Abstract
Microbial fuel cell (MFC) technology is gaining popularity as a viable and sustainable method of energy and power generation, while simultaneously reducing environmental pollution levels. However, MFCs are not economically feasible due to their low energy productivity and the high cost of producing anode materials. In this study, an anode made of an enset corm biosynthetic graphene derivative (E-rGO) was installed in the dual chamber of an MFC. In addition, a composite anode of E-rGO/Fe3O4 was developed to remove Cr (VI) and Pb (II) ions from wastewater while simultaneously generating energy. The synthesized materials were analyzed using UV–Vis, SEM–EDS, XRD, FTIR, RAMA, and TGA spectroscopy to investigate the materials’ optical, morphological, and structural properties, molecular vibrational states, structural defects, and thermal stability. CV and EIS were also utilized to investigate the electrochemical characteristics of the synthesized materials. For Cr (VI), the anodes made using E-rGO, and E-rGO/Fe3O4 NCs had remediation efficiencies of 70.6%, and 79.2%, while for Pb (II), they were 65.1%, and 73.8%. Furthermore, the composite anode (E-rGO/Fe3O4 NCs) delivered a maximum power density of (39.77 mW/m2) and a current density of (1171 mA/m2), higher than E-rGO modified anode electrode power density (8.75 mW/m2) and current density (609 mA/m2), respectively. The final results of the produced anodes revealed that Enset corm biomass is a viable and affordable material for improving MFC anode performance. The outcome of the manufactured anodes suggests that enset corm biomass is a viable and economical material to improve MFC anode efficiency.
Similar content being viewed by others
Introduction
Utilizing accessible electroactive microbes for sustainable energy production from renewable waste and waste recycling provides an eco-friendly approach to address energy demands1,2,3. Researching simple, affordable electrochemical methods for energy transformation and waste treatment represents a progressive path to generate electricity and manage waste through microorganisms4. Within bioelectrochemical systems, Microbial Fuel Cells (MFCs) technology currently represents a vital and interesting method for managing waste and contaminants such as metal ions, dyes, organic phosphate, and inorganic substances. These function by converting chemical energy into electricity through biocatalysts. This method tackles the energy crisis and climate change via the generation of eco-conscious energy5,6,7. Electroactive microbial communities act as catalysts, oxidizing the provided organic substrate to generate protons and electrons8,9. Numerous electroactive bacterial species have been discovered in marine and freshwater sediments, as well as in various substrates such as manure, sludge from aerobic/anaerobic wastewater treatment, and wastewater from different sources10. Microbes form biofilms around and on the anode, enabling them to produce electricity by transferring electrons to the anode using different mechanisms. These mechanisms involve long-distance electron transport via conductive pili, short-range transfer with redox-active molecules, and electron transfer through shuttling molecules. Additionally, microbial growth creates the biofilm on the anode and uses energy for respiration. During respiration, microbes release electrons and protons. The produced electrons flow through an external circuit to the cathode, generating electricity and reducing electron acceptors. Meanwhile, protons move from the anode to the cathode through the proton exchange membrane (PEM) to complete the circuit11. Microorganisms can also transfer electrons to electron acceptors that exist in an insoluble state, significantly reducing the toxic effect of pollutants from wastewater12,13. Employing electroactive microorganisms for the breakdown or removal of contaminants at contaminated locations presents a cost-effective and eco-friendly alternative to conventional remediation techniques. Nevertheless, a crucial challenge lies in ensuring sufficient interaction between the pollutants and the microbes, potentially hindering the reaction rate14. Moreover, the efficiency of MFCs is limited and hindered by a numerous factors such as electrical conductivity15,16, carrier materials (porosity and surface area)17,18, biocompatibility19,20, proton exchange membrane21, stability of electrode22,designed method MFCs23, availability, and cost24 of the anode electrode and the rate of substrate degradation/oxidationprocess25.
To address this challenge, a broad range of functionalized materials and modification strategies have been advanced over the past decade26,27. The excellent conductivity, effective electron transport characteristics, attractive chemical reactivity, and strong mechanical and thermal durability of carbon-derived materials such as carbon nanotubes (CNTs)—including multi-wall carbon nanotubes (MWCNTs) and single-wall carbon nanotubes (SWCNTs), carbon black, graphene, and its derivatives—make them popular choices for anode electrodes. These materials, along with various metal-based anodes like gold, copper, nickel, titanium, and aluminum, can provide MFC technology with superior electrochemical efficiency. This is due to their high electrical conductivity (resulting in a higher rate of electron flow) and good mechanical stability28.
However, the high cost of MWCNTs and certain biotoxicity on cells of SWCNTs are limited in MFC3,29,30,31,32. In addition, the high surface area and good biocompatibility of commercial graphene and it derivatives used to fabricate MFC anodes are limited due to the high cost and unaffordability33. Similarly, metal-based materials were costly, corroded over time, and had poor bacterial adhesion because they were not compatible with microorganisms3,34. To increase energy output, Nitisoravut et al.35 investigated stainless steel as an anode material; however, because of insufficient microbial attachment, its power density ultimately stabilized at 23 mW/m2. On the other hand, metal oxide-based materials show better microbial adherence to the anode and increased electron transfer efficiency36. To address this challenge, currently, researchers have begun synthesizing Porous carbon-based electrode materials, graphene and its derivatives from waste biomass materials for anode preparation as a cost-effective solution, beyond that these materials are widely used in super capacitors and sensor due to their exceptional electrical conductivity, high specific surface area, variable pore structure, and amazing physicochemical stability4,37.
Graphene oxide (GO) has been produced from agricultural waste materials using a variety of synthetic methods, making it a more affordable option that is readily available, cost-effective, and stable as a biocompatible carbon-rich material compared to commercial alternatives. For example, Yaqoob et al.38, prepared GO derived from cellulose waste as anodes for MFC. The maximum current density and power density achieved were 24.56 mA/m2 and 0.11 mW/m2, respectively, and the removal efficiency of Pb2+ ions was 53.50%. Nevertheless, the electrical conductivity of GO is limited by the breakdown of the conjugated sp2 hybrid system. This disruption occurs when hydrophilic functional groups, such as hydroxyl and epoxy groups, bind to graphene sheets. These groups disrupt the electronic structure of graphene and prevent efficient electron flow. Several published articles are referring to the natural biomass anode, which addresses several conventional issues, such as showing poor mechanical and electrical stability, along with reducing the cost of the anode39. Through the removal of certain functional groups that include oxygen, reduced graphene oxide (rGO) lessens these difficulties. This improves its mechanical durability, exceptional thermal resistance, extensive electroactive surface, electrical conductivity, chemical stability, and biocompatibility throughout the film-making process40. In addition, doping or bonding metal oxides to naturally occurring carbon-rich materials could lead to breakthroughs in the field of MFCs. Metal oxide-based nanoparticles (NPs) such as zinc oxide, manganese oxide, cobalt oxide, titanium dioxide, and iron oxide have recently been investigated as cost-effective anode electrode modifiers for MFCs41,42. Yaqoob et al.38, to recent research, ZnO and waste-sourced GO show promise for efficiently eliminating hazardous metal ions from wastewater and producing energy at the same time. However, the electrical conductivity of GO hinders the energy and power density of the material. Furthermore, among the various metal oxides, magnetite (Fe3O4) stands out as a promising anode material for MFC because of its abundance, cost-effectiveness, strong biocompatibility, and high affinity. These properties are highly beneficial for the advancement of long-life, high-power MFCs43,44. Fe3O4 has been researched for its potential uses in energy conversion and storage, such as in fuel cells and batteries. It has been researched for its possible uses in water treatment, such as in magnetic separation and catalysis. Recently, GO/Fe3O4 NCs have been created, which combine the properties of both materials. These NCs have shown benefit in energy conversion and treatment applications, such as in fuel cells, batteries, and water treatment systems. In water treatment usage, GO/Fe3O4 NCs have been employed as adsorbents, where they have shown high effectiveness in removing heavy metals and organic pollutants from water. The magnetite nanoparticle in the nanocomposites can assist in enhancing the magnetic separation of the adsorbent, while the graphene oxide provides a high surface area for adsorption45. However, the low conductivity of Fe3O4 may somewhat limit the performance of MFCs; combining Fe3O4 nanoparticles with rGO carbon material composites can effectively address the poor electron transfer behavior associated with Fe3O4. This hybrid approach not only improves conductivity but also increases the biocompatibility with external electrolyte microbes, making it a desirable anode material for MFCs. Several studies have shown that hybridization of metal oxides with commercially available graphene (G) and its derivative (rGO), improves the interaction between the electrode surface and bacteria, resulting in improved electron transfer efficiency (ETE), energy density, and power density in MFC applications13,46. The high surface area and excellent electrical conductivity of the NCs make it an ideal material for use in microbial fuel cells, which can generate electricity from organic waste and provide a sustainable source of energy. Furthermore, the ability of the NCs to effectively remove heavy metals from wastewater through microbial fuel cells demonstrates its potential for use in waste water treatment. This can help to address the growing issue of water pollution caused by industrial waste and provide a sustainable solution for water purification.
