Abstract
Extracting uranium from water is of great significance for environmental protection and nuclear energy development. Herein, the Amidoximation Polyethylenimine—Monomer (PEI-MO-AO) with a porous block was synthesized. The adsorbent was formed by crosslinking polyethyleneimine and the polymers of terephthalaldehyde and diaminomaleonitrile and the following amidoximation enhanced the adsorption performance. Accordingly, the porous structure endows the PEI-MO-AO a good hydrophilicity. The adsorption results exhibited that the maximum capacity reached 218 mg·g−1 at a pH of 6 and can adsorb uranium over a larger pH range. Besides, it maintained a distribution coefficient of 8760 mL·g−1 for uranium despite the high-intensity interference of other ions. After six consecutive adsorption cycles, the removal rate of uranium ions sustained at 81.6%, and the uranium adsorption capacity maintained a relatively elevated value of 189.31 mg·g−1. The PEI-MO-AO porous network polymeric material exhibited significant potential as an adsorbent, offering novel insights into the domain of uranium extraction and removal.
Similar content being viewed by others
Introduction
Rising industrial energy demand and heavy reliance on fossil fuel have exacerbated environmental degradation, accelerating the alternative energy needs1. Nuclear energy stands out as a pivotal solution to global energy challenges, owing to its clean, efficient operation and absence of greenhouse gas emissions2. However, the terrestrial uranium reserves are severely limited and inadequate to meet the growing demands. On the contrary, the ocean represents a vast reservoir of uranium, containing an estimated 4.5 billion tons3. Paradoxically, the nuclear industry operations generate substantial volumes of radioactive uranium-containing wastewater, which poses a severe environmental challenge. Uranium exhibits exceptional mobility compared to natural heavy metals and possesses an extremely long half-life, leading to persistent ecological contamination and associated hazards4. Consequently, the development of effective uranium extraction methodologies from solutions has become critical for both resource recovery and environmental protection5.
To address these challenges, various methods have been developed for uranium treatment, including membrane separation6, chemical precipitation7, ion exchange8, biological treatment9, and adsorption10. Among these uranium treatment techniques, the adsorption method stands out as the most promising approach due to its remarkable efficiency, cost-effectiveness, and eco-friendliness11. Currently, numerous adsorbent materials have been developed, primarily encompassing porous carbon-based materials12, metal-organic framework materials13, porous aromatic framework materials14, and composites15. Although significant progress has been made, numerous challenges persist, including the easy detachment of adsorbing groups, as well as the inherent limitations of the materials themselves, which result in a sharp decline in performance and poor recyclability after multiple uses. Therefore, further exploration and innovation are essential to overcome these shortcomings. Polyethylenimine (PEI) has garnered attention in multiple fields, including dye adsorption16, phosphate removal17, enrichment of rare earth elements18, and identification of aromatic derivatives, owing to its strong chelating properties, hydrophilicity, biocompatibility, and abundant amine groups on its macromolecular chain19. Notably, PEI-based composites have been widely reported for uranium extraction. For instance, Su et al. prepared a polyethyleneimine-modified lucerne adsorbent by grafting and used it for the adsorption of uranium in seawater20, while Ao et al. prepared a polyethyleneimine chitosan/α-MnO2 nanorod honeycomb composite material via post-grafting freezing, which has excellent elasticity and ultra-lightweight properties, and has an adsorption capacity of up to 301.9 mg·g−1 of uranium21. Similarly, Guo et al. prepared a polyethylenimine -functionalized graphene oxide/molybdenum disulfide composite aerogel with a uranium adsorption capacity of 184.53 mg·g−1 using grafting functionalization22. Despite these advancements, studies on synthesis strategies for PEI-based materials remain limited.
Functionalized polymer materials, distinguished by easy formation of three-dimensional network structure, hydrophilicity, water retention capabilities, and vast specific surface area, have emerged as prominent uranium adsorbents. However, not all functionalized modifications can enhance uranium adsorption capacity. Among functional groups, the amidoxime group is widely used in extracting uranium because of its good selectivity and high adsorption capacity23, which can significantly enhance uranium adsorption efficiency through augmenting its specific surface area when integrated into a porous matrix. Therefore, incorporating the amidoxime group into polyethyleneimine polymers can effectively enhance the uranium adsorption performance of adsorbent. This synergy enables uniform distribution of the amidoxime groups within a three-dimensional cross-linked hydrophilic network, facilitating the migration of UO22+ into the interior of the adsorbent and promoting synergistic interactions24. Accordingly, this synthesis strategy addresses challenges related to material shaping, cost, and recyclability, offering promising prospects. Capitalizing on the modifiable characteristics of polyethylenimine, the amidoxime group is introduced to enhance both the adsorption strength and selectivity.
