Abstract
The choice of general anesthetic drugs is crucial. We aimed to investigate the effects of different doses of remimazolam combined with propofol in patients with ischemic cerebrovascular disease. A total of 105 patients were selected and divided into three groups by the random number table method (n = 35), group P, group R1, and group R2. Group P received a continuous propofol infusion. In contrast, Group R1 and Group R2 received continuous infusion of 0.1 mg/kg∙h and 0.2 mg/kg∙h of remimazolam, respectively, combined with propofol. MAP, HR, and BIS, extubation time, and 30-day postoperative mortality rate were recorded. Neurocognitive function and neurofunctional outcomes were followed up 1 day before, 1, 3, 7, 30, 90, and 180 days after. Compared with group P, the incidence of intraoperative hypotension in group R1 was lower, the fluctuation of mean blood pressure (ΔMAP) was decreased, the intraoperative dosage of propofol and ephedrine was decreased, the recovery time and extubation time were shortened, and the CAM-CR score on the 1st and 7th day after surgery was reduced. Compared with the R1 group, the incidence of intraoperative hypotension in group R2 was increased, the ΔMAP was increased, and the recovery time and extubation time were shortened. Remimazolam combined with propofol general anesthesia for interventional treatment of ischemic cerebrovascular disease can achieve satisfactory sedation depth, fewer adverse reactions, and does not affect patients’ postoperative neurocognitive function recovery and neurological function outcome. Compared with propofol alone, the hemodynamics of group R1 are more stable, and the recovery of group R2 is faster. Remimazolam may have a potential brain protective effect in Patients undergoing endovascular treatment for ischemic cerebrovascular disease.
Similar content being viewed by others
Introduction
Ischemic cerebrovascular disease is one of the most common neurological diseases in the world, the first cause of disability and the third cause of death, second only to heart disease and tumors1,2.
In recent years, RCT research evidence shows that different anesthesia methods (general anesthesia, local anesthesia, or awake sedation anesthesia) have no significant effect on the clinical prognosis of patients with ischemic cerebrovascular interventional therapy. More and more neurointerventional doctors are willing to choose general anesthesia because the patient is more conducive to the implementation of surgery, complicated and time-consuming surgery. General anesthesia is painless and comfortable, and patients do not need to experience bad experiences such as tension, anxiety, and even fear, which is more acceptable to patients. However, most of the patients with ischemic cerebrovascular disease are elderly, with fragile organ functions and many complications, which brings great challenges to anesthesia management. The physiological effects of anesthesia on patients with ischemic cerebrovascular disease, especially hypotension, may reduce cerebral blood flow, aggravate cerebral ischemia, and even cause irreversible brain damage. Therefore, perioperative blood pressure monitoring and management is critical. Propofol is the most widely used intravenous anesthesia drug in neurosurgery, which has the advantages of reducing cerebral oxygen consumption and cerebral metabolism. Still, its dose-related circulatory inhibition is also large3,4. Remimazolam is A new type of ultra-short-acting benzodiazepine, which mainly acts on γ-aminobutyric acid A receptor. It has fast and short-acting time, and it is not easy to accumulate after long-term infusion. It can be antagonized by flumazenil. Several studies have shown that remimazolam reduces the occurrence of hypotension during anesthesia in elderly patients compared to propofol5,6,7. Soejima T et al.8 found that after induction with remimazolam, cerebral blood volume was not changed, the cerebral blood flow velocity (CBFV) of the middle cerebral artery was reduced by 11%, and the mean blood pressure (MAP) was decreased by 17%, but it was within the range of automatic regulation of cerebral vessels. As a new sedative, remimazolam is not widely used in patients with vulnerable organ functions. The purpose of this study was to observe the effects of remimazolam combined with propofol on hemodynamics, postoperative neurological recovery, and neurological outcomes in patients undergoing interventional surgery for ischemic cerebrovascular diseases, and to provide clinical reference.
Materials and methods
The study was approved by the ethics committee of the First Affiliated Hospital of Guangxi Medical University (2020 No. 019) and conducted according to the Declaration of Helsinki. The trial was registered in the Chinese Clinical Trial Registry (date of registration: 06/08/2020, registration number: ChiCTR2000035282) and conducted according to the Consolidated Standards of Reporting Trials statement. Written informed consent was obtained from all participants after having been provided with detailed information about the study aims, procedures, and risks before enrolling for the study.