To the best of our knowledge there is no comprehensive report on the performance of E-rGO/Fe3O4 NCs as an anode electrode in MFCs. Therefore, in this article, we proposed a novel sustainable and bio-inspired anode material, i.e., Enset/Enseteventricosum plant underground carbon-rich corms waste derived (E-rGO), as well as an E-rGO with magnetite (Fe3O4) containing nanocomposites. The corms of the enset plant are a major by-product in the agricultural areas of Ethiopia. Additionally, in the literature, there were few efforts made on the removal of Cr (VI) and Pb (II) from those ions wastewater supplemented via MFCs. Hence, the removal of Cr (VI) and Pb (II) ions and energy generation using the synthesized E-rGO and E-rGO/Fe3O4 anodes were also reported in this study.
Experimental
Materials and chemicals
The materials included wastewater effluents obtained from a nearby pond at Mattu University, sweet potato waste material (a gift from the local market in Mattu town, Ethiopia), and enset/enseteventricosum corm waste material (a gift from Kaffa Zone, Southwest Ethiopia). Chemicals used for the experiments included, Ferric chloride (97%), Ferrous chloride (99%), Potassium permanganate, Sodium nitrate purchased from Sigma-Aldrich Chemical Co. Ltd., Sulfuric acid (98%), Hydrochloric acid (37%, Merck), Nitrogen (N2) gas, Sodium hydroxide (NaOH, Merck), and Lead(II) nitrate solution (R&M Chemicals). All chemicals, nutrients, and substrates used in the MFC experiments were analytical grade and used without further purification. It should be noted that all measurements were taken at room temperature, and doubly distilled water was used for the preparation of all solutions and for conducting electrochemical experiments.
Synthesis of E-GO
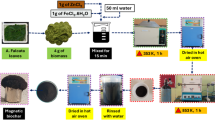
Synthesis of GO derived from the underground corms of enset/enseteventricosum was performed based on previous reports, with some modifications47. First, corms isolated from enset/enseteventricosum were dried in an oven set at 105 °C for 24 h after being washed with deionized water. The dried underground corms were finely ground in a grinder and then calcined in a furnace under a nitrogen atmosphere at 900 °C for 3 h to obtain calcined corm carbon for E-GO synthesis. A standard procedure involved blending 5 g of enset/enseteventricosumcorm powder with 6 g of NaNO3 in 150 mL of concentrated H2SO4, followed by continuous stirring for 60 min. Then 15 g of KMnO4 was slowly added to the solution in an ice bath at 0–5 °C for 3 h with stirring. The reaction mixture was stirred at room temperature for another 24 h to complete the oxidation process, resulting in a color change from black to purplish brown. After this time, 150 mL of distilled water was added, and the temperature was increased to 90 °C for 20 min to obtain a dark brown solution. After cooling to room temperature, another 200 mL of water was slowly added, and the reaction was stopped by dropping H2O2 (20 mL) to remove excess KMnO4. The synthesized E-GO was washed with distilled water and ethanol until pH neutral (pH = 7) and dried in an oven at 40 °C for 36 h.
Reduction of E-GO
E-GO was reduced using sodium borohydride as a reducing agent. First, 100 mg of E-GO powder was dispersed in 100 mL of distilled water, and the graphene oxide was exfoliated into sheets by ultrasonication for 1 h to obtain a stable water dispersion. Then, 200 mg of NaBH4 was introduced into the dispersion under magnetic stirring, and the mixture was kept stirred at 125 °C for 3 h. Eventually, a black precipitate (E-rGO) was obtained, isolated by filtration, and dried at 60 °C under vacuum48,49.
Synthesis of E-rGO/Fe3O4 nanocomposites
E-rGO/Fe3O4 nanocomposites were prepared using a previously reported one-pot co-precipitation method with several modifications and optimized E-rGO/Fe3O4 ratios46,48,50.The reduction of GO and the concurrent production of Fe3O4 nanoparticles were done under controlled conditions. Using a Qsonica ultrasonicator (USA), E-GO (50 mg) was first dispersed in deionized water (150 mL) and then ultrasonically treated for 2 h. Before being added to the E-GO dispersion, 176 mg of FeCl3.6H2O and 130 mg of FeCl2.4H2O were separately thoroughly dissolved in 50 mL of deionized water. The pH was gradually raised to 10 by adding a NaOH solution. For 2 h, the reaction was kept at 80 °C while being stirred. Following completion, the E-rGO/Fe3O4 nanocomposite was separated by magnetic means, rinsed several times with ethanol and distilled water, and then dried at 60 °C. The conversion of enset/enseteventricosum corm-derived reduced graphene oxide (E-rGO) and E-rGO/Fe3O4 nanocomposites is shown in Scheme 1.
Anode preparation
The anode, which is essential to the MFC system, facilitates electron transport. Facilitating the movement of electrons toward the cathode compartment guarantees that the circuit is completed. The anode electrode was modified using the modification reported in previous studies51,52,53. Bare graphite electrodes were first submerged in distilled water for a full day as part of the MFC setup. After that, they underwent alteration after being rinsed with a 200 mM phosphate buffer solution (PBS). Equal amounts of composite (E-rGO NPs and E-rGO/Fe3O4 NCs) and polyvinyl alcohol (PVOH) polymer binder solutions were then made utilizing a drop-cast coating technique to strengthen the connection between the bare graphite electrode and the synthesized nanocomposite catalysts. In a beaker, the necessary quantity of nanocomposites was added. The required amount of nanocomposites was placed in a beaker and water that had been deionized. To create a uniform solution, the mixture was put in an ultrasonic water bath and left there for 15 min; a ratio of 15:85 (wt/wt% of PVOH to E-rGO and E-rGO/Fe3O4 nanocomposite catalyst) was dissolved separately in 10 mL deionized water and magnetically stirred for 1 h at 90 °C. Before coating the bare graphite electrode, the mixture was sonicated to ensure uniform distribution of the nanocatalyst. 300 μL of the suspension was coated on the bare graphite electrode surface by the drop casting method using a micro syringe to improve the interface stability and binding energy and then dried in an oven at 60 °C for 12 h to dry. The graphite rods with dimensions of 12 cm × 0.5 cm (height × radius) served as anode and cathode electrodes in both chambers. The surface area of both synthesized electrodes (E-rGO and E-rGO/Fe3O4) was 40 cm2.
Material characterizations
The synthesized materials (E-rGO and E-rGO/Fe3O4) were characterized using a variety of techniques. Surface topography and elemental composition were performed using scanning electron microscopy-energy dispersive X-ray (JSM-6500F, FE-SEM). The crystal structure of the samples was examined using an XRD (Bruker D2-phaser X-ray diffractometer). Uv–vis spectroscopy (Azzota SM-1600 SPECTROPHOTOMETER, USA) was utilized for optical properties. Fourier transform infrared (FT-IR, JASCO FT/IR6200) was applied to identify the unknown chemical composition of the materials. A Raman spectroscopy (IHR550) with a 532 nm laser was used to evaluate molecular vibrational states and structural defects. Thermo gravimetric analysis (TGA, DTG-60H detector (Shimadzu, Japan) was used to determine the thermal stability of the materials. The conductivity of the wastewater samples, temperature, and pH were measured using the electrical conductivity meter (ECM) (Alpha-800 conductivity meter), thermometer (GH, ZEAL LTD, England), and a pH meter (EUTECH instrument-700 USA). Electrochemical measurements were conducted using EC-Lab for Windows v10.36 software in a three-electrode, one-compartment system connected to an electrochemical workstation (Biologic VSP-300, Canada). Working, reference, and counter electrodes were Ag/AgCl and Pt wire, respectively, within a potential window between 0 V and + 1.5 V using the EC-Lab software for Windows v10.36. Gr, E-rGO, and E-rGO/Fe3O4 modified graphite electrodes were tested in an aqueous electrolyte solution of 2 M H2SO4 at a scan rate of 5 mV/s. Measurements using electrochemical impedance spectroscopy (EIS) were conducted over the frequency range 100 kHz–10 mHz, with a 10 V applied potential vs. Ag/AgCl electrode. Atomic absorption spectrophotometer (BUCK SCIENTIFIC Model 210, USA) was used to determine the reduction efficiency of Cr (VI) and Pb (II) ions.