Herein, a PEI-based bulk adsorbent, PEI-MO-AO was synthesized via using long-chain monomers to modify polyethyleneimine and form a network structure. The adsorbent was prepared through crosslinking the monomer (MO) with polyethyleneimine and curing with terephthalaldehyde, followed by reduction and amidoximation. Subsequently, the porous structure and chemical groups of PEI-MO-AO were characterized by scanning electron microscopy and Fourier transform infrared spectroscopy, respectively. Other properties such as adsorption conditions, kinetics, thermodynamics, and selectivity were analyzed through experiments with controlled variables. Under the optimal conditions, the PEI-MO-AO material underwent six adsorption-desorption experiments to evaluate its reproducibility. Finally, X-ray photoelectron spectroscopy (XPS) was utilized to analyze the PEI-MO-AO adsorbent, providing insights into its adsorption mechanism.
Experiment
Materials
Diaminomaleonitrile (DM, AR), p-Phthalaldehyde (PC, AR), Acetic anhydride (AA), and Cyanobenzaldehyde (CBA, AR) were purchased from Shanghai Haohong Scientific Co., Ltd. Polyethylenimine (PEI, AR), Dimethyl sulfoxide (DMSO, AR), Hydroxylamine hydrochloride (NH2OH·HCl, AR) and Tetrahydrofuran (THF, AR) were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. All reagents were used without further purification.
Preparation of monomer (MO)
The MO was synthesized via the condensation reaction and the steps are as follows. Firstly, 1.22 g of DM and 1.34 g of PC were added into a 50 mL three-necked flask, followed by the addition of 20 mL of DMSO and a magnetic stirrer. Then, 1.0 mL of AA was added as the catalyst after the solution had became homogeneous. After 24 h of reaction with a temperature of 50℃, the product was directed into a container with distilled water, resulting in a flocculent substance. The product MO was obtained by filtration and washing.
Preparation of polyethylenimine-monomer (PEI-MO)
The PEI-MO was prepared as follows. Firstly, 0.5 g PEI was dissolved in 10 mL DMSO and formed a homogeneous solution. Then, MO was added and the reaction continued for 12 h. Next, 0.3 g PA was added and the mixture began to solidify. The solidified product was frozen for 24 h to maintain its shape. Afterwards, it was transferred to distilled water. This process was repeated five times over two days. Finally, the product was freeze dried to obtain PEI-MO.
Preparation of amidoximation polyethylenimine-monomer (PEI-MO-AO)
PEI-MO was dissolved to the sodium cyanoborohydride solution (0.5 wt%) to synthesize PEI-MO-R. Subsequently, PEI-MO-R (1 g), sodium hydroxide (0.75 g), methanol (10 mL), and hydroxylamine hydrochloride (1.25 g) were added to 20 mL distilled water for reaction. After the amidoximation process, the product was washed and dried to obtain the final product PEI-MO-AO. In order to obtain the optimal ratio, the products containing different monomers were named PEI-MO-AO-1 to PEI-MO-AO-6, as shown in Table S1. The preparation of PEI-MO-AO is shown in Fig. 1.
Calculation methods
The adsorption capacity (qe) and removal rate (R) were measured by the fallowing formulas25:
where the \(\:{c}_{0}\) and \(\:{c}_{e}\) mean the original and equilibrium uranium (VI) concentrations (mg/L) respectively, \(\:m\) is the weight of the adsorbent (mg) and V is the volume of uranium solution (mL).
After adsorption, the adsorption capacity of adsorbent per unit mass for different initial concentrations of uranium at three different temperatures was calculated. Langmuir (3) and Freundlich (4) equations were used to fit the adsorption data.
The linear fitting expression of Langmuir model is as follows:
The linear fitting expression of Freundlich model is as follows:
where: Ce: Uranium concentration at adsorption equilibrium, mg g−1; Qe: Uranium adsorption capacity at adsorption equilibrium, mg g−1; Qm: Saturated adsorption capacity, mg g−1; KL: Equilibrium constant related to bond strength, L mg−1; KF: approximate index of adsorption capacity; 1/n: function of adsorption strength during adsorption.
Through the study of adsorption thermodynamics, we can understand the degree and power of uranium adsorption by adsorbent, and further judge the heat exchange behavior of adsorption process. The thermodynamic parameter values can be calculated from the Van’t Hoff isothermal equation and the Gibbs free energy definition. The specific equation is as follows:
where Kd: thermodynamic equilibrium constant, mL g−1; C0: initial Uranium concentration, mg g−1;Ce: Uranium concentration at adsorption equilibrium, mg g−1; ΔS0: change in standard entropy, J mol−1 K−1; R: gas constant, J mol−1 K−1; ΔH0: standard enthalpy change, kJ mol−1; T: the absolute temperature of the solution, K; ΔG0: change in standard Gibbs free energy, kJ mol−1.
In order to further study the adsorption behavior of uranium ion, quasi-first-order (8) and quasi-second-order (9) adsorption kinetic models were used to fit the adsorption kinetic data.
The linear fitting expression of the quasi-first-order adsorption kinetics model is as follows:
The linear fitting expression of the quasi-second-order adsorption kinetics model is as follows:
where Qe: Uranium adsorption capacity at adsorption equilibrium, mg g−1; Qt: Uranium adsorption capacity adsorbed over a period of time, mg g−1; k1: equilibrium rate constant of quasi-first-order kinetics, min−1; k2: equilibrium rate constant of quasi-second-order kinetics, g mg−1 min−1; t: contact time, min.
