Abstract
The current study aimed to identify how manipulated sleep restriction affects dietary choices and physical activity (PA). Young adults were administered one week of well-rested sleep levels (WR: 8–9 h/night) and sleep-restriction (SR: 5–6 h/night) in their (naturalistic) at-home setting, using a randomized cross-over design. Participants made consequential bids for snacks/beverages following SR and WR in an auction task. Other primary outcome measures included daily self-reported dietary intake and actigraphy-measured PA. Multivariate regression analyses examined main and moderator impacts of SR on primary outcome measures. In total, 118 treatment participants completed the study (M = 20 years old, n = 65 females). SR predicted increased self-reported daily caloric intake and importance of taste (over healthiness) in auction bids, but only in participants with higher baseline cognitive or behavioral control characteristics (p < .05). Furthermore, SR predicted reduced average hourly PA (p < .01), increased sedentary behaviors (p < .01), and more prolonged bouts of sitting (p < .01) for all participants. While prior literature suggests dietary choices may mediate the link between SR and obesity, we did not find altered dietary choices. Rather, our results suggest decreased PA may also contribute to the link between SR and obesity.
Similar content being viewed by others
Introduction
Rising obesity rates are a global health concern affecting more than 670 million individuals1, and modifiable lifestyle behaviours such as physical inactivity and poor diet are implicated as key contributors2. Yet another global health problem is insufficient sleep3,4, which has been estimated to cost 1–3% of annual economic GDP4. Moreover, such figures likely underestimate the true economic cost given that insufficient sleep has spillover effects that are difficult to measure and identify. For example, insufficient sleep may contribute to weight and obesity via negative impacts on both physical activity5,6 and dietary choices7,8. Insufficient sleep may also impact the timing of dietary intake in ways that promote higher BMI12,13, which highlights the potential importance of chrononutrition in studying health outcomes and interventions14,15. It is not uncommon for individuals with poor sleep hygiene to also have poor dietary and/or PA habits16,17,18, such that these health behaviours are likely interrelated, but it is often difficult to establish the direction of causation using field data.
A review of the literature connecting sleep parameters and PA found inconsistent and insignificant associations at the individual level19. However, longitudinal and cross-sectional evidence suggests that physical inactivity is associated with increased insomnia and poorer sleep quality20,21. While sleep seems to impact dietary choices, diet has also been shown to impact sleep (e.g22.). Evidence from intervention studies, which are best suited to identify causal relationships, suggests insufficient sleep causes overeating (see meta-analysis in Fenton et al.23, and restricted nightly sleep reduces physical activity (e.g6,24,25.). Additional studies with exogenous (i.e., experimenter prescribed) variation in sleep duration are still needed to help better understand causal relationships within this unholy trinity of damaging health behaviors.
This paper presents unique data from a preregistered at-home cross-over sleep protocol that examines dietary choice and physical activity under naturalistic conditions with prescribed nightly sleep durations. Our main study objectives were to examine the causal impact of restricted sleep levels on dietary intake, dietary choices, and PA levels. Previous research just highlighted has mostly examined these key questions separately or examined them observationally or with methodologies that make causation harder to attribute to insufficient sleep itself6, is an exception). As a result, our examination of how experimentally manipulated sleep restriction affects dietary choices (i.e., self-reported 24-hours dietary recall, and also consequential snack/beverage decision making) and objectively measured physical activity is a focused effort to bring together our understanding of how sleep effects these two key components of the weight and obesity question (i.e., energy homeostasis) that looms large in modern society.
Dietary choice was studied with self-reported dietary recall data and via choices from a consequential (i.e., incentivized) auction task designed to examine preferences for snacks and drinks that differed in macronutrient and hedonic attributes. That is, we generated data in our study on self-reported daily energy intake, and we complemented this with consequential bid data on snacks and beverages collected at the end of each treatment week, which speaks to how sleep restriction may affect momentary dietary choices. Finally, actigraphy-measured PA data were collected and converted into metabolic equivalent tasks (METs) to examine daily PA intensity under sleep-restriction (SR) and well-rested (WR) conditions (see also McElroy et al.25).
Our preregistered hypotheses focused on the impact of SR on dietary reports, food preference choices, and PA levels. We hypothesized that daily caloric, fat, and sugar intake based on self-reports would be higher during the SR compared to the WR treatment week. We also hypothesized that SR would promote higher bids in the snack and beverage auction task for items with higher calorie content. Finally, we hypothesized that average hourly PA would be lower during the SR compared to WR treatment week.
Materials and methods
Details of the randomized cross-over design protocol were preregistered on the Open Science Framework (OSF) (registrations https://doi.org/10.17605/OSF.IO/NSPRK, https://doi.org/10.17605/OSF.IO/BKH5J, and https://doi.org/10.17605/OSF.IO/EXV4D). The study was reviewed and approved by the ethics board at the first-author’s institution.
Participants
Data collection took place between August 2021-April 2023, with participant screening, randomization, and enrollment occurring each semester (4 semesters total) at one of the author’s institutions (University) where the experiments were conducted. Participants were prescreened for eligibility and to collect demographic and self-reported sleep measures. Inclusion criteria for the study were: between 18 and 40 years of age, no self-reported diagnosed or suspected sleep disorder, no self-reported eating disorders or dietary restrictions, non-extreme diurnal preference, and no risk of major depressive or generalized anxiety disorder. Diurnal preferences were assessed using the reduced-form morningness-eveningness questionnaire26, risk of major depressive disorder was assessed using the PHQ-2 questionnaire27, and risk of generalized anxiety disorder was assessed using the GAD-7 questionnaire28. Sample characteristics are shown in Appendix Table A1.