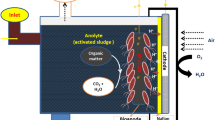
MFCs setup and operation
In addition to electrode material, the choice of designs of microbial fuel cell (MFC) is another factor that significantly influences the performance, functionality, and application suitability of MFCs. In this study, a double-chambered setup was preferred over a single-chambered MFC setup. This is due to, in a double-chambered setup, the cathode is typically isolated from the anode, often using a proton exchange membrane (PEM) or a salt bridge. This isolation ensures that oxygen can be effectively reduced at the cathode without influencing the microbial activity in the anode compartment, which is crucial for maximizing microbial electrochemical reactions and achieving better energy output. In a single-chambered system, the proximity of the cathode to the anode can cause issues with the back diffusion of oxygen from the cathode to the anode with PEM and contamination in the anode chamber, which disrupts the microbial electrochemical reactions, as microbes are adapted to use organic compounds as electron donors and not oxygen. Furthermore, the anode chamber in a heavy metal ion remediation system can have several advantages over the cathode chamber, depending on the specific design and application of the system, such as enhanced ion exchange, increased removal efficiency, reduced energy consumption, and improved water quality54,55,56. The height of DMFCs is 12 cm. The diameter of the anode chamber and cathode chamber was 6.5 cm. The operational volume was kept at 750 mL; however, each chamber had a combined capacity of about 1000 mL. The chamber of anode contained 150 g of sweet potato waste and 150 ppm chromium and lead ions treated pond wastewater. The cathode chamber contained 50 mM KMnO4 and buffer with 0.1 M phosphate. Both the cathode and the anode were stabilized with a phosphate buffer solution, maintaining a pH of 7 and 6.45, respectively. The compartment for the anode was sealed, without an external intake, and the cathode was provided with external oxygen. The anode was sealed because it did not require an external substrate. For each reaction, a prepared anode electrode was used, and a graphite rod of similar size was used as the cathode electrode. The technique described in a prior work was used to prepare salt bridges53. That is, 3 g of agar was added to 100 mL of boiling water and stirred until the agar dissolved. To this mixture was added 12 g of KCl. The solution was filled into a high-density polyethylene plastic pipe 9 cm long and 2 cm in diameter. One end of the compression fitting was tightly covered with a glass stopper. The solution was allowed to cool for nearly 2 h. To demonstrate the electrocatalytic activity of the nanocomposites compared to bare graphite rod electrodes, three double-chambered MFCs were constructed. Bare graphite rods, E-rGO, and E-rGO/Fe3O4 modified electrodes were used as anodes for three different reactions, and graphite rods were used as cathodes for all reactions. The MFCs were operated continuously for 30 days at room temperature. In all cases (GR, E-rGO, E-rGO/Fe3O4), the electrodes were connected using copper wires with an external resistance of 1000 Ω. The physicochemical properties of the pond wastewater and the Cr (VI) and Pb (II) ion-supplemented pond wastewater are shown in Table 1 before MFCs operation.
Evaluation of MFCs: electrochemical analysis and remediation performance
Electrochemical characterization began by obtaining a stable open-circuit voltage (OCV) and then drawing a polarization curve by varying the external resistance (10,000, 1000, 500, 300, and 100–50 Ω) at 30-min intervals. The voltage drop across the external resistance was recorded every 30 min using a digital multimeter (DT830D, Haoyue, China) for data collection. Conversion from voltage to current (measured in milliamps) was accomplished by applying Ohm’s law. Mathematical formulas were used to calculate internal resistance (r), current density (CD), power density (PD), and the voltage output (V) (1–5).
where V is the voltage output, I is the current, R is the external resistance, r is the internal resistance, E is the electromotive force (emf), and A is the cross-sectional area. In the graphite electrode, the cross-sectional area was calculated using the straight cylinder formula (A = 2πr2 + 2πrh) where, h is the height and r is radius of the graphite electrode. The electromotive force is expressed in terms of open circuit voltage (OCV). Polarization curves were generated when the reaction reached a quasi-steady state at the optimum voltage. For the examination of Cr and Pb, 1.0 mL of solution was taken out of the anode chamber at each sampling interval of 15 days. Equation (6) was used to get the reduction efficiency (%).
where RE% = remediation efficiency, Minitial = initial concentration of the metal (Pb), and M final = final concentration of the metal after MFC operation.
Results and discussion
Characterization of E-rGO and E-rGO/Fe3O4 materials
SEM–EDS analysis
The morphological properties of the synthesized E-rGO and E-rGO/Fe3O4 nanocomposites were investigated using SEM. Figure 1a shows that there is sufficient surface area for the functionalization of thin layered sheets of 2D E-rGO with interconnected networks57. To increase the surface area and facilitate the electron transfer process, Fe3O4 nanoparticles were synthesized and anchored to the E-rGO surface (Fig. 1b). In this study, the Fe3O4 nanoparticles strongly adhered to the E-rGO nanosheets, resulting in electrostatic interactions between the negatively charged E-rGO nanosheets and the positively charged Fe3+58. The chemical composition of each of the produced nonmaterial’s was analyzed using energy dispersive X-ray spectroscopy (EDS); EDS analysis of E-rGO confirmed the presence of C and O elements (Fig. 1c); EDS analysis of E-rGO/Fe3O4 confirmed the presence of C, Fe, and O components in the composite, and the final product chemical composition was obtained, demonstrating the uniform distribution of magnetic nanoparticles on the E-rGO sheet (Fig. 1d). Elemental mapping is a powerful technique often used in material science, especially with tools like Energy Dispersive X-ray Spectroscopy (EDS or EDX) in conjunction with Scanning Electron Microscopy (SEM), as shown in Fig. 1e–g. It revealed the elemental homogeneity, segregation, or clustering of C, Fe, and O elements.
XRD and UV–Vis analyses
X-ray diffraction (XRD) is one of the widely used nondestructive techniques to analyze crystallite size, orientation, and average spacing of atomic layers. In this study, XRD studies were performed to confirm the formation of synthesized E-GO, E-rGO, and E-rGO/Fe3O4 (Fig. 2). The XRD pattern of E-GO showed a high and strong peak with a (001) lattice reflection at 2θ = 10°, as shown in Fig. 2a. This is explained by the carbonized carbon’s efficient oxidation38. The effective conversion of GO to rGO by using NaBH4 as reducing agent is confirmed by a broad XRD signal with a reflection peak at a 2θ value of 25.7° and lattice reflection of (002), indicating formation of rGO57. Furthermore, the (002) plane of GO disappears in rGO, suggesting in situ reduction of GO to rGO59. A similar broad peak was observed in the angle range between 15 and 30 during the synthesis of rGO using heated coconut shells60. In Fig. 2a, the XRD pattern of binary E-rGO/Fe3O4NCs, 2θ = 30.5°, 35.57°, 43.55°, 54.1°, 57.34°, 63.00°, and 74.66°, which correspond to (220), (311), (400), (422), (511), (440), and (533) crystal planes of face-centered cubic (fcc) structure of Fe3O4. These peaks confirm the presence of Fe3O4 nanoparticles as revealed by (JCPDS card number 19-0629). In addition, a broad diffraction peak at about 26.7° corresponding to the (002) plane of E-rGO was observed, providing further evidence of successful hybridization between the reduction of E-GO nanosheets and Fe3O4 nanoparticles. This peak also indicates that Fe3O4 NPs are attached to the surface of E-rGO nanosheets. Scherrer’s formula was used to estimate the crystallite size (D) of the magnetite powder in the synthesized samples58.
where K is the crystallite shape factor (0.9), k is the wavelength of the Cu Kα line (0.15406 nm), β is the full width at half maximum (FWHM) of the XRD peak, and λ is the Bragg angle. The crystallite sizes for E-rGO and E-rGO/Fe3O4 powders were 29.1 and 22.7, respectively. The hybridization of E-rGO and Fe3O4, as indicated by this result, resulted in a slight decrease in crystallite size. Figure 2b shows the absorption peaks of the synthesized E-rGO and E-rGO/Fe3O4 using a UV–vis spectrophotometer; the absorption peak around 245 nm wavelength for E-rGO is attributed to the π → π * transition of aromatic C=C. The peak for E-rGO shifts to 265 nm, indicating the formation of the binary complex indicating the successful formation of the binary complex.
