mortality of colistin monotherapy versus colistin-fosfomycin combination therapy for the treatment of carbapenem-resistant Enterobacteriaceae (CRE) infections: A propensity score analysis. J. Infect. Public Health 17(5), 727–734 (2024).
Katip, W., Rayanakorn, A., Oberdorfer, P., Taruangsri, P. & Nampuan, T. Short versus long course of colistin treatment for carbapenem-resistant Acinetobacter baumannii in critically ill patients: A propensity score matching study. J. Infect. Public Health 16(8), 1249–1255 (2023).
Kim, S. H. et al. Synergistic effects of colistin-rifampin-based triple antimicrobial combination therapy against Carbapenem-resistant Pseudomonas aeruginosa: A time-kill assay. J. Antimicrob. Chemother. 80(3), 738–745 (2025).
Lodise, T. P. Jr., Lomaestro, B. & Drusano, G. L. Piperacillin-tazobactam for Pseudomonas aeruginosa infection: Clinical implications of an extended-infusion dosing strategy. Clin. Infect. Dis. 44(3), 357–363 (2007).
Coşkun, U. & Dagcioglu, Y. Evaluation of toxin-antitoxin genes, antibiotic resistance, and virulence genes in Pseudomonas aeruginosa isolates. Rev. Assoc. Med. Bras. 69(1), 51–55 (2023).
Author information
Authors and Affiliations
Contributions
S. X: Methodology, Software, Validation, Visualisation, Formal Analysis, Investigation, Data Curation, Writing – Original Draft Preparation; D. Z: Methodology, Software, Validation, Visualisation, Formal Analysis, Investigation, Data Curation, Writing – Original Draft Preparation; X. L: Methodology, Writing – Review & Editing; L. H: Validation, Resources, Writing – Review & Editing; S. Z: Conceptualisation, Methodology, Validation, Visualisation, Formal Analysis, Resources, Writing – Review & Editing, Supervision, Project Administration. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Ethical approval and consent to participate
The research protocol was reviewed and approved by Ruijin Hospital Ethics Committee and Xiangya hospital Ethics Committee (reference number: KY2023-083, 202212318). The need for informed consent was waived by the Review Boards of Ruijin Hospital and Xiangya Hospital due to the observational retrospective nature of the study. The study was conducted in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xiao, S., Zhu, D., Liang, X. et al. Risk factors for multidrug resistant and carbapenem resistant Pseudomonas aeruginosa bloodstream infections among inpatients in Central and East China. Sci Rep 15, 20719 (2025). https://doi.org/10.1038/s41598-025-07820-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-07820-x