Abstract
The kidneys are one of the primary organs affected by amyloid A (AA) amyloidosis in mixed-breed cats. The distribution of amyloid deposits within the kidneys varies among individuals; however, the underlying cause is unknown. This study investigated the association between serum AA (SAA) polymorphisms and the pattern of renal amyloid deposition in five mixed-breed cats. Histological analysis of the kidneys revealed amyloid deposits in the renal glomeruli and renal papillae in all cases. In contrast, the amyloid deposition pattern differed in the medulla, with widespread deposition from the corticomedullary junctional area to the inner medulla in two of the five cats. These amyloids were mainly located in the basement membrane of the renal tubules, extending towards the lumen and into the interstitium. Conversely, in the other three cats, amyloid deposition in the medulla was sparse and the deposits were localized in the perivascular stroma in the corticomedullary junction. Genetic analysis identified four SAA alleles involving six amino acid substitutions (Q1E, I29K, D42E, Q45R, P48R, and A51V). Mass spectrometry and immunohistochemistry revealed that AA amyloid derived from SAA with Q45 was predominantly deposited in the cortex and papilla, as well as in the perivascular stroma of some parts of the outer medulla. On the other hand, AA amyloid derived from SAA with R45 was specifically observed around the tubules in the renal inner to outer medulla. Except for Q45R, no substitutions were associated with distribution patterns. These findings suggest that the SAA polymorphism sequence is associated with the site of AA amyloid deposition in the kidney. Moreover, this study is the first report of such a complex pattern of amyloid deposition in the organ of the same individual, emphasizing that the primary structure of the amyloid precursor protein determines amyloid distribution.
Similar content being viewed by others
Introduction
Amyloid A (AA) amyloidosis, caused by serum AA (SAA)-derived amyloid deposition, is one of the most common systemic amyloidosis in humans and animals1. In contrast to other types of systemic amyloidosis, which have been documented in only a limited number of animal species, AA amyloidosis has been documented in a remarkably broad range of mammalian and avian species2. In humans, the kidneys are the primary target organs for AA amyloidosis3. The intrarenal distribution of deposits varies among patients and can be divided into three types4. Glomerular-dominated deposition is the most common presentation of AA amyloidosis5 and generally causes proteinuria6. Other types of deposition, predominantly in blood vessels or the tubular interstitium, have also been described4 but these have minimal or no proteinuria levels5. The diversity of glomerular or vascular AA deposition has been explained by conformational differences resulting from different lengths of SAA fragments that comprise the amyloid7. However, the influence of intrinsic factors such as amino acid variations remains poorly understood.
The diversity in intrarenal amyloid distribution in AA amyloidosis has also been reported in animals2,8. In cows, while some individuals exhibit a predominant glomerular distribution of amyloid deposition, others show predominant deposition in the medullary tubulointerstitium9. However, the factors responsible for differences in renal deposition distribution have not been elucidated in animals. In both humans and animals, the distribution of amyloid deposition within the kidneys is closely related to clinical manifestations, particularly glomerular deposition, which leads to lethal nephrotic syndrome9. Therefore, elucidating the factors influencing amyloid distribution is essential for accurately diagnosing and managing AA amyloidosis.
In feline AA amyloidosis, the kidneys, liver, and spleen are known to be the principal organs affected10. The diversity of AA amyloid deposition between organs has been observed in two domestic cat breeds, the Siamese and the Abyssinian, which develop hereditary AA amyloidosis; the liver is predominantly affected in the Siamese, and the renal medulla in the Abyssinian11,12. To date, two studies have investigated the relationship between amino acid substitutions in SAA and the distribution of amyloid deposition in feline AA amyloidosis13,14. Niewold et al. detected specific amino acid substitutions in the SAA of Siamese cats with liver-dominated familial AA amyloidosis13. In contrast, Tei et al. investigated SAA sequences in Japanese domestic cats (mixed-breed) and found no relationship between the inter-organ diversity of AA amyloid deposits and SAA polymorphisms14.
As observed in cattle and humans, the pattern of deposition diversity within the kidneys may also occur in cats. In a recent report on renal changes in AA amyloidosis in nine domestic shorthair cats, eight showed AA deposition in the cortex and medulla15. In addition, another study examining amyloid deposits in the kidneys of 19 cats revealed that their distribution varied across the cortex, glomeruli, and medulla16.
Building on these observations, in the present study, we investigated the intrarenal diversity of amyloid deposits and SAA polymorphisms in five cats with AA amyloidosis.