FT-IR and Raman spectra analyses
To confirm the production of E-rGO and E-rGO/Fe3O4 nanocomposites, FTIR analysis of the nanocomposites was performed, as shown in Fig. 3a. The characteristic peaks of the synthesized E-rGO spectra are C–H stretching, C=C stretching, C–H bending, C–O stretching of alkoxy groups, and C–O stretching vibration of C–O–C at 3060, 1566, 1339, 1098, 1224, and 599.28 cm−1, respectively, C–OH stretching of alcohol groups, identified by OH out-of-plane bending61,62,63,64. These functional groups are also present in E-rGO-Fe3O4, but the bonding positions are red-shifted, and the peak sharpness is altered, especially for the aromatic C=C bond. This suggests that the different functional groups in E-rGO-Fe3O4 act in different coordination contexts. The presence of Fe–O stretching vibrations in the E-rGO/Fe3O4NCs was observed around 564 cm−1. The characteristic peak at 820 and 794–883 cm−1 is due to C-H binding. Along with the simultaneous reduction of GO and Fe3⁺ ions, the decreased peak intensities of OH and C=O stretching vibrations in GO sheets verify the electrostatic interaction between Fe3⁺/Fe2⁺ ions and E-rGO sheets. It is proposed that the shift in peak location and the alteration in C=C bonding in E-rGO favor covalent bonding60,65.
Raman analysis of E-rGO and E-rGO/Fe3O4NCs was performed, and the corresponding results are shown in Fig. 3b. The Raman spectrum of E-rGO shows the presence of two intrinsic peaks at 1346 cm−1 and 1575 cm−1 attributed to the D and G bands, respectively. The G band is associated with the in-plane bond stretching motion of the C sp2 atom pairs (E2g phonons) associated with in-plane bond stretching motions, while the D band indicates the transition from sp2-hybridized carbon to sp3-hybridized carbon. The intensity of the D band is higher than the G band due to defects and partially disordered structures in E-rGO46, the E-rGO Raman spectrum shows two broad peaks at 1339 cm−1 and 1591 cm−1 corresponding to the D and G modes, respectively: the G band represents the in-plane coupled stretching motion of a pair of C sp2 atoms (E2g phonons), while the D band is the K-point of a ring breathing mode or A1g symmetry corresponding to phonons62; the D and G bands were also seen in the Raman spectra of E-rGO/Fe3O4NCs, but were stronger. This suggests that more defects were formed in the E-rGO nanosheets. The main causes are the accumulation of magnetite nanoparticles and the chemical reduction of graphene oxide by NaBH4; in the case of E-rGO/Fe3O4 nanocomposites, some extra peaks were observed at low Raman shift values (224, 289, 395, 485, and 648 cm−1) which may correspond to vibrational modes of Fe–O bonds in Fe3O4 nanoparticles and Fe–C bonds on the E-rGO surface66. Furthermore, the ID/IG ratio of E-rGO/Fe3O4 increased from 1.27 to 1.39, and the G band of E-rGO/Fe3O4 was blue shifted by 0.12 cm−1 relative to E-rGO, suggesting that the charge transfer and interaction between E-rGO and Fe3O4 may be responsible for the disordered graphitic crystal structure was suggested.
Thermal stability analysis
The thermal stability of E-rGO and E-rGO/Fe3O4 NC was investigated using TGA, as shown in Fig. 4). Due to loosely attached water molecules that are present between the sheets and are visible up to 200 °C, the TGA curve of E-rGO exhibits a mass loss before reaching 100 °C. The second loss at 100 °C is attributed to the decomposition of surface functional groups such as carboxylic acids, hydroxides, and epoxides49. The first major loss following this produces CO, CO2, and byproducts of the reduction process. The loss of C=O and C–O single-bonded oxygen groups, which is seen between 200 and 590 °C, and the release of reduction process byproducts translate into the third mass loss. The mass loss after 590 °C is due to the rapid decomposition of oxygen species, suggesting the thermal stability of rGO up to 800 °C67. The evaporation of absorbed water is responsible for the modest mass loss below 100 °C as compared to E-rGO. After that, a slow mass loss takes place between 200 and 600 °C, which is explained by the elimination of water vapor and unstable oxygen-containing functional groups from the sample as a result of their disintegration. E-rGO/Fe3O4 NCsa few weight loss took place between 350 and 530 °C, while the major weight loss was observed after 550 °C, which was attributed to the interface change between oxygen-based material and metal ions68, which can be attributed to the Fe3O4 oxidation to Fe2O3. This reason was also supported by other69.
Electrochemical characterization
CV and EIS analysis
As shown in Fig. 5a, the electrocatalytic activity of the prepared materials was evaluated using CV in a 1 M H2SO4-supported electrolyte solution. The results confirmed that no obvious redox peak or current was observed at the graphite anode electrode due to its low electrical conductivity. In contrast, CV plots of bare graphite electrodes modified with E-rGO and E-rGO/Fe3O4 NCs show easily identifiable redox peaks, which could be assigned to electrochemical reactions from the electrolyte. Additionally, the E-rGO/Fe3O4NCs modified electrode exhibits improved electrochemical and capacitive behavior, as evidenced by a significantly greater area under the CV curve than the E-rGO modified electrode70. EIS is now being used as a nondestructive technique to provide useful information about the electrochemical properties of electrode surfaces and the reactions occurring there. EIS test was performed with an applied potential of 10 V and a frequency range of 100 kHz–10 MHz. In EIS, the applied sinusoidal voltage is typically small, often in the range of 5–10 mV (RMS). This small amplitude is used to ensure the system stays within the linear region of its response, meaning the current response is directly proportional to the applied voltage. If you increase the AC amplitude significantly (e.g., from 10 to 100 mV or more), here’s what happens: System Becomes Nonlinear: The assumption of linearity breaks down, and the response (current) is no longer proportional to the input (voltage). This can distort the Nyquist plot, introducing non-ideal features or shifting the semicircle and line. Change in Charge Transfer Resistance (Rct): At higher overpotentials, electrochemical kinetics may change, sometimes causing a decrease in Rct due to faster reaction rates. However, depending on the material and reaction, it might also increase if side reactions or degradation start. Additionally, at higher applied voltages, Faradaic processes (real redox reactions) can dominate, leading to additional or nonlinear arcs and Warburg-like distortions in the Nyquist plot. This alters not only the plot’s size but also its shape, complicating interpretation. Table 2 and Fig. 5b display the corresponding fitting electrochemical parameters Rct and Rs of graphite modified E-rGO and E-rGO/Fe3O4. While all electrodes showed similar Rs values, 1.27–1.46 ohms. Rs represents solution resistance, with lower values indicating better electrolyte ionic conductivity. The Rct at the electrode–electrolyte interface was determined by the semicircle’s diameter, while the second straight line corresponded to the diffusion resistance, known as the Warburg impedance (W) of the electrolyte; a smaller Rct indicates a faster electron transfer rate44,71,72. The bare graphite electrode exhibited a larger semicircle than the other modified electrodes, indicating a higher Rct at the electrode/electrolyte interface due to the slower electron transfer rate Rct values decreased when the bare Gr was modified, as well as when E-rGO and E-rGO/Fe3O4 were modified, 123, 62, and 51 ohms, respectively; the E-rGO/Fe3O4 modified electrode exhibited a smaller Rct and showed superior electrochemical activity due to the larger specific surface area facilitating diffusion and charge transfer processes73. Additionally, in the corresponding circuit, CPE denotes the constant phase element compensating for the solution/electrode interface’s double-layer capacitance. The equivalent circuit is shown in Fig. 5b:
Electrochemical surface area analysis
The electrochemical surface area (ECSA) is a measure of the accessible surface area of an electrode that actively participates in electrochemical reactions. The ECSA of the prepared materials was evaluated using cyclic voltammetry (CV) in a 1 M H2SO4-supported electrolyte solution, as shown in Fig. 6. It performs cyclic voltammetry within a non-Faradaic potential region, where no redox reactions occur. Measure the current response at various scan rates (e.g., 10–100 mV/s). The average capacitive current (ΔJ = (Ja − Jc)/2) against the scan rate (ν) to determine the double-layer capacitance. The slope of the linear plot gives Cdl.
Cdl: Measured double-layer capacitance (mF/cm2), Cs: Specific capacitance of a smooth surface (typically 20–60 µF/cm2 depending on the material and electrolyte).When comparing E-rGO to E-rGO/Fe3O4 (magnetite-loaded E-rGO), the ECSA of pure E-rGO/Fe3O4 greater cdl value compared to the others.