The partition coefficient (Kd, mL g−1), selectivity coefficient (SU/M) of adsorbent for different ions per unit mass were calculated, so as to explore the adsorption and selection ability of adsorbent for uranium ions with the same concentration of co-existing ions.
The expression of the distribution coefficient Kd is:
The expression of the selectivity coefficient SU/M is:
where C0: initial solution uranium concentration, mg L−1; Ce: concentration of uranium in solution at adsorption equilibrium, mg L−1; V: volume of uranium solution, mL; m: Mass of adsorbent, g; Kd(U): partition coefficient of uranium ion, mL g−1; Kd(M): partition coefficient of coexisting ions, mL g−1.
The Eluent Efficiency was calculated to explore the reusability of the adsorbent.
where Cd is the concentration of uranium ion in desorption solution after desorption (mg L−1).
Characterization method
The uranium concentration was measured by Inductively Coupled Plasma Mass Spectrometer (ICP-MS, Agilent 7800). The microstructures of the materials were investigated by a scanning electron microscopy (SEM, S-4800). PerkinElmer Fourier Transform Infrared Spectroscopy (FT-IR) was applied to analyze the chemical bonding and structure of the materials. Chemical elemental composition was represented by an X-ray photo-electron spectroscopy (XPS, Thermo escalab 250Xi). Thermal properties are investigated by using thermogravimetric analysis on a thermobalance (TG, Q50 TA instrument). The water contact Angle is measured by Video optical contact Angle measuring instrument (OCA 100).
Results and discussion
Fabrication and characterizationof PEI-MO-AO
The preparation process of adsorbent is as follows. First, MO was synthesized through a condensation reaction. Then, p-Phthalaldehyde was solidified and the pores were connected by reduction. Subsequently, amidoximation was conducted. Through its characterization and performance testing, it can be well applied to the actual adsorption work. To confirm the successful synthesis of materials, FT-IR characterization was conducted on the entire process of PEI-MO-AO preparation. Figure S1 confirms the conversion of DM and PC to MO. After the condensation reaction, the C≡N characteristic peak of DM and the C=O peak of PC are observed at 2243 cm−1 and 1696 cm−1 in the MO spectrum26respectively. Compared to the DM spectrum, the cyano peak in MO exhibits a significant red shift, which is attributed to the conjugation effect produced by the introduction of the benzene ring and the carbon-nitrogen double bond, indicating the successful synthesis of MO. FT-IR spectra of the PEI-MO-AO preparation process is shown in Fig. 2a. The PEI-MO prepared by crosslinking has obvious characteristic peaks of PA, MO and PEI, including the cyano peak at 2201 cm−1, the C=N peak at 1631 cm−1, and the hydroxyl characteristic region in the range of 3000 cm−1 to 3750 cm−127. After the amidoxime reaction, the cyano peaks of PEI-MO-AO spectrum completely disappeared and the absorption peaks of C=N, C–N and N–O bonds appear at 1631 cm−1, 1360 cm−1 and 965 cm−128, respectively, which preliminarily indicate that the synthesis of PEI-MO-AO is successful29. In addition, XPS spectra can also demonstrate the successful synthesis of PEI-MO-AO. The full-scan XPS spectra of PEI-MO-AO are shown in Fig. S3a, where the peaks at 536.2 and 398.1 eV correspond to the O 1s and N 1s energy levels. The high-resolution XPS spectrum of O 1s (Fig. S3b) can be split into three peaks of 530.93, 531.45 and 532.59 eV, which belong to O–H, C=O and N–O. Meanwhile, Fig. S3c shows the N 1s high-resolution XPS spectrum of PEI-MO-AO, the four characteristic peaks at 398.75, 399.30, 399.85, and 401.20 eV, belong to –NH/NH2, NH-C = N-OH, C–N, –NH2+, respectively. The peak of hydroxyl oxygen in the amidoxime group observed at 531.34 eV and the peak of C=N observed at 399.30 eV reveals critical evidence of surface elemental composition and chemical bonding changes to confirm polymer synthesis.
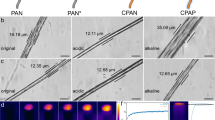
Scanning electron microscopy (SEM) was used to characterize the microstructure of PEI-MO-AO. As shown in Fig. 2b, the digital photograph of the adsorbent is shown in the upper left corner, showing a relatively deep earthy yellow color. This is mainly due to the formation of C=N bonds and the π–π conjugation with the benzene ring after amidoximation of the monomer, which absorb a large amount of purple light and appear as a complementary yellow color. The pore structure of PEI-MO-AO is magnified 230 times in Fig. 2b, it can be obviously observed that a relatively uniform mesh structure with pore sizes on the order of 10 micrometers could be clearly seen, which is due to the braiding of large molecular chains. Besides, to get the crystalline structure information of the polymer PEI-MO-AO, an X-ray diffraction analysis was performed. As shown in Fig. 2c, it can be seen that the polymer does not appear obvious crystal type diffraction peak, showing a continuous wide peak at around 22°, indicating that the polymer do not form a crystal structure.