Results
Histopathology
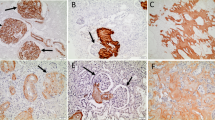
Severe amyloid deposition was histologically observed in the kidneys and livers of all 5 cats. There was severe deposition in the liver, mainly in the space of Disse, with no obvious differences in distribution among individuals (Supplementary Fig. 1). In the kidney, all cases showed amyloid deposition in glomeruli and interstitial connective tissue in the cortex, as well as diffuse deposition in the tubular interstitium of papilla (Supplementary Figs. 2–6). Cases with severe amyloid deposition in the renal papilla were accompanied by necrosis of the renal papilla. Within the renal medulla, amyloid distribution varied among cases (Table 1). In cases 1 and 2, severe amyloid deposition was observed in the medulla, particularly from the outer stripe of outer medulla (OSOM) to the inner medulla (IM) (Fig. 1, Supplementary Fig. 2 d). These amyloid deposits were primarily observed along the basement membrane of the renal tubules, and the deposition that extended into the lumen caused epithelial cells sloughing (Fig. 1c). In contrast, medullary amyloid deposition was milder in cases 3–5 (Fig. 1b). Amyloid deposits were primarily observed in the perivascular interstitium, where they extended from the basement membrane into the surrounding interstitium in a patchy or smudgy manner (Fig. 1d). These deposits resulted in expansion of the interstitial space. Notably, the changes were mainly in the OSOM (Supplementary Fig. 4). Immunohistochemistry (IHC) revealed that all of the hepatic and renal amyloid deposits were positive for the commercial anti-Pan-SAA antibody, indicating AA amyloidosis (Supplementary Figs. 1–6). Other than amyloid deposition, renal infarction was observed in cases 1, 2, and 4. Case 3 had lymphoma in the kidney and liver.
Amyloid deposition in the renal medulla in case 1 (a, c, e) and case 4 (b, d, f). Cat. (a, b) Amyloid deposits are highlighted by the Congo red stain (CR). Bars, 100 μm. (c) In case 1, amyloid deposition is primarily observed along the basement membrane of the renal tubules and causes epithelial cell sloughing. Bar, 50 μm. Inset: Amyloid deposits exhibit yellow-green birefringence under polarized light. CR. (d) In case 4, amyloid deposition extends into the renal tubule interstitium. Inset: Amyloid deposits exhibit yellow-green birefringence under polarized light. Bar, 50 μm. CR. (e, f) Amyloid deposits show moderate immunolabeling. Bars, 50 μm. Immunohistochemistry for Pan-SAA.
SAA genes in five cats
Reverse transcription-polymerase chain reaction (RT-PCR) and Sanger sequencing, based on cloning of the coding region of SAA, revealed two or three SAA pleomorphic sequences in each case (Supplementary Fig. 7). A summary of the results is shown in Fig. 2. Among the five cases, there were a total of six amino acid substitutions (Q1E, I29K, D42E, Q45R, P48R, and A51V), resulting in four SAA variants with the following amino acid combinations: SAAv1 (Q1-I29-D42-R45-P48-A51), SAAv2 (Q1-K29-E42-Q45-R48-A51), SAAv3 (E1-I29-D42-Q45-P48-A51), and SAAv4 (E1-I29-D42-R45-P48-V51). Case 1 had SAAv1, SAAv3, and SAAv4; case 2 had SAAv2, SAAv3, and SAAv4; case 3 had SAAv3 and SAAv4; and cases 4 and 5 had SAAv2 and SAAv3. Focusing on the 45th residue, SAAv1 and SAAv4 have arginine (SAAR45), and SAAv2 and SAAv3 have glutamine (SAAQ45). In other words, cases 1–2 with severe medullary amyloid deposition had both SAAR45 and SAAQ45. Among cases 3–5 with milder amyloid deposition in the medulla, case 3 had both SAAR45 and SAAQ45, while cases 4–5 had only SAAQ45.
SAA sequences in each case. In total, six amino acid substation sites are detected and highlighted in black; Q1E, I29K, D42E, Q45R, P48R, and A51V. Four different SAA sequences (SAAv1, SAAv2, SAAv3, and SAAv4) were identified. All five cases possessed multiple SAA alleles: case 1 expressed SAAv1, SAAv3, and SAAv4; case 2 expressed SAAv2, SAAv3, and SAAv4; case 3 expressed SAAv3 and SAAv4; and cases 4 and 5 expressed SAAv2 and SAAv3. The sequence description after residue 81 is truncated, as no substitutions were observed.