Sleep protocol
Young adult treatment participants were prescribed one week (7 nights) each of well-rested sleep levels (WR: 8–9 h per night attempted sleep) and sleep-restricted sleep levels (SR: 5–6 h per night attempted sleep) in randomly assigned order with a washout week of ad lib sleep levels in between. Sleep occurred in one’s at-home setting, and participants kept daily sleep diaries29. Participants were asked not to nap during the prescribed sleep (SR and WR) weeks, although any naps were included in the total sleep time measures we report. Each participant’s daily 24-hours sleep duration was measured using a wrist-worn actigraphy device (Actiwatch Spectrum Plus: Philips Healthcare), which also provided an objective measure of PA during non-sleep hours across the 3-week protocol—devices were set to sample activity data at 30-second epochs. Actigraphy data were downloaded to the manufacturer software (Actiware), where rest intervals (nighttime and any daytime naps) were scored by the experimenters using validated protocols (e.g., Goldman et al.30). The manufacturer’s software algorithm then scored each 30-second epoch for sleep based on a validated algorithm that used wrist motion as a proxy for gross motor movements. For short, in the text we refer to average 24-hours total sleep time outcomes as average “nightly” sleep in places, but all objectively measured sleep during the protocol is captured for our analysis. As noted in our preregistration plans, we required at least 5 days (out of 7 for a given treatment week) of valid actigraphy data to include in the analysis, and our final sample for analysis includes only participants for whom we have valid data for both treatment weeks. No restrictions were placed on participants regarding daily dietary intake or daily PA during the protocol—only sleep duration was prescribed by the experimenters. Objective and subjective measures related to sleep protocol differences by treatment are in Appendix Table A2.
Measures
The snack/drink auction task
The auction task presented participants with a total of 4 distinct snacks and 4 distinct beverages in a series of consequential auctions (see full experiment instructions in Appendix B). Participants rated their hunger and thirst levels, time since last food or drink (except water) consumption, and they also provided subjective ratings of the healthiness and tastiness of each snack/drink items prior to submitting auction bids. Appendix Tables A3 and A4 show the summary information on objective profile and subjective ratings for each snack and drink item.
The snack items were labeled A, B, C, and D. One auction scenario involved endowing the participant with Snack (A) The A→B snack auction asked the participant to make a bid to trade snack A for snack (B) The high-bid auction winner consumed snack B at the end of the session, and the auction payment was subtracted from the participant’s other cash earnings—in addition to a fixed payment for study completion, participants also received cash compensation from other incentivized task not reported in this paper. All other participants consume their endowment snack A. Bids were constrained to be between -USD$2.00 and + USD$2.00 with negative bids indicating a preference for the endowed item. These bids were the primary outcome measure for this task.
Bids were elicited separately for all possible comparisons with Snack A as the endowment: A→B, A→C, and A→D snack auction scenarios. Afterwards, snack B was presented as the endowment snack and bids for B→A, B→C, and B→D auction scenarios were submitted, etc. This implied a total of 12 snack auction bids from each participant, although some were redundant, namely, both the A→B and B→A auctions measure one’s relative preference for A compared to B, and we discuss later our approach to analysis only of unique item comparisons. Bids were elicited for a similar set of 12 beverage auctions and, after all bidding was completed, one randomly selected snack and one beverage auction were played out to determine consumption at the end of the laboratory session.
Dietary intake
To measure dietary intake, daily 24-hour recalls were collected each day of the protocol and analyzed using the validated Automated Self-Administered 24-hour (ASA24) Dietary Assessment Tool (U.S. 2020 version). The ASA-24 was developed by the National Cancer Institute31and is commonly used in interventional or clinical research (e.g32.). The primary outcomes extracted were daily calories (Kcals), fat, sugar, and caffeine, as well as the timing of intake—these data were assessed by a trained researcher familiar with the methodology (author NK). In relation to meal timing, we examined total Kcal energy and number of days on which food was consumed during the estimated circadian night as secondary outcome measures. Because we did not have physiological data (e.g., saliva samples) to assess melatonin onset for establishing one’s circadian evening, we estimated a 10-hour circadian night using actigraphy-based data from a similar sample33. The 10-hour window started two hours prior to habitual sleep onset of 01:13 (see Sletten et al.34).
Actigraphy-based sleep and PA
Passive but objective data collection was used to assess sleep duration and PA using actigraphy. Sleep measures included a binary SR indicator variable denoting the treatment assignment and average total nightly sleep (in minutes, to include any daytime naps), TST, from scored actigraphy data. Some of the sensitivity analysis also included subtracting one’s self-reported optimal nightly sleep from the actigraphy measured sleep to create a Personal Sleep Deprivation measure.
PA measures were constructed from the actigraphy’s raw activity count data, which were sampled at 30 s epochs. Scoring of PA data used all valid 30-second data epochs not within a scored rest interval (see McElroy et al.25). Raw activity counts were used to construct average hourly activity counts. Because others have validated the use of the Actiwatch device used to differentiate between PA intensity levels35,36,37, we therefore used the cutoffs from Lee and Tse36 to score PA into sedentary, mild, moderate, and vigorous activity intensity levels—we halved the counts-per-minutes cutoffs to align with our data collection at 30-second epochs. Finally, we also used the raw activity count data to construct variables describing prolonged sitting bouts and planned PA bouts, based on evidence of ≥ 30 consecutive minutes of sedentary PA intensity38, or ≥ 10 consecutive minutes of moderate-to-vigorous PA intensity39.