Notably, the hydrophilic properties of adsorbents significantly influence the adsorption efficiency and the water contact angle tests were carried out to analyze the hydrophilicity of the adsorbent. Figure 2d shows the change in contact angle over time and the contact angles of PEI-MO-AO with all proportions changed from 180° to 0° within 400 ms. Notably, as the proportion of the MO increases, the slope of the fitted curves changes faster and the time taken was shorter, indicating that MO has a positive impact on the hydrophilicity of the material and promote the contact between the solution and the material30. This can be attributed to the large number of amidoxime groups on the MO, which increase the affinity for water and facilitate the adsorption rate of the material. The above results indicate that the material has strong hydrophilicity, which is beneficial for the adsorption process. The stability of adsorbents in the environment is a necessary condition for their full utilization. PEI-MO-AO is treated by various reagents such as THF, DMF, DMSO, HCl (0.1 M) and NaOH (0.1 M) solution for 24 h. As shown in Fig. 2e, there are almost no change in the peak patterns, and the material is able to maintain its chemical properties well even in organic solvents, which implies that they have high chemical stability31. Moreover, thermogravimetric analysis was also used to evaluate the thermal stability of PEI-MO-AO. As shown in the Fig. 2f, the properties of the samples do not change significantly, which implies that they have high chemical stability. From Fig. S2, it can be found that there is an obvious tendency of weight loss at 80 °C and 300 °C and the weight decreases sharply at 300 °C, stabilizing at 500 °C, which can be attributed to the loss of water and the decomposition of the polymer network framework, respectively32. Based on the TGA and DTG analysis, it can be concluded that the polymers exhibit good thermal stability, indicating their suitability for use in harsher environments. Additionally, the pore structure of the material was also analyzed. As shown in Fig. S4, the median pore diameter is 1996.42 nm, indicating a broader distribution in terms of volume. And the material has a high porosity of 88.058%, which further illustrates the porous structure of this material. This pore structure is beneficial for the adsorption process, as it provides a large surface area and channels for the adsorbate to diffuse into the material, thereby enhancing the overall adsorption efficiency.
Characterization of uranium adsorption properties
In order to optimize the use of raw materials and adsorption effect, batch adsorption experiments were conducted to determine the best proportion of PAO and HA in the adsorbent. As shown in Fig. 3a, with the increase of MO, the uranium adsorption capacity of PEI-MO-AO slowly increases. However, when the MO: PA ratio reaches 3:3, the adsorption capacity hardly changes much or even slightly decreases with the ratio of MO increasing. This trend is due to that the addition of MO leads to the increase of structural density, resulting in altered pore structure and reduced specific surface area. In summary, PEI-MO-AO-4 is selected as the best ratio of the subsequent experiments.
Based on the above analysis, the influence of solid-to-liquid ratio was systematically investigated. According to Fig. 3b, PEI-MO-AO demonstrates a continuous enhancement in uranium removal efficiency with the increase of solid-to-liquid ratio. When the ratio reaches 0.4 g L−1, the removal rate stabilizes at 91% with diminished growth momentum. Notably, despite the persistent improvement in removal efficiency, the adsorption capacity exhibits a progressive decline. Through comparative analysis of the removal rate enhancement and adsorption capacity reduction, 0.4 g L−1 is identified as the optimal ratio. Below this threshold, increasing the ratio substantially improves adsorption performance, while exceeding this value yielded only marginal benefits. So the optimized ratio of 0.4 g L−1 is subsequently employed for further investigations.
The pH of the environment has an important influence on the existing forms of uranium (Fig. 3d), which influences the adsorption effect of uranium33.When the pH is lower than 5, UO22+ is the primary form of uranium. Between pH 5 and 6, some UO22+ converts to (UO2)3(OH)5+. When the pH exceeds 6, UO22+ tends to combine with CO32+. According to Fig. 3c, when the pH is less than 6.0, the adsorption capacity of PEI-MO-AO increases sharply (from 29.77 to 218.81 mg g−1); When the pH is higher than 6.0, the adsorption capacity decreases significantly (from 218.81 to 78.68 mg g−1). The change of adsorption capacity is ascribed to the existing forms of U(VI). In acid environments the adsorption sites are positively charged, which leads to electrostatic repulsion between the adsorption sites and the uranium, hindering the adsorption process34. On the other hand, when the pH exceeds 6, the main existing forms of uranium ions become negatively charged, and the transformation generates electrostatic repulsion with negative sites in the adsorbent, leading to a diminished adsorption effect35. The uranium adsorption capacity reached its peak at pH 6.0, and it shows a relatively high adsorption capacity at pH 8.0 (close to the pH of actual seawater), indicating that this functional adsorbent PEI-MO-AO can be used to extract uranium from acidic wastewater containing uranium ions. For better experimental performance, subsequent experiments were conducted at the optimal pH of 6.