Distribution of variant SAA-derived amyloids
To determine the respective renal distribution of amyloid derived from SAAR45 and SAAQ45 by IHC, we generated two antibodies: anti-SAAQ45 antibody and anti-SAAR45 antibody. The anti-SAAQ45 and anti-SAAR45 antibodies specifically detect SAAQ45 and SAAR45, respectively (Supplementary Fig. 8). IHC with the anti-SAAQ45 antibody revealed positive reactions for amyloid deposits in the glomeruli and renal papilla in all cases (Fig. 3a, e, Supplementary Fig. 2-5b, h). Medullary amyloid deposits in cases 1 and 2 were negative for the anti-SAAQ45 antibody (Fig. 3c, Supplementary Fig. 2e) and positive for the anti-SAAR45 antibody (Fig. 3d, Supplementary Fig. 2f). In cases 1 and 2, some of the amyloid deposits in the renal papillae were also positive for the SAAR45 antibody (Fig. 3e, Supplementary Fig. 2 h). In cases 3–5, all renal amyloid deposits were positive for anti-SAAQ45 (Supplementary Fig. 3–5b, e, h) and negative for anti-SAAR45 antibody (Supplementary Fig. 3–5c, f, i). Despite the variation in the intensity of immunolabeling, no noticeable distribution differences were observed between antibodies in hepatic amyloid deposits (Supplementary Fig. 6).
Differences in the distribution of SAAQ45-derived and SAAR45-derived amyloid in the renal glomeruli, medulla, and papilla. Case 1, cat, immunohistochemistry. (a, b) Amyloid deposits in the glomeruli are positive for SAAQ45 (a) but negative for SAAR45 (b). Bars, 200 μm. (c, d) Amyloid deposits in the outer medulla are negative for SAAQ45 (c) but positive for SAAQ45 (d). Bars, 500 μm. (e, f) In the renal papilla, both SAAQ45-positive amyloid (e) and SAAR45-positive amyloid (f) are present. Bars, 200 μm.
To corroborate the immunohistochemical findings, amyloid deposits in the glomeruli, medulla, and papilla of case 1 were analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS). Mass spectrometry (MS) demonstrated that SAA is the predominant component of amyloid deposits in all areas (Supplementary Table 1). To predict the AA amyloid-origin variant in each region, we focused on the 45th and 51 st amino acid residues (Table 2). Tryptic peptides with R45 originating from SAAv1 or SAAv4 were detected in the medulla. In contrast, tryptic peptides with Q45, originating from SAAv3, were detected in glomeruli. Tryptic peptides with A51 (SAAv1 or SAAv3) were detected in all areas. The chymotryptic peptide containing Q45 and A51 (SAAv3) was detected in amyloid deposits in both the glomeruli and renal papilla. Although this fragment was also found in the medulla, a peptide containing R45 and A51 (SAAv1) was simultaneously detected in the medullary amyloid deposits. Given these results, it was concluded that the medullary amyloid deposits contain SAAv1 origin AA amyloid, whereas the glomerular and papillary amyloid deposits were of SAAv3 origin in case 1. The MS results of the papillary amyloid partially overlapped with those of the outer medulla, which was expected because the two were mixed during microdissection.
To assess whether the findings observed in case 1 were consistent across other subjects, we extended the LC-MS/MS analysis to renal amyloid deposits from cases 2–5. In case 2, glomerular amyloid deposits were derived from SAAv3, and the medullary deposits were derived from SAAv4 (Table 3). In cases 3–5, which had minor or no medullary deposits, SAAv3 peptide fragments were highly detected in all regions.
The tryptic peptides specific to SAAv2, GNYEAAQR (residues 39–46 of SAAv2) and GGAWAAK (residues 49–55 of SAAv2), were not detected in any of the samples. Furthermore, peptides with Ile29 (SAAsv1, v3, v4) were detected in all samples, but no peptides with Lys29 (SAAv2) were detected, suggesting that there were no SAAv2-derived amyloids.
In summary, among the six amino acid substitutions identified (E1Q, I29K, D42E, Q45R, P48R, and A51V), the combination I29-D42-P48—corresponding to SAAv1, SAAv3, and SAAv4—was consistently detected in amyloid deposits across all cases, irrespective of their renal distribution. These substitutions are therefore considered unrelated to the intrarenal amyloid distribution. In all cases (1–5), glomerular deposits were derived from the SAAv3 (E1-Q45-A51), while medullary deposits in cases 1 and 2 originated from either SAAv1 (Q1-R45-A51) or SAAv4 (E1-R45-V51). These findings suggest that the E1Q and A51V substitutions do not influence the renal distribution of amyloid, whereas the 45th residue appears to play a key role in determining the localization of amyloid deposits.