Key participant control measures
In addition to demographics from the screening survey, additional measures that we considered important to take into account in our analysis were collected during Session 1: the Three-Factor Eating Questionnaire (TFEQ: Stunkard and Messick40, , self-reporting of one’s height and weight (to calculate body mass index [BMI]), a baseline measure of one’s future orientation41, and one’s score on a cognitive reflection task42. See Appendix Figure A2 for the distributions of these measures in our participants.
Procedures
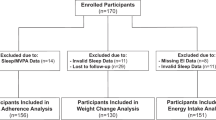
Eligible participants were randomly assigned to a SR-WR or WR-SR ordering of the prescribed sleep treatment weeks. The experimenters conducted the randomization, which assigned half the viable participants to each treatment order. Depending on enrollments and attrition for each cohort, we disproportionately enrolled participants in later cohorts from whichever treatment order was unrepresented in the sample, which was how we achieved equal numbers of enrolled participants for each treatment order (see also the participant flow diagram in Fig. 1). We preregistered a target sample size of n = 100 participants based on an a priori power analysis using G*Power to guarantee power = 0.80 to detect small-medium sized effects in the data. The final sample was n = 118 participants completed the 3-week randomized crossover protocol.
After random treatment order assignment, a detailed email invited participation in the 3-week study, which required 3 in-lab sessions of 1.5 h each. Details were given regarding sleep tracking, sleep level assignments each week, and required daily online dietary intake reports. Participants were recruited in Cohorts of 11–16 participants. For a given cohort, all laboratory sessions took place on a common day of the week, i.e., Tuesday, Wednesday, or Thursday, at either 3:00pm or 3:30pm. The sleep protocol largely followed Dickinson et al.29.
After an initial laboratory session to initiate the study, the other two lab sessions occurred at the end of each treatment week (i.e., the end of Weeks 1 and 3). Participants were asked to refrain from eating or drinking anything but water for 3 h prior to these lab sessions and were informed that they would be consuming a snack and a drink as part of the session. Instructions, baseline measures and instruments to capture TFEQ and CRT scores, and assignment of actigraphy devices took place during Session 1, while decision tasks were administered during Sessions 2 and 3. Additionally, Session 3 concluded the study.
Data analysis and statistical modeling
In analyzing snack/drink bids in the laboratory auction task, our data are structured as a panel set with 12 observations per participant (6 unique snack and 6 unique beverage auction bids). Random effects generalized least squares (GLS) models were estimated to account for the panel nature of the data, and all analysis included robust standard errors clustered on the participant. Estimations were conducted separately for the snack versus beverage auctions, and we constructed variables to describe the difference between calories (and other objective macronutrients) and subjective taste/health rating of the auction item relative to the endowment item. Using this approach, a positive coefficient on the Calorie Difference variable would indicate that higher calorie content was preferred because it predicts a higher auction bid. Similar macronutrient variables were created for the pairwise differences in Calories, Fat, Sodium, Sugar, Protein, as well as the subjective differences in one’s Taste and Healthiness ratings between the two items. Models included cohort fixed effects and repeat administration effects (suppressed from the results table for space considerations). Our main preregistered hypotheses test results are shown in Table 2, with sensitivity analysis relegated to the online Appendix. The key SR effect of interest is the interaction term between the binary SR indicator variable and the Calories(or Taste and Health) difference variables.
Although the panel data set is comprised of 12 observations per participant, each participant made 24 total bids (12 snack and 12 beverage auctions). However, only half of these auctions represent unique pairwise item comparisons as noted in section C1 above. Because of this, a bootstrapped estimation procedure was used to address the issue of whether our results depend on which of unique auctions are used in the regressions. Specifically, we conducted 1000 bootstrapped regression where the model was estimated each time after randomly selecting one auction from each set of redundant pairs (e.g., the A◊B or the B◊A auction, but not both). The results we present are the average coefficient estimates from these 1000 bootstrapped regressions. Analysis was performed using STATA software (version 18), and statistical significance thresholds are reported at p = .01 and p = .05 levels. For preregistered hypotheses, these thresholds are for the appropriate 1-tailed test. Otherwise, 2-tailed test thresholds are reported. More details on analyses specific to each task are given in the Results section.
Dietary intake analysis was conducted using daily macronutrients extracted from ASA24 reports for the 6 days that were fully within a treatment condition (i.e., dietary reports from days transitioning from one week to another were excluded from analysis). Similarly, for the PA analysis only epochs fully within a treatment condition were analyzed. Epoch-level physical activity count data were converted to metabolic equivalency task (METs) intensity levels using published calibration cutoffs specific to the devices we used36. Key hypotheses were tested for the daily dietary intake outcomes and the average hourly PA by examining the significance of the binary indicator variable, SR, in predicting the key dependent variable outcome measure. Random effects (GLS) models were estimated with robust standard errors using STATA software (version 18).
Aside from analysis related to the preregistered hypotheses, exploratory analyses were conducted on the auction data and ASA24 data. The exploratory analysis was aimed at examining whether participant sex or other behavioral and cognitive-related traits moderated an SR effect in the data. For the auction bid analysis, split samples were estimated for comparison groups using the 100 iterations of the bootstrap estimation method described above, while for the ASA24 data the exploratory analysis was conducted by adding a series of binary indicator variables and their interactions with the binary SR treatment indicator variable to regression specifications. The binary indicator variables separated participants into one or the other categorization of the characteristic trait (e.g., Obese versus Non-Obese, More Controlled Eaters versus Less Controlled Eaters, etc.). Similar analysis of potential moderating effects were conducted on the PA data, but these were preregistered are therefore not considered exploratory.