Study design and patients
This randomized, double-blind, controlled trial enrolled consecutive patients undergoing interventional therapy for cerebrovascular stenosis (balloon dilatation, stent insertion, or balloon dilatation + stent insertion) under general anesthesia at the Department of Anesthesiology, The First Affiliated Hospital of Guangxi Medical University, Nanning, China between August 2020 and January 2022. The inclusion criteria were as follows:1 The degree of intracranial artery stenosis confirmed by DSA was ≥ 70%2, the estimated time of stenosis (disease change or symptom onset until stenosis was found on imaging) was greater than 48 h3, age 18–79 years old4, BMI 18–30 kg/m25, ASA grade II-IV. The exclusion criteria were as follows:1 emergency surgery2, concurrent aneurysm embolization or coronary stenting3, mental disorders, inability to communicate, inability to cooperate, or coma4, allergy to narcotic drugs, analgesics, or addiction to narcotic drugs5, participation in other clinical investigators within the last 3 months.
Randomization and grouping
Patients were randomly assigned into three groups: propofol group (group P), remimazolam 0.1 mg/kg∙h combined with propofol group (group R1), and remimazolam 0.2 mg/kg∙h combined with propofol group (group R2), with 35 cases in each group. The random number table method ensured equal distribution in the three groups. Randomization was done by opening a sealed envelope just before entry to the operating room. All patients, data collectors, and data analyzers were blinded to the group allocation.
Anesthesia induction and maintenance
The patient fasted for 6 h before surgery, did not drink for 2 h, and did not receive any sedation, analgesics, or anticholinergic drugs on the morning of surgery. ECG, HR, BP, SpO2, BIS, and body temperature were monitored after entry into the room. Peripheral veins were opened, 6 ml/kg of compound sodium lactate Ringer’s solution was injected, and 6–8 L/min of oxygen was given through the mask.
Anesthesia induction: With MOAA/S score equal to 0 and BIS 40–60 as sedative targets, propofol TCI 1.0–3.0 µg/ml in group P, remimazolam 0.06 mg/kg in groups R1 and R2, and then combined with propofol TCI 0.6–3.0 µg /ml. Three groups were given intravenous injections of fentanyl 2 µg /kg, remifentanil TCI 2.0 ~ 3.0 ng /ml, and cisatracurium 0.2 mg/kg.
Anesthesia maintenance: Propofol TCI 1.0–3.0 µg/ml in group P, remimazolam 0.1 mg/kg∙h or 0.2 mg/kg∙h combined with propofol TCI 0.6 ~ 3.0 µg/ml in groups R1 and R2, respectively. During the operation, BIS 40–60, VT 6–8 ml /kg, PETCO2 35–45 mmHg were maintained, MAP fluctuated within ± 20% of the baseline value, and body temperature was 36–37℃. When HR > 100 beats/min, Esmolol 5 ~ 10 mg intravenously; HR < 45 beats/min, atropine 0.25 ~ 0.5 mg intravenously; When MAP > 20% of the basic value, urapidil was injected intravenously 10 ~ 15 mg; When MAP <-20% of the baseline value, 5 to 10 mg ephedrine is injected intravenously, and when the blood pressure is lowered and continuous administration of vasoactive agent is required, norepinephrine is injected intravenously constant pump with adjust dosage according to blood pressure. Fentanyl 1 ~ 2 µg/kg and ondansetron 5 mg were injected intravenously 10 min before the end of the operation for postoperative analgesia and prevention of postoperative nausea and vomiting. Anesthetic drug infusion was stopped after the operation, and patients were transferred to PACU for anesthetic resuscitation. The patient’s MOAA/S score was assessed every minute, and the tracheal catheter was removed after the patient recovered from spontaneous breathing (tidal volume greater than 6 ~ 8 ml/kg, respiratory rate greater than 10 times/min, pulse oxygen saturation of more than 92% within 10 min of air inhalation), became conscious, muscle strength recovered as before surgery and hemodynamic stability.
Outcome measures
The primary indicators were ΔMAP (absolute value of the difference between MAP and baseline MAP at each time point), the incidence of hypotension, and the modified Rankin scale score. The Secondary indicators were the incidence of perioperative stroke, the perioperative neurocognitive function, and the peri-procedural mortality. Anesthesia recovery time (the time when the intravenous anesthetic infusion was stopped until the MOAA/S score was equal to 5 points), extubation time (the time when the intravenous anesthetic infusion was stopped until the tracheal catheter was removed), and stay time in the anesthesia recovery room were recorded. Adverse events such as intraoperative hypertension, bradycardia, tachycardia, postoperative nausea and vomiting, intraoperative awareness, delayed recovery, and perioperative stroke were recorded, as well as postoperative hospital stay and 30-day mortality were recorded.