Discussion
Based on IHC and MS results, all five cats were diagnosed with AA amyloidosis. Genetic analysis revealed that all cats had two or three SAA alleles. While there were multiple substitutions in the SAA sequence, IHC and MS highlighted the importance of the substitution at position 45th (Q/R). SAAQ45-derived amyloid deposition was observed mainly in the glomeruli, OSOM, and papilla. In contrast, the SAAR45-derived amyloid was restricted to the medulla and presented a prominent deposition along the basement membrane of the renal tubules. Summary of this amyloid distribution is shown in Supplementary Table 2. Functionally, the glomerulus is a mass of capillaries and produces primitive urine17 while renal tubules in the medulla reabsorb necessary components from this urine18. Given that both urinary pH and protein concentration undergo rapid changes during their passage through the nephrons, protein concentration in urine changes quickly as it passes through the nephrons, it is highly possible for different biochemical properties of the amyloid or precursor protein to change the deposition site. To our knowledge, this is the first report to show different distributions of distinct polymorphic precursor protein-derived amyloids within the same individual. This study suggests that a few differences in the primary structure of amyloid precursor proteins dynamically affect the intrarenal distribution of amyloid deposition.
Histologically, in cases 1 and 2, SAAR45-derived amyloid was detected along the basement membrane of renal tubules. In contrast, in cases 3–5, SAAQ45-derived amyloid exhibited milder deposition in the medulla, primarily originating from the vascular basement membrane and spreading into the interstitium. These histological differences suggest the possibility of predicting the origin of SAA. Additionally, renal infarctions were observed in three of the five cases, coinciding with relatively severe amyloid deposition. Potential factors include compromised blood flow due to amyloid deposition leading to focal ischemic lesions, or conversely, enhanced amyloid deposition in areas already affected by such lesions. Further studies involving a larger number of cases are needed to elucidate these mechanisms.
The substitution of residue 45 may have influenced the biochemical properties of SAA, the structure of the AA fibrils, or both. A previous study determined the cryo-electron microscopy (cryo-EM) structure of amyloid fibrils derived from a sequence (UniProt accession: Q1T770) homologous to SAAv3 in this study19. This structure reveals that Glu45 and Arg46 in SAAv3 organize strand β7, contributing to cross-β spine formation. Additionally, Arg46 establishes a hydrogen bond with Asp32, creating a β-arch that bridges two ~ 25-residue-long meandering tails. The substitution of glutamine at position 45 with arginine introduces a sequence of highly hydrophilic Arg45-Arg46 residues, which could alter the β-arch environment and potentially impact fibril structure. Future research should focus on the structural analysis of fibrils derived from feline SAAR45 to better understand these effects.
Notably, peptides specific to SAAv2 were not detected from all cases. In other words, SAA variants containing Ile29-Asp42-Pro48 were identified within amyloid deposits, whereas variants with Lys29-Glu42-Arg48 were not. According to the cryo-EM structure of feline AA fibrils19 Ile29 is located on strand β4 at the beginning of the central β-arch, while the hydrophobic side chain of Ile29 faces the inside of the β-arch and forms hydrophobic bonds with the side chains of Pro48, Trp52, and Ala54. Therefore, substituting the 29th hydrophobic residue isoleucine for the basic hydrophilic residue lysine and the 48th hydrophobic residue proline for basic hydrophilic residue arginine is expected to significantly affect the conformation around this region. The 42nd residue, whether aspartic acid or glutamic acid, is an acidic and hydrophilic amino acid, and a single-residue substitution should not significantly alter its structure. On the other hand, in the feline amyloid A structure, the 42nd amino acid forms a β-turn with the 43rd residue, suggesting that this region plays a crucial role in determining the amyloid structure. However, it is possible that technical factors such as enzymatic digestion caused differences in detection sensitivity in this region. The role of Ile29, Asp42, and Pro48 in feline AA amyloidosis should be investigated in the future.