Results
Protocol validity
The full set of n = 118 (n = 65; 55% female) treatment participants represent the “intent-to-treat” sample used for the main analysis. The participant flow of our study is presented in Fig. 1, and Table 1 summarizes condition and participant characteristics (see also Appendix Table A1 for additional participant characteristic measures by treatment condition). Note that the Fig. 1 participant flow diagram highlights that we also recruited a smaller set of Control-condition participants (n = 37) as part of our preregistration plans. These Control-condition participants were administered two weeks of WR sleep levels in place of one SR and one WR week, and this group served mainly to validate the cross-over sleep manipulation protocol and so we did not include these in our analysis (see Appendix A for additional information on the Control participants). Figure 2 documents the validity of the sleep restriction protocol by comparing differences between objective and subjective measures across the SR and WR treatment weeks of the protocol.
Assessment of protocol validity. Participants were asked to not nap during the SR or WR week of the study, but any rare naps in the data were scored and included in the measure of average 24 h sleep time. Significance is based on a one-sample Z-test of the difference between actigraphy measured average nightly sleep duration during the SR and WR treatment weeks (See Appendix Table A2), with associated Cohen’s D effect size shown. A similar result is found using a one-sample t-test.
Food/beverage auctions
Results showed that the Calorie difference predicts beverage bids but not snack bids, but the statistically insignificant interaction term effect for Calorie Dif * SR data fails to support the preregistered hypothesis that SR would increase one’s inclination to bid more for additional calories. The statistically significant and positive coefficient estimates on Taste Difference in models 1 and 3 of Table 2 indicate that participants submitted significantly higher bids for items deemed to be tastier, with a large effect size. However, SR did not alter how these hedonic attribute effects on bids. These results are robust to three sensitivity analyses that vary the sleep control measure and accounts for potential sample selection (see Appendix Table A5 for the selection equation results, and Tables A6, A7, and A8 for the sensitivity analysis results).
Dietary intake
Regarding ASA dietary intake reports, the Table 3 regression analysis results highlight that the only significant predictor of daily calories or fat consumption is the binary Female indicator variable, which found that females, compared to males, reported about 430 fewer daily calories and 17 fewer fat grams consumed. The data fail to support our preregistered hypothesis of a main SR effect on reported calories consumed, and this was robust to sensitivity analysis shown in Appendix A (Tables A9, A10, and A11).
Exploratory analysis of dietary choices (auction bids and ASA24 dietary reports)
The key exploratory analysis findings are summarized in Table 4 (see Appendix A, Tables A12, A13, A14, A15, A16, A17, A18, A19, and A20 for full results of the exploratory analysis). For the auction task, SR increased the relative bid premium for tastiness over healthiness of beverages for male participants. SR also increased the relative tastiness bid premium for snacks in Low Emotional eaters, and in those who self-reported being hungrier. Statistically significant moderators all had small effect sizes (ηp2) on the outcome measures examined. Regarding the ASA24 data, SR was found to increase daily caffeine intake and nighttime reports (but without increasing nighttime calories). In addition, daily Kcal, fat, and sugar reported were all higher in the SR compared to WR condition for Non-Obese, High Cognitive Reflection, and Controlled Eaters. Other findings were less robust where a characteristic moderated an SR effect for only one of the macronutrients examined.
Physical activity outcomes
The daytime PA data were analyzed using multivariate regression models to assess both average hourly physical activity levels based on raw activity count data, and to examine PA intensity using METs conversion specific to the actigraphy devices we used36. Figures 3 and 4 summarize the PA results via coefficient plots (full results and sensitivity analysis are shown in the Appendix, Tables A21, A22, A23, A24, and A25, and Figure A1). SR predicted significantly lower average hourly PA compared to WR, and this result is robust to sensitivity analysis results shown also in Fig. 3 (see Appendix Table A21 for full results). Analysis of participant subgroups was also conducted. We had preregistered the hypotheses that the SR impact on PA would differ by BMI classification and TFEQ eating characteristics, but the data fail to support those hypotheses because the estimated SR effect was general—that is, SR lowered PA across all subgroups examined (see summary Figure A1 in Appendix A, supported by full results shown in Appendix Tables A22 and A23). When converting the data to generally accepted PA intensity categories46, the data in Fig. 4 (upper panel) show that SR significantly increased the proportion of wake-hour time classified as sedentary PA and lowered the proportion of time spent in all other intensity categories (light, moderate, and vigorous PA). Finally, we examined the impact of SR relative to WR on bouts of prolonged sitting and planned activity. Here, Fig. 4 (lower panel) highlights that SR predicted increased instances of prolonged sitting compared to the WR week, and the effect size is large (ηp2 = 0.133), but there was no significant difference in planned PA episodes during SR compared to WR (see Appendix A, Tables A24 and A25, for full estimation results supporting Figs. 3 and 4).
Analysis of SR impact on daytime actigraphy-measured PA. Coefficient plots show the point estimate and the 95% (thick line) and 99% (thin line) confidence intervals. Control variables include demographic, a binary order (of week) indicator variable, and cohort fixed effects. Robust standard errors are clustered on the participant (n = 118 treatment participants, n = 236 panel data set observations). Partial eta-squared effect sizes are for the comparable ordinary least squares model with additional control variables. Physical activity per minute is calculated from all treatment week non-sleep epochs, starting the morning after the first treatment night until the start of the laboratory session one week later. Partial eta-squared effect sizes are for comparable OLS regressions (does not differentiate weighted from non-weighted regression for the IPW corrected models).