Cyclability performance of E-rGO
As shown in Fig. 7, the cyclability performance of enset corm biosynthetic graphene derivative (E-rGO) material modified electrode in energy conversion was demonstrated. The high electrical conductivity and stability and stability of the E-rGO material make it an effective electrode for various energy conversion applications. The study provides valuable insights into the potential of E-rGO material for future research and development in the field of energy conversion. To assess the cyclability of the E-rGO electrode, up to 200 repetitive cyclic voltammetries were performed as shown in Fig. 7. No significant change (less than 10% of variation in current density values) in electrocatalytic activity (i.e., peak current density and reversibility) can be appreciated after the measurement. Furthermore, the cyclability is linked to the unique properties of the enset corm-derived E-rGO. For example, functional group and Morphology, the presence of hydroxyl and carboxyl on the E-rGO surface, confirmed by FTIR analysis shown in Fig. 3a, which enhances electrolyte accessibility and ion transport. The interconnected network and high surface area of the E-rGO nanosheets, observed in SEM images Fig. 1a, provide a stable and conductive framework that minimizes structural degradation.
Performances of E-rGO and E-rGO/Fe3O4 modifiedanodes in MFCs
Open circuit voltage and current versus time
The efficiency of Gr, E-rGO, and E-rGO/Fe3O4 modified electrodes in DMFC was evaluated by OCV and CCV tests. Investigating the catalytic activity of the prepared materials is very important to improve the performance of the MFC, and OCV is an important criterion. The open circuit voltage experiments were performed without connecting the anode and cathode to a specific load. Each experiment lasted 30 days with organic waste (sweet potato waste) as substrate. The voltages generated at the bare Gr, E-rGO, and E-rGO/Fe3O4 modified electrode MFC increased gradually but consistently during the first few days, reaching maximum open circuit voltages of 439 mV, 500 mV, and 960 mV, respectively, over 15 days, according to Fig. 8a. However, it eventually increased as a result of the formation of adherent biofilms and the absorption of nutrients under anaerobic conditions in the anode compartment. This process increased current production, followed by an increase in open circuit voltage (OCV)74.
The maximum OCV of 900 mV achieved by the E-rGO/Fe3O4 NCs modified Gr may be due to the effective attachment of the electro-generating bacteria on the modified anode electrode and the formation of a conductive biofilm. This could also be due to the high charge density capacitance after bio-anode formation. These interactions promoted conductive biofilm formation by increasing the active surface area at the electrode surface, leading to higher fast electron transfer rates during MFC operation. The operation of the electrodes continued until the voltage trend decreased. After day 15, all electrodes exhibited a decreasing trend in voltage generation. This was due to an insufficient supply of organic substrate. The organic substrate provided was only enough for 15 days to achieve maximum voltage generation. After that, the low carbohydrate content for the oxidation process reduced bacterial activity, resulting in a lower generation of electrons. The maximum current corresponding to an external resistance of 50 Ω is shown in Fig. 8b for bare Gr, E-rGO, and E-rGO/Fe3O4 NCs modified electrodes, respectively. 1.97 and 3.74, respectively38.
Polarization behavior and internal resistance
Another crucial parameter for characterizing MFC performance had been shown the polarization curve shown in Fig. 9, which indicates that the voltage as a function of current and power density75.The proper resistance and current relationship can be found with the aid of the polarization curve. Before conducting the polarization study, the external resistance was removed, and all cells were kept in open-circuit mode until a stable open-circuit voltage (OCV) was obtained for 24 h. To record voltage, current density, and power density, each anode electrode of the DCMFC was connected to a resistance ranging from 10 KΩ to 50 Ω. Each cell was maintained at each resistance level for 30 min to obtain consistent readings. Because of the absence of biofilm in the early stages of operation, microorganisms are unable to withstand the loading caused by low resistance. Smaller resistances are employed to encourage high-density biofilm formation and reduce the steady-state period after biofilm formation. It is important to note that with large external resistance, the demand for electron production is lower than the amount the microorganism can supply because the conducting channels are shorter. As a result, larger external resistances resulted in longer stationary periods than those with lower resistances. Furthermore, microbial oxidation of substrate wastewater was expected to occur at a higher rate when a low resistance was used in the circuit than when a high resistance was used, This is because electrons flow faster through a low resistance than a high resistance74. Thus, circuit connections with high external resistance generated less power but stabilized the voltage faster. However, with low external resistance, power generation is high, but voltage stabilization is slow. Moreover, voltage instability occurred significantly faster under low external resistance. This instability at low resistance was attributed to the rapid electron transfer to the cathode, leading to increased power output and current density despite reduced potential stability. Likewise, some studies have reported similar output trends76,77.As shown in Fig. 9a,b, maximum power densities of 8.75 and 39.77 mW m−2were obtained for MFC with E-rGO and E-rGO/Fe3O4 anodes, respectively, with an effect of with 434, and 416 Ω internal resistances, respectively. The corresponding current densities were 609 mA m−2 and 1171 mA m−2 with an external resistance of 50 Ω, respectively. The ability of the NC-modified electrode to facilitate rapid electron transfer rates between bacterial colonies and the electrode surface resulted in notable increases in current and power density performance overall. Specifically, electrodes modified with E-rGO/Fe3O4NCs exhibited good electrochemical performance and showed promise as anodic modifiers; the improved electrochemical performance of E-rGO/Fe3O4 may be related to the low internal resistance and efficient charge transfer capacity of rGO78. Indirectly increasing the surface area of the anodes increased the porosity of the NCs and promoted the growth of bacteria and biofilms. These results suggest that the synergistic effects of E-rGO and Fe3O4 play an important role in improving MFC performance by improving the electron transfer process and enhancing electrochemical activity. Notably, the produced anode material is used to simultaneously generate electricity and reduce hexavalent chromium and divalent lead from concentrated wastewater. This is due to the fact that E-rGO and Fe3O4NPs form a complex under near-neutral solution, which improves the specific capacitance and cycling stability at the graphite anode surface. In addition, Fe3O4 and E-rGO provided numerous active surface sites and surface areas for microbial attachment. Similar trends in polarization studies have been described in a previous study79. The main limitation of our study from polarization curve, the polarization resistance and mass (concentration) resistance is dominated in our modified electrode. This can be due to several reasons. Firstly, the modified anode may be a higher resistance to electron transfer, which can lead to higher polarization resistance. Secondly, the modified anode may have a higher concentration of reactants which can lead to higher mass (concentration) resistance80,81. The theoretical aspect relation of voltage and current with the external loads are shown in Fig. 9c,d. The current density efficiency of the MFC anodes and a comparison with other anodes reported to date are shown in Table 3.
Energy generation and bioremediation of Cr (VI) and Pb (II) ions supplemented wastewater
Numerous microbes (electroactive bacterial species) have been found in marine, freshwater sediments, various substrates, including manure, sludge from aerobic/anaerobic wastewater treatment, and wastewater from different sources, can generate electricity by oxidizing waste materials10. Furthermore, microbial action is critical to the high performance of MFC in electron transport and contaminant ion removal. The most prevalent electron-producing microbial groups are Actinobacteria, Firmicutes, Proteobacteria phyla, fungi, algae, and yeasts that can generate electricity. Additionally, Geobacter spp., Rhodoferax ferrireducens, Aeromonas hydrophila, Clostridium butyricum, Shewanella spp., E. coli, etc., are commonly reported microbes in MFCs. The microbes form a biofilm around the anode and transfer the electrons by utilizing different mechanisms, such as long-range via conductive pili, short-range mechanisms through redox-active molecules, and electron transfer via shuttling molecules. Ordinarily, the anode is not considered part of the aquatic atmosphere in the chamber; microbes’ growth forms the biofilm on the surface of the anode and uses power for respiration purposes. Throughout the respiration process, microbes release electrons and protons that transfer to the anode and cathode. Metal reduction from soluble to insoluble state was carried out by bacterial species in MFC. In the process of respiration, it produces the electrons, which simultaneously carry out the redox reaction to degrade the metal86,87. For example, the Geobacter genus Habepili is as conductive and actively expressed as metals. In this experiment, sweet potatoes were used as an organic substrate, and several microorganisms were grown. Initially, in the anode chamber, the carbohydrate sweet potato (C6H10O5) is converted into glucose (C6H12O6) and oxidized by the bacteria, resulting in the production of electrons and protons. The electrons are transferred to microorganisms present in the wastewater. The microorganisms can use these electrons to reduce the heavy metal ions in the wastewater. Simultaneously, the electrons flowing through the external circuit can also be used to power the fuel cell, which can generate electricity that can be used to drive other processes or power devices. The protons migrate to the cathode through the proton exchange membrane and are reduced. This investigation revealed the following oxidation and reduction processes:88
The electrons and protons produced are transferred to the cathode, where they are involved in oxidation. Furthermore, soluble metals were efficiently converted to insoluble metals. At the end of the operation, the analyzed sludge contained insoluble metals. The reduction of Cr (VI) and Pb (II) ions by microorganisms can be driven by the electrons provided by the negative electrode in the fuel cell. The electrons can be transferred from the electrode to the microorganisms, which can then use them to reduce the metal ions in the wastewater. This process can be facilitated by the presence of specific microorganisms that are capable of reducing heavy metals.