The adsorption rate significantly impacts the adsorption capacity of adsorbents. Thus, the effect of adsorption time on the PEI-MO-AO adsorbent in uranium solutions with different concentrations (50, 75 and 100 ppm) was investigated. As shown in Fig. 4a, as the initial solution concentration increases, the adsorption capacity also increases, and the curves tend to stabilize after 400 min, reaching the maximum adsorption value. Figure 4b,c and Table S2 show that the R2 values for the pseudo-second-order adsorption curve fits (R2 = 0.9924, 0.9916, 0.9934) under three different concentrations are all higher than those for the first-order kinetic curves (R2 = 0.8931, 0.9296, 0.9534), indicating that the adsorption of PEI-MO-AO is more consistent with the second-order kinetic model36,37. This proves that the adsorption process is mainly controlled by chemical rates. In addition, Morris–Weber model was used to analyze the adsorption data. Figure S5 shows that the adsorption process is mainly divided into two stages, including external diffusion and osmotic equilibrium stages. As shown in Table S3, the value of K1 is greater than K2 and R12 is closer to 1, indicating good adaptability. However, the R22 is low and the intercept is large, which means this stage might be a composite stage of multiple diffusion or equilibria. In summary, the adsorption process of PEI-MO-AO is chemical adsorption, in which the main rate-control step is the diffusion of uranium ions into the interior38.
Moreover, to evaluate the adsorption capacity of PEI-MO-AO and explore its adsorption process, the adsorption capacity was tested at different concentrations and temperatures to analyze the adsorption isotherm. As shown in Fig. 4d, as the temperature increases, the adsorption capacity also increases, indicating that the process is endothermic. To conduct a deep analysis of the adsorption process and understand its mechanism, the temperature curves were fitted using Langmuir, Freundlich, Dubinin-Radushkevich, and Temkin models, and the calculated data are shown in Figs. 4e,f, S6, S7 and Table S4. According to Table S4, the R2 value of the Langmuir model is significantly higher than that of the other fitting curves, indicating that the adsorption of PEI-MO-AO is closer to uniform monolayer adsorption39. The maximum adsorption capacities calculated based on the Langmuir model are 328.95 mg·g−1, 344.83 mg·g−1, and 361.01 mg·g−1 respectively. The thermodynamic analysis of PEI-MO-AO adsorption behavior was conducted through evaluations of the temperature-dependent equilibrium constant (Fig. S8, Table S5). The negative ΔG values, ranging from − 25.822 to − 44.113 kJ·mol−1, confirm the spontaneous nature of uranium adsorption, with spontaneity increasing at elevated temperatures. The calculated ΔH value of 519.25 kJ mol−1 significantly exceeds the 20.9 kJ·mol−1 threshold for physical adsorption, indicating the predominance of chemical adsorption through coordination bond formation. Additionally, the positive ΔS value of 1.83 kJ·mol−1·K−1 suggests an increase in system disorder during adsorption, which synergistically drives the spontaneous process40. These thermodynamic parameters verify that uranium capture by PEI-MO-AO primarily occurs through chemically controlled mechanisms.
(a) Adsorption kinetics curve (pH = 6, C0 = 100 ppm, V = 500 mL, m = 200 mg); (b) Pseudo-first-order dynamics model fitting curve; (c) Pseudo-second-order dynamics model fitting curve; (d) Adsorption thermodynamic curve (pH = 6, V = 500 mL, m = 200 mg); (e) Langmuir model fitting curve; (f) Freundlich model fitting curve.
The uranium selectivity of the adsorbent is crucial to ensure its application in real environments, which is explored through ionic competition experiments. To analyze the effect of ionic competition, common cation (K+, Ca2+, Na+, Mg2+, Ba2+, Sr2+, Co3+, Bi2+) competition experiments were carried out and the results are shown in Fig. 5a. PEI-MO-AO demonstrates 79.3% U(VI) adsorption capacity in competitive systems, marginally lower than the 90% achieved in single-ion solutions, confirming interference from coexisting cations while the competing ions exhibit weak adsorption capacities. Distribution coefficients (Fig. 5b) further validated this selectivity, with the Kd value (8760 mL·g−1) being ten-fold higher than those of competing ions. Table S6 quantitatively confirms the superior U(VI) selectivity of PEI-MO-AO in mixed-ion environments through calculated separation factors. The high selectivity of uranium can be attributed to the following points. First, the surface-grafted amidoxime groups exhibit specific coordination toward U(VI), forming stronger chelation with UO22+ compared to high-valent ions (e.g., Al3+, Th4+). Secondly, the high charge density and linear geometry of UO22+ enhance its compatibility with the electrostatic environment and steric confinement within the material’s pores, whereas ions like Fe2+ face adsorption barriers due to their larger hydrated radii or mismatched coordination geometries. Additionally, low-charge cations (e.g., K+, Ca2+) show negligible competition due to weak electrostatic interactions. The salt resistance of PEI-MO-AO was evaluated through uranium removal tests in NaCl and NaNO3 solutions (Fig. 5c and Fig. S9). Both electrolytes caused gradual efficiency reductions across 0–2 M concentrations, with NaNO3 showing marginally greater impact due to enhanced interference of nitrate. Remarkably, 85% removal efficiency persisted at seawater-level salinity (0.5–0.7 mol·L−1), confirming its potential application value. Despite the resistance of ions, the reusability of the adsorbent is crucial for estimating its practicality and cost. Firstly, as shown in Fig. S10, the uranium elution effects of different kinds of solutions on PEI-MO-AO were investigated41. After desorption for 12 h, both HCl and HNO3 show high elution rates, and HNO3 was selected for subsequent cycling experiments. Figure 5d shows the uranium removal rate and elution rate of PEI-MO-AO in the process of six adsorption-elution cycles. Although both the removal rate and elution rate gradually decrease with the cycle progress, the adsorbent still maintains good adsorption effect after six cycle tests, which indicates that the PEI-MO-AO adsorbent has good recyclability. As shown in Table 1, PEI-MO-AO exhibits a maximum adsorption capacity of 218 mg·g−1, offering some advantages over comparable materials.