Analysis of SR impact on PA intensity (upper panel), prolonged sitting and planned PA (lower panel). Coefficient plots show point estimates with 95% (thick line) and 99% (thin line) confidence intervals. Robust standard errors are clustered on the participant (n = 118 treatment participants, 2 observations each). Partial eta-squared effect sizes are from the comparable ordinary least squares models. PA intensity categories were constructed using METS cutoffs from the actigraphy activity counts data (cutoffs derived in Lee and Tse36. A bout of prolonged sitting was defined as ≥ 30 consecutive minutes within the sedentary METS classification (see Bellettiere et al.38), while a bout of planned PA was defined as ≥ 10 consecutive minutes of moderate or vigorous METS activity39. Results are similar if using actigraphy measured average nightly sleep instead of a binary SR indicator variable.
Discussion
Overall, our findings suggest that sleep restriction had little impact on food choice or actual dietary intake. This finding contrasts with the results of recent meta-analyses showing that sleep restriction interventions increase daily caloric intake by 252.8 kcal47 and 364 kcal48 relative to recommended sleep duration. Nevertheless, almost all studies included in these reviews were conducted in controlled laboratory settings and assessed impacts on ad libitum caloric intake during one meal or over one day. The small number of included studies conducted in free-living settings focused on small samples (n = 12–14) of healthy men or women. The review by Zhu et al.47 included both total sleep deprivation and partial sleep restriction studies, the latter of which may be more akin to real-world sleep disturbances albeit these studies were not examined separately. Furthermore, inter-individual (i.e., parallel group) designs may be limited given the large variability in energy intake increases resulting from insufficient sleep due to inter-individual and sex differences49. Finally, most of these reviews have focused on adults more broadly rather than young adults as was the focus in the current research.
Data from our consequential snack and beverage bid data did not support our general hypothesis that SR would increase bids for high calorie content items. While examinations of SR impacts on general cognitive control have found that impulsivity increases during SR50,51, which presumably would affect more momentary dietary choices, researchers have not found clear evidence that SR increases choices for higher-calories snacks. Nedeltcheva et al.52 reported that SR increased caloric intake overall from snacks, but with no significant increase in daily kcalories. Schmidt et al.6 also reported no significant overall daily food intake during SR compared to a WR condition. Our auction data can reveal additional nuances of dietary preference effects during SR. For example, our data showed that subjective taste mattered most in auction bids, and exploratory analyses found some evidence that males, non-emotional eaters, or those who self-reported being hungrier, may respond to SR with a more disproportionate focus on taste, rather than healthiness, in dietary choices. If taste engages reward processing areas in the brain more so than an item’s healthiness53, then any SR-driven increase in the salience of a food/drink item’s positive reinforcement may further widen the preference of taste over healthiness, at least in some subsets of individuals.
We also failed to support the hypothesis that SR would lead to a general increase in daily calories, fat, and sugar consumed. This is consistent with others’ findings that SR did not lead to increase daily energy expenditure (e.g52..; Schmidt et al.6). However, exploratory results from ASA24 dietary recall data showed an increase in reported daily fat, sugar, and total kcalories among Non-Obese, High Cognitive Reflection, and Controlled Eaters participants. In other words, the negative effects of SR on daily dietary choices may be isolated to those who have a baseline higher level of certain behavioral or cognitive control related characteristics, which is an intriguing finding that merits further study.
With respect to PA, our data showed a robust and general effect whereby SR lowered the within-participant average hourly PA, increased the proportion of wake-time spent in a sedentary METs classification, decreased the proportion of wake time spent in moderate-to-vigorous METS classifications, and increased estimated bouts of prolonged sitting. These findings replicate those reported by McElroy et al.25 in a unique sample of young adults, using a more robust scoring of actigraphy-derived PA outcomes measures. As we prescribed varied nightly sleep across treatment weeks, this increases our confidence in assigning causation to SR for any resulting changes in PA and/or dietary choices.
The finding that SR led to unaltered food and macronutrient intake combined with reduced PA is consistent with those recently reported by Schmid et al.6. If these behaviors were maintained with more chronic sleep restriction, they would be expected to result in weight gain and associated morbidities due to an altered caloric balance. Thus, our data suggest SR might have an indirect effect on weight gain, via reduced PA, as well as the direct effect proposed by others. Future studies would need to test this notion directly.
These findings have broad implications worth noting. First, recent reviews of health promotion programs delivered in higher education institution settings such as universities typically target both risk (alcohol and other substance use, smoking) and protective factors (nutrition, mental health, physical activity), yet few include a focus on sleep54,55). Such programs should aim to include interventions or health promotion activities focused on improving sleep-related outcomes. Implications may also generalize into other areas, such as occupational settings. For example, Workplace Health Promotion Programs (HPPs) most typically focus on weight loss and smoking cessation, but such programs should also consider expanding their focus to include adequate sleep targets. Our results suggest reducing the prevalence of chronically insufficient sleep, in addition to other direct health cost benefits to an organization, may also indirectly help address weight and obesity via increased daily PA. In the broader policy arena, the lack of a sleep health focus in national public health agendas, already considered a costly policy oversight56, is even more damaging than previous thought.