This study has some limitations. First, only formalin-fixed tissue was available for analysis, which made it difficult to purify amyloid fibrils and restricted biochemical analyses, such as determining SAA cleavage points and structural analysis. Consequently, we could not determine whether the divergent amyloid distribution was due to amino acid substitutions in SAA affecting either the properties of the precursor protein or the fibrils. Isolation and structural analysis of amyloid fibrils derived from each region of fresh tissue should reveal the effects of amino acid substitutions in greater detail. Second, only the kidney and liver were analyzed in this study; therefore, the diversity of amyloid deposition in other organs was not evaluated. Although no obvious differences in distribution were found in the liver, a divergent distribution may exist in other hot spots of AA amyloid-deposition, such as the gastrointestinal tract and endocrine glands. It is expected that future studies will provide a more comprehensive understanding by examining the effects of SAA variants on amyloid distribution in organs other than the kidneys in greater detail and by increasing the number of cases.
In conclusion, this study raises the hypothesis that differences in the primary structure of precursor proteins affect the distribution of amyloid deposition by showing that polymorphic allele-derived AA amyloids exhibit different renal distributions within individuals. Since different renal distributions of amyloid deposits could lead to distinct clinical manifestations, understanding the cause of these varying distributions is crucial. The SAA polymorphisms in mixed-breed cats, which are likely complex due to extensive interbreeding, present a valuable model for analyzing the relationship between detailed disease pattern assessment and SAA variants. Future in-depth analysis of disease risk alleles in these animals can enhance our understanding of the association between specific alleles and the disease phenotypes.
Materials and methods
All tissue samples were clinical specimens obtained by veterinarians or veterinary technicians for diagnostic purposes and therefore did not require approval from the local ethics committee.
Case information and histopathological analysis
Five mixed-breed cats (cases 1–5) were analyzed in this study. All cats were born, kept, and died in Japan. The ages of cases 1, 4, and 5 were 5, 3, and 4 years old, respectively. Information on the ages of cases 2 and 3 was not available. Case 1 was a spayed female, case 2 was an intact female, and cases 3–5 were neutered males. There was no blood relationship between them. All cats were dead naturally and necropsied at animal hospitals or the authors’ laboratories within 24 h after death. For all cases, the liver and kidney were formalin-fixed, paraffin-embedded (FFPE). The following pathological analyses were performed under the supervision of a Japanese College of Veterinary Pathologists-licensed veterinary pathologist (TM). Frozen liver samples were also available for cases 1, 2, and 4. The FFPE tissues were cut into 3-µm sections, and stained with hematoxylin and eosin and Congo red. Amyloid deposits were indicated as Congo red-positive material showing yellow to green birefringence under polarized light. The degree of amyloid deposition in each area was scored using Congo red-stained specimens under high-power magnification (10 fields). In the cortex, deposition was assessed according to the percentage of affected glomeruli: –, no deposition; +, deposition in 1–25% of glomeruli; ++, 26–50%; and +++, more than 50%. In the medulla and papillae, the evaluation was based on the percentage of the interstitial area involved: –, no deposition; +, deposition in less than 5% of the interstitial area; ++, 5–20%; and +++, more than 20%.
Frozen liver samples were also available for cases 1, 2, and 4. The FFPE tissues were cut into 3-µm sections, and stained with hematoxylin and eosin and Congo red. Amyloid deposits were indicated as Congo red-positive material showing yellow to green birefringence under polarized light.
Sequencing of serum amyloid A gene
RT-PCR and sequencing were performed to determine the SAA sequence. Primers were designed using Primer Blast in NCBI. Two SAA isoforms have been registered in cats: SAA1 (NCBI accession: XM_045038064) and SAA2 (NCBI accession: NP_001295969.1). New primers that theoretically bind to the coding regions of both SAA1 and SAA2 were designed: forward, 5’-CTTTTCACGGGCCTCGTCTT-3’; reverse, 5’-TCAGTACTTGTCAGGCAGGC-3.’ Total RNA was extracted from the frozen liver samples of cases 1, 2, and 4 using the RNeasy Mini Kit (QIAGEN N.V., Venlo, Netherlands). For cases 3 and 5, RNA was extracted from FFPE liver tissue sections using an innuPREP FFPE total RNA Kit (Analytik Jena, Überlingen, Germany). One-step RT-PCR was performed using a PrimeScript One-Step RT-PCR Kit (Takara Bio, Shiga, Japan). RT-PCR was performed as follows: reverse transcription at 50 °C for 30 min; 35 cycles of 94 °C for 30 s, 60 °C for 30 s, and 72 °C for 1 min; and a final extension at 72 °C for 3 min. The product size was analyzed using agarose gel electrophoresis, and target DNA fragments (446 bp) were purified from the gel using NucleoSpin Gel and PCR Clean-up (MACHEREY-NAGEL, Düren, Germany). Nucleotide sequences were determined using the contracted service of Eurofins Genomics (Tokyo, Japan). Each amino acid sequence was translated from the sequenced codons using EMBOSS Transeq20.