Based on electrochemical considerations, Microorganisms receive electrons directly from electrodes and reduce chromium from the highly toxic oxidation state of Cr (VI) to the less toxic oxidation state of Cr (III). Similarly, microorganisms can change Pb ions from the soluble Pb (II) state to the insoluble Pb state. The reduction of Cr (VI) (chromium in + 6 oxidation state, typically Cr₂O₇2⁻ or CrO₄2⁻ in aqueous solutions) to Cr (III) (chromium in + 3 oxidation state) is an important reaction in wastewater treatment. The reduction process involves electron transfer and is typically facilitated at the electrode surface. The half-reaction for the reduction of Cr (VI) is as follows:
Also, Lead ions (Pb2⁺) can be reduced to Pb (metallic lead), and the anode metal reduction reaction is represented as follows:
Previous studies have demonstrated that the use of MFCs for metals remediation is an innovative way to reduce water pollution. The choice of anode material and proton exchange source had a significant impact on both energy production and pollutant removal89,90,91. In this study, both modified and bare electrodes were used as anodes, including bare Gr, E-rGO/Gr, and E-rGO/Fe3O4, and their efficiencies were compared, as shown in Table 4. Bioremediation of effluent with Cr (VI) and Pd (II) ions was performed using DMFC for 30 days. The results showed that E-rGO and E-rGO/Fe3O4 electrodes provided 40 cm2 of surface area for bacterial growth and achieved purification efficiencies of 70.6% and 79.2% of Cr (VI), respectively. These electrodes showed higher Cr (VI) purification efficiencies than bare Gr anodes (63.5%); anodes fabricated with E-rGO and E-rGO/Fe3O4NCs showed purification efficiencies of 65.1% and 73.8%, respectively. In contrast, unmodified graphite anodes showed 55.5% removal efficiency for Pb (II). The removal effectiveness of the materials was influenced by the conductivity of the materials utilized: compared to E-rGO and bare graphite (Gr), the E-rGO/Fe3O4 anode electrode showed more stability against bacterial respiration. By the time the reaction reached the midpoint (30 days), the E-rGO/Fe3O4 anode electrode showed more stability against bacterial respiration. Overall, both electrodes showed better biocompatibility for bacterial growth and their respiration processes. It is interesting to note that the highest reduction rates of Cr (VI) and Pd (II) were observed in previously published studies92. Microorganisms receive electrons directly from electrodes and reduce chromium from the highly toxic oxidation state of Cr (VI) to the less toxic oxidation state of Cr (III). Similarly, microorganisms can change Pb ions from the soluble Pb (II) state to the insoluble Pb state. Table 5 and Fig. 10 show a comparative profile with the previous literature that was tabulated based on bioelectrochemical fuel cells with MFC results and the proposed harmful pollutant bioremediation and energy generation mechanism.
Conclusions and future recommendations
The present study highlights the enseteventricosum corm waste-derived synthesis of E-rGO and E-rGO/Fe3O4 NCs for the fabrication of the anode for MFCs. More over the present study successfully showed the synthesis of the enseteventricosum corm waste-derived synthesis of E-rGO and E-rGO/Fe3O4 anode for improving the electron transportation, which led to high energy performance. In the present study, sweet potato waste was used as an organic substrate with novel fabricated anodes to achieve the high performance of MFCs. The prepared anodes were electrochemically supported by considering different approaches to validate the performance of energy generation and wastewater treatment. Different material characterizations and MFCs operational results indicated that both E-rGO and E-rGO/Fe3O4 modified electrodes are highly stable (thermal and chemical) for MFCs operation. In the present study, the DCMFCs were operated by using two electrodes, E-rGO and E-rGO/Fe3O4, with sweet potato as an organic substrate. Each MFCs operation took 30 days and showed the highest current efficiency within 15 days. On the 15th day, the E-rGO showed 609 mA/m2 and E-rGO/Fe3O4 showed 1171 mA/m2. Similarly, the achieved remediation efficiency of E-rGO was 70.6%Cr (VI) and 65.1% Pb (II), while the E-rGO/Fe3O4 showed a remediation of 79.2% Cr (VI) and 73.8%Pb (II). According to these results, biocompatible carbon-based E-rGO/Fe3O4 nanocomposites anodes are a more promising material to reduce cost, time, and environmental hazard for future scalable renewable energy conversion and toxic metal ion and organic pollutants removal by using MFC.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Dessie, Y., Tadesse, S. & Eswaramoorthy, R. Review on manganese oxide-based biocatalyst in microbial fuel cell: Nanocomposite approach. Mater. Sci. Energy Technol. 3, 136–149 (2020).
Sushma, S. & Anand, H. Designing the shape of graphite anode for microbial fuel cells to increase its efficiency. Int. Res. J. Eng. Technol 4, 553–556 (2017).
Yaqoob, A. A., Ibrahim, M. N. M. & Guerrero-Barajas, C. Modern trend of anodes in microbial fuel cells (MFCs): An overview. Environ. Technol. Innov. 23, 101579 (2021).
Temesgen, T. et al. Advancements in biomass derived porous carbon materials and their surface influence effect on electrode electrochemical performance for sustainable supercapacitors: A review. J. Energy Storage 73, 109293 (2023).
Bhatia, S. K., Joo, H.-S. & Yang, Y.-H. Biowaste-to-bioenergy using biological methods–a mini-review. Energy Convers. Manage. 177, 640–660 (2018).
Hein, J. R., Evans, J. & Jones, P. Mobile methodologies: Theory, technology and practice. Geogr. Compass 2, 1266–1285 (2008).
Zhuo, T. et al. Micro-pressure promotes endogenous phosphorus release in a deep reservoir by favouring microbial phosphate mineralisation and solubilisation coupled with sulphate reduction. Water Res. 245, 120647 (2023).
Du, Z., Li, H. & Gu, T. A state of the art review on microbial fuel cells: A promising technology for wastewater treatment and bioenergy. Biotechnol. Adv. 25, 464–482 (2007).
Logan, B. E. Exoelectrogenic bacteria that power microbial fuel cells. Nat. Rev. Microbiol. 7, 375–381 (2009).
Ramírez-Vargas, C. A. et al. Microbial electrochemical technologies for wastewater treatment: Principles and evolution from microbial fuel cells to bioelectrochemical-based constructed wetlands. Water 10, 1128 (2018).
Sonawane, J. M., Yadav, A., Ghosh, P. C. & Adeloju, S. B. Recent advances in the development and utilization of modern anode materials for high performance microbial fuel cells. Biosens. Bioelectron. 90, 558–576 (2017).
Yang, G. et al. Anode potentials regulate Geobacter biofilms: New insights from the composition and spatial structure of extracellular polymeric substances. Water Res. 159, 294–301 (2019).
Yaqoob, A. A., Ibrahim, M. N. M., Yaakop, A. S., Umar, K. & Ahmad, A. Modified graphene oxide anode: A bioinspired waste material for bioremediation of Pb2+ with energy generation through microbial fuel cells. Chem. Eng. J. 417, 128052 (2021).
Bala, S. et al. Recent strategies for bioremediation of emerging pollutants: A review for a green and sustainable environment. Toxics 10, 484 (2022).
Palanisamy, G. et al. A comprehensive review on microbial fuel cell technologies: Processes, utilization, and advanced developments in electrodes and membranes. J. Clean. Prod. 221, 598–621 (2019).
Hindatu, Y., Annuar, M. & Gumel, A. Mini-review: Anode modification for improved performance of microbial fuel cell. Renew. Sustain. Energy Rev. 73, 236–248 (2017).
An, X., Wang, Y., Yu, C. & Hu, X. Biochar-bacteria coupling system enhanced the bioremediation of phenol wastewater-based on life cycle assessment and environmental safety analysis. J. Hazard. Mater. 480, 136414 (2024).
Santoro, C., Arbizzani, C., Erable, B. & Ieropoulos, I. Microbial fuel cells: From fundamentals to applications. A review. J. Power Sources 356, 225–244 (2017).
Tan, R. K. L. et al. Graphene as a flexible electrode: Review of fabrication approaches. J. Mater. Chem. A 5, 17777–17803 (2017).
Yaqoob, A. A. et al. Recent advances in metal decorated nanomaterials and their various biological applications: A review. Front. Chem. 8, 341 (2020).