Our study is not, of course, without limitations. We cannot empirically distinguish from self-report ASA24 data whether systematic underreporting may exist during SR, perhaps offsetting increased intakes during SR. However, the fact that SR also showed a null effect on bidding behavior in the snack/drink auction task may help mitigate the concern that the null effect of SR on dietary intake is spurious. Separate analysis showed that SR did not differentially impact ASA24 reports across weeks due to general fatigue or effort effects, and analysis also documents a null SR effect on macronutrients in cross-sectional analysis of each protocol week (1 and 3) separately. In the auction task, participants generally did not review the nutritional labels, and so incomplete information on macronutrients somewhat limits our ability to fully address the role of SR in these auction data. Also, while we assessed participant hunger/thirst prior to administration of the snack and beverage auction task, these were not validated scales, but rather a simple 9-point Likert scale to indicate one’s current level of hunger or thirst (1 = lowest, 9 = highest).
There are also limitations associated with using actigraphy-measured activity counts as a proxy for overall PA36,57 or to infer sitting behaviors that implies both physical inactivity andposture. However, this technology is usually considered more objective than PA self-reports, and it has precedence in the literature (e.g24,58.). Despite these limitations, our PA data analyses all converge to a common finding that SR negatively impacts these PA-related behaviors across all participant subgroups.
The young-adult study population was mostly college students who may have later bedtimes, and thus the findings may not generalize. For example, food-related reward signals may be heightened under sleep restriction more generally in adolescents whose inhibitory control processes are less developed59. Future research will help identify the importance of brain development on how SR impacts these critical behavioral areas. We also did not have baseline ventral striatum responsivity measures in our sample, which may moderate a link between SR and dietary choice60.
Conclusions
The goal of this paper was to better understand the link between insufficient sleep, dietary choice, and physical activity, which are considered key contributors to the global weight and obesity crisis. Our most general result is that mild but chronic SR significantly reduced average hourly PA and increased sedentary behaviors. Other results indicate that individuals higher in control-related characteristics may be those most susceptible to negative dietary choice impacts of SR. This study was intended as a complement to more highly controlled in-lab sleep and dietary choice studies, but a key benefit lies in its ecologically validity, which more easily allows findings to transfer externally to real-world settings. Overall, our findings are consistent with the conclusion that negative SR effects on PA may be a pathway connecting poor sleep to weight and obesity, while SR effects on dietary choice may be more conditional on individual characteristics.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
Di Angelantonio, E. et al. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 388(10046), 776–786 (2016).
Guthold, R., Stevens, G. A., Riley, L. M. & Bull, F. C. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1· 9 million participants. Lancet Global Health. 6(10), e1077–e1086 (2018).
Gordon, S., Vandelanotte, C., Rayward, A. T., Murawski, B. & Duncan, M. J. Sociodemographic and behavioral correlates of insufficient sleep in Australian adults. Sleep. Health. 5(1), 12–17 (2019).
Hafner, M., Stepanek, M., Taylor, J., Troxel, W. M. & Van Stolk, C. Why sleep matters—the economic costs of insufficient sleep: a cross-country comparative analysis. Rand Health Q. 6(4), 11 (2017).
Axelsson, J. et al. Sleepiness as motivation: a potential mechanism for how sleep deprivation affects behavior. Sleep 43(6), zsz291 (2020).
Schmid, S. M. et al. Short-term sleep loss decreases physical activity under free-living conditions but does not increase food intake under time-deprived laboratory conditions in healthy men. Am. J. Clin. Nutr. 90(6), 1476–1482 (2009).
Klock, M. D., Jakobsdottir, S. & Drent, M. L. The role of leptin and Ghrelin in the regulation of food intake and body weight in humans: a review. Obes. Rev. 8(1), 21–34 (2007).
Spiegel, K., Knutson, K., Leproult, R., Tasali, E. & Cauter, E. V. Sleep loss: a novel risk factor for insulin resistance and type 2 diabetes. J. Appl. Physiol. 99(5), 2008–2019 (2005).
Markwald, R. R. et al. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc. Natl. Acad. Sci. 110(14), 5695–5700 (2013).
Taheri, S., Lin, L., Austin, D., Young, T. & Mignot, E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med., 1(3), e62 (2004).
Zerón-Rugerio, M. F., Doblas-Faxeda, S., Diez-Hernández, M. & Izquierdo-Pulido, M. Are emotional eating and other eating behaviors the missing link in the relationship between inadequate sleep and obesity?? A systematic review. Nutrients 15(10), 2286 (2023).
Baron, K. G., Reid, K. J., Kern, A. S. & Zee, P. C. Role of sleep timing in caloric intake and BMI. Obesity 19(7), 1374–1381 (2011).
Culnan, E., Reid, K. J., Zee, P. C., Crowley, S. J. & Baron, K. G. Meal timing relative to DLMO: associations with BMI and body fat. Sleep. Health. 7(3), 339–344 (2021).
Almoosawi, S. et al. Chronotype: implications for epidemiologic studies on chrono-nutrition and cardiometabolic health. Adv. Nutr. 10(1), 30–42 (2019).
Davis, R., Rogers, M., Coates, A. M., Leung, G. K. & Bonham, M. P. The impact of meal timing on risk of weight gain and development of obesity: a review of the current evidence and opportunities for dietary intervention. Curr. Diab. Rep. 22(4), 147–155 (2022).
Kase, B. E., Liu, J., Wirth, M. D., Shivappa, N. & Hebert, J. R. Associations between dietary inflammatory index and sleep problems among adults in the united states, NHANES 2005–2016. Sleep. Health. 7(2), 273–280 (2021).
Štefan, L., Sporiš, G., Krističević, T. & Knjaz, D. Associations between sleep quality and its domains and insufficient physical activity in a large sample of Croatian young adults: a cross-sectional study. BMJ Open., 8(7), e021902 (2018).