The incidence and severity of postoperative delirium were evaluated by the 3D-CAM scale and CAM-CR scale 1 day before, 1st day after, 3rd day after, and 7th day after. A Mini-mental State Examination Scale, Modified Rankin Scale (mRS), National Institute of Health stroke scale (NIHSS) score, and Barthel Index (BI Index) were performed 1 day before, 1st day after, 3rd day after, 7th day after, 30 days after, 90 days after, and 180 days after. MMSE Scale (standard deviation method to calculate the incidence of perioperative neurocognitive disorder (PND)), mRS Score, National NIHSS score, and BI Index assessed cognitive function, neurological disability, and ability to perform daily living.
Statistical analysis and sample size calculation
IBM SPSS Statistics version 21 was used for data analysis. The measurement data were tested for normal distribution using Kolmogorov-Smirnov test. The distribution is expressed as mean ± SD for normal data and median and interquartile spacing for skewness data [M (IQR)]. One-way ANOVA was used to measure normality and the Kruskai-Walis test was used to measure skewness. Count data are expressed as percentages (%) using the χ2 test or the Fisher exact probability method (if applicable). Part of the data belonged to follow-up data, and the independence hypothesis was unsatisfied at each time point. Longitudinal data analysis method was adopted and repeated measurement ANOVA was used. P < 0.05 was considered statistically significant. Statistical mapping was performed using GraphPad Prism 8 software.
The sample size was calculated based on a pilot study. In this pilot study, the mean values of ΔMAP in groups P, R1, and R2 were 32.60 ± 9.35 mmHg, 22.60 ± 9.25 mmHg, and 31.80 ± 9.11 mmHg, respectively. Assuming a two-sided α = 0.05 and β = 0.20, with the sample size ratio N3/N2/N1 = 1, and a 30% reduction in ΔMAP for patients using remimazolam, based on a significance level of 0.05 (two-sided) and a power of 0.90, the minimum sample size for each group was calculated to be 19 cases. Assuming a 20% loss to follow-up rate for the study subjects, the required sample size for each group would be N1 = N2 = N3 = 19 ÷ 0.8 ≈ 24 cases. In the study, 35 patients were included in each of the three groups.
Results
Baseline clinical characteristics of the study participants
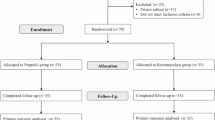
According to the inclusion and exclusion criteria, 95 patients were included in the final analysis, with 34 patients in the P group, 31 in the R1 group, and 30 in the R2 group (Fig. 1). There was no significant difference in gender, age, height, weight, BMI, ASA grade, comorbidities, smoking history, preoperative mRS Score, NIHSS score, and BI index among the three groups (Table 1).
Primary outcomes
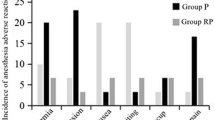
Compared with group P, the incidence of intraoperative hypotension in group R1 was lower (P < 0.05, Table 2), and the fluctuation of mean blood pressure (ΔMAP) was decreased (P < 0.05, Table 2). Compared with the R1 group, the incidence of intraoperative hypotension was increased in the R2 group (P < 0.05), and the ΔMAP was increased (P < 0.05). There was no significant difference in mRS Scores among the three groups 1 day before surgery, 1 day after surgery, 3 days after surgery, 7 days after surgery, 30 days after surgery, 90 days after surgery, and 180 days after surgery (P > 0.05).
Secondary outcomes
Compared with group P, the CAM-CR score of group R1 was lower on day 1 and day 7 after surgery (P < 0.05). Compared with group R1, the CAM-CR score in group R2 was higher on day 1 after surgery (P < 0.05). There was no significant difference in the incidence of postoperative delirium among the three groups (P > 0.05). There was no significant difference in the incidence of PND, MMSE score, and BI index among the three groups 1 day before surgery, 1 day after surgery, 3 days after surgery, 7 days after surgery, 30 days after surgery, 90 days after surgery and 180 days after surgery (P > 0.05) (Table 3).