Cloning and sequencing of heterozygous SAA
The amplified PCR products were cloned using pGEM®-T Easy Vector Systems (Promega, Madison, WI, USA) and sent to a sequencing service (Greiner Bio-One, Frickenhausen, Germany). Amino acid sequences were translated from the sequenced codons using EMBOSS Transeq20. The determined SAA sequences were compared to the SAA sequences registered in UniProt by BLAST21.
Immunohistochemistry
Two antibodies recognizing an SAA amino acid sequence (Asp32-Gly50), which is highly conserved across species, were produced using the contracted service of Cosmo Bio Co. (Tokyo, Japan), following protocols compliant with ARRIVE guidelines (Animal Research: Reporting of In Vivo Experiments) and approved by the company’s animal experiment committee22. One was designed for the SAA sequence with Gln45 (DKYFHARGNYDAAQRGPGG), named the anti-SAAQ45 antibody (catalog number, AF21040687-001; order number, PEP6038). The other was designed for the SAA sequence with Arg45 (DKYFHARGNYDAARRGPGG), named the anti-SAAR45 antibody (the catalog number, AF23031668-002; order number, PEP11054). In addition, a commercially available anti-SAA polyclonal antibody (PAA885Hu01; Cloud-Clone Corp., USA) was also used as an anti-pan-SAA antibody. For antigen retrieval, tissue sections were autoclaved at pH 7.0 for 20 minutes at 121°C. Sections were then incubated with primary antibodies for 120 minutes at room temperature. Horseradish peroxidase-conjugated polymer anti-rabbit IgG antibody (Dako, Santa Clara, CA, USA) was used as the secondary antibody, and 3,3’-diaminobenzidine tetrahydrochloride (DAB) was employed as the chromogen to visualize the positive reaction. The primary antibody was omitted as a negative control. These processes were performed manually. The negative activity is checked in the kidney of a cat without amyloid deposition (Supplementary Fig. 6).
Cross-validation of the newly produced antibody
In this study, two antibodies were produced by immunizing peptides with the sequence located at Asp32-Gly50 in Felis catus mature SAA (UniProt accession: P19707). Although this region of SAA is evolutionarily conserved in animals23 residue 45 is known to be diverse. For example, the 45th amino acid residues of SAA in cows (UniProt accession: P35541) and quails (UniProt accession: A0A8C2TJQ9) are glutamine (SAAQ45) and arginine (SAAR45), respectively. Therefore, preliminary experiments were conducted to verify the cross-reactivity of the new antibodies to SAA with the 45th residue.
Congo red staining and IHC with commercial anti-Pan-SAA antibody (PAA885Hu01, Cloud-Clone Corp.) and newly produced anti-SAA antibodies were performed on amyloid deposits in cow (kidney) and quail (duodenum). Amyloid deposition in these animals has been identified as AA in previous studies9,23. The conditions for IHC are the same as those described above.
Amyloid deposits in bovine and quail were positive for commercial anti-SAA antibodies (Supplementary Fig. 1). The anti-SAAQ45 antibody showed strong positive immunoreactions to bovine amyloid but was negative for quail amyloid. The anti-SAAR45 antibody strongly reacted with quail amyloid but was negative or weakly positive for bovine amyloid. The results demonstrate that the anti-SAAQ45 antibodies and anti-SAAR45 antibodies specifically detect SAAQ45 and SAAR45, respectively.
Tissue microdissection and mass spectrometry
LC-MS/MS was performed on amyloid deposits collected from Congo red-stained sections to determine the protein profile of amyloid deposits in cases 1–5. As in the previous studies24,25. Congo red-positive amyloid deposits approximately 500,000 µm2 each, were collected under a stereomicroscope. In case 1, five samples of amyloid deposits were collected from each of three sites: the glomerulus, inner stripe of the outer medulla, and papilla. Three samples from each collection were digested with trypsin, and two samples were digested with chymotrypsin before LC-MS/MS analysis, as described previously24,25.
For cases 2–5, the amyloid deposit samples were collected from the following sites: case 2, amyloid deposits in the glomeruli and inner stripe of the outer medulla; cases 3 and 4, glomeruli and papilla; and case 5, glomeruli. For cases 2–5, collected samples were digested by trypsin and subjected to LC-MS/MS analysis, as described previously24,25.