Theorell-Haglöw, J. et al. Sleep duration is associated with healthy diet scores and meal patterns: results from the population-based epihealth study. J. Clin. Sleep Med. 16(1), 9–18 (2020).
Atoui, S. et al. Daily associations between sleep and physical activity: A systematic review and meta-analysis. Sleep Med. Rev. 57, 101426 (2021).
Boyle, J. T., Nielson, S. A., Perlis, M. L. & Dzierzewski, J. M. Move your feet and sleep: A longitudinal dynamic analysis of self-reported exercise, sedentary behavior, and insomnia symptoms. Sleep. Health. 10(3), 321–326 (2024).
Hofman, A., Voortman, T., Ikram, M. A. & Luik, A. I. Time spent in physical activity, sedentary behavior, and sleep: associations with self-rated sleep quality in middle-aged and older adults. Sleep. Health. 8(6), 701–704 (2022).
Peuhkuri, K., Sihvola, N. & Korpela, R. Diet promotes sleep duration and quality. Nutr. Res. 32(5), 309–319 (2012).
Fenton, S., Burrows, T. L., Skinner, J. A. & Duncan, M. J. The influence of sleep health on dietary intake: a systematic review and meta-analysis of intervention studies. J. Hum. Nutr. Dietetics. 34(2), 273–285 (2021).
Bromley, L. E., Booth, I. I. I., Kilkus, J. N., Imperial, J. M., Penev, P. D. & J. G., & Sleep restriction decreases the physical activity of adults at risk for type 2 diabetes. Sleep 35(7), 977–984 (2012).
McElroy, T., Dickinson, D. L. & Vale, S. The impact of restricting sleep duration on physical activity: secondary analysis of a randomized crossover study. Health Psychol. 43(8), 561–569 (2024).
Adan, A. & Almirall, H. Horne & Östberg morningness-eveningness questionnaire: A reduced scale. Pers. Indiv. Differ. 12(3), 241–253 (1991).
Kroenke, K., Spitzer, R. L. & Williams, J. B. The patient health Questionnaire-2: validity of a two-item depression screener. Med. Care. 41(11), 1284–1292 (2003).
Spitzer, R. L., Kroenke, K., Williams, J. B. & Löwe, B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch. Intern. Med. 166(10), 1092–1097 (2006).
Dickinson, D. L., Drummond, S. P. & McElroy, T. The viability of an ecologically valid chronic sleep restriction and circadian timing protocol: an examination of sample attrition, compliance, and effectiveness at impacting sleepiness and mood. PLoS ONE. 12(3), e0174367 (2017).
Goldman, S. E. et al. Poor sleep is associated with poorer physical performance and greater functional limitations in older women. Sleep, 30(10), 1317-1324 (2007).
Subar, A. F. et al. The automated Self-Administered 24-hour dietary recall (ASA24): a resource for researchers, clinicians and educators from the National Cancer Institute. J. Acad. Nutr. Dietetics. 112(8), 1134 (2012).
Evans, M. E. et al. A decade of dietary assessment methodology research at the National institutes of health, 2012–2021. J. Nutr. 153(5), 1627–1635 (2023).
Dickinson, D. L., Drummond, S. P. & Dyche, J. Voluntary sleep choice and its effects on bayesian decisions. Behav. Sleep. Med. 14(5), 501–513 (2016).
Sletten, T. L., Vincenzi, S., Redman, J. R., Lockley, S. W. & Rajaratnam, S. M. Timing of sleep and its relationship with the endogenous melatonin rhythm. Front. Neurol. 1, 137 (2010).
Kemp, C. et al. Assessing the validity and reliability and determining cut-points of the Actiwatch 2 in measuring physical activity. Physiol. Meas. 41(8), 085001 (2020).
Lee, P. & Tse, C. Y. Calibration of wrist-worn ActiWatch 2 and actigraph wGT3X for assessment of physical activity in young adults. Gait Posture. 68, 141–149 (2019).
Neil-Sztramko, S. E., Rafn, B. S., Gotay, C. C. & Campbell, K. L. Determining activity count cut-points for measurement of physical activity using the Actiwatch2 accelerometer. Physiol. Behav. 173, 95–100 (2017).
Bellettiere, J. et al. Associations of sitting accumulation patterns with cardio-metabolic risk biomarkers in Australian adults. PLoS ONE. 12(6), e0180119 (2017).
Haskell, W. L. et al. Physical activity and public health: updated recommendation for adults from the American college of sports medicine and the American heart association. Circulation 116(9), 1081 (2007).
Stunkard, A. J. & Messick, S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J. Psychosom. Res. 29(1), 71–83 (1985).
Hershfield, H. E., Cohen, T. R. & Thompson, L. Short horizons and tempting situations: lack of continuity to our future selves leads to unethical decision making and behavior. Organ. Behav. Hum Decis. Process. 117(2), 298–310 (2012).
Primi, C., Morsanyi, K., Chiesi, F., Donati, M. A. & Hamilton, J. The development and testing of a new version of the cognitive reflection test applying item response theory (IRT). J. Behav. Decis. Mak. 29(5), 453–469 (2016).
Dickinson, D. L., Brookes, J., Ferguson, C. & Drummond, S. P. The impact of self-selected short sleep on monetary risk taking. J. Sleep Res. 31(3), e13529 (2022).
Straus, L. D., Drummond, S. P., Nappi, C. M., Jenkins, M. M. & Norman, S. B. Sleep variability in military-related PTSD: A comparison to primary insomnia and healthy controls. J. Trauma. Stress. 28(1), 8–16. https://doi.org/10.1002/jts.21982 (2015).