Cheng, T. et al. Numerical study of proton exchange membrane fuel cells with airfoil cross flow field. J. Power Sources 631, 236232 (2025).
Erable, B., Byrne, N., Etcheverry, L., Achouak, W. & Bergel, A. Single medium microbial fuel cell: Stainless steel and graphite electrode materials select bacterial communities resulting in opposite electrocatalytic activities. Int. J. Hydrogen Energy 42, 26059–26067 (2017).
Zhu, L. et al. Optimization of power generation and sewage treatment in stacked pulsating gas-liquid-solid circulating fluidized bed microbial fuel cell using response surface methodology. Int. J. Hydrogen Energy 101, 161–172 (2025).
Rahimnejad, M., Adhami, A., Darvari, S., Zirepour, A. & Oh, S.-E. Microbial fuel cell as new technology for bioelectricity generation: A review. Alex. Eng. J. 54, 745–756 (2015).
Kaur, R., Marwaha, A., Chhabra, V. A., Kim, K.-H. & Tripathi, S. K. Recent developments on functional nanomaterial-based electrodes for microbial fuel cells. Renew. Sustain. Energy Rev. 119, 109551 (2020).
Kirubaharan, C. J. et al. Facile fabrication of Au@ polyaniline core-shell nanocomposite as efficient anodic catalyst for microbial fuel cells. Electrochim. Acta 328, 135136 (2019).
Dessie, Y., Tilahun, E. & Wondimu, T. H. Functionalized carbon electrocatalysts in energy conversion and storage applications: A review. Heliyon (2024).
Yang, W. & Chen, S. Biomass-derived carbon for electrode fabrication in microbial fuel cells: A review. Ind. Eng. Chem. Res. 59, 6391–6404 (2020).
Thepsuparungsikul, N., Ng, T., Lefebvre, O. & Ng, H. Different types of carbon nanotube-based anodes to improve microbial fuel cell performance. Water Sci. Technol. 69, 1900–1910 (2014).
Magrez, A. et al. Cellular toxicity of carbon-based nanomaterials. Nano Lett. 6, 1121–1125 (2006).
Peng, Z. et al. Advances in the application, toxicity and degradation of carbon nanomaterials in environment: A review. Environ. Int. 134, 105298 (2020).
Gusev, A. et al. Acute toxic and cytogenetic effects of carbon nanotubes on aquatic organisms and bacteria. Nanotechnol. Russ. 7, 509–516 (2012).
Cai, T. et al. Application of advanced anodes in microbial fuel cells for power generation: A review. Chemosphere 248, 125985 (2020).
Yaqoob, A. A. et al. Recent advances in anodes for microbial fuel cells: An overview. Materials 13, 2078 (2020).
Shahid, K., Ramasamy, D. L., Haapasaari, S., Sillanpää, M. & Pihlajamäki, A. Stainless steel and carbon brushes as high-performance anodes for energy production and nutrient recovery using the microbial nutrient recovery system. Energy 233, 121213 (2021).
Firdous, S. et al. The performance of microbial fuel cells treating vegetable oil industrial wastewater. Environ. Technol. Innov. 10, 143–151 (2018).
Temesgen, T. et al. Optimization of water hyacinth stem-based oxygen-functionalized activated carbon for enhanced supercapacitors. ACS Omega 9, 30725–30736 (2024).
Yaqoob, A. A. et al. Cellulose derived graphene/polyaniline nanocomposite anode for energy generation and bioremediation of toxic metals via benthic microbial fuel cells. Polymers 13, 135 (2020).
Hung, Y.-H., Liu, T.-Y. & Chen, H.-Y. Renewable coffee waste-derived porous carbons as anode materials for high-performance sustainable microbial fuel cells. ACS Sustain. Chem. Eng. 7, 16991–16999 (2019).
Jawaid, M., Ahmad, A., Ismail, N. & Rafatullah, M. Environmental remediation through carbon based nano composites (Springer, 2021).
Song, T.-S. et al. Cobalt oxide/nanocarbon hybrid materials as alternative cathode catalyst for oxygen reduction in microbial fuel cell. Int. J. Hydrogen Energy 40, 3868–3874 (2015).
Bhowmick, G., Das, S., Verma, H., Neethu, B. & Ghangrekar, M. Improved performance of microbial fuel cell by using conductive ink printed cathode containing Co3O4 or Fe3O4. Electrochim. Acta 310, 173–183 (2019).
Rana, N. & Bassi, A. Modern Nanotechnology: Volume 1: Environmental Sustainability and Remediation, 125–144 (Springer, 2023).
Song, R. B. et al. Graphene/Fe3O4 nanocomposites as efficient anodes to boost the lifetime and current output of microbial fuel cells. Chem. Asian J. 12, 308–313 (2017).
Meral, K. & Metin, Ö. Graphene oxide–magnetite nanocomposite as an efficient and magnetically separable adsorbent for methylene blue removal from aqueous solution. Turk. J. Chem. 38, 775–782 (2014).
Ma, J., Shi, N. & Jia, J. Fe3O4 nanospheres decorated reduced graphene oxide as anode to promote extracellular electron transfer efficiency and power density in microbial fuel cells. Electrochim. Acta 362, 137126 (2020).
Alam, S. N., Sharma, N. & Kumar, L. Synthesis of graphene oxide (GO) by modified hummers method and its thermal reduction to obtain reduced graphene oxide (rGO). Graphene 6, 1–18 (2017).
Shin, H. J. et al. Efficient reduction of graphite oxide by sodium borohydride and its effect on electrical conductance. Adv. Func. Mater. 19, 1987–1992 (2009).
BhaskaraRao, B. et al. Fe3O4 nanoparticles intercalated reduced graphene oxide nanosheets for supercapacitor and lithium-ion battery anode performance. J. Mater. Sci.: Mater. Electron. 34, 1910 (2023).
Habibi, F., Seyyedi, M. & Ayati, B. Synthesis and application of reusable and magnetic RGO/Fe3O4 nanocomposites in BR46 removal from an aqueous solution; future prospects of an efficient adsorption platform. J. Mater. Environ. Sci. 13, 900–913 (2022).
Harshiny, M., Samsudeen, N., Kameswara, R. J. & Matheswaran, M. Biosynthesized FeO nanoparticles coated carbon anode for improving the performance of microbial fuel cell. Int. J. Hydrogen Energy 42, 26488–26495 (2017).
Patade, S. et al. Bioremediation of dye effluent waste through an optimised microbial fuel cell. Int. J. Adv. Res. Biol. Sci. 3, 214–226 (2016).
Dessie, Y. & Tadesse, S. Optimization of polyvinyl alcohol binder on PANI coated pencil graphite electrode in doubled chamber microbial fuel cell for glucose biosensor. Sens. Bio-Sens. Res. 36, 100484 (2022).
Obileke, K., Onyeaka, H., Meyer, E. L. & Nwokolo, N. Microbial fuel cells, a renewable energy technology for bio-electricity generation: A mini-review. Electrochem. Commun. 125, 107003 (2021).
Amend, J. P. & Shock, E. L. Energetics of overall metabolic reactions of thermophilic and hyperthermophilic Archaea and Bacteria. FEMS Microbiol. Rev. 25, 175–243 (2001).
Kim, M. H. et al. An analysis of the performance of an anaerobic dual anode-chambered microbial fuel cell. J. Power Sources 196, 1909–1914 (2011).
Kailasa, S. et al. High sensitive polyaniline nanosheets (PANINS)@ rGO as non-enzymatic glucose sensor. J. Mater. Sci.: Mater. Electron. 31, 2926–2937 (2020).
Kahsay, M. H., Belachew, N., Tadesse, A. & Basavaiah, K. Magnetite nanoparticle decorated reduced graphene oxide for adsorptive removal of crystal violet and antifungal activities. RSC Adv. 10, 34916–34927 (2020).
Rana, U. & Malik, S. Graphene oxide/polyaniline nanostructures: transformation of 2D sheet to 1D nanotube and in situ reduction. Chem. Commun. 48, 10862–10864 (2012).
Nugraheni, A. Y., Nasrullah, M., Prasetya, F. A., Astuti, F. & Darminto, D. Materials science forum. 285–289 (Trans Tech Publication).
Yang, Y., Zhang, W., Liu, R., Cui, J. & Deng, C. Preparation and photocatalytic properties of visible light driven Ag-AgBr-RGO composite. Sep. Purif. Technol. 190, 278–287 (2018).
Tienne, L. G. P. et al. Reduced graphene oxide synthesized by a new modified Hummer’s method for enhancing thermal and crystallinity properties of Poly(vinylidene fluoride). J. Mater. Res. Technol. 18, 4871–4893 (2022).