Compared with group P, the amount of propofol in groups R1 and R2 was decreased (P < 0.05), the amount of ephedrine in group R1 was reduced (P < 0.05), and the recovery time and extubation time in group R2 were shortened (P < 0.05). Compared with the R1 group, the recovery and extubation times in the R2 group were shortened (P < 0.05). There were no significant differences in BIS, operation time, anesthesia time, urine volume, infusion volume, intraoperative body temperature, PACU residence time, postoperative hospital stay, and 30-day postoperative mortality among the three groups. (Table 4). There was no significant difference in the incidence of adverse events such as intraoperative bradycardia, postoperative nausea and vomiting (PONV), intraoperative awareness, delayed recovery, and perioperative stroke among the three groups (P > 0.05) (Table 5).
Discussion
In this study, it was found that most patients with ischemic cerebrovascular disease who underwent intravascular interventional therapy were elderly (mean age 62.6 years), with more complications before surgery, including 81.1%, 87.4%, 41.1%, 33.7%, and 31.6% patients with hypertension, atherosclerosis, dyslipidemia, diabetes mellitus, and previous stroke history, respectively, about 70% of the patients with ASA III~IV grade. Preoperative cognitive decline (MMSE score < 21) occurred in 15.8% of patients. These characteristics indicate that patients with ischemic cerebrovascular disease have weak brain function and a high risk of perioperative neurological complications (postoperative delirium, perioperative stroke).
General anesthesia drugs, such as propofol, have dose-related circulatory inhibition, resulting in lower blood pressure9. In patients with ischemic cerebrovascular disease, cerebral vascular autoregulation is impaired. During general anesthesia, it is more susceptible to cerebral hypoperfusion and even further damage to nerve function10,11. Therefore, the selection of anesthetic drugs and perioperative blood pressure management are critical12. Due to its hemodynamic stability, Etomidate is considered one of the most suitable anesthetic induction agents. Still, long-term use may inhibit adrenal function and is not ideal for anesthetic maintenance13. The light inhibition of hemodynamics is a good feature of remimazolam14. Studies have shown that remimazolam is less likely to inhibit circulation and respiration than propofol5,15,16. Our study found that the incidence of intraoperative hypotension and ΔMAP in the R1 group were the lowest among the three groups. The possible reason is that the dosage of propofol in group R1 was reduced. In contrast, the depth of anesthesia in group R2 was deeper (the mean BIS value of the three groups was 47.5 in group P, 47.5 in group R1, and 41.0 in group R2). Nakayama, et al.17 reported two super-elderly (95 and 103 years old) undergoing general anesthesia with remimazolam at the induction doses of 1.2 mg/kg∙h and 1.0 mg/kg∙h and maintenance doses of 0.2 mg/kg∙h and 0.1 mg/kg∙h for hip fracture surgery. The surgery was completed successfully. For elderly or critically ill patients, the appropriate dose for induction and maintenance of anesthesia may be much lower than expected. The application of remimazolam combined with propofol in patients with ischemic cerebrovascular disease should be carefully considered according to BIS or vital signs. With the increase of remimazolam dose, attention should be paid to the reduction of the dosage of propofol, and it may be reasonable to appropriately increase the BIS range (such as BIS 50 ~ 60).
The recovery time and extubation time of the R2 group were shorter than those of the P group and R1 group. The possible reason18,19 may be related to the influence of the combined anesthesia of benzodiazepine sedative drugs and propofol on the pharmacokinetics of propofol. Remimazolam exerts sedative and hypnotic effects by specifically activating GABAAR and takes effect within 1 to 3 min after intravenous injection. It is mainly metabolized into the inactive CNS7054 metabolite by non-specific esterases. When remimazolam is combined with propofol for anesthesia, the concentration of propofol decreases as the concentration of remimazolam increases. At low concentrations, propofol is eliminated in a first-order manner in the body, thus reducing the elimination half-life. Remimazolam is eliminated through first-order pharmacokinetics, and there is no clear relationship between body weight and elimination clearance. Long-term infusions or higher doses do not lead to accumulation or prolonged effects. Therefore, patients in the R2 group with a higher concentration of remimazolam regained consciousness more quickly. At the same time, the superior performance of remimazolam during the recovery period may change people’s long-held perception of benzodiazepines. At present, there is a lack of clinical evidence regarding the neurotoxicity of general anesthetic drugs. The fact that remimazolam accelerates anesthesia recovery may suggest that it has a beneficial effect on the recovery of brain function in ischemic cerebrovascular disease. To further verify the impact of remimazolam on neurological function, this study conducted continuous evaluations of perioperative neurological function in patients from the first day after surgery to three months postoperatively.