Walters, E. M. et al. The impact of prolonged exposure on sleep and enhancing treatment outcomes with evidence-based sleep interventions: A pilot study. Psychol. Trauma: Theory Res. Pract. Policy. 12(2), 175 (2020).
Norton, K., Norton, L. & Sadgrove, D. Position statement on physical activity and exercise intensity terminology. J. Sci. Med. Sport. 13(5), 496–502 (2010).
Zhu, B., Shi, C., Park, C. G., Zhao, X. & Reutrakul, S. Effects of sleep restriction on metabolism-related parameters in healthy adults: a comprehensive review and meta-analysis of randomized controlled trials. Sleep Med. Rev. 45, 18–30 (2019).
Al Khatib, H. K., Harding, S. V., Darzi, J. & Pot, G. K. The effects of partial sleep deprivation on energy balance: a systematic review and meta-analysis. Eur. J. Clin. Nutr. 71(5), 614–624 (2017).
Chaput, J. P. et al. The role of insufficient sleep and circadian misalignment in obesity. Nat. Reviews Endocrinol. 19(2), 82–97 (2023).
Demos, K. E. et al. Partial sleep deprivation impacts impulsive action but not impulsive decision-making. Physiol. Behav. 164, 214–219 (2016).
Rossa, K. R., Smith, S. S., Allan, A. C. & Sullivan, K. A. The effects of sleep restriction on executive inhibitory control and affect in young adults. J. Adolesc. Health. 55(2), 287–292 (2014).
Nedeltcheva, A. V. et al. Sleep curtailment is accompanied by increased intake of calories from snacks. Am. J. Clin. Nutr. 89(1), 126–133 (2009).
Hare, T. A., Camerer, C. F. & Rangel, A. Self-control in decision-making involves modulation of the VmPFC valuation system. Science 324(5927), 646–648 (2009).
Sweeting, H., Thomson, H., Wells, V. & Flowers, P. Evolution of ‘whole institution’ approaches to improving health in tertiary education settings: a critical scoping review. Res. Papers Educ. 38(4), 661–689 (2023).
Tafireyi, C. G. S. & Grace, J. M. The physical activity and health promotion activities of global university students: a review of reviews. Global Health Promotion. 29(4), 63–73 (2022).
Barnes, C. M. & Drake, C. L. Prioritizing sleep health: public health policy recommendations. Perspect. Psychol. Sci. 10(6), 733–737 (2015).
Keadle, S. K., Shiroma, E. J., Freedson, P. S. & Lee, I. M. Impact of accelerometer data processing decisions on the sample size, wear time and physical activity level of a large cohort study. BMC Public. Health. 14(1), 1–8 (2014).
Ramakrishnan, R. et al. Accelerometer measured physical activity and the incidence of cardiovascular disease: evidence from the UK biobank cohort study. PLoS Med., 18(1), e1003487 (2021).
Simon, S. L., Field, J., Miller, L. E., DiFrancesco, M. & Beebe, D. W. Sweet/dessert foods are more appealing to adolescents after sleep restriction. PloS One, 10(2), e0115434. (2015).
Satterfield, B. C., Raikes, A. C. & Killgore, W. D. Rested-baseline responsivity of the ventral striatum is associated with caloric and macronutrient intake during one night of sleep deprivation. Front. Psychiatry. 9, 749 (2019).
Acknowledgements
The authors are grateful for valuable comments from seminar participants at CSIRO, Monash University, the University of Auckland, and Arizona State University. The authors thank Jayjit Roy for writing and sharing the STATA code to run the bootstrap estimation procedure used in analyzing the auction data. Valuable research assistance was provided by Lauren Mulholland, Luca Coleman, Ethan Granholm, and Parker Reid.
Funding
This study was funded by the U.S. National Science Foundation (grant SES #2018001). The funders had no role in the design or execution of the study, analysis, interpretation of the data or the decision to submit results for publication.
Author information
Authors and Affiliations
Contributions
The study was designed by all authors (DLD, NK, and SPAD). Ethics applications, OSF preregistrations and clinical trial registration were submitted by DLD. Recruitment and data collection were done by DLD. Data cleaning was done by DLD and NK. Analysis was conducted by DLD. The manuscript was written, edited, and evaluated for scientific content by all authors, and all authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Human ethics and consent to participate
The study was approved by the Institutional Review Board at Appalachian State University, in accordance with the Declaration of Helsinki. Informed consent was obtained from all participants in this study.
Registrations
The sleep protocol and study procedures were preregistered prior to enrollment of participants (Open Science Framework: https://doi.org/10.17605/OSF.IO/NSPRK (August 24, 2021) and https://doi.org/10.17605/OSF.IO/BKH5J (August 24, 2021), respectively). A secondary data preregistration was completed to analyze the physical activity (PA) data from the actigraphy data collection, which was done after data collection but prior to the PA data analysis (https://doi.org/10.17605/OSF.IO/EXV4D (October 5, 2023). Clinical trials registration was retrospective (ClinicalTrials.gov, identifier NCT05728983)—completed after commencing enrollment of participants but prior to final participant enrollments (February 14, 2023).
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Dickinson, D.L., Kakoschke, N. & Drummond, S.P.A. The impact of insufficient sleep on dietary choices and physical activity behaviors: evidence from a randomized cross-over trial. Sci Rep 15, 24513 (2025). https://doi.org/10.1038/s41598-025-08289-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-08289-4