Hosseine, M., Naghib, S. M. & Khodadadi, A. Label-free electrochemical biosensor based on green-synthesized reduced graphene oxide/Fe3O4/nafion/polyaniline for ultrasensitive detection of SKBR3 cell line of HER2 breast cancer biomarker. Sci. Rep. 14, 11928 (2024).
Emiru, T. F. & Ayele, D. W. Controlled synthesis, characterization and reduction of graphene oxide: A convenient method for large scale production. Egypt. J. Basic Appl. Sci. 4, 74–79 (2017).
Charandabinezhad, S. R., Asgharzadeh, H. & Arsalani, N. Synthesis and characterization of reduced graphene oxide/magnetite/polyaniline composites as electrode materials for supercapacitors. J. Mater. Sci.: Mater. Electron. 32, 1864–1876 (2021).
Mishra, A. K. & Ramaprabhu, S. Nano magnetite decorated multiwalled carbon nanotubes: A robust nanomaterial for enhanced carbon dioxide adsorption. Energy Environ. Sci. 4, 889–895 (2011).
Ms, N. A. D. et al. Investigation of chemical bonding and supercapacitivity properties of Fe3O4-rGO nanocomposites for supercapacitor applications. Diam. Relat. Mater. 104, 107756 (2020).
Li, W., Jiang, X., Yang, H. & Liu, Q. Solvothermal synthesis and enhanced CO2 adsorption ability of mesoporous graphene oxide-ZnO nanocomposite. Appl. Surf. Sci. 356, 812–816 (2015).
Kim, H.-K., Roh, K. C. & Kim, K.-B. In situ electrochemical dilatometric study of Fe3O4/reduced graphene oxide nanocomposites as anode material for lithium ion batteries. J. Electrochem. Soc. 162, A2308 (2015).
Kuila, T. et al. Facile method for the preparation of water dispersible graphene using sulfonated poly (ether–ether–ketone) and its application as energy storage materials. Langmuir 28, 9825–9833 (2012).
He, Z. & Mansfeld, F. Exploring the use of electrochemical impedance spectroscopy (EIS) in microbial fuel cell studies. Energy Environ. Sci. 2, 215–219 (2009).
Demeku, A. M. et al. Surface electroactive sites of tungstated zirconia catalysts for vanadium redox flow batteries. ACS Appl. Mater. Interfaces. 16, 7047–7056 (2024).
Xue, J. et al. A novel catalyst of titanium boride toward V3+/V2+ redox reaction for vanadium redox flow battery. J. Alloys Compd. 875, 159915 (2021).
Masoudi, M., Rahimnejad, M. & Mashkour, M. Enhancing operating capacity of microbial fuel cells by using low-cost electrodes and multi anode-cathode connections in a membrane-less configuration. Int. J. Hydrogen Energy 46, 8226–8238 (2021).
Agarry, S. Bioelectricity generation and treatment of petroleum refinery effluent by Bacillus cereus and Clostridium butyricum using microbial fuel cell technology. Niger. J. Technol. 36, 543–551 (2017).
Mateo, S., Cañizares, P., Rodrigo, M. A. & Fernandez-Morales, F. J. Driving force of the better performance of metal-doped carbonaceous anodes in microbial fuel cells. Appl. Energy 225, 52–59 (2018).
Sarma, P. J. & Mohanty, K. Epipremnum aureum and Dracaena braunii as indoor plants for enhanced bio-electricity generation in a plant microbial fuel cell with electrochemically modified carbon fiber brush anode. J. Biosci. Bioeng. 126, 404–410 (2018).
Mondal, S., Rana, U. & Malik, S. Reduced graphene oxide/Fe3O4/polyaniline nanostructures as electrode materials for an all-solid-state hybrid supercapacitor. J. Phys. Chem. C 121, 7573–7583 (2017).
Covarrubias-García, I. et al. Reduced graphene oxide decorated with magnetite nanoparticles enhance biomethane enrichment. J. Hazard. Mater. 397, 122760 (2020).
Park, J. et al. A review of understanding electrocatalytic reactions in energy conversion and energy storage systems via scanning electrochemical microscopy. J. Energy Chem. 91, 155–177 (2024).
Tesfaye, T., Shuka, Y., Tadesse, S., Eyoel, T. & Mengesha, M. Improving the power production efficiency of microbial fuel cell by using biosynthesized polyanaline coated Fe3O4 as pencil graphite anode modifier. Sci. Rep. 15, 587 (2025).
Wang, Y., Zhao, C. E., Sun, D., Zhang, J. R. & Zhu, J. J. A graphene/poly (3, 4-ethylenedioxythiophene) hybrid as an anode for high-performance microbial fuel cells. ChemPlusChem 78, 823–829 (2013).
Peng, X. et al. Enhanced performance and capacitance behavior of anode by rolling Fe3O4 into activated carbon in microbial fuel cells. Biores. Technol. 121, 450–453 (2012).
Karra, U. et al. Performance evaluation of activated carbon-based electrodes with novel power management system for long-term benthic microbial fuel cells. Int. J. Hydrogen Energy 39, 21847–21856 (2014).
Yaqoob, A. A., Guerrero-Barajas, C., Ibrahim, M. N. M., Umar, K. & Yaakop, A. S. Local fruit wastes driven benthic microbial fuel cell: A sustainable approach to toxic metal removal and bioelectricity generation. Environ. Sci. Pollut. Res. 29, 32913–32928 (2022).
Yaqoob, A. A. et al. Outlook on the role of microbial fuel cells in remediation of environmental pollutants with electricity generation. Catalysts 10, 819 (2020).
Yaqoob, A., Fadzli, F., Ibrahim, M. & Yaakop, A. Benthic microbial fuel cells: A sustainable approach for metal remediation and electricity generation from sapodilla waste. Int. J. Environ. Sci. Technol. 20, 3927–3940 (2023).
Yaqoob, A. A. et al. A glimpse into the microbial fuel cells for wastewater treatment with energy generation. Desalin. Water Treat. 214, 379–389 (2021).
Luo, H.-P., Liu, G.-L., Zhang, R.-D. & Jin, S. Comparison of power generation in microbial fuel cells of two different structures. Huan jing ke xue Huanjing kexue 30, 621–624 (2009).
Shabani, M. et al. A critical review on recent proton exchange membranes applied in microbial fuel cells for renewable energy recovery. J. Clean. Prod. 264, 121446 (2020).
Roy, H. et al. Microbial fuel cell construction features and application for sustainable wastewater treatment. Membranes 13, 490 (2023).
Lin, C.-W., Chung, Y.-P., Liu, S.-H., Chen, W. T. & Zhu, T.-J. Optimizing the parameters of microbial fuel cells using response surface methodology to increase Cr (VI) removal efficiency and power production. Process Saf. Environ. Prot. 172, 369–378 (2023).
Wu, X. et al. Effect of acclimatization on hexavalent chromium reduction in a biocathode microbial fuel cell. Biores. Technol. 180, 185–191 (2015).
Li, F., Jin, C., Choi, C. & Lim, B. Simultaneous removal and/or recovery of Cr (VI) and Cr (III) using a double MFC technique. Environ. Eng. Manage. J. 18, (2019).
Wang, H., Song, X., Zhang, H., Tan, P. & Kong, F. Removal of hexavalent chromium in dual-chamber microbial fuel cells separated by different ion exchange membranes. J. Hazard. Mater. 384, 121459 (2020).
Yaqoob, A. A. et al. Utilizing biomass-based graphene oxide–polyaniline–ag electrodes in microbial fuel cells to boost energy generation and heavy metal removal. Polymers 14, 845 (2022).
Acknowledgements
We thank the Mattu University department of chemistry for conducting the experiments.
Author information
Authors and Affiliations
Contributions
G.S.W. conceived the research. D.A.Z., A.M.D., T.T.T., and G.S.W. conducted experiments. G.S.W., D.A.Z., N.M.D., B.A.H., A.M.D., C.T.H., E.T.G., T.A., A.H.F. and analyzed the results. G.S.W., D.A.Z., E.T.G., and N.M.D. prepared the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Weldegrum, G.S., Zemedagegnehu, D.A., Demeku, A.M. et al. Facial synthesis of reduced grapheneoxide/magnetite nanocomposite for energy generation and toxic metal bioremediation via microbial fuel cells. Sci Rep 15, 27879 (2025). https://doi.org/10.1038/s41598-025-08458-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-08458-5
Keywords
This article is cited by
-
Facile synthesis of Ag/PANI/rGO nanocomposite for electrochemical detection of toxic heavy metals in water
Discover Electrochemistry (2025)