Although several recent studies have compared the safety and sedative effects of remimazolam with those of propofol20,21, few studies have focused on the issue of postoperative delirium, and there has been no report of remimazolam being used in patients with ischemic cerebrovascular disease. This study found that the degree of postoperative delirium in the R1 group was relatively mild. There were no differences among the three groups in terms of the incidence of postoperative delirium, postoperative recovery of neurocognitive function, and the occurrence of cognitive dysfunction. Whether the degree of postoperative delirium in group R1 was milder was related to better hemodynamic and cerebral perfusion during the perioperative period remains to be further confirmed by research.
Our research has some limitations. Firstly, this study is a single-center design, which weakens the external validity of the trial. All the patients in this study are from China. Further research is needed to confirm our performance in other populations. Secondly, BIS is one of the most commonly used methods for monitoring anesthesia depth; however, the effect of BIS on the anesthesia depth of a single remimazolam or remimazolam combined with other anesthetic drugs remains unclear. Our research found that the BIS value of remimazolam combined with propofol can remain between 40 and 60. However, the optimal BIS range for patients with ischemic cerebrovascular disease needs to be further verified by clinical data. In the future, by increasing the sample size, the strength of the results can be enhanced.
Conclusion
This study adopted a prospective, double-blind, randomized controlled approach to compare the perioperative hemodynamics, anesthesia recovery, perioperative neurocognitive recovery, adverse reactions and events, and neurofunctional prognosis among three groups of patients. The aim was to explore the impact of remimazolam combined with propofol on patients undergoing interventional surgery for ischemic cerebrovascular disease and to determine the optimal dose of remimazolam. The study found that both doses of remimazolam (0.1 mg/kg∙h and 0.2 mg/kg∙h) were safe and effective for general anesthesia in patients with ischemic cerebrovascular disease; 0.1 mg/kg∙h remimazolam provided more stable hemodynamics, while 0.2 mg/kg∙h remimazolam significantly shortened the recovery time of patients; remimazolam could reduce the degree of postoperative delirium in patients with ischemic cerebrovascular disease, but did not affect the neurofunctional outcome of patients at 3 months postoperatively. Remimazolam may have a potential brain protective effect in Patients undergoing endovascular treatment for ischemic cerebrovascular disease.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Lu, J., Liu, W. & Zhao, H. Headache in cerebrovascular diseases. Stroke Vascular Neurol. 5 (2), 205–210 (2020).
Tabeeva, G. R. [Headache and cerebrovascular diseases]. Zhurnal nevrologii i psikhiatrii Imeni. SS Korsakova. 121 (2), 114–121 (2021).
Ebert, T. J., Muzi, M., Berens, R., Goff, D. & Kampine, J. P. Sympathetic responses to induction of anesthesia in humans with Propofol or etomidate. Anesthesiology 76 (5), 725–733 (1992).
Yang, M. & Zhang, Y. Propofol addiction: the mechanism issues we need to know. Anesthesiology Perioperative Sci. 2 (1), 6 (2024).
Liu, T. et al. Effect of remimazolam induction on hemodynamics in patients undergoing valve replacement surgery: A randomized, double-blind, controlled trial. Pharmacol. Res. Perspect. 9 (5), e00851 (2021).
Yokose, M. et al. Hypotension after general anesthesia induction using remimazolam in geriatric patients: protocol for a double-blind randomized controlled trial. PloS One. 17 (9), e0275451 (2022).
Lu, K. et al. Remimazolam versus Propofol for deep sedation/anaesthesia in upper Gastrointestinal endoscopy in elderly patients: A multicenter, randomized controlled trial. J. Clin. Pharm. Ther. 47 (12), 2230–2236 (2022).
Soejima, T. et al. Change in cerebral circulation during the induction of anesthesia with remimazolam. J. Anesth. 37 (1), 92–96 (2023).
Philip, A. B., Brohan, J. & Goudra, B. The Role of GABA Receptors in anesthesia and Sedation: an Updated Review (CNS drugs, 2024).
Baker, J. R. et al. Cerebral Blood Flow Dynamics in Neurogenic Orthostatic Hypotension: A Systematic Review and Meta-Analysis. Hypertension (Dallas, Tex: 2024. (1979).