To clarify the protein composition of amyloid deposits, the MS/MS data of tryptic/chymotryptic samples were collated to theoretical fragment patterns of tryptic/chymotryptic peptide sequences from the UniProt database using Mascot Server (Matrix Science Inc., London, UK). The in silico proteolytic enzymes “semiTrypsin” and “none” in Mascot Server’s drop-down list were selected for tryptic and chymotryptic samples, respectively. Statistically significant peptides were extracted using Mascot’s probability-based scoring algorithm. Furthermore, peptide fragments with significant (“pep_expect” < 0.05) values were selected.
Furthermore, to identify which SAA variant originated amyloid deposits, MS/MS data were also analyzed using a database consisting of SAAsv1−v4 sequences described below. The origin of the detected peptides was determined based on a combination of substitution residues 29 (I/K), 45 (R/Q) and 51 (A/V), which can classify each SAA variant. In case 1, since the SAAv2 gene was not identified, consideration of the 29th amino acid substitution was ignored. Specifically, the deposited SAA variant was identified by analyzing whether the tryptic peptide containing residue 45 was GNYDAAR (residues 39–45 of SAAv1 and SAAv4), GNYEAAQR (residues 39–46 of SAAv2) or GNYDAAQR (residues 39–46 of SAAv3), whether the tryptic peptide containing residue 51 was GPGGAWAAK (residues 47–55 of SAAv1 and SAAv3), GGAWAAK (residues 49–55 of SAAv2) or RGPGGVWAAK (residues 46–55 of SAAv4), and whether the chymotryptic peptide containing residues 45 and 51 was DAARRGPGGAW (residues 42–52 of SAAv1), DAAQRGPGGAW (residues 42–52 of SAAv3), or DAARRGPGGVW (residues 42–52 of SAAv4). The reliability of peptide detection was evaluated using the peptide scores provided by the Mascot Server (Matrix Science Inc.).
Data availability
The research data are available from the corresponding author upon reasonable request.
References
Buxbaum, J. N. et al. Amyloid nomenclature 2024: update, novel proteins, and recommendations by the international society of amyloidosis (ISA) nomenclature committee. Amyloid 31, 249–256. https://doi.org/10.1080/13506129.2024.2405948 (2024).
Moccia, V. et al. AA amyloidosis in vertebrates: epidemiology, pathology and molecular aspects. Amyloid 32, 3–13. https://doi.org/10.1080/13506129.2024.2417219 (2025).
Karam, S., Haidous, M., Royal, V., Leung, N. & Renal AA amyloidosis: presentation, diagnosis, and current therapeutic options: a review. Kidney Int. 103, 473–484. https://doi.org/10.1016/j.kint.2022.10.028 (2023).
Sen, S. & Sarsik, B. A proposed histopathologic classification, scoring, and grading system for renal amyloidosis: standardization of renal amyloid biopsy report. Arch. Pathol. Lab. Med. 134, 532–544. https://doi.org/10.5858/134.4.532 (2010).
Westermark, G. T., Sletten, K. & Westermark, P. Massive vascular AA-amyloidosis: a histologically and biochemically distinctive subtype of reactive systemic amyloidosis. Scand. J. Immunol. 30, 605–613. https://doi.org/10.1111/j.1365-3083.1989.tb02468.x (1989).
Thorne, J. et al. Serum amyloid A Protein-Associated kidney disease: presentation, diagnosis, and management. Kidney Med. 4, 100504. https://doi.org/10.1016/j.xkme.2022.100504 (2022).
Banerjee, S. et al. Amyloid fibril structure from the vascular variant of systemic AA amyloidosis. Nat. Commun. 13, 7261. https://doi.org/10.1038/s41467-022-34636-4 (2022).
Iwaide, S. et al. Classification of amyloidosis and protein misfolding disorders in animals 2024: A review on pathology and diagnosis. Vet. Pathol. 62, 117–138. https://doi.org/10.1177/03009858241283750 (2025).
Murakami, T. et al. Atypical AA amyloid deposits in bovine AA amyloidosis. Amyloid 19, 15–20. https://doi.org/10.3109/13506129.2011.637145 (2012).
Ferri, F. et al. AA-amyloidosis in cats (Felis catus) housed in shelters. PLoS One. 18, e0281822. https://doi.org/10.1371/journal.pone.0281822 (2023).
Godfrey, D. R. & Day, M. J. Generalised amyloidosis in two Siamese cats: spontaneous liver haemorrhage and chronic renal failure. J. Small Anim. Pract. 39, 442–447. https://doi.org/10.1111/j.1748-5827.1998.tb03753.x (1998).