Adsorption mechanism
To elucidate the uranium adsorption mechanism, XPS analysis was conducted on PEI-MO-AO before and after uranium adsorption. As shown in Fig. 6a, the full-scan spectra revealed characteristic peaks for C 1s (285.4 eV), N 1s (398.1 eV), and O 1s (536.2 eV). Compared to the spectra before adsorption, the specific double peaks (381.5, 392.0 eV) of U4f5/2 and U4f7/2 are observed, which proves the uranium coordination. The further peak fitting of U4f showed peak at 381.55 eV, 381.45 eV, 391.90 eV, and 392.45 eV (Fig. 6b), suggesting that the adsorption process involves a transformation from U (VI) to U (IV)41,49. Furthermore, Fig. 6c,d show that the O 1s spectrum is divided into two peaks at 531.34 eV and 530.93 eV, which correspond to the hydroxyl oxygen in the amidoxime group and the oxygen in water molecules, respectively. However, the peak of hydroxyl oxygen shifted to 531.78 eV, suggesting its involvement in uranium adsorption50. After uranium adsorption, the high-resolution spectra of N1s (Fig. 6e,f) shows similar shifts and the shifts are observed in C–N from 399.85 eV to 400 eV and in C=N from 399.30 eV to 399.46 eV51,52. Similarly, the –NH2+ group in the amidoxime and polyethylenimine shifts from 401.20 eV to 401.45 eV53. Additionally, the -NH/NH2 peak in the polyethylenimine chain increases from 398.75 eV to 398.85 eV. In conclusion, the XPS spectral results show that the complexation of PEI-MO-AO to uranium is mainly based on the N and O atoms of the amidoxime group and polyethylenimine.
Conclusion
This study developed a novel bulk adsorbent PEI-MO-AO with a porous structure by using a reducing agent as a pore-forming connecting agent, which solves the structural problem of adsorbents that are difficult to recover. Besides, the porous structure solved the hydrophilic problem of the adsorbent. Adsorption experiments show that the adsorbent demonstrates good uranium adsorption capacity of 218 mg·g−1 (pH = 6, C0 = 100 ppm). Furthermore, the kinetic and thermodynamic analysis shows high conformity of Langmuir monolayer adsorption behavior and pseudo-second-order kinetics, confirming that the adsorption process is chemically dominant monolayer adsorption. PEI-MO-AO exhibits a good removal rate for uranium ions in the ion competition experiments, and the distribution coefficient of U(VI) ions reach a higher value of 8760 mL·g−1 compared to other ions, indicating its excellent selectivity. After six cycles, it retained 81.6% uranium removal with 189.31 mg·g−1 capacity. Mechanistic analysis via XPS identified that uranium is mainly adsorbed based on the N and O atoms in the adsorbent. Accordingly, through simple weaving and crosslinking, PEI-MO-AO can be used as a potential adsorbent for uranium extraction and provides a new idea for the field of uranium extraction.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Clarkson, T. C. et al. Environmental controls on very high δ238U values in reducing sediments: implications for neoproterozoic seawater records. Earth Sci. Rev. 237, 104306 (2023).
Ma, J. et al. Sunlight polymerization of poly(amidoxime) hydrogel membrane for enhanced uranium extraction from seawater. Adv. Sci. 6 (13), 1900085 (2019).
Feng, S. et al. Highly efficient extraction of uranium from seawater by natural marine crab carapace. Chem. Eng. J. 430, 133038 (2022).
Zhong, X. et al. Highly efficient enrichment mechanism of U(VI) and Eu(III) by covalent organic frameworks with intramolecular hydrogen-bonding from solutions. Surf. Sci. 504, 144403 (2020).
Sholl, D. S. et al. Seven chemical separations to change the world. Nature. 532, 435–437 (2016).
Liu, T. et al. Metal–organic framework-intercalated graphene oxide membranes for selective separation of uranium. Anal. Chem. 93, 16175–16183 (2021).
Singh, D. K. et al. Development of a phosphate precipitation method for the recovery of uranium from lean tenor alkaline leach liquor. Hydrometallurgy. 171, 228–235 (2017).
Cheng, Y. et al. Polyamine and amidoxime groups modified bifunctional polyacrylonitrile-based ion exchange fibers for highly efficient extraction of U(VI) from real uranium mine water. Chem. Eng. J. 367, 198–207 (2019).