Fu, P. et al. Effect of ferroptosis on chronic cerebral hypoperfusion in vascular dementia. Exp. Neurol. 370, 114538 (2023).
Barnes, S. C., Beishon, L. C., Hasan, M. T., Robinson, T. G. & Minhas, J. S. Cerebral haemodynamics, anaesthesia and the frail brain. Anesthesiology Perioperative Sci. 1 (3), 19 (2023).
Zhang, H. et al. The application and pharmaceutical development of etomidate: challenges and strategies. Mol. Pharm. 21 (12), 5989–6006 (2024).
Hu, B. et al. Comparison of Remimazolam Tosilate and Etomidate on Hemodynamics in Cardiac Surgery: A Randomised Controlled Trial. Drug design, development and therapy. 17, 381-8. (2023).
Sun, Q. et al. The effects of remimazolam combined with sufentanil on respiration, circulation and sedation level in patients undergoing colonoscopy. BMC Anesthesiol. 24 (1), 252 (2024).
Fechner, J. et al. Anaesthetic efficacy and postinduction hypotension with remimazolam compared with propofol: a multicentre randomised controlled trial. Anaesthesia 79 (4), 410–422 (2024).
Nakayama, J., Ogihara, T., Yajima, R., Innami, Y. & Ouchi, T. Anesthetic management of super-elderly patients with remimazolam: a report of two cases. JA Clin. Rep. 7 (1), 71 (2021).
Schüttler, J. et al. Pharmacokinetics and pharmacodynamics of remimazolam (CNS 7056) after continuous infusion in healthy male volunteers: part I. Pharmacokinetics and clinical pharmacodynamics. Anesthesiology 132 (4), 636–651 (2020).
Wiltshire, H. R., Kilpatrick, G. J., Tilbrook, G. S. & Borkett, K. M. A placebo- and midazolam-controlled phase I single ascending-dose study evaluating the safety, Pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056): part II. Population Pharmacokinetic and pharmacodynamic modeling and simulation. Anesth. Analg. 115 (2), 284–296 (2012).
Zhang, X., Li, S. & Liu, J. Efficacy and safety of remimazolam besylate versus Propofol during hysteroscopy: single-centre randomized controlled trial. BMC Anesthesiol. 21 (1), 156 (2021).
Chen, S. et al. The efficacy and safety of remimazolam Tosylate versus Propofol in patients undergoing colonoscopy: a multicentered, randomized, positive-controlled, phase III clinical trial. Am. J. Translational Res. 12 (8), 4594–4603 (2020).
Acknowledgements
The authors have nothing to report.
Funding
This work was supported by Guangxi Science and Technology Base and Talent Special Project (No. AD25069060), Guangxi Key Research and Development Program (No. AB24010066), National Natural Science Foundation of China (No. 82460239), the Clinical Research “Climbing” Program of the First Affiliated Hospital of Guangxi Medical University (No. YYZS2021004), Special Fund for Clinical Application Research and Training of China Health Promotion Foundation (No. 2020-ZCXY-1168), Special Fund of Neurotoxicity of General Anesthetics and Its Prevention and Treatment Innovation Team of the First Affiliated Hospital of Guangxi Medical University (No. YYZS2022001), and the Youth Science Foundation of Guangxi Medical University (No. GXMUYSF202309).
Author information
Authors and Affiliations
Contributions
All authors participated in the whole process of this study and approved the final version. Tianxiao Liu, Liyin Qin, Yubo Xie, Jing Chen: planning, conducting, reporting, conception, design, acquisition of data, data analysis, interpretation of data, and writing of the manuscript. Maolin Su, Yunting Wei, Huabo Yu, Hao Wei: conducting, acquisition of data and data analysis. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, T., Qin, L., Su, M. et al. Remimazolam and propofol combination in ischemic cerebrovascular disease endovascular treatment: a randomized study. Sci Rep 15, 21083 (2025). https://doi.org/10.1038/s41598-025-08403-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-08403-6
Keywords
This article is cited by
-
Association between a remimazolam–propofol combination for maintenance of anesthesia and extubation time: a propensity score analysis
JA Clinical Reports (2025)
-
Remimazolam alleviates cerebral ischemia–reperfusion injury of rats by inhibiting NF-κB/NLRP3 inflammasome pyroptosis
Scientific Reports (2025)