Boyce, J. T., DiBartola, S. P., Chew, D. J. & Gasper, P. W. Familial renal amyloidosis in Abyssinian cats. Vet. Pathol. 21, 33–38. https://doi.org/10.1177/030098588402100106 (1984).
Niewold, T. A., van der Linde-Sipman, J. S., Murphy, C., Tooten, P. C. & Gruys, E. Familial amyloidosis in cats: Siamese and Abyssinian AA proteins differ in primary sequence and pattern of deposition. Amyloid 6, 205–209. https://doi.org/10.3109/13506129909007328 (1999).
Tei, M. et al. Variation of amino acid sequences of serum amyloid a (SAA) and immunohistochemical analysis of amyloid a (AA) in Japanese domestic cats. J. Vet. Med. Sci. 80, 164–172. https://doi.org/10.1292/jvms.17-0447 (2018).
Moccia, V. et al. Histological evaluation of the distribution of systemic AA-amyloidosis in nine domestic shorthair cats. PLoS One. 18, e0293892. https://doi.org/10.1371/journal.pone.0293892 (2023).
Palizzotto, C. et al. Renal amyloid-A amyloidosis in cats: characterization of proteinuria and biomarker discovery, and associations with kidney histology. J. Vet. Intern. Med. 38, 205–215. https://doi.org/10.1111/jvim.16920 (2024).
Terasaki, M., Brunson, J. C. & Sardi, J. Analysis of the three dimensional structure of the kidney glomerulus capillary network. Sci. Rep. 10, 20334. https://doi.org/10.1038/s41598-020-77211-x (2020).
Nawata, C. M. & Pannabecker, T. L. Mammalian urine concentration: a review of renal medullary architecture and membrane transporters. J. Comp. Physiol. B. 188, 899–918. https://doi.org/10.1007/s00360-018-1164-3 (2018).
Schulte, T. et al. Cryo-EM structure of ex vivo fibrils associated with extreme AA amyloidosis prevalence in a Cat shelter. Nat. Commun. 13, 7041. https://doi.org/10.1038/s41467-022-34743-2 (2022).
Madeira, F. et al. The EMBL-EBI job dispatcher sequence analysis tools framework in 2024. Nucleic Acids Res. 52, W521–w525. https://doi.org/10.1093/nar/gkae241 (2024).
UniProt, C. UniProt: the universal protein knowledgebase in 2023. Nucleic Acids Res. 51, D523–D531. https://doi.org/10.1093/nar/gkac1052 (2023).
du Percie, N. et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. PLoS Biol. 18, e3000410. https://doi.org/10.1371/journal.pbio.3000410 (2020).
Nakayama, Y. et al. Experimentally induced, and transmissible AA amyloidosis in Japanese quail (Coturnix japonica). Vet. Pathol. 54, 912–921. https://doi.org/10.1177/0300985817723692 (2017).
Murakami, T. et al. Identification of novel amyloidosis in dogs: α-S1-casein acquires amyloidogenicity in mammary tumor by overexpression and N-terminal Truncation. Vet. Pathol. 60, 203–213. https://doi.org/10.1177/03009858221148511 (2023).
Iwaide, S. et al. Fibrinogen Aα-chain amyloidosis outbreaks in Japanese squirrels (Sciurus lis): a potential disease model. J. Pathol. 261, 96–104. https://doi.org/10.1002/path.6150 (2023).
Acknowledgements
This research was supported by JSPS KAKENHI (Grant No. 23H02380) and the Program on Open Innovation Platform with Enterprises, Research Institute, and Academia (OPERA) from the JST. The LC-MS/MS analyses and Mascot analysis were performed at the Tokyo University of Agriculture and Technology for Smart-Core-facility Promotion Organization.
Author information
Authors and Affiliations
Contributions
NK and TM conceptualized the paper. NK, MK, MI, HK, SI, YI, MH, YK, and NSM performed the experiments and validated the study. NK, MK, and TM performed the formal analysis. KN and MT provided FFPE samples. MT and TA provided records of clinical information. NK and TM wrote the original draft and outlined study. All authors reviewed and TM edited. TM handled administrative tasks and acquired funding.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kobayashi, N., Kaneda, M., Ikeda, M. et al. Polymorphisms in SAA alter intrarenal amyloid distribution of AA amyloidosis in cats. Sci Rep 15, 21553 (2025). https://doi.org/10.1038/s41598-025-07983-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-07983-7