Banala, U. K. et al. Microbial interactions with uranium: towards an effective bioremediation approach. Environ. Technol. Innov. 21, 101254 (2021).
Liu, T. et al. Ligand-assistant iced photocatalytic reduction to synthesize atomically dispersed Cu implanted metal-organic frameworks for photo-enhanced uranium extraction from seawater. Small. 19, 2208002 (2023).
Qasem, N. A. A. et al. Removal of heavy metal ions from wastewater: a comprehensive and critical review. NPJ Clean. Water. 4, 1–15 (2021).
Liu, R. et al. Polyacrylate/phytic acid hydrogel derived phosphate-rich macroporous carbon foam for high-efficiency uranium adsorption. J. Water Process. Eng. 53, 103659 (2023).
Zhao, Z. et al. Defect controlled MOF-808 for seawater uranium capture with high capacity and selectivity. J. Mol. Liq. 367, 120514 (2022).
Xu, Z. et al. Synchronous construction of high sulfonic acid grafting degree and large surface area in conjugated microporous polymer adsorbents for efficient removal of uranium (VI). Sep. Purif. Technol. 309, 122953 (2023).
Sun, Z. et al. One-pot hydrolysis/amidoximation and self-assembly to polyamidoxime-based composite hydrogels for high-efficiency uranium capture. Colloids Surf. A. 655, 130323 (2022).
Wong, S. et al. Adsorption of anionic dyes on spent tea leaves modified with polyethyleneimine (PEI-STL). J. Clean. Prod. 206, 394–406 (2019).
Suzaimi, N. D. et al. Enhancing the performance of porous rice husk silica through branched polyethyleneimine grafting for phosphate adsorption. Arab. J. Chem. 13, 6682–6695 (2020).
Nie, D. et al. Composites of multiwalled carbon nanotubes/polyethyleneimine (MWCNTs/PEI) and molecularly imprinted polymers for dinitrotoluene recognition. Sens. Actuators B. 224, 584–591 (2016).
Ayalew, Z. Synthesis and application of polyethyleneimine (PEI)-based composite/nanocomposite material for heavy metals removal from wastewater: A critical review. J. Hazard. Mater. Adv. 8, 100158 (2022).
Su, Q. et al. Polyethyleneimine-functionalized luffa cylindrica for efficient uranium extraction. J. Colloid Interface Sci. 530, 538–546 (2018).
Zhou, L. et al. Polyethyleneimine incorporated chitosan/α-MnO2 Nanorod honeycomb-like composite foams with remarkable elasticity and ultralight property for the effective removal of U(VI) from aqueous solution international. J. Biol. Macromol. 218, 190–201 (2022).
Guo, D. et al. Recovery of uranium (VI) from aqueous solutions by the polyethyleneimine-functionalized reduced graphene oxide/molybdenum disulfide composition aerogels. J. Taiwan Inst. Chem. Eng. 106, 198–205 (2020).
Liu, X. et al. Temperature-sensitive amidoxime-based hydrogels for fast and efficient adsorption of uranium ions. Sep. Purif. Technol. 340, 126630 (2024).
Gao, F. et al. Artemisia gum reinforced amidoxime gel membrane promotes rapid extraction of uranium from seawater. Sep. Purif. Technol. 330, 125548 (2024).
Lin, K. et al. Kelp inspired bio-hydrogel with high antibiofouling activity and super-toughness for ultrafast uranium extraction from seawater. Chem. Eng. J. 430, 133121 (2022).
Yang, X. et al. Simultaneous alkaline hydrolysis and non-solvent induced phase separation method for polyacrylonitrile (PAN) membrane with highly hydrophilic and enhanced anti-fouling performance. J. Membr. Sci. 635, 119499 (2021).
Tan, H. et al. Bioinspired surface engineering of amidoxime functionalized polyvinylidene fluoride membrane for efficient uranium extraction from seawater and wastewater. Sep. Purif. Technol. 360, 131194 (2025).
Bai, J. et al. Processable Amidoxime functionalized porous hyper-crosslinked polymer with highly efficient regeneration for uranium extraction. J. Mol. Liq. 362, 119745 (2022).
Li, R. et al. Hierarchically structured layered-double-hydroxides derived by ZIF-67 for uranium recovery from simulated seawater. J. Hazard. Mater. 338, 167–176 (2017).
Bi, C. et al. Efficient uranium adsorbent prepared by grafting Amidoxime groups on dopamine modified graphene oxide. Prog. Nucl. Energy. 155, 104515 (2023).
Chaithanya, M. S. et al. Distribution, chemical speciation and human health risk assessment of metals in soil particle size fractions from an industrial area. J. Hazard. Mater. Adv. 9, 100237 (2023).
Catherine, H. N. et al. Surface interaction of tetrabromobisphenol a, bisphenol a and phenol with graphene-based materials in water: adsorption mechanism and thermodynamic effects. J. Hazard. Mater. Adv. 9, 100227 (2023).
Jiao, G. J. et al. Efficient extraction of uranium from seawater by reticular polyamidoxime-functionalized oriented holocellulose bundles. Carbohydr. Polym. 300, 120244 (2023).
Godiya, C. B. et al. Amine functionalized sodium alginate hydrogel for efficient and rapid removal of Methyl blue in water. Int. J. Biol. Macromol. 144, 671–681 (2020).
He, Y. et al. Amidoxime functionality of carbon black by plasma technology for efficient uranium extraction from aqueous solution and simulated seawater. Sep. Purif. Technol. 356, 129931 (2025).
Li, J. et al. Symbiotic aerogel fibers made via in-situ gelation of aramid nanofibers with polyamidoxime for uranium extraction. Molecules. 24, 1821 (2019).
Huang, Z. et al. Bifunctional phosphorylcholine-modified adsorbent with enhanced selectivity and antibacterial property for recovering uranium from seawater. ACS Appl. Mater. Interfaces. 12, 16959–16968 (2020).
Huang, G. et al. Efficient removal of uranium (VI) from simulated seawater with hyperbranched polyethylenimine (HPEI)-functionalized polyacrylonitrile fibers. New. J. Chem. 42, 168–176 (2018).
Ahmad, M. et al. Preparation of novel bifunctional magnetic tubular nanofibers and their application in efficient and irreversible uranium trap from aqueous solution. ACS Sustain. Chem. Eng. 8, 7825–7838 (2020).
Estes, S. L. et al. Enthalpy of uranium adsorption onto hematite. Environ. Sci. Technol. 54, 15004–15012 (2020).
Pan, H. B. et al. Carbonate-H2O2 leaching for sequestering uranium from seawater. Dalton Trans. 43, 10713–10718 (2014).
Younes, A. A. et al. Uranium sorption from aqueous solutions using polyacrylamide-based chelating sorbents. Sep. Sci. Technol. 53, 2573–2586 (2018).
Massoud, A. et al. Sorption characteristics of uranium from sulfate leach liquor by commercial strong base anion exchange resins. J. Radioanal. Nucl. Chem. 322, 1065–1077 (2019).
Dacrory, S. et al. Innovative synthesis of modified cellulose derivative as a uranium adsorbent from carbonate solutions of radioactive deposits. Cellulose. 27, 7093–7108 (2020).
Hassanein, T. F. et al. Synthesis of polyamide 6/nano-hydroxyapatite hybrid (PA6/n-HAp) for the sorption of rare Earth elements and uranium. J. Environ. Chem. Eng. 9, 104731 (2021).
Youssef et al. Uranium (VI) sorption from aqueous solution using commercial anion exchange resins; kinetics, isotherm, and thermodynamic investigations. J. Radioanal. Nucl. Chem. 333, 1975–1989 (2024).
Youssef, W. M. et al. Uranium capture from aqueous solution using palm-waste based activated carbon: sorption kinetics and equilibrium. Environ. Monit. Assess. 196, 428 (2024).
Masoud, A. M. et al. Dithiocarbamate functionalised crosslinked polyacrylamide for separation and pre-concentration of uranium (VI) ions from sulphate medium. Int. J. Environ. Anal. Chem. 1–23 (2024).
Li, H. et al. Zwitterion functionalized graphene oxide/polyacrylamide /polyacrylic acid hydrogels with photothermal conversion and antibacterial properties for highly efficient uranium extraction from seawater. Adv. Funct. Mater. 33, 2301773 (2023).
Su, M. et al. Graphene oxide functionalized with nano hydroxyapatite for the efficient removal of U(VI) from aqueous solution. Environ. Pollut. 268, 115786 (2021).
Li, L. et al. Effects of chain conformation on uranium adsorption performance of amidoxime adsorbents. Sep. Purif. Technol. 307, 122777 (2023).
Ahmad, Z. et al. A membrane-supported bifunctional poly(amidoxime‐ ethyleneimine) network for enhanced uranium extraction from seawater and wastewater. J. Hazard. Mater. 425, 127995 (2022).
Li, Y. et al. Synergistic strategy design of (malonamide-amidoxime) bifunctional branching network crosslinked membrane and application in uranium (VI) resource recovery. Chem. Eng. J. 461, 142013 (2023).
Acknowledgements
We acknowledge the financial support from Key Research and Development Plan Guided Project of Heilongjiang Province (GZ20230004), Harbin Science and Technology Plan Self-funded Project (2022ZCZJCG031), Key Project of Natural Science Foundation of Heilongjiang Province (ZD2021E005).
Author information
Authors and Affiliations
Contributions
H.Y.: Conceptualization, writing-original draft. L.H.: Formal analysis, investigation. Z.Z.: Formal analysis. H.W.: Investigation. S.F.: Methodology, investigation, writing-original draft. X.L.: Investigation, writing and editing. J.B.: Writing-review and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yan, H., Huo, L., Zhang, Z. et al. Preparation of functionalized polyethyleneimine materials and studies on its uranium adsorption properties. Sci Rep 15, 22354 (2025). https://doi.org/10.1038/s41598-025-08545-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-08545-7
Keywords
This article is cited by
-
Balsa wood/ZIF-8 composite material for efficient uranium adsorption from aqueous solutions
Journal of Radioanalytical and Nuclear Chemistry